Abstract
The current rise in the interest in physical phenomena at nano spatial scale is described hereby as a natural consequence of the scientific progress in manipulation with matter with an ever higher sensitivity. The reason behind arising of the entirely new field of nanoscience is that the properties of nanostructured materials may significantly differ from their bulk counterparts and cannot be predicted by extrapolations of the size-dependent properties displayed by materials composed of microsized particles. It is also argued that although a material can comprise critical boundaries at the nano scale, this does not mean that it will inevitably exhibit properties that endow a nanomaterial. This implies that the attribute of “nanomaterial” can be used only in relation with a given property of interest. The major challenges faced with the expansion of resolution of the materials design, in terms of hardly reproducible experiments, are further discussed. It is claimed that owing to an unavoidable interference between the experimental system and its environment to which the controlling system belongs, an increased fineness of the experimental settings will lead to ever more difficulties in rendering them reproducible and controllable. Self-assembly methods in which a part of the preprogrammed scientific design is substituted with letting physical systems spontaneously evolve into attractive and functional structures is mentioned as one of the ways to overcome the problems inherent in synthetic approaches at the ultrafine scale. The fact that physical systems partly owe their properties to the interaction with their environment implies that each self-assembly process can be considered a co-assembly event.
NANOSCIENCE AND NANOMATERIALS: HOW IT ALL BEGAN
Nano is a word whose echo seems to reverberate everywhere these days, from the popular media to scientific journals and magazines [1,2]. The number of scientific articles on nanoscale phenomena has been growing at an exponential rate throughout the last 30 years, and currently more than 60 countries support national projects or programs aiming at the development of nanotechnologies [3]. In view of the huge promises nanotechnologies offer in terms of becoming a main drive for the future high-tech business and economic growth, the race for dominance in control of material structure, properties and behavior at the ultrafine scale, involving developed and third-world countries alike [4], has become one of the most exciting ones in the history of science and humanity.
Although a surge in the interest in material nanostructures is a relatively recent phenomenon, lasting effectively a few decades only, nanomaterials have been present in the atmosphere and in the ground as dust particles and nanominerals during the entire evolution of life [5]. In fact, the majority of mineral particles suspended in the atmosphere are smaller than 100 nm, which is the upper size limit for the grain of a nanomaterial. Slow processes such as natural precipitation, weathering effects and biological debris formation, and abrupt events such as volcanic eruptions and mechanical grinding associated with earthquake-generating faults have all been discerned as origins of atmospheric nanoparticles. Nanosized particles are, however, not limited to our planet solely. Diamond nanoparticles were found in the airborne dust of Mars, and are thought to have formed in presolar supernovae [6].
Hence, if nanoparticles have been with us since the dawn of the human race, how come that the entire rise in the interest in physical effects at nano scale presents only a recent phenomenon?
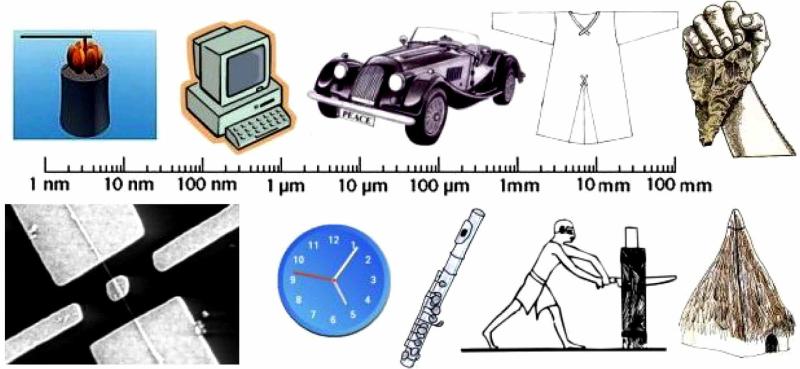
The reason is the following. Namely, the progress of humanity is underlain by a continual advance in the sensitivity of human interactions with their physical surrounding (Fig. 1). What we name today as progress in the amount of information can be regarded as a progress in the human sensitivity to “read” and produce critical boundaries at ever smaller scales. Gregory Bateson defined information as a difference that makes a difference [7], and it is a standard view of systems theory to observe boundaries and differences as the most fundamental units of human cognition and the material order [8]. Millennia ago, the most sophisticated creativity in “materials science” could have been the production of ceramic bricks and tiles with the critical boundaries on the order of a few centimeters, but with the art of producing clock mechanisms and modern machines, the critical boundaries moved down to millimeter and micrometer scales. Thus, as humanity evolves, the critical boundaries for human tasks shift to smaller scales. Nowadays, science is slowly approaching the limits of Moore’s law in miniaturizing semiconductors and electronic circuits, whereby many other devices require a functional shift from microscale to nanoscale boundaries in order to gain superiority on the market.
Fig. (1).
Critical length scales for some of the key inventions from the history of humanity. As one shifts from prehistoric huts, cutting tools and garments to modern musical instruments, automobiles and mechanical clocks to computers, nanocopters and nanomotors, the critical lengths shift from millimeter to mi-crometer to nanometer scale, respectively.
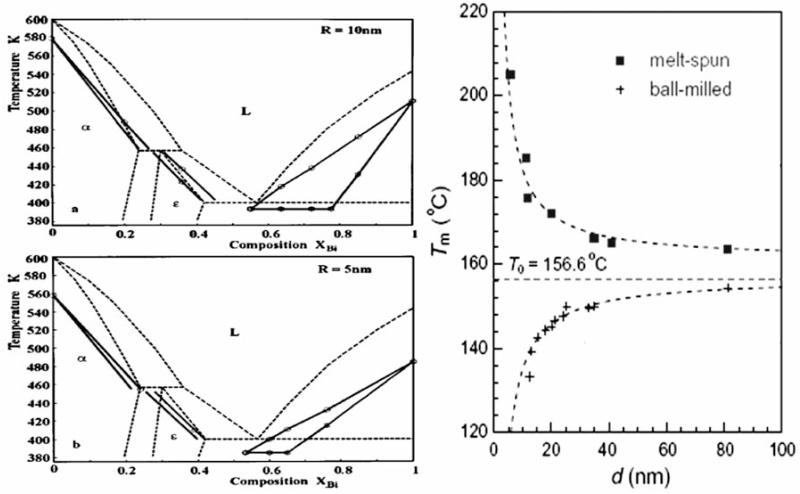
A detailed exploration of numerous nanosystems has led us to conclude that in most cases size-dependent properties of material systems at the nano scale could not be predicted by simple extrapolations starting from the micro scale. Hence, the major reason behind the formation of an entire scientific field solely dedicated to research of physical phenomena at the nano spatial scale is that the properties of material systems become significantly different once they are dispersed in the nano range. Despite exhibiting identical chemical compositions, bulk materials and their nanosized counter-parts thus can possess thoroughly different properties. For example, as a result of a higher surface energy of smaller particles, they act as more efficient catalysts comparing to their bulk counterparts. Quite often, however, specific polymorphs are almost thoroughly unstable in their nanosized form despite their stability in the system composed of microsized grains. For example, surface enthalpies and free energies of small particles are normally larger than those of the bigger ones, which implies that nanoparticles would get hydrated more easily than the microsized ones. Also, it means that as the surface enthalpy is inversely proportional to metastability of the coarse phase, reducing the particle size can lead to an unpredictable stability of phases other than the coarse one. A specific alumina phase, γ-Al2O3, thus becomes more stable in nanosized form with respect to α-Al2O3, whereas γ-Fe2O3 becomes stable with respect to α-Fe2O3 [9]. Despite the common sense expectation that the smaller the nanoparticle is, the more soluble it would be, this is not always the case. There are certain compositions for which a reverse case applies [10]. Many other situations in which the properties of substances with identical chemical compositions drastically differ based on whether the grain size falls in the nano or micro range have previously been assessed by the author [11]. Adsorption isotherms, melting point, electrical and mechanical properties, eutectic temperature and entire phase diagrams of single compounds and alloys (Fig. 2), Curie point and critical conditions for any phase transformation are all subject to change as the solid phase becomes nanostructural. This implies that many classical physical relationships, including Laplace, Hall-Petch, Katz-Thompson and Gibbs-Thomson relations, need to be revised before one applies them in describing the behavior of a nanoparticulate system [12].
Fig. (2).
Different phase diagrams for Pb-Bi alloy with the particle size of 5 and 10 nm [13] (left). Variation in the melting point of indium nanoparticles embedded in aluminum matrix [14] (right). Note how the melting point increases with a decrease in the particle size in case of the system prepared by melt spinning, whereas the trend is opposite in case of the system obtained using a ball milling technique.
Although nano is the word of a striking omnipresence in the world of hard sciences, it may be astonishing to realize that this word is not so strictly defined when used as a prefix in the materials science terminology. The current definition is that materials with the grain size in the range of 1 - 100 nm are considered as nanomaterials. But what happens with materials that, despite having the grain size in the given range, exhibit properties equivalent to those of bulk materials? For example, take a look at a magnetic material with the grain size below the superparamagnetic size limit. By looking at the particle size only, one might conclude that such a material should exhibit superparamagnetic effects, but this is not necessarily the case. Namely, only when there is little or no overlap between the magnetic moments of the neighboring particles will the superparamagnetic behavior be preserved. Once the particles become agglomerated (and it is their natural tendency, owing to both high surface energies and the magnetic attraction), interactions of magnetic moments across the particle-particle interface will become significant enough to cancel out the superparamagnetic effects of the overall system, which will thus end up with having a finite remanent magnetization [15,16]. Concerning this, a large disparity in scientific circles exists over whether such a material could be named nanomaterial or only if one manages to prevent their agglomeration by permanently keeping the particles at a given distance between each other could this attribute be employed. The latter way of thinking essentially suggests that structural features are less important than structural properties. In the end, even when probing the structure of a material, we are looking at selected properties, which we then convert into a structural picture. Accordingly, it is the critical boundaries occurring at the nano scale with respect to a measured property of interest that define whether a material could be considered as nanomaterial or not. In addition, the attribute of “nano” can be given only in relation with the given property. This means that a material structure that can be considered as nano from one angle does not necessarily need to be so from the view-point of another property thereof. To put it different, having critical boundaries is the necessary but not the only precondition for a material to receive a prefix nano. Interaction between the nanosized units is equally crucial for determining the nano character of the respective material system.
THE PROBLEMS OF REPRODUCIBILITY ENTAILING FINE EXPERIMENTAL APPROACHES
The crucial question in this descent of scientific interest towards ever smaller size scales is the following: how far can we go before reaching the limits? One thing is for certain, and that is that the practical limit is going to be reached significantly before the physical limits of measurements, defined by Heisenberg’s uncertainty principle, although it will be less strictly defined and subject to improvements over time. The first signs of reaching the actual limits are actually something that most experimentalists have faced at one or another point of their careers. This is often disguised in terms of irreproducible experiments.
As we could have seen after expounding reasons behind the current rise in interest in physical effects at the nano scale, the scientific attention has been continuously descending towards ever finer details of the material world. Resolution of the scientific probing of natural systems has been increasing and lately in its wake producing the trend of miniaturization of electronic devices. However, in order for this miniaturization to be successful, merely probing the systems at an ultrafine resolution is not enough. It is necessary to control the purposefully implemented modifications in them. That is, it is the continuous interference between the human user and these tiny devices that ought to be predictable and reproducible. With that, however, come numerous difficulties and challenges.
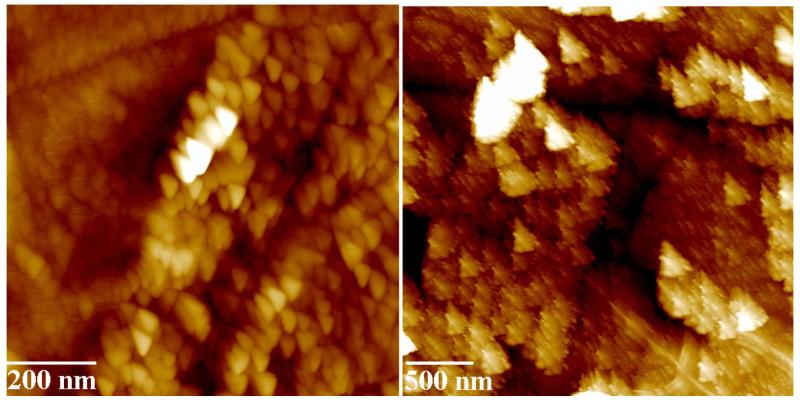
First of all, there are natural limits that prevent us from attaining a perfect control at the ultrafine scale. The most fundamental limit is defined by Heisenberg’s uncertainty principle, which in its weak interpretation tells us that an interaction between the measuring apparatus and the measured system, during which the measured system becomes changed, is the precondition for every measurement (Fig. 3). However, environmental effects such as adsorption of gases, protracted heat dissipation and an increasing friction-to-power ratio of mechanical gearings with the decrease in size are usually invoked in the context of limited miniaturization of electronic devices. As a reply to overly optimistic ideals of molecular machining [17], “fat and sticky fingers” problem is invoked as a consequence of insufficiently fine tools implemented with the purpose of manipulating with physical systems at atomic and molecular scale [18]. Self-assembly methodologies are, however, posed as a response with much prospect to overcome the problem of limited efficiency of mechanical interference with the synthesized systems [19]. The essential feature of these methods lies in the partial substitution of an exceeding interference with the designed systems (so as to guide their evolution in the preconceived direction) with letting physical systems spontaneously evolve into attractive and functional structures. Yet, another question emerges. It is how reproducible these self-assembly settings would be once the complexity of the systems in question gets exceedingly high. Sooner or later, limits of reproducible settings would be transgressed, which would result in uncertain and irreproducible accomplishments.
Fig. (3).
Nanosized particles shown in these atomic force micrographs, are, in fact, not triangularly shaped as it appears. Just as images obtained using an electron microscope are the results of an interaction between the electron beam (controlled by lens properties and other settings) and the examined material, and as every visually perceived object is an intersection of the biological and cognitive predispositions of us as observers and the objects of our surrounding, the images obtained using an atomic force microscope are a convolution of the tip shape and the examined morphology. The microscope tip for some reason became triangularly shaped in this case, causing the observed particles to appear similarly triangular. As the critical dimensions of material systems become finer than ever, such a reflection of the properties of the measuring system in the measured data will become more present than ever.
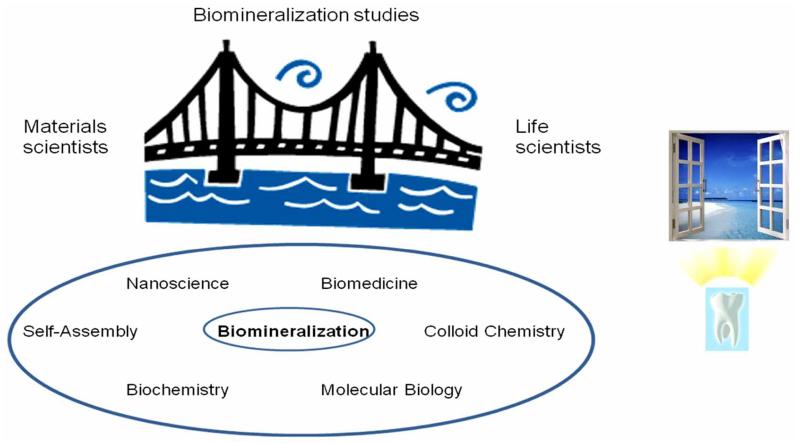
For example, nanoscience and bioscience are slowly but inevitably approaching each other, and there is more and more materials scientists beginning to apply biological or biochemical systems in their studies, and vice versa. Some research topics, such as biomineralization, can be seen not only as bridges between materials science and life sciences, but as intersections of diverse physicochemical subfields of interest as well (Fig. 4). But with the implementation of biosystems in the classical materials science methodology, the precision requirements to maintain the same level of reproducibility become significantly more stringent. Proteins are in their structural complexity one step below the living systems, and it is well known how the latter are subject to the key principle of chaos, that is, to a significant and essentially uncontrollable sensitivity of evolution upon the mildest changes in the initial conditions of the experimental settings [20].
Fig. (4).
A fruitful communication between materials scientists and life scientists is often hindered because of the different terminology, experimental methods and approaches to research used. Some scientific topics, such as biomineralization studies, need to adopt the language of both in order to be successful. Typically, such fields can be in addition seen as lying at the intersection of various other, narrow or broad scientific areas (left). Openness towards correlating individual scientific areas and topics with distant and seemingly not linkable fields and perspectives presents a step in the direction of realizing an enormous importance of the tiniest scientific endeavors. For example, teeth, one of the prototypes of an intricate organization of multiple mineral tissues, could be through an inexhaustible complexity of their structural organization and biosynthetic pathways seen as a window to the wonders of science and Nature.
Furthermore, the measurement uncertainty described in quantum theory and the indeterminate evolution described in chaos theory are not as unrelated as was previously thought. The imperfect, probabilistic nature of all measurements at the atomic scale introduces enough uncertainty to make even the simplest multi-body systems behave unpredictably after finite amounts of time. For example, the fundamental uncertainty in describing the initial state implies that the motion of a frictionless snooker ball becomes completely unpredictable after only about a dozen collisions [21]. Hence, although the ultimate precision limit outlined by Heisenberg’s uncertainty principle may seem irrelevant for even the most sophisticated chemical experiments nowadays, we should be aware that its randomizing effects inevitably “climb up” to the microscopic levels where they impose substantial obstacles to attempts to predict and control the evolution of physical systems on a fine scale.
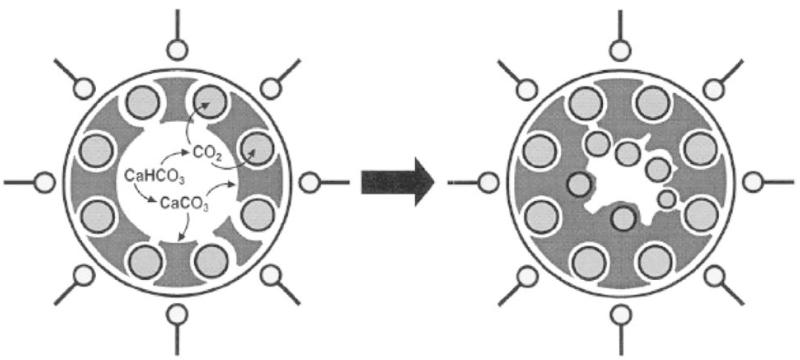
Then, there is the problem of some seemingly banal factors, such as the presence of gases in liquid systems. As being based on weak interactions, including hydrogen bonding and hydrophobic forces, self-assembly effects typically take place in wet conditions. And as more and more materials are produced using soft chemical methods that involve precipitation from the solution, the effect of dispersed and dissolved gases will prove to be of an immense importance. Furthermore, in view of the transition from traditional chemical engineering approaches to small scale studies, the requirements for having ultra-small batch sizes will inevitably show up and the necessity to have a more precise control over the gaseous content of the systems will follow. There are many cases in which the gas content significantly influences the reaction taking place in the system. For example, the first nucleation stage in precipitation of ferrites involves an oxidation of ferric ions. The oxygen content of the solution can thus crucially determine the kinetics of the overall process, particle size, microstructure and crystal stoichiometry of the precipitated material, and thereupon its properties, including the magnetic ones [22]. Another example comes from the precipitation of calcium phosphate materials, such as the ones used for reparation of hard tissues. In those cases, the presence of carbon dioxide in the solution can significantly influence the process, and that not only through pH changes (remember that perfectly pure, distilled water does not have pH 7, but pH 5.7 at atmospheric conditions owing to the effect of dissolution of carbon dioxide and the formation and dissociation of carbonic acid), but through the incorporation of carbonate ions in the crystal lattice [23]. Yet another example comes from the recent synthesis of vaterite in reverse micelles of SDS/hexane/water microemulsion [24]. The formation of calcium carbonate is triggered by a continuous purging of nitrogen gas through the microemulsion. This leads to a gradual removal of carbon dioxide bubbles, which are, however, unable to escape from the dispersed water phase and become trapped at the oil-water interface where they form a gas-water interface (Fig. 5). The latter subsequently acts as a nucleation surface for the formation of calcium carbonate. Finally, gas bubbles (strictly speaking, these are all cavities, as bubbles are defined as possessing a boundary of a different phase beyond which the same phase as the one comprising the bubble exists [25]) are known for their ability to intensify hydrophobic forces, as shown in the case of a ten-fold decrease in flocculation rates upon the removal of dissolved gas in a colloid system composed of paraffin and stearic acid. Generally speaking, water under atmospheric conditions contains approximately 5 mM of dissolved gas, which adds up to a single gas molecule per each 3 nm (note that oils contain ten times more than that). The effect of dissolved gas on various chemical transformations in wet conditions is mostly unknown, except for the fact that their presence can be comparable to defects in a solid body [26].
Fig. (5).
Mechanism for the formation of calcium carbonate particles in reverse micelles with gaseous cavities playing a crucial role in the process. Realizing that the effects of neglected variables are critical for the evolution of an experimental system is what makes chemical research more exciting than a plot of a mystery novel. It simultaneously points out how small deeds, the ones that are despite their seeming unimportance capable of significantly contributing to the evolutionary path of the world, may be what really matters in life. Because, after all, the entire scheme of science can be from a pragmatic philosophical perspective said to represent nothing but a metaphor of human experiences. The image shown is reproduced and adapted from Ref. [24].
Just like every stone contains literally all the naturally occurring elements of the periodic table, dust and other particulate or ionic impurities are always present in any experimental system, in smaller or larger extent. There they produce effects on nucleation similar to the one of carbon dioxide bubbles. They normally present undesirable surfaces that make heterogeneous nucleation energetically more favorable [27]. In case of precipitation of compounds with a versatile spectrum of adoptable phases and stoichiometries, such as calcium phosphate, the amount, identity and nature (dispersed, dispersedly adsorbed or clumped on the vessel walls, etc.) of extraneous impurities or seed particles can have a decisive role in defining the mechanism of crystallization and the final phase composition of the precipitate. The stability of some compounds is highly sensitive to the slightest amount of impurities, and carbonic acid is often taken as example, as a single molecule of water is shown to be enough to induce a fairly quick dissociation of carbonic acid (the half-life of which is in perfectly pure gaseous state estimated at 180,000 years) to carbon dioxide and water [28]. If we consider impossibility to perfectly isolate a system from its environment, we can easily conclude that there can be no perfect conditions for measuring the basic parameters of homogeneous nucleation, such as the induction time, also known as lag nucleation time. As, strictly speaking, vacuum presents the only correct environment for estimating homogeneous nucleation of solution (and that in perfectly pure state of the latter), whereas under such low pressure liquid state could not be sustained, it can be concluded that there could no experimental conditions for attaining this aim. As a matter of fact, the induction time for homogeneous nucleation should reach a practical infinity more often than it does in reality, and our ability to experimentally estimate it at a fine scale could be, in that sense, seriously questioned.
Then, as the reaction vessels shrink in size in parallel with the growth in complexity of the components and interactions that are to be induced and controlled therein, it will be harder and harder to eliminate the effects of the environment. This is quite similar to the problem faced by engineers in attempts to minimize electronic circuits and mechanical devices below certain limits, when minor environmental effects can spoil the working of the system, such as an adsorbed foreign particle that changes the weight of the lever and causes a disruptive imbalance. For example, most chemists still employ glass vessels for their reactions. However, if a reaction that is to be induced and controlled is too fine, the effect of leakage of ions from the glass container walls can prove to be detrimental for achieving control over the reaction. As many fine colloidal processes, such as sedimentation, are sensitive to the underlying surface, different types of glass are known to produce sediments of different stability [29]. This difference in composition of the reaction container can be obvious if the beakers were obtained from different suppliers or (if practically identical vessels were used at the start) it can be triggered by their unequal aging which through different forms of leakage changed the surface composition over time. Not only different identity of the functional groups of the surface and its charge, but the various textures of silica surfaces in contact with metastable solutions are also known to drastically affect the stability of the latter, in a way that a minor difference in porosity of the surface material sometimes determines whether the solution will remain metastable or will crash [30]. This effect of unpredictable stability during aging is apparent to anyone who has ever stored (or ran a reaction using) a metastable solution, such as stimulated body fluid (SBF), supersaturated with respect to more than one calcium phosphate phase, in glass bottles. Many experimentalists claim that plastic reaction vessels present an inerter and safer choice, but this is not so. Namely, it has been well documented that rubber, polyethylene and Teflon all undergo absorption and chemical degradation effects over time and may significantly disrupt experimental reproducibility [31]. Another downside of polymeric surfaces is that they are normally less smooth compared with the glass. There are also examples of reactions unexpectedly catalyzed by the contact between a partially degraded, microporous surface of the plastic container and reactants. Needless to say, perfect cleaning procedures cannot exist, and just like the perfect vacuum is unattainable, every cycle of washing diminishes the traces of the previous compounds contained, but inevitably leaves them present within a finite range. This is why Tadashi Kokubo, the inventor of the method for assessing the bioactivity of materials based on immersing them in SBF and monitoring the extent of precipitation of hydroxyapatite on their surface, claims that a polyethylene vessel in which a precipitate formed once or a scratch is visible has to be discarded and not used for any future storage of SBF [32].
The effect of leakage of ions from the glass container resembles the problems faced upon the attempts to grow one crystalline phase on another at low supersaturations, when the epitaxial interaction anticipated can be obscured as the result of dissolution of atomic ingredients of the seed phase into the solution. These ion exchange reactions between the introduced seed and solution are often rapid and may significantly contribute to the equilibrium state [33]. Then, even though a condenser system is applied, evaporation effects in exceedingly small systems can never be overcome, because even tiny precipitation of the solvent on the vessel walls will produce changes in concentration of the dissolved or dispersed entities. Finally, fluctuations of temperature, which each water bath exhibits in one extent or the other, produce changes at the level of pH and ionic activity products, which all subjects the system to certain uncontrollable inconstancies during the reaction time.
Although magnetic stir bars are regularly used to agitate the system and ensure a more homogeneous distribution of its content, their effect on the evolution of the systems in question can often be surprising. Crystallization kinetics of some diamagnetic compounds, such as calcium carbonate [34] and zinc sulfate [35], is enigmatically influenced by an external magnetic field, for example. A magnetic stir bar can also present an undesirable nucleation surface, and that particularly when one works at lower supersaturations when the crystallization process is typically surface adsorption-controlled. On the other hand, at higher supersaturations when crystallization is typically bulk diffusion-controlled, the system can be markedly influenced by the stirring rate. In fact, running crystallization experiments at various stirring rates is regularly applied to find out whether bulk diffusion presents a crucial step in the process. If its effect on crystal growth rates is observed it may signify a bulk diffusion-controlled process, although it is worth noting that this method could not be readily applied in case of nanoparticles for which the stirring rate normally has little or no effect on the thickness of the diffusion layer [33].
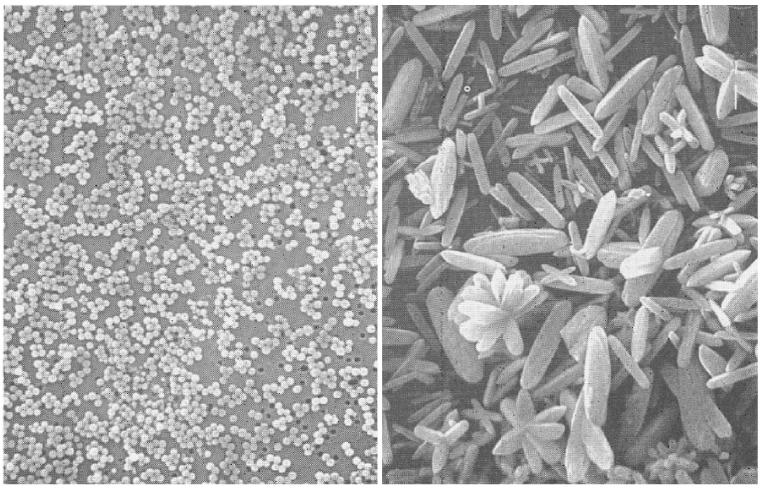
It has frequently been observed that identical experiments in terms of the initial chemical compositions ran under slightly different conditions (i.e., small batch vs. large batch [36,37], stirred vs. non-stirred [38], open vessel vs. closed vessel [39]) may produce thoroughly different outcomes (Figs. 6, 7). Experimentalists sometimes produce substantial amounts of material by repeating an enormous number of experiments, each one of which yields just tiny quantities [40]. The reason is that they found it impossible to multiply the batch size without modifying the properties of the product. Problems like this become particularly critical during the transfer of small-scale, lab syntheses to large-scale, industrial fabrication levels.
Fig. (6).
An example demonstrating the sensitivity of product properties to small changes in experimental conditions. Shown are differently shaped particles obtained by aging identical aqueous solutions of lanthanum nitrate and urea in a closed (left) and open vessel (right). Reprinted with permission from Ref. [39].
Fig. (7).
Another example of a drastic change in morphology following a minute modification of the experimental conditions [36]. Both morphologies shown were obtained after deswelling an identical, thixotropic mixture of cholesterol and carboxymethyl cellulose in an ultrasound field. The image on the left, however, corresponds to the particles prepared within a ten times smaller batch size compared with the morphology shown on the right. The same ultrasound intensity applied on a smaller volume in the former case breaks the material apart into smaller fragments, whereas it merely produces bubbles on a cholesteric surface in the system of a larger volume.
In view of the increasing sensitivity of fine chemical reactions upon the tiniest changes in the experimental conditions, even the effects normally neglected in chemical practice, such as gravity [41], may occasionally turn out to present a key factor that determines whether the system will wind up in state A or state B. Despite previously expressed disbelief in the possible effects of gravity on the stability of dispersions of nanoparticles, the effect was evidenced as significant and that particularly when one works with high-density nanoparticles, such as the ones composed of gold [42]. As more and more synthetic methods rely on weak chemical interactions, maintaining delicate sets of conditions for each one of them will be required, which inevitably entails the challenges of unrepeatable experimental results.
Now, if one thinks that only experimental settings will be increasingly subject to irreproducible situations, whereas the corresponding theoretical calculations could still be carried out without difficulties, he would be wrong. Namely, in about the same extent as the experimental conditions will be subject to uncontrollable variations, the theoretical calculations will prove to be uncertain. Even simple systems involving a phosphate buffer and a few types of ions that tend to be precipitated in the presence of a foreign phase produce incalculable ion exchange, association and dissociation effects. As the experimentalists would come up with the requirements to control the experimental conditions at extremely fine sets of conditions (such as pH and ionic concentrations at the third digit), the theorists would hardly be able to find the way to calculate the composition of the system that leads to these conditions. Such a situation is no wonder to anyone aware of the fact that theoretical calculations and experimental control have mostly been holding hand-in-hand throughout the centuries during which empirical science intensively developed.
However, we should be aware that synthetic chemists exist exactly owing to inability of theorists to perfectly calculate and predict all types of chemical transformations. Chemistry has frequently been considered as lagging behind the calculus complexity in physics, but this is so only because chemists have long ago gave up on the importance of predictive calculations in the design of their experiments and smartly, for the sake of efficiency, decided to substitute theory with experimental trial-and-error. In fact, due to an enormous amount of approximations that needs to be employed prior to getting any meaningful output from these calculations, the real-life meaning thereof has been practically lost. Even the most basic calculations involving ion exchange effects in the presence of buffers and a phase transition are, in fact, so complex that should one try to bring up a perfectly precise result, he would find himself in a tangled web of iterative relationship, the attempts to untangle which would certainly not pay off.
In view of this, serious chemists sometimes leave out complex equations from their presentations because of pure aesthetic reasons [43]. In chemistry, calculations are most frequently not necessary, precisely because approximations are so many that the same effect could be achieved with a mere qualitative description. In fact, such an imprecise and qualitative character of chemistry in couple with its hard science character has been taken as the sign of its inherent beauty [44]. Expecting from chemical research to satisfy the same level of precision as research in physics may have a retarding and futile effect on the quality of the final outcomes, particularly in view of many cost-effective methods for fabrication of attractive materials, involving simple experimental settings [45], simply waiting “around the corner” to be discovered. On the other hand, it may be argued that the more engineering and the less fundamental the approach in chemical research, the greater the magnitude of the black box between knowable phenomena and controllable parameters on one side and eventually collected outcomes on the other. Tendencies to satisfy the practical research aspects, such as obtaining the desired material structure and properties, imply a larger size of this black box (because one essentially does not bother to explain the mechanism of the formation, but is satisfied with having a straight relationship between the external parameters that control the synthesis conditions and the final outcome), whereas “brave” plunging into exploration of fundamental interactions involved in the evolution of the system within given boundary conditions means that this black box may be eventually shrunk, although we will certainly get to it if we descend deep enough into fine details of our explanations. Anyhow, as the challenge to sink deeper and deeper into controlling physical behavior and chemical transformations within ultrafine systems becomes more pressing, an eventual encounter of physical, calculative precision and chemical, aesthetic “intuitiveness” will need to take place, which will probably contribute to setting physical chemistry at the top of the pyramid of natural sciences.
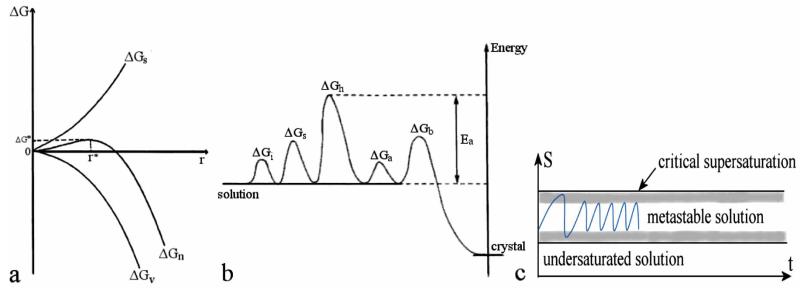
When it comes to a fruitful coordination between theoretical and empirical knowledge, the most important thing to remember is that we should resist becoming slaves of the theoretical explanations of the experimental phenomena. We should remember that each one of the explanations in usage is only one out of an endless number of possible ones. In other words, we should bear in mind that every scientific description is merely a map that helps us orient on the real territory, but always approximates certain features of the latter. For example, I have heard many students referring to the standard explanation of nucleation phenomena (Fig.9a) in the following manner. They claim that the unfavorable surface energy term, proportional to the squared radius of the nucleus, grows at lower radii faster than the favorable bulk energy term, proportional to the cubed radius of the nucleus, but after a critical size, corresponding to a unity (as only unity stands equal when squared and cubed), is reached, this trend becomes reversed. My question that tends to point out the model character of this description is how Nature really differs between sizes that fall below 1 on the given scale and those that exceed this limit. As Nature does not think in numbers, the explanation based on mathematics should be in this as in any other case accepted merely as descriptive. Nonetheless, to make the model quantitatively fit the reality, radius of the nucleus has to be linked to a few additional parameters, such as surface tension, supersaturation ratio, temperature and volume of the growth units.
Fig. (9).
(a) Change of the free surface (ΔGs) and bulk (ΔGv) energies and their sum (ΔGn) for the growth of a nucleus of radius r; (b) multiple energy barriers that ions need to surpass during the crystal growth; and (c) change in saturation of a solution during a titration of ions aimed at attaining the conditions of a constant composition of the solution and a continuous crystallization of the solid phase. Notice that the transition from an embryo (r < r*) to a stable nucleus (r > r*) is an asymmetric process with respect to time (that is, approaching r* from the embryo side takes longer than to “slide” down the energetic barrier “hill”), which under a constant supply of precursor ions implies an oscillatory character of variations in the solution composition.
To get back to the old track of the discourse, I will refer to a striking example of the failure to experimentally verify Mpemba effect, according to which the warmer the water, the faster it freezes when cooled down with an equal rate [46]. If simple experiments such as this one could not be reproducibly performed because of the lack of control over the composition of pure water and the convection of heat [47], how is it going to be with more complex experimental settings? Complexity of the system investigated for Mpemba effect is such that it is hard to imagine a simpler one, both in terms of composition and volume. In reality, however, neither temperature nor composition of this basic chemical could be kept at controlled levels. If one works in a water bath, regular fluctuations of not only temperature but of many values dependent thereon (such as pH and, therefore, ionic strength and activities of ionic species, the level of supersaturation, etc.) may be detected, and the situation is even worse if one uses air heaters owing to their even lower heat capacity, directly corresponding to a lower heat amortization capacity. In fact, calculating and explaining pH changes in comparatively simple systems such as carbonated distilled water present enormously complex tasks if one wants to reach the ideal of perfection, and if one uses tap water, the situation becomes thoroughly chaotic, owing to practically insolvable cooperative ion association effects. Maintaining pH throughout prolonged periods of time at a stable value at the third digit has been previously re-ported [48], although until these results become reproduced or averaging procedures applied during the measurement revealed, one could not really say whether this is possible or not.
It is a strange fact that the simplicity of Mpemba effect is such that it requires an enormous complexity of experimental settings to study it in detail. However, such may be the general nature of the evolution of human knowledge where simplicity and complexity are always neatly balanced. Every simplicity floats on an underlying complexity, and vice versa. Particularly in view of the modern requirements of “greening” chemical methods for the sake of promoting their and our sustainability, chemists ought to stick to simple methods in their synthetic approaches. I believe that although many chemists insist on using complex mixtures and equipment in their approach, there is an endless sea of open possibilities that unimaginably simple procedures can offer [49]. One of them has recently been presented as the first known method for the production of stable dispersions of uniform and well-defined particles of cholesterol [50]. However, the simplicity of certain aspects of scientific methodologies that we may or may not apply should not obstruct our awareness of the underlying complexity of the nature of things. This love of complexity would certainly prevent us from making immature and vulgar generalizations in our hypothetic thinking.
Calibration problems, essential for a reliable reproduction of experiments, are caused not only by the natural measurement errors, but in large extent by other effects, including most problematically temperature. Namely, every sample has a unique pH vs. temperature relationship, which implies that even though a pH electrode may be hypothetically perfectly calibrated, the pH-meter would be unable to correct pH values for temperature effects with a perfect precision. Then, heat content is never uniformly distributed throughout the sample, which implies variations in temperature, and different sensors in the system (such as pH electrode and temperature sensor) will respond to different environments. Every electrode also leaks some of its electrolyte content into the solution during a continuous measurement, thereby manifesting the general principle that there is no measurement without an interaction in which the measuring device modifies the properties of the measured system. The greater the leakage rate, the more precise the measurement, which reflects the general trend of all measurements: the greater the interference with the measured system, the more information will be gathered, but the more changes will be introduced in the system as well, which at the same time makes the measurement results less reliable. Finally, every electrode presents a foreign surface which can influence nucleation, and that becomes particularly critical when one works at low supersaturations. The latter will be increasingly exploited in future because many natural self-assembly processes occur exactly under conditions of low supersaturation when the control over the phase transformation is moved away from the spontaneous physicochemical propensities of the system to subtler interactions conveyed by weak chemical forces.
Apparently, most advanced self-assembly techniques are based on promoting metastable conditions for a spontaneous evolution of the system into desired state. Now, every suspension is inherently metastable, and it is only a matter of time when the critical conditions for segregation of phases will be reached. Some suspensions, like Faraday’s gold sols, kept in the British Museum, have been stable for more than a hundred years, some of them can undisturbed reach geological times, but some of them begin segregating immediately after their preparation, being thus in need of a stabilizing agent or modified particle characteristics. Practical reasons require finding conditions that will promote the quickest possible transition from the metastable to the final state, and as the complexity of the targeted structures increases over time, this norm will be harder and harder to attain. There is a synergy of weak physical and chemical forces acting in parallel that defines this transition, and sometimes the least suspected ones can present crucial factors, making chemistry more interesting than any detective story. Gravity is, as previously mentioned, one the often neglected weak forces that may have a crucial effect on the behavior of particles in suspension. It predisposes the system to undergo either sedimentation or creaming as part of the segregation mechanism, and in view of the different interfaces that the segregating particles will be in contact with (vessel wall/solvent or air/solvent), the self-assembled morphologies thus formed may be completely different. Some synthesis techniques, such as Langmuir-Blodgett film preparation, rely on the self-assembly of thin layers of particles at air-water interface, and gravity can often be the one to spoil the tendency of other weak forces to move the particles close to this interface.
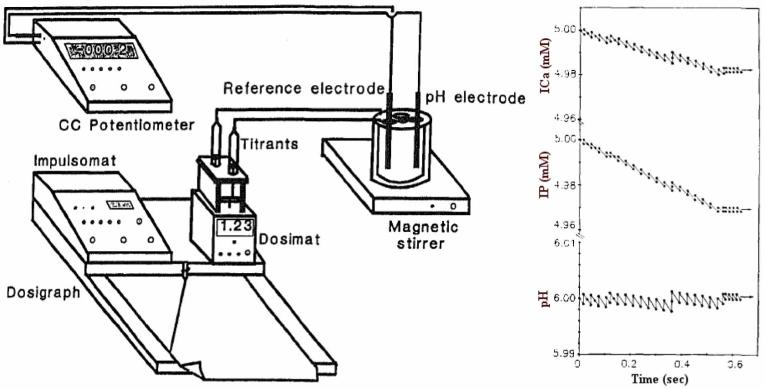
It presents a huge challenge to embark on the research voyage aimed at attaining a perfect control over physicochemical conditions within relatively small systems. Some self-assembly reactions take place at very slow rates, requiring thus a need to maintain control over these conditions throughout extended periods of time. Also, some of these self-assembly processes occur within a tiny window of conditions, and their reproducibility could be thus brought into question. To cope with these challenges, we have recently witnessed an expansion of the so-called “constant composition” approaches, utilized for the purpose of promoting constant and reproducible sets of fine conditions during extended periods of time (Fig. 8). The method was developed by Nancollas and Tomson in 1978 [51], and is applied nowadays by many research groups [52-54]. However, it can be argued that albeit given a perfectly precise response of the electrode, the potentiostat and other sensors applied, the constant composition approach nevertheless presents a misnomer. Contrary to the way the precipitation using a constant composition approach may be imagined [55], not a single experimental setting of this kind provides conditions for a literal constant composition precipitation. In addition to practical problems such as the necessity of introducing titrants at an ever higher concentration in order to make up for the volume increase and ionic concentration decrease following the titration (unless solvent evaporation volume is miraculously set to match the volume of titrants added) and a hardly definable effective concentration of the ions in solution at which the rate of the crystal growth is comparable to the rate of the addition of titrants, there are seemingly unavoidable obstacles inherent in the very thermodynamic and kinetic principles that govern nucleation and crystal growth. Namely, as every nucleation event requires constituents of the system to surpass an energetic barrier, it is highly unlikely that one can manage to lead the elements of the system transcend it in a continuous fashion. Instead, nucleation events will during a constant supply of precursor ions occur in waves, so that the composition of the entire system will correspondingly fluctuate. Even if one starts from a low supersaturation, intending to induce merely an attachment of ions onto an already existing crystal surface, the standard kinetic framework forces us to conceive an energetic barrier, the surpassing of which will most likely take place in waves during a constant titration (Fig. 9).
Fig. (8).
Scheme of the constant composition approach, developed by Tomson and Nancollas, together with the predicted minor variations in concentration of titrated reactants and pH in view of the imperfect response of the potentiostat that triggers the addition of titrants. Reprinted with permission from Ref. [55].
Critical supersaturation is hardly definable in case of complex mixtures where ionic activities can drastically vary depending on the effects of additives, ion-pairing, hydration of the crystal surface, temperature, the dominant mechanism of growth (sometimes surface nucleation competes with growth through a mere attachment of ions, such as screw dislocation mechanism), and other kinetic factors. Nucleation events also start occurring before a critical supersaturation level is reached, with nucleation rate typically exponentially increasing until the system approaches a sudden runaway in the crystallization process at the critical point. Not only is in case of complex mixtures hard to estimate the nucleation rate at a given supersaturation lower than the critical level, but its function versus relative supersaturation tends to be different depending on the system in question. Thus, it exponentially increases towards the critical point in case of homogeneous nucleation, grows as f(x) = 1 − cosx function towards the critical point in case of heterogeneous nucleation in the presence of extraneous particles with variable nucleation efficiencies, and it can even approach the critical conditions with a constant level in case of heterogeneous nucleation on particles with equal nucleation efficiencies.
Furthermore, although the classical nucleation model predicts that the nucleation rate should continuously increase with increasing saturation, in reality every phase transition follows a nonclassical model. According to it, there are two antagonistic effects taking place. Namely, the higher the supersaturation, the higher will be the thermodynamic driving force for nucleation, inducing the latter to proceed at a higher rate (although this rate normally decreases as nucleation proceeds due to depletion of growing units from the mother phase). But on the other hand, moving the system state away from equilibrium modifies the transfer of matter between the growing embryos and the mother phase, and since this transfer has to be ensured for the nucleation to proceed, this shift can significantly delay nucleation in some cases. In case of formation of glass, for example, an ultrafast cooling promotes the latter effect, leaving the system in the metastable state with an extremely slow transformation to the stable, crystalline form. If one would make an attempt to condense water vapor at the temperature of liquid nitrogen, at first it may seem that such a transition would proceed momentarily. But it is not so [56]. Namely, at this low temperature, the equilibrium pressure of water vapor (po) is so low that one collision between its molecules (which is the first step to the formation of an embryo that then has to advance forming a stable nucleus or to simply dissipate) occurs every 1016 years. To induce nucleation, one would have to increase supersaturation ratio (p/po) to ~ 1012 by compressing the system and increasing its concentration, for instance. In contrast, condensation of water vapor at atmospheric conditions occurs in the supersaturation range p/po = 5 – 8. As we see, this antagonistic effect implied by the fact that the mother phase has to ensure a reasonable supply of matter to the embryo for nucleation to proceed becomes most critical in far-from-equilibrium conditions, although complex kinetic conditions within a system may sometimes predispose it to exhibit such far-from-equilibrium characteristics even thought its state lies close to equilibrium. The fact that nucleation rate is subject to variations, both before and after the critical point is reached, and depends on the system in question, contributes to difficult predictions of nucleation and crystal growth rates in any real systems.
Notice also that the termination of growth in case of complex mixtures does not normally occur exactly at the supersaturation limit, but somewhere in the metastable zone, which may be, for example, due to an accumulation of surface defects, overgrowth of a more soluble phase or the effect of additives, such as macromolecules, which may block the growing faces (at both high and low concentrations) or hinder the diffusion of ions (at higher concentrations only). These are the reasons why shaded boundaries are drawn as surrounding saturation and critical supersaturation limits in Fig. (9c). Calculations based on solubility product are thus hardly applicable whenever there is a substantial complex formation, although even in case of simple solutions the solubility product is subject to change depending on the amount of defects, size and morphology of the precipitated crystals. As external surface is formed after the first crash of the solution, magnitude of the waves decreases (Fig. 9c). Apparently, one would need to avoid any further nucleation and induce merely attachment of ions onto already existing crystalline surfaces in order to minimize the magnitude of these waves and approach the “constant composition” conditions. However, as free energy of the crystal formation is a combination of multiple factors besides nucleation, including bulk diffusion (ΔGb), adsorption (ΔGa), dehydration (ΔGh), surface diffusion (ΔGs) and active site incorporation (ΔGi) (Fig. 9b), the energy barriers involved in the process would still possess a time-asymmetric character, which would result in a wave-like change in the solution composition.
Each one of these energy barriers is tied to a certain induction time required for the system to surpass it, and is in addition subject to change as the reaction proceeds and the phase composition and interaction between the components of the system get modified. These lag-time effects connected to each one of the energy barriers of precipitation process (with a particular emphasis on nucleation) produce sudden changes in the composition of the system, thereby uncontrollably interfering with the system. By producing tiny and sudden waves of change in composition, the constant composition conditions become constantly perturbed during titration. This hinders any attempts to enable constant composition conditions through finding out the precise effective concentration of the titrants at which the rates of precipitation and titration will precisely match. The latter, however, still presents standard but overly simplified approach behind conceiving experimental settings of this nature.
Hence, under any “constant composition” conditions, precipitation will occur in waves, and it is only magnitude and phase of these waves over time that are the subject of uncertainty, depending on kinetic and thermodynamic factors that define the synergy between nucleation and crystal growth. In addition, as the precipitation reaction proceeds and the contact area between the solution and the solid phase gets increased, the interfacial free energy as the driving force of the phase transition is subject to change, implying a shift of the already narrow and hardly definable window of conditions that promote a balanced continuous delivery of ions to the system and their precipitation. As the width of the metastable saturation zone and the critical supersaturation limit are subject to change as the titration proceeds, a variable delivery of precursor ions through titration has to take place in order to approximate “constant composition” conditions with respect to the working solution. Moreover, the titrant is normally introduced in form of droplets (dialysis may in that sense present a more sensitive method of ionic supply), which is another effect contributing to the oscillatory character of precipitation, not to mention the spatial gradient in composition thus established, which might have a critical effect in a dynamic setting of continuous precipitation like this. Namely, it is well known that adding reagents of an exceedingly high concentration to a solution can induce local overcoming of critical conditions for a phase transformation, even though an equalized system as whole would have remained far from the critical point. Adding an acid or a base in form of droplets to a metastable solution of a sparingly soluble salt, such as SBF, so as merely to adjust the pH within a stable range, may thus cause an irreversible local precipitation of calcium phosphate. Even when agitation is intensive and the rate of addition of reactants extremely slow, locally inhomogeneous distribution of reactants will exist, frequently favoring precipitation of compounds even below their solubility limit, which is the effect routinely employed in co-precipitation syntheses of nanosized magnetic ceramic particles [57]. In case of solutions or suspensions of biomolecules, such as proteins, which are known to possess “ridged” free energy landscapes and thus can exist in a wide array of metastable states, the effect of local gradients of chemical conditions following titration or any other interference with the system would be even more drastic. For example, peptide dispersions frequently require a precise pathway of change in the ionic strength, pH of the solvent or temperature to attain a given state, so that not only a precise sequence of adding components to a protein-comprising solution is important, but different rates of adding a solvent to a protein powder upon its dissolution can often result in suspensions of thoroughly different properties in terms of the particle size, morphology and the overall stability of the dispersion. Moreover, chemical parameters of a reaction system, including pH and ionic strength, are in every complex, multiphase system not uniformly distributed, but show variations around interfaces, which may have a critical effect in the presence of extraordinarily sensitive entities, such as peptides or other biomolecules. The concentration of protons is, for example, known to show variations of a couple of orders of magnitude as one approaches a solid surface in contact with the solution, and the scale of this effect may extend up to the centimeter scale [58]. Hence, to provide conditions in which titrant solutions will be pumped into the system in exact phase with the precipitation of ions looks from today’s perspective as fantastic as finding the exact de Broglie’s wavelength for a solid body to pass through the wall via producing the Ramsauer-Townsend minimum. Strictly speaking, as of today, the concept of constant composition precipitation may present only an approximate one.
Additional challenge to the ideals of perfectly controlled, “constant composition” titration comes from phase transitions that occur in the solid state. Some compounds exhibit complex polymorphic and other phase transitions upon precipitation, and calcium phosphate compounds are some of them. For example, empirically observed Ostwald-Lussac’s rule dictates that the most soluble phase for which the supersaturation limit is exceeded will be the first to precipitate, successively followed by precipitation of the less soluble phases [59]. This rule is merely a confirmation of the kinetic argument according to which the most probable way for a system to transform from state A to state B in the absence of a sufficient amount of energy that would enable its immediate “leap” thereto is through passing through transient stages with energy levels positioned between the starting and the ending state, which is why life as a transitory stage in the reaction of burning in the oxygenated atmosphere to yield primarily CO2, H2O and ash can also be seen as an example of Ostwald’s rule. In case of an ordinary precipitation of calcium phosphate, this rule dictates that although hydroxyapatite may be thermodynamically the most stable and the least soluble phase under given conditions, amorphous calcium phosphate will normally be the first to precipitate, but only to be subsequently, in the solid phase, transformed usually (at neutral pH and in aqueous media) first into octacalcium phosphate, and then to either monophasic hydroxyapatite or a mixture of a tricalcium phosphate polymorph and hydroxyapatite. (However, as Ostwald-Lussac’s rule is a rule, and not a law, it cannot be used to predict with absolute certainty the exact transformation pathways leading to the final products, and large variations observed in case of precipitation of hydroxyapatite clearly demonstrate this.) If coupled with a continuous supply of external ions, such a transformation will cause hardly controllable variations in the solution composition, as some ions, such as phosphate, will be released into the solution during this transformation [60] (as Ca/P molar ratio is in the range of 1 – 1.5 for the amorphous phase, whereas it equals 1.667 for stiochiometric hydroxyapatite) [61], whereas others, like hydroxyl and calcium ions, will be additionally attracted to the solid surface. Thus, the induction time often correlated with the nucleation of hydroxyapatite corresponds not to the onset of the formation of the solid phase (which is amorphous calcium phosphate in most cases) but to the point in time when the formation of hydroxyapatite formation from the amorphous precursor takes place. This time can vary anywhere from seconds and minutes to geological times depending on the level of supersaturation and the corresponding metastability of the solution. Nevertheless, such a prolonged time span of precipitation events certainly makes the aim of molding a solution into a “constant composition” precipitation mode a utopian one.
An important fact often disregarded in routine calculations of conditions for crystallization is that no matter how sparingly soluble a salt may be, precipitation and dissolution always present simultaneously occurring phenomena. Even the tiniest degree of dissolution/re-precipitation is enough to modify the particle size distribution and morphology over the course of their remaining in the solution. One of the reasons why this feedback effect is frequently neglected is that, as in many other physical systems in which cause and effect and entwined within a close loop, it induces incalculable nonlinear effects in the evolution of the system. However, as chemical calculations are inherently not predisposed to satisfy the criteria of perfect precision (as they tackle ensembles, not individual entities), approximations like this rarely produce drastic effects on the experimental design. Nevertheless, this is not to say that as the fineness of the experimental settings and sensitivity required to control them increase in parallel these approximations would not need to cede place to more detailed and careful calculations.
Be that as it may, even when there are no phase transformations in the solid state during precipitation, every precipitate is, more or less, prone to exhibiting ripening effects whereby normally, as in the case of Ostwald ripening, smaller particles tend to dissolve on the account of the growth of bigger ones. Such an active change of the morphology, structure, particle size and, as we see, sometimes even the chemical identity of the precipitate over aging time subtly modifies the critical supersaturation conditions for crystal nucleation and growth (as nature of the solid surfaces in contact with the solution partly determines the mechanism of crystal nucleation and growth and the overall kinetics thereof). A similar morphological change over the course of aging of the precipitate can be induced by aggregation effects, and as opposing the classical LaMer’s model based on combining a short nucleation burst, followed by the growth of nuclei by diffusion, it is well established nowadays that the majority of uniform, micro-sized colloidal particles comprise a complex substructure, as their formation at a certain stage involves an aggregation of the primary subunits into the actual particles [62,63]. Hence, the fact that any precipitate is not an unchangeable body, but is instead in constant state of morphological and structural fluctuation presents another factor that renders the ideal of continuously modifying the solution content in precise feedback with the precipitation events highly inoperative.
The problem of weak reproducibility and controllability that gets intensified as science approaches the limits in its interference with physical structures and processes at the ultrafine scale is tightly related to the essential point of Heisenberg’s principle of uncertainty. It tells us that as structural and interactive details of the physical processes studied get finer, the harder it gets to interfere with them in a well controlled way. Just like it gets harder and harder to manipulate with pieces of something as they get smaller and smaller, the same trend applies for any experimental control. As entities we tend to manipulate get to be finer, the only way to “keep pace” with them is to increase the sensitivity of our “touch”, which, however, has obvious limits that lie far closer to the macroscopic size scale than the fundamental limits outlined by Heisenberg’s uncertainty principle.
In essence, this principle can be, in view of this analogy, regarded as the general form of the so-called “fat and sticky finger” problem. Both of these, however, can be seen as the consequence of an ever more difficult demarcation between the system and its environment. Notice that the observer in attempt to study the system while exhibiting as little modifying interference with the system as possible belongs to this environment. In fact, all contemporary synthetic or characterization methods trying to achieve this demarcation suffer from these problems. Unless the fuzziness of the boundaries between the system and environment is prevented, ireproducible results will inevitably follow.
But, some of the most prosperous modern synthetic methodologies do not want to interfere too much with the system/environment boundary and keep things overly under control, but let them spontaneously evolve in certain extent. These methods are inherently connected to the concept of self-assembly. However, it was exactly in view of an inability to separate the system from its environment and precisely establish a boundary between the two at the fine scale that I recently proposed each self-assembly to be a misnomer [64]. Namely, as a self-assembly of each particular system crucially depends on the environment that the system is linked to, each self-assembly could be regarded as a co-assembly. The latter implies that the system and its environment inevitably change in synchrony with each other. What follows is in some extent a broadening of the scope of this thesis by supporting it with additional examples and arguments. As complementary to the original presentation of this thesis, I will focus here on the assembly of biochemical systems. This is so because the stability of biological systems rests on a complex network of self-assembly interactions underlain by weak physicochemical forces. In that sense, the contemporary trend of fabrication of inorganic materials using self-assembly principles could be seen as a fundamental form of biomimetics.
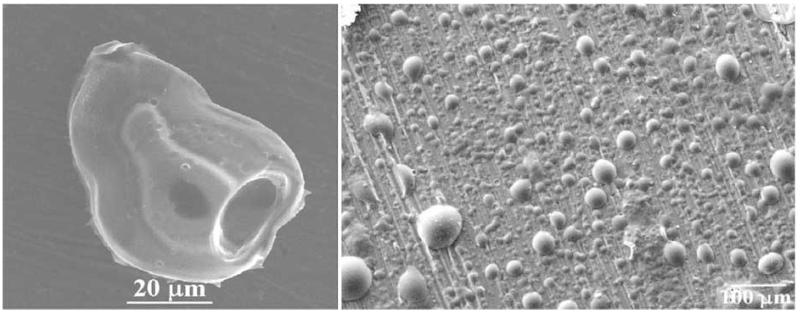
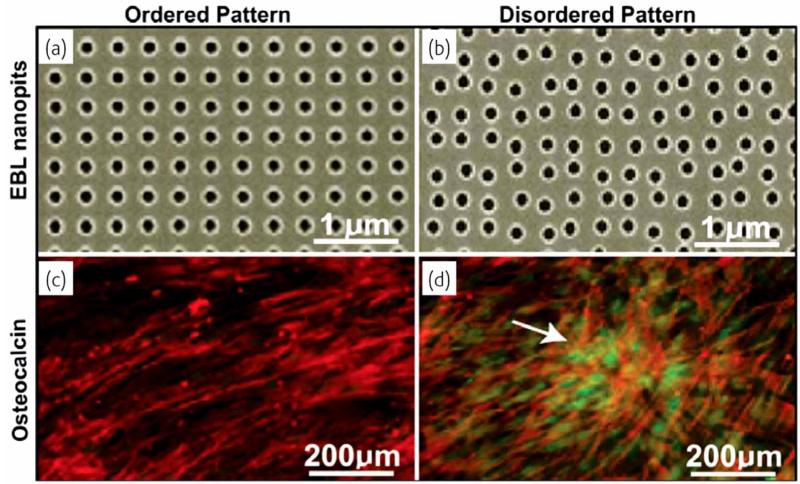
However, before proceeding to the discussion of the co-assembling nature of self-assembly phenomena, it should be added that the principles of biomimicry and the problems of irreproducibility are inherently linked together. Namely, the way biosynthesis proceeds in the living world is not through a perfect reproductive control but through a high selectivity relative to an array of the given products, some of which satisfy the posed requirements and some of which do not [65]. This principle is equally valid irrespective of the scale of the processes in question, from genetic evolution (that follows Darwinian model with mistakes inherent to DNA replication) to biological fabrication of various tissues at the micro scale. As can be seen from Fig. (10), a perfect order can sometimes be detrimental for reaching a satisfying biocompatibility between an artificial material and a living system.
Fig. (10).
Different nanostructural patterns obtained using electron beam lithography (EBL), and the corresponding images of the expression of the bone-specific extracellular matrix protein osteopontin within differentiated mesenchymal stem cells. Note that the disordered pattern of the biomaterial constituents stimulates the protein expression more than the ordered pattern does. Reprinted with permission from Ref. [66].
Moreover, a perfect uniformity in structure and properties is known to be a feature of colonies of malign cells, as compared to the natural diversity in shape and behavior present in healthy tissues and cell cultures. From this point of view one can deduce the cause behind a greater compatibility of natural and structurally less uniform materials, such as rocks or wood, comparing to pure metals or plastics, the latter of which are known to be potentially carcinogenic (something readily seen by analogously correlating the uniformity of cancerous cells and polymers). Biomolecules with highly repetitive molecular structures are also associated with simpler and more primitive functions within the organism. Fatty acids thus mostly act as membranes, involved in providing a protective coating of processes taking place inside the cell or an organelle; glycogen is used as storage of sugary energetic sources; collagen with its repetitive Gly-X-Y sequence serves to build the structurally supportive framework of the body, etc. On the other hand, complex proteins and nucleic acids possess significantly less redundant molecular structures, which provides them with a highly coded nature, so that even single-point mutations can thoroughly damage their biological functionality.
In that sense, attaining a perfect control over material structure at an exceptionally small scale may not be that essential for the progress in production of novel and superiorly functional materials. A piece of control and a piece of unpredictable development should thus be an ideal of human endeavors in any aspect of their creativity. This brings us again to beauty of the concept of partial control and partial spontaneous assembly of systems evolving in our hands, be it materials we fabricate, young scholars we educate or the biosphere that we will hopefully keep on progressively sustaining.
CLARIFYING THE CONCEPT OF SELF-ASSEMBLY
Although the concept of templating is firmly established in the field of fine particle design, it is questionable whether the imageries of molding and casting can be applied at the level of weak physicochemical interactions. The classical, mechanical descriptions valid in macroscopic and microscopic domains are applicable less and less as one descends first to the nanoscopic level and then to the one of atomic interactions. Likewise, a modification of picturesque representations of physical phenomena is necessary in the framework of fine particle design.
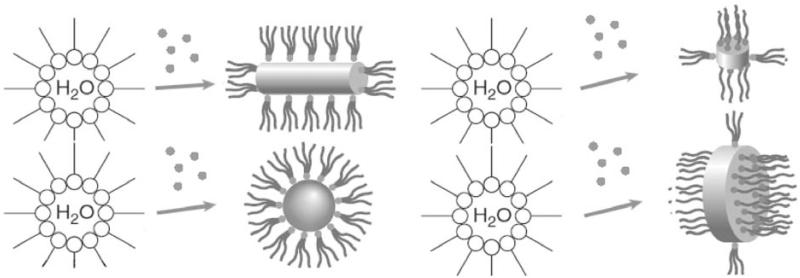
In fact, when it comes to design of fine particles using reverse micelles or other mesoscopic assemblies as templates, we cannot pick any available chemical composition and tailor its morphology based on the morphology of the parent structure. In case of some compounds the interaction may indeed produce templating effects, but in case of other materials a disparity between the parent and the produced structure will be noticed. In fact, the majority of interactions between the synthesized solid material and structurally flexible mesoscopic assemblies are mutual, so that a feedback synergy becomes established all until an equilibrium is reached. It is not only that the mesoscopic surfactant assemblies impose spatial restrictions on the growing particles, but the crystal growth force is also sufficiently intensive so as to affect the topology of the surfactant assemblies (Fig. 11).
Fig. (11).
Upon the introduction of particles that they are meant to assemble, reverse micelles frequently undergo a drastic change in shape. In that sense, the grown, assembled or aggregated particles direct the morphological change on amphiphilic aggregates, whereas the amphiphilic aggregates guide the growth, assembly or aggregation of the particles in the course of this co-assembling interaction. Partially reprinted from Ref. [67].
Reverse micelles have found a special place on my menu of critics in this context, especially due to my long-time involvement in the study thereof [68]. Many examples may be given in support of the hypothesis that their role is not purely to provide a template for the growth of nuclei within, but much more than that [69]. Sometimes there is the resemblance in morphology but a significant discrepancy in size, and sometimes neither size nor shape could be accommodated into the “template” paradigm. For example, acicular BaSO4 particles nucleated in reverse micelles grow up to 5 nm-sized spheres, but then due to an unexplained cooperative surfactant interaction become aggregated forming 5 nm wide filaments. Even more interestingly, and contrary to both standard and intuitive conceptions, the more intensive the interaction between the surfactant (AOT) and the nanoparticles, the more aggregation/alignment takes place. Namely, higher anion concentrations promoting a higher negative charge and a more repulsion with the negatively charged AOT molecules in emulsion provide the only conditions for obtaining discrete particles, whereas conditions promoting a higher level of the electrostatic attraction between the surfactant and nanoparticles lead to aggregation effects and the formation of fibers [70].
Should a purely templating role of reverse micelles and other mesoscopic structural frameworks be accepted, it would be impossible to explain many situations in which particles of thoroughly different morphologies comparing to the ones adopted by the parent micelles are obtained [71-73]. And not only that, but the fact that keeping the shape and size of the micelles constant but employing surfactant of a different identity often results in drastic changes in the identity of the obtained material could not be explained from the passive-role paradigm. In response to that, the membrane surface of reverse micelles is, more and more, referred to in the descriptions of chemical mechanisms of syntheses taking place within these aqueous-amphiphilic aggregates.
In the previous section we have seen that, strictly speaking, it is impossible to thoroughly isolate any system from the context of its existence and evolution. As the control of materials properties descends down to an ever lower scale, and as materials science phenomena become increasingly permeated with difficultly controllable biological entities and interactions, the challenge to eliminate the effects of the system’s surrounding will be ever harder to achieve.
This can easily be witnessed by recalling a simple fact that all the properties of material systems equally depend on their intrinsic properties and on the interaction with the environment. For example, diffusion properties of particles and the stability of their dispersions are determined as much by the intrinsic nature of the particles as by the medium in which they are dispersed. Peptide conformations are preconditioned by their native primary structure, but the proper folding frequently requires their native environment in which their biological functionality takes hold. All the topological features of a stone are shaped equally from within as from without. The history of the interplay between the inner potentials outlined by the stone’s composition and crystal structure and environmental factors manifested during its formation and aging, such as temperature, pressure, humidity, pH and friction, defines the actual properties of the stone. Crystals have a natural tendency to grow such that their visible faces correspond to the most energetically stable atomic planes in the crystal lattice, but at the same time the environmental factors add up their own influence to the physicochemical conditions of the crystal growth. Thence, it is well known that the level of supersaturation in the medium surrounding the growing crystals defines the crystal growth rate and the surface topography (i.e., in general, relatively low levels of supersaturation produce smoother surfaces), but the latter are also dependent on the initial properties of the crystal surface (that is, whether it can be considered as flat, kinked or stepped). Mechanical properties of materials depend not on their structure alone, but on the measurement contexts and settings as well. Mechanical performance of bone thus, for example, may drastically vary when indentations are carried out in dry vs. wet conditions, or in sodium vs. calcium buffers [74]. Changes in the measurement context become significant when evaluating bone quality, which makes sense in view of a fine support bone obtains from the surrounding skeletal, tendon and muscular structures in the body. And so on.
Every chemical reaction depends on the context in which it is induced and in which it evolves, and this complexity of the context of chemical reactions explains why it is impossible to perfectly predict chemical activities of the reacting species and other chemical factors involved in determining the reaction kinetics. All chemical reactions are triggered only within a specific window of conditions provided by the environment of the reacting species, but on the other hand each reaction modifies its environment, resulting in a feedback interaction that normally settles down in an equilibrium state. However, even in a perfect equilibrium state the reaction still occurs, although in a well balanced manner. In essence, there are no static states within any physical system. Every static balance is essentially a dynamic equilibrium. For example, every solid phase in equilibrium with the solution does not rest unchangingly. Instead, the processes of precipitation and dissolution incessantly occur in synchrony, and as a result, even relatively simple precipitation reactions need to be investigated from two directions: crystallization and dissolution [75]. Only then could a decent view at the phase transformation studied be formed.
Le Châtelier’s principle lies at the heart of chemical transformations, and is based on a feedback equilibrium in which the activities of products affect the ones of reactants and vice versa. Likewise, Newton’s law of action and reaction underlies the basic laws of physics, and in sociology and psychology we are aware that explanations of traits of individuals and communities can be explained only at the intersection of the properties of their existential contexts and their intrinsic predispositions. In fact, the ideas of linear causality can be traced back to Aristotle who postulated that ’we regard our knowledge as complete only if we know the initial cause’, and through such an assumption established the foundations of logic. However, the historical refinement of human knowledge has corresponded to transitions from linearized models of physical systems to those that invoke interdependent variables and circular causality.
Aristotle’s general theory of movement (that in its derivative form corresponds to Newton’s law of inertia) was thus replaced by Newton’s law of reciprocal action that emphasized mutual action of objects in contact [76]. Independence of space and time on the movement of objects (i.e., mass) in Newton’s classical physics was replaced by the dependence of space and time coordinates on the observer’s position in Einstein’s special theory of relativity. Furthermore, the definition of space-time coordinates affected by the presence of mass in the latter theory was replaced by the mutual effects within mass (energy) – space-time relationship through introducing the concept of space-time ‘warping’ in the presence of mass. Independence of physical particles has been shown as illusory from the quantum perspective, and in the quantum field theory particles are represented as quantized states of the field that extends throughout the whole space. As quantum descriptions of atomic entities in terms of relations that figure between entities and their environments replaced the classical representations of atoms as entities that are isolated, autonomous and independent on the context of their existence, neither can the living beings be seen any-more as isolated bodies that could be described by René Descartes’ definition of substance as “a being that so exists as to require nothing else for its existence”. Instead, they can be seen as harmonies of interactions spread between them and the rest of the world, all in accordance with the old Christian norm that “we have got only what we give”. Numerous other fields of science nowadays witness transitions from applying unilateral, single-variable and strictly hierarchical models to acknowledging holistic and networked causalities.
Biological self-assembly processes are the prototypes of reactions that do not take place spontaneously and all by themselves, but are inherently guided by the already existing structures and processes. For example, the re-assembly of the capsid proteins of the tobacco mosaic virus occurs only when the viral RNA is present in the reconstitution mixture [77]. However, even then, if the viral RNA gets sheared, the length of the resulting rod-shaped protein assemblies would be proportional to the length of the RNA, suggesting an intrinsic dependency of this assembly process on another structure. In fact, Omnis Cellula e Cellula principle seems to apply for all cellular processes conceivable. Without the preexisting “template” for the formation of novel entities, the latter would be deprived of the conditions for their proper assembly.
Many similar examples in which the nature of the “seed” determines structural properties of the resulting assemblies may be given [77]. To assemble virion particles of bacteriophage T4 that contain 40 different proteins, a number of additional proteins that are eventually not present in the viral assembly are required. Thus, the crucial role that various assemblyases (that is, proteins that catalyze correct assembly processes but do not become a part of the assembled entity) play is given more and more emphasis in the modern biochemical analyses. Chaperons as proteins that assist in the proper folding of other peptides may present another example of “catchers in the rye” in the world of biochemistry [78].
Reverse micelle-assisted routes for the preparation of nanostructured materials may be said to be inspired by the biomineralization processes taking place within intracellular vesicular departments [79]. However, even then, the narrow window of solution conditions seems to be controlled by the surrounding cellular organization. Ions are released into vesicles by a precise controlled pumping, complexation and enzymatic activity, so that calcium concentrations frequently surpass the ones in the surrounding cytoplasm by many orders of magnitude. A difference in pH also has to be established and precisely maintained since, for example, a threeunit difference has to exist across the vesicular membrane in order for the exchange of hydroxyl ions to take place. Also, vesicles are attached to the inner cell wall or some other stabilizing structure, such as organelle or a filament, with microtubules involved in this anchoring. Therefore, vesicles in which the synthesized particles are formed and shaped are themselves templated against some other scaffolds. And these cycles of mutual scaffolding have their ends and beginning merged into one.
Even when the extracellular foam of vesicles is used for biomineralization purposes, such as in the case of the formation of a porous continuous silica mesh of diatoms along the external membrane of vesicular foam, the organization of vesicles is not guided by surface tension restrictions, but by specific morphogenic processes. As such, vesicles are under cellular control just about as much as they evolve by spontaneous assembly under the given conditions. Furthermore, vesicles of spherical or elliptical shapes are known to occasionally give rise to cubic or hexagonally shaped particles during biomineralization.
No matter where we impose boundaries to the environment surrounding an assembling system – a few atomic layers or a few macromolecular radii around it – we can discern well coordinated changes in the system and its environment during the process of co-assembly. Thus, although protein molecules were traditionally represented in terms of their folded primary structure, it is nowadays well established that the surrounding water molecules and hydrogen bonds in which they may figure are essential part of the structure of most, if not all, proteins (Fig. 12). As Philip Ball, a co-editor of Nature magazine, asserts, “the structure and dynamics of this hydration shell seem to feed back onto those aspects of the protein themselves so that biological function depends on a delicate interplay between what we have previously regarded as distinct entities: the molecule and its environment” [80]. Even in the case of fibrous proteins, such as collagen (in which backbone hydrogen bonding between polypeptide chains in its triple-stranded structure does not present the major stabilizing force, as opposed to the case of α–helices and β–sheets), additional enthalpic contributions come from water molecules that form a “scaffold” around the surface of the collagen triple helix, implying that water nonetheless serves an intimate role in stabilizing this protein [81]. As far as the structure of DNA is concerned, water has a crucial role in screening the electrostatic repulsion between the charged phosphate groups and thereby stabilizing the helical structure of the molecule [82].
Fig. (12).
Stereo diagram of the rubredoxin backbone (left, middle) and the structure of prealbumin (right), showing the role of water critically involved in promoting the native conformation of these peptides. Black circles denote iron atoms, whereas empty circles denote water molecules. Dotted lines signify hydrogen bonds. Reprinted with permission from Ref. [83]
The key-and-lock metaphor has presented a dominating one in representing enzymatic reactions in the living domain. Its flaw is that it inherently comprises an assumption of the absence of conformational changes in both the substrate and the enzyme during their recognition and processing. However, the current models normally take into account the conformational adjusting of the enzyme to facilitate the interaction with a substrate. Moreover, it is known that in many cases the substrate molecules also modify their tertiary structure as they enter the active site of catalysis. This indicates that, in general, mutual structural adjusting of the interacting entities conditions chemical modifications in the biological domain.
It is said that ribosomes and other ribonucleoprotein complexes are the only intracellular entities that would self-assemble without any influence of the surrounding medium. However, even though they seemingly do not require a direct interaction with additional cellular components, this does not say that proper physicochemical environment is not required for their formation. A stable organization of the cell is inherently dependent on the properties of its cytoskeleton, composed in large extent of microtubules. After the synthesis of tubulin protein molecules, they get organized in arrays and thus form microtubules, but the assembly of which is determined by the organization of specialized microtubule-organizing centers (Fig. 13). These centers are, on the other hand, in the state of a constant associative interaction with other cytoskeletal components, including actins and myosins.
Fig. (13).
A scheme showing the assembly of macromolecular units into cytoskeletal polymers, and their association into various supramolecular structures, such as filaments or bundles. Note that microtubule-associated proteins (MAPs) and actin-binding proteins (ABPs) are required for a proper assembly of these supramolecular structures that include microtubules (MTs), intermediate filaments (IF) and actin filaments (AFs). Many peptide assemblies and tertiary structures, including the pathological conformations of prions, could not be, therefore, reproduced in vitro, out of their native, intracellular environments owing to their proper assembly or conformational change being preconditioned by the co-assembling activity of other molecular species. Reprinted with permission from Ref. [84].
Proteinaceous particles, known as prions, present another fascinating example of the propagation of allosteric shifts, which in biological environment take shape of an infectious disease, resulting in the formation of amyloid fibrous plaque. Their infectious nature presents evidence of the thesis that a change in the tertiary structure of a protein can be propagated from molecule to molecule. At the same time, it evidences the necessity of introducing epigenetic co-assembly interactions into the classical repertoire of genetic factors and natural selection as involved in determining the phenotype of a species throughout the evolution. No wonder then that template-like reproduction of protein conformations that prions exhibit in context of neurodegenerative diseases has been connected to the inheritance of acquired traits, postulated in Lamarckian theory of evolution [85].
It has recently been shown that not only does gene expression of osteoblasts lead to formation of new bone, but the presence of collagen, calcium and other ions in the osteoblast cell cultures enhances the expression of necessary markers for osteogenesis [86,87]. Thus, it may be a general observation that living systems modify their environment, whereas the environment modifies them. We may, therefore, close the circle between causes and effects in the interaction between a cell/organism and environment within this and most probably every other case in the biological realm. Genetic constitution of the organism thus presents only a chain in the network of intra- and extra-cellular interactions in which each cause is an effect and vice versa [88,89]. Genetic information is crucial to provide the correct sequence of amino acids in a protein, but it does not seem to be sufficient to provide the information on the pathways for the assembly events in which all the cellular components participate. Furthermore, any postulation of a one-to-one correspondence between individual genes and particular phenotypic traits would be incorrect. Instead, individual phenotypic traits are determined by a multitude of interacting genes, whereas single genes normally have multiple regulatory functions in various structural domains of the organism. It is also known that a single gene-regulated protein may control the expression of more than one gene, and vice versa: many gene-regulated proteins often control the expression of a single gene [90]. This is why the role of epigenetic pathways in gene expression presents an increasing subject of molecular biological studies. Just as the flow of information associated with replication-transcription-translation chain of events shows heritability, the same can be said for other epigenetic assembly processes, despite the fact that they normally involve weak and not covalent interactions like the former.
Thus we come to the principle of autopoiesis as the one describing life in all of its morphogenetic aspects [91,92]. It says that the purpose of all biological entities is a continual involvement in the production of all other entities within the given system. This principle seems to be valid irrespective of whether we consider macromolecules in a cell, cells in the body, organisms in an ecosystem, or ecosystems in the biosphere. Whereas in the world of mechanics Newton’s law of action and reaction illustrates the fundamental principle of change according to which it becomes impossible to exert an impulse on a body without the body exerting an equal force in the opposite direction, in the biological domain autopoietic features of living species reflect the fact that every living system presents a harmony of processes in which no distinction between the ‘creator’ and the ‘created’ could be easily made.
This is how we come back to the starting point that the boundary between the system and its environment will be harder and harder to establish for the purpose of precise control over ever finer experimental details and settings. This point of view is in sympathy with the modern awakening of the interest in holistic nature of physical phenomena, in which the delicate correlations between the most distant parts of the biological whole are increasingly being pointed out. Just like the context is crucial in defining the meaning of any text or system enwrapped in it, the same can be said for the most advanced chemical design settings today. In fact, influence of the environment in which they evolve will prove to be of an ever increasing importance. As humans are inextricably related to their natural substrates, the preservation of which largely threatens the ideals of sustainable development nowadays [93], the consideration of environmental settings will be vital for the proper control of fine experiments of the future.
DICHOTOMY OF ASSEMBLING INTERACTIONS
So far we have seen that every “self-assembly” process is inherently dependent on the surrounding physical structures and sets of conditions, and can be, therefore, regarded as a form of co-assembly. A principle similar to Newton’s law of action and reaction, according to which an ordering process within a system has to be coupled with corresponding changes in the system’s closest surrounding, can be envisaged herein.
Albeit this, when we particularly consider the self-assembly of inorganic and polymeric systems in the context of modern synthetic chemistry, a useful dichotomy can be invoked [94]. According to it, two types of self-assembly mechanisms may be distinguished. To the first category belong all the assembly processes in which the underlying structure is stable enough to impose its own structural features to the one of the assembled system. In this case, a close resemblance between the template and the assembled morphology could be observed. The relative inflexibility of the substrate in this assembly mechanism normally leads to difficulties in the attempts to post-synthetically separate the two phases. Due to the strong interaction imposed by the substrate, these problems can be indeed significant.
To the second category belong all the interactions between the substrate and the assembled phase in which mutual re-shaping occurs. Whereas in the former case, the morphology of the assembled phase may be predicted by knowing the underlying morphology, this is certainly not the case for the co-assembly mechanisms in which a feedback loop is established between the co-structured phases until equilibrium is reached with the co-existence of two unexpected and hardly explainable morphologies. Whenever we have the case of acicular particles co-existing within elliptical micelles, we cannot be sure whether it was the uniaxial crystal growth that elongated the micelles or it was the elongation of micelles that directed the preferential growth of the comprising crystals.
To satisfy both the ideals of predictability and facile phase separation, we need to find a middle way that corresponds to neatly balanced rigidity and flexibility (Fig. 14). Thus we may overcome both weak and phase-blending interactions inherent to the synergistic approach and strong and phase-adhering interactions of the scaf-folding approach. In view of the simultaneous thermodynamic openness and iterative operational closeness of every biological entity, the balance between flexibility and rigidity can be recognized as crucial for enabling healthy development of every aspect of one’s creativity.
Fig. (14).
A kinesin molecule as it carries a vesicle during its “walk” along a microtubule. The mechanistic connotation of the image is, however, underlain by an array of molecular recognition interactions and the corresponding co-assembly phenomena. Hence, the soft chemical aspect of spontaneous assembly ought to be integrated with the hard chemical aspect of litho-graphic and other rigid methods of control of the systems in question in order to maximize the synthetic, processing and functional effectiveness of scientific endeavors. Reprinted with permission from Ref. [95].
There is also a special set of interactions between the self-assembling system and the substrate in which an almost complete inertia of the latter is required to induce a proper assembly. This does not mean that the parent structure is not subject to change, but signifies a mild set of modifications taking place at a narrow scale, due to an apparent inflexibility of the substrate. Even though changes at the level of the substrate as a whole may not be readily detectable, the closest atomic layers surrounding self-assembling entities are inevitably subject to organized change, as the co-assembling nature of each self-assembly suggests. Theoretically, one can imagine a substrate that with its complete, vacuum-like inertness forces the entities to rely on their own intrinsic propensities to self-assemble, but these situations are so rare that may be considered as an exception that merely confirms the rule. Usually, the self-assembling entities are adsorbed on a substrate or dispersed within a gaseous or liquid medium, which implies weak interactions existing between the self-assembling system and its environment. It is exactly these weak interactions that in most situations guide the self-assembling process along its complex kinetic pathway.
The observation that not a single system could be precisely demarcated from its environment so that the latter does not exhibit any effect upon the evolution of the former brings us to the link between the concept of co-assembly and the aforementioned challenge of attaining control over physicochemical synthetic and functional processes at nano and sub-nano scales. I have previously mentioned a few examples, including the effect of gaseous particles adsorbing on the ultrafine surface of the system components, functional characteristics of biomolecules arising only in specific biological environments, and the impossibility of providing conditions for a perfect homogeneous nucleation because the container walls will always present a potential nucleation surface. In the end, the very human interference with these fine experiments, apparently acting as their creative impetus, can be, strictly speaking, considered as a form of environmental effect. As there always needs to be a form of such interference, its fineness would determine the successfulness of the design of matter at the ultrafine scale. After all, “fat and sticky finger” problem, posed as a general obstacle to the development of nanomolecular machines using the concept of atom-by-atom manipulation, can be considered as the problem tied with inevitable effects of environment on the evolution of the physical systems under control. To quote Richard E. Smalley, “Much like you cannot make a boy and a girl fall in love with each other simply by pushing them together, you cannot make precise chemistry occur as desired between two molecular objects with simple mechanical motion along a few degrees of freedom in the assembler-fixed frame of reference. Chemistry, like love, is more subtle than that” [96].
Thus, at the very end, we arrive at a mere confirmation of the starting point of this paper. It is that the fineness and sensitivity of human interaction with the natural substrate (a.k.a. matter) determine the fineness and sensitivity of the material structures and devices that will come out of this interaction. This idea can be correlated with the concept of co-creation, earlier proposed by the author, and applicable to many, if not all, aspects of human experience, including perception, cognitive reflections, biological evolution, psychosocial traits, and the progress of human knowledge [97,98]. According to the idea of co-creation, each aspect of human experience arises through the interplay between the creativity of human mind and environmental incentives. In the course of this interaction, both are subject to mutual change. Mind draws Nature, and Nature draws human mind, as I love to say.
CONCLUSIONS
The major difficulties that science will face in descending towards interaction with ever smaller and finer physical phenomena are related to the inability to demarcate and keep control over the boundary that separates the system from its environment below certain size limits. The fundamental limit of this interference is outlined by Heisenberg’s uncertainty principle, although other effects will present a more immediate obstacle in attempts to expand the limits of human control at the small scale. The problems of irreproducible experiments and incalculable reaction settings are the signs that the limit has been approached. This inability to perfectly separate a system for its environment explains why each self-assembly process presents essentially a co-assembly event. The real challenge will, nonetheless, be dwelling upon this boundary in our research, rather than looking for safe areas of knowledge in which mere conformations of preexisting paradigms may take place. The ethical character of scientific research, an encompassing of which requires an even larger breadth compared with the one this paper occupies, will be increasingly shown as a vital part thereof as the pressure to “publish or perish” coupled with less and less reproducible experiments will invite ever more researchers to compromise a honest quest for knowledge with the desire to prove their own scientific qualities and advance in career. However, once we realize that experiments that do not fit our preconceptions are the starting points of exciting new discoveries that will not merely confirm the existing knowledge, but may possibly lead to groundbreaking insights, we may learn how to joyfully and patiently approach them. The more we are ready to risk and possibly fall in the research adventures of ours by walking along these limiting boundaries where the coasts of affirmed knowledge and the sea of unsettling ideas and experimental conditions meet, the more of the treasures of knowledge will we be able to bring to the world.
ACKNOWLEDGEMENTS
The writing of this work took place during the author’s involvement in the research project supported by the NIH grant R01-DE017529. Dr. Stefan Habelitz is gratefully acknowledged for valuable discussions and support.
REFERENCES
- [1].Uskoković V. Nanomaterials and nanotechnologies: approaching the crest of this big wave. Curr. Nanosci. 2008;4:119–129. [Google Scholar]
- [2].Uskoković V. Nanotechnologies: what we do not know. Tech. Soc. 2007;29(1):43–61. [Google Scholar]
- [3].Li X, Chen H, Dang Y, Lin Y, Larson CA, Roco MC. A longitudinal analysis of nanotechnology literature: 1976-2004. J. Nanopart. Res. 2008;10:3–22. [Google Scholar]
- [4].Kay L, Shapira P. Developing Nanotechnology in Latin America. J. Nanopart. Res. 2008;11(2):259–278. doi: 10.1007/s11051-008-9503-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hochella MF., Jr. Nanoscience and Technology: The next revolution in the earth sciences. Earth Planet Sci. Lett. 2002;203:593–605. [Google Scholar]
- [6].Hochella MF, Jr., Lower SK, Maurice PA, Penn RL, Sahai N, Sparks DL, Twining BS. Nanominerals, Mineral nanoparticles, and earth systems. Science. 2008;319:1631–1635. doi: 10.1126/science.1141134. [DOI] [PubMed] [Google Scholar]
- [7].Bateson G. Hampton Press; Cresskill, NJ: 1979. Mind and nature: a Necessary Unity. [Google Scholar]
- [8].Brown GS. Laws of form. Cognizer Press; Portland, OR: 1969. [Google Scholar]
- [9].Navrotsky A, Mazeina L, Majzlan J. Size-Driven structural and thermodynamic complexity in iron oxides. Science. 2008;319:1635–1638. doi: 10.1126/science.1148614. [DOI] [PubMed] [Google Scholar]
- [10].Tang RK, Wang LJ, Nancollas GH. Size-effects in the dissolution of hydroxyapatite. an understanding of biological demineralization. J. Mater. Chem. 2004;14:2341–2346. [Google Scholar]
- [11].Uskoković V. Theoretical and practical aspects of colloid science and self-assembly phenomena revisited. Rev. Chem. Eng. 2007;23(5):301–372. [Google Scholar]
- [12].Andrievskiy RA. Size-dependent interface effects in properties of nanomaterials; Presented at the 10th YUCOMAT Conference; Herceg-Novi, Montenegro. 2008.Sep 8-12, [Google Scholar]
- [13].Jesser WA, Shneck RZ, Gile WW. Solid-Liquid Equilibria in Nano Particles of Pb-Bi Alloys. Phys. Rev. B. 2004;69 144121/1-13. [Google Scholar]
- [14].Lu K, Jin ZH. Melting and superheating of low-dimensional materials. Curr. Opin. Solid State Mater. Sci. 2001;5:39–44. [Google Scholar]
- [15].Uskoković V, Drofenik M, Ban I. The Characterization of nanosized nickel-Zinc ferrites synthesized within reverse micelles of CTAB/1-hexanol/water microemulsion. J. Magn. Magn. Mater. 2004;284:294–302. [Google Scholar]
- [16].Uskoković V, Drofenik M. Synthesis of relatively highly magnetic nano-sized NiZn-ferrite in microemulsion at 45 °C. Surf. Rev. Lett. 2005;12(1):97–100. [Google Scholar]
- [17].Drexler KE. Engines of creation: the coming era of nanotechnology. anchor. New York, NY: 1986. [Google Scholar]
- [18].Smalley RE. Of chemistry, love and nanobots. Sci. Am. 2001;285(3):68–69. doi: 10.1038/scientificamerican0901-76. [DOI] [PubMed] [Google Scholar]
- [19].Tirrell MV, Katz A, editors. Self-assembly in materials synthesis. MRS Bull. 2005;30 [Google Scholar]
- [20].Uskoković V. Questions and challenges for the upcoming trends in practical colloid science. In: Emelio A, editor. Progress in Colloid and Surface Science Research. Scarpetti, Nova Science Publishers; Hauppauge, NY: 2007. pp. 1–51. [Google Scholar]
- [21].Emanuel K, Edward N. Lorenz (1917-2008) Science. 2008;320(5879):1025. doi: 10.1126/science.1159438. [DOI] [PubMed] [Google Scholar]
- [22].Uskoković V, Drofenik M. A mechanism for the formation of nanostructured NiZn ferrites via a microemulsion-assisted precipitation method. Coll. Surf A. 2005;266:168–174. [Google Scholar]
- [23].Uskoković V, Kim M-K, Li W, Habelitz S. Enzymatic processing of amelogenin during continuous crystallization of apatite“. J. Mater. Res. 2008;32:3184–3195. doi: 10.1557/JMR.2008.0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Walsh D, Lebeau B, Mann S. Morphosynthesis of calcium carbonate (Vaterite) microsponges. Adv. Mater. 1999;11(4):324–328. [Google Scholar]
- [25].Atkins P, de Paula J. Physical Chemistry. 7th ed. Oxford University Press; Oxford, UK: 2002. [Google Scholar]
- [26].Ninham B. Self-Assembly-Some Thoughts. In: Robinson BH, editor. Self-Assembly. IOS Press; Amsterdam: 2003. [Google Scholar]
- [27].Melikhov IV, Lazić S, Vuković Ž . The effect of dissolved impurity on calcium phosphate nucleation in supersaturated medium. J. Coll. Interface Sci. 1989;127(2):317–327. [Google Scholar]
- [28].Loerting T, Tautermann C, Kroemer RT, Kohl I, Hallbrucker A, Mayer E, Liedl KR. On the surprising kinetic stability of carbonic acid (H2CO3) Angew Chem. Int. Ed. 2000;39(5):891–894. doi: 10.1002/(sici)1521-3773(20000303)39:5<891::aid-anie891>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- [29].Uskoković V. Insights into morphological nature of precipitation of cholesterol. Steroids. 2008;73:356–369. doi: 10.1016/j.steroids.2007.12.005. [DOI] [PubMed] [Google Scholar]
- [30].Canham LT, Reeves CL, Loni A, Houlton MR, Newey JP, Simons AJ, Cox TI. Calcium phosphate nucleation on porous silicon: factors influencing kinetics in acellular simulated body fluids. Thin Solid Films. 1997;297:304–307. [Google Scholar]
- [31].Nagy IP, Bazsa Gy. Artifacts caused by plastics in chemical experiments: Absorption, degradation, catalysis and irreproducible behavior. React. Kin Catal. Lett. 1991;45(1):15–25. [Google Scholar]
- [32].Kokubo T, Takadama H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials. 2006;27:2907–2915. doi: 10.1016/j.biomaterials.2006.01.017. [DOI] [PubMed] [Google Scholar]
- [33].Zhang JW, Nancollas GH. Mechanisms of growth and dissolution of sparingly soluble salts. In: Hochella MF Jr., White AF, editors. Mineral-Water Interface Geochemistry. [Google Scholar]; Reviews in Mineralogy. 1990;23:365–396. [Google Scholar]
- [34].Madsen HEL. Influence of magnetic field on the precipitation of some inorganic salts. J. Crystal Growth. 1995;152(1):94–100. [Google Scholar]
- [35].Reitas AMBF, Andgraf FJGL, Yvlt JN, Iuletti MG. Influence of magnetic field in the kinetics of crystallization of diamagnetic and paramagnetic inorganic salts. Crystal Res. Tech. 1999;34(10):1239–1244. [Google Scholar]
- [36].Uskoković V. Composites comprising cholesterol and carboxymethyl cellulose. Coll. Surf. B. 2008;61:250–261. doi: 10.1016/j.colsurfb.2007.08.014. [DOI] [PubMed] [Google Scholar]
- [37].Bunker CE, Harruff BA, Pathak P, Payzant A, Allard LF, Sun YP. Formation of cadmium sulfide nanoparticles in reverse micelles: extreme sensitivity to preparation procedure. Langmuir. 2004;20:5642–5644. doi: 10.1021/la049607+. [DOI] [PubMed] [Google Scholar]
- [38].Debuigne F, Jeunieau L, Wiame M, Nagy JB. Synthesis of organic nanoparticles in different W/O microemulsions. Langmuir. 2000;16:7605–7611. [Google Scholar]
- [39].Matijević E. Colloid science of ceramic powders. Pure Appl. Chem. 1988;60(10):1479–1491. [Google Scholar]
- [40].Matijević E, Babu SV. Colloid aspects of chemical-mechanical planarization. J. Coll. Interface Sci. 2008;320(1):219–237. doi: 10.1016/j.jcis.2007.11.057. [DOI] [PubMed] [Google Scholar]
- [41].Okubo T, Tsuchida A. Microgravity effects on thermodynamic and kinetic properties of colloidal dispersions. Ann. N.Y. Acad. Sci. 2002;974:164–175. doi: 10.1111/j.1749-6632.2002.tb05906.x. [DOI] [PubMed] [Google Scholar]
- [42].Dokou E, Barteau MA, Wagner NJ, Lenhoff AM. Effect of gravity on colloidal deposition studied by atomic force microscopy. J. Coll. Interface Sci. 2001;240(1):9–16. doi: 10.1006/jcis.2001.7626. [DOI] [PubMed] [Google Scholar]
- [43].Matijević E. Monodispersed colloids: art and science. Langmuir. 1986;2:12–20. [Google Scholar]
- [44].Polanyi M. The value of the inexact. Phil. Sci. 1936;13:233–234. [Google Scholar]
- [45].Uskoković V. Surface charge effects involved in the control of stability of sols comprising uniform cholesterol particles. Mater. Man Proc. 2008;23(6):620–623. [Google Scholar]
- [46].Jeng M. Hot water can freeze faster than cold? Am. J. Phys. 2006;74:514. [Google Scholar]
- [47].Ball P. Does hot water freeze first? Phys. World. 2006:19. [Google Scholar]
- [48].Wang L, Guan X, Du C, Moradian-Oldak J, Nancollas GH. Amelogenin promotes the formation of elongated apatite microstructures in a controlled crystallization system. J. Phys. Chem. C. 2007;111(17):6398–6404. doi: 10.1021/jp0675429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Matijević E. Preparation and characterization of well defined powders and their applications in technology. J. Eur. Ceram. Soc. 1998;18:1357–1364. [Google Scholar]
- [50].Uskoković V, Matijević E. Uniform particles of pure and silica coated cholesterol. J. Coll Interface Sci. 2007;315(2):500–511. doi: 10.1016/j.jcis.2007.07.010. [DOI] [PubMed] [Google Scholar]
- [51].Tomson MB, Nancollas GH. Mineralization kinetics; a constant composition approach. Science. 1978;200:1059–1060. doi: 10.1126/science.200.4345.1059. [DOI] [PubMed] [Google Scholar]
- [52].Koutsopoulos S, Dalas E. Hydroxyapatite crystallization in the presence of serine, tyrosine and hydroxyproline amino acids with polar side groups. J. Crystal Growth. 2000;216:443–449. [Google Scholar]
- [53].Chow LC, Markovic M, Takagi S. A dual constant-composition titration system as an in vitro resorption model for comparing dissolution rates of calcium phosphate biomaterials. J. Biomed. Mater. Res B: Appl. Biomater. 2003;65B:245–251. doi: 10.1002/jbm.b.10009. [DOI] [PubMed] [Google Scholar]
- [54].Tang R, Henneman ZJ, Nancollas GH. Constant composition kinetics study of carbonated apatite dissolution. J. Crystal Growth. 2003;249:614–624. [Google Scholar]
- [55].Ebrahimpour A, Zhang J, Nancollas GH. Dual constant composition method and its application to studies of phase transformation and crystallization of mixed phases. J. Crystal Growth. 1991;113:83–91. [Google Scholar]
- [56].Mutaftschiev B. The atomistic nature of crystal growth. Springer; Berlin: 2001. [Google Scholar]
- [57].Arai Y. Chemistry of powder production. Springer; Berlin: 1996. [Google Scholar]
- [58].De Aza PN, Guitian F, Merlos A, Lora-Tamayo E, De Aza S. Bioceramics-simulated body fluid interfaces: pH and its influence of hydroxypatite formation. J. Mater. Sci. Mat. Med. 1996;7:399, 402. [Google Scholar]
- [59].Lu X, Leng Y. Theoretical analysis of calcium phosphate precipitation in simulated body fluid. Biomaterials. 2005;26(10):1097–1108. doi: 10.1016/j.biomaterials.2004.05.034. [DOI] [PubMed] [Google Scholar]
- [60].Lazi S. Microcrystalline hydroxyapatite formation from alkaline solutions. J. Crystal Growth. 1995;147:147–154. [Google Scholar]
- [61].Orme CA, Giocondi JL. Model systems for formation and dissolution of calcium phosphate minerals. In: Behrens P, Bäuerlein E, editors. Handbook of Biomineralization. Vol. 2. Wiley; Weinheim: 2007. [Google Scholar]
- [62].Privman V, Goia DV, Park J, Matijević E. Mechanism of formation of monodispersed colloids by aggregation of nanosize precursors. J. Coll. Interface Sci. 1999;213:36–45. doi: 10.1006/jcis.1999.6106. [DOI] [PubMed] [Google Scholar]
- [63].Matijević E. Nanosize precursors as building blocks for monodispersed colloids. Coll. J. 2007;69(1):29–38. [Google Scholar]
- [64].Uskoković V. Isn’t self-assembly a misnomer? multi-disciplinary arguments in favor of co-assembly. Adv. Coll. Interface Sci. 2008;141(1-2):37–47. doi: 10.1016/j.cis.2008.02.004. [DOI] [PubMed] [Google Scholar]
- [65].Viney C, Bell FI. Inspiration versus duplication with biomolecular fibrous materials: learning nature’s lessons without copying nature’s limitations. Curr. Opin. Solid State Mater. Sci. 2004;8:165–171. [Google Scholar]
- [66].Stevens MM. Biomaterials for bone tissue engineering. Mater. Today. 2008;11(5):18–25. [Google Scholar]
- [67].Yin Y, Alivisatos P. Colloidal nanocrystal synthesis and the organic-inorganic interface. Nature. 2005;437:664–670. doi: 10.1038/nature04165. [DOI] [PubMed] [Google Scholar]
- [68].Uskoković V, Drofenik M. Synthesis of materials within reverse micelles. Surf. Rev. Lett. 2005;12(2):239–277. [Google Scholar]
- [69].Uskoković V, Drofenik M. Reverse micelles: inert nano-reactors or physico-chemically active guides of the capped reactions. Adv. Coll. Interface Sci. 2007;133(1):23–34. doi: 10.1016/j.cis.2007.02.002. [DOI] [PubMed] [Google Scholar]
- [70].Hopwood JD, Mann S. Synthesis of barium sulfate nanoparticles and nanofilaments in reverse micelles and microemulsions. Chem. Mater. 1997;9:1819–1828. [Google Scholar]
- [71].Boutonnet M, Kizling J, Stenius P. The preparation of monodisperse colloidal metal particles from micro-emulsions. Coll Surf. 1982;5(3):209–225. [Google Scholar]
- [72].Lin JC, Dipre JT, Yates MZ. Microemulsion-directed synthesis of molecular sieve fibers. Chem. Mater. 2003;15:2764–2773. [Google Scholar]
- [73].Hua R, Zang C, Shao C, Xie D, Shi C. Synthesis of barium fluoride nanoparticles from microemulsion. Nanotechnology. 2003;14:588–591. [Google Scholar]
- [74].Bertassoni L, Habelitz S, Kinney J, Marshall S, Marshall G. Biomechanistic perspective on the remineralization of dentin. Caries Res. 2009;43:70–77. doi: 10.1159/000201593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Tang R, Nancollas GH. New mechanism for the dissolution of sparingly soluble mineral. Pure Appl. Chem. 2002;74(10):1851–1857. [Google Scholar]
- [76].Ulanowicz RE. On the nature of ecodynamics. Ecol. Complex. 2004;1:341–354. [Google Scholar]
- [77].Grimes GW, Aufderheide KJ. Basel; Karger: 1991. Cellular aspects of pattern formation: the problem of assembly. [PubMed] [Google Scholar]
- [78].Gebeshuber IC, Drack M, Aumayr F, Winter H, Franek F. Scanning probe microscopy: from living cells to the subatomic range. In: Bhushan B, Fuchs H, editors. Applied Scanning Probe Methods II. Springer-Verlag; New York, NY: 2005. [Google Scholar]
- [79].Mann S. Biomineralization: Principles and concepts in bioinorganic materials chemistry. Oxford University Press; Oxford, UK: 2001. [Google Scholar]
- [80].Ball P. Water as an active constituent in cell biology. Chem. Rev. 2008;108(1):74–108. doi: 10.1021/cr068037a. [DOI] [PubMed] [Google Scholar]
- [81].Cooper A. Thermodynamics of Protein Folding and Stability. In: Allen G, editor. Protein: A Comprehensive Treatise. Vol. 2. JAI Press; Stamford, CN: 1999. pp. 217–270. [Google Scholar]
- [82].Peyrard M. Nonlinear dynamics and statistical physics of DNA. Nonlinearity. 2004;17:R1–R40. [Google Scholar]
- [83].Richardson JS. The anatomy and taxonomy of protein structure. Adv. Protein Chem. 1981;34:167–339. doi: 10.1016/s0065-3233(08)60520-3. [DOI] [PubMed] [Google Scholar]
- [84].Ramirez G, Alvarez A, Garcia-Abreu J, Gomes FCA, Moura-Neto V, Maccioni RB. Regulatory roles of microtubule-associated proteins in neuronal morphogenesis. involvement of the extracellular matrix. Braz. J. Med. Biol. Res. 1999;32(5):611–618. doi: 10.1590/s0100-879x1999000500015. [DOI] [PubMed] [Google Scholar]
- [85].Chernoff YO. Mutation processes at the protein level: is lamarck back? Mut. Res. 2001;488(1):39–64. doi: 10.1016/s1383-5742(00)00060-0. [DOI] [PubMed] [Google Scholar]
- [86].Varanasi V, Saiz E, Loomer PM, Ancheta B, Uritani N, Tomsia AP, Marshall SJ, Marshall GW. Direct relationship between enhanced gene and matrix protein expression by differentiating pre-osteoblasts exposed to bioactive glass ions. Acta Biomater. 2009 doi: 10.1016/j.actbio.2009.05.035. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Hench LL. Genetic design of bioactive glass. J. Eur. Ceram. Soc. 2009;29:1257–1265. [Google Scholar]
- [88].Maturana H, Verden-Zöller G. Biology of Love. In: Opp G, Peterander FF, editors. Focus Heilpadagogik. Ernst Reinhardt; München: 1996. [Google Scholar]
- [89].Bunnell P. changing the question. Cyber Hum. Know. 2004;11(4):5–10. [Google Scholar]
- [90].Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Garland Science; Oxford, UK: 2007. Molecular biology of the cell. [Google Scholar]
- [91].Maturana H, Varela F. Shambhala; Boston, MA: 1987. The Tree of knowledge: the biological roots of human understanding. [Google Scholar]
- [92].Romesin HM. Autopoiesis, structural coupling and cognition: a history of these and other notions in the biology of cognition. Cyber Hum. Know. 2002;9(3-4):5–34. [Google Scholar]
- [93].Uskoković V. Of sustainability, elephants and prefab sprouts. Int. J. Sustain Soc. 2008;1(1):85–102. [Google Scholar]
- [94].Cölfen H, Mann S. Higher-order organization by mesoscale self-assembly and transformation of hybrid nanostructures. Angew Chem. Int. Ed. 2003;42:2350–2365. doi: 10.1002/anie.200200562. [DOI] [PubMed] [Google Scholar]
- [95].Biomolecular Self-Assembling Materials: Scientific and Technological Frontiers. National Academy of Sciences; Washington, DC: 1996. [PubMed] [Google Scholar]
- [96].Baum R. Nanotechnology: drexler and smalley make the case for and against ‘molecular assemblers’. Chem. Eng. News. 2003;81:48. [Google Scholar]
- [97].Uskoković V. On science of metaphors and the nature of systemic reasoning. world futures. J. Gen. Evol. 2009;65:241–269. [Google Scholar]
- [98].Uskoković V. On the light doves and learning on mistakes. Axiomathes. 2009;19:17–50. [Google Scholar]