Abstract
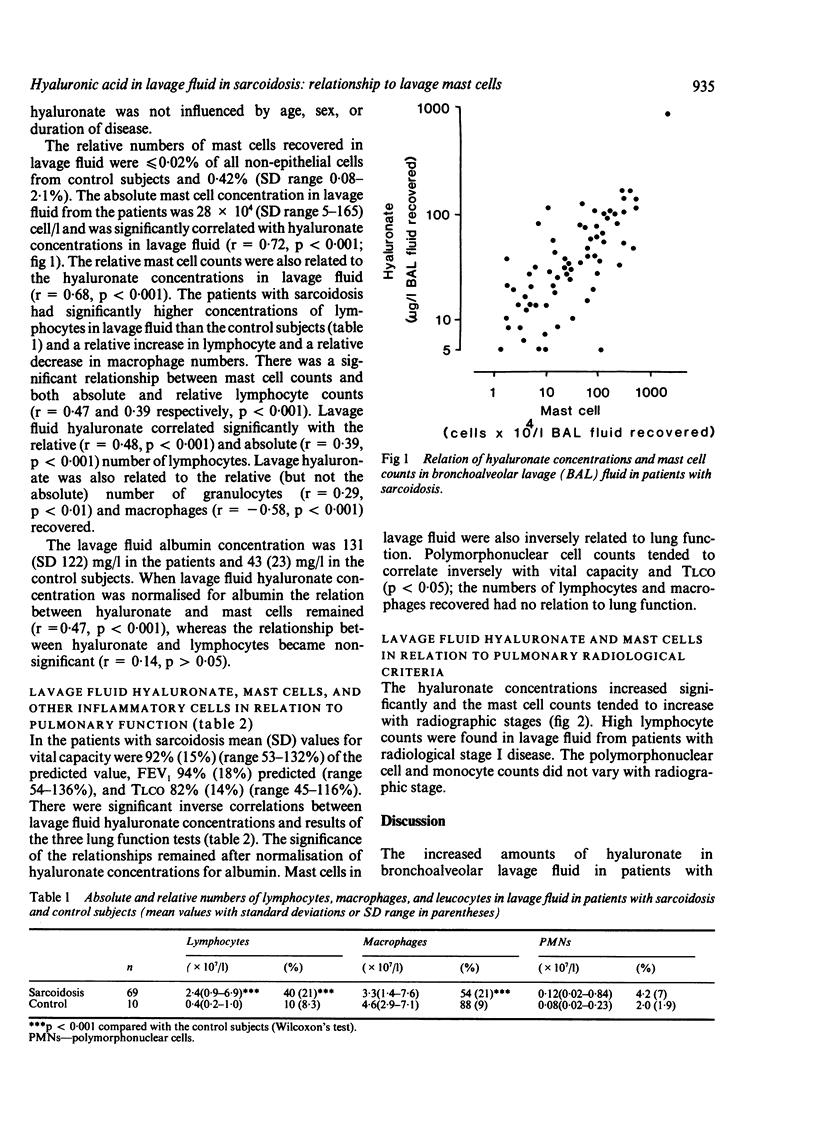
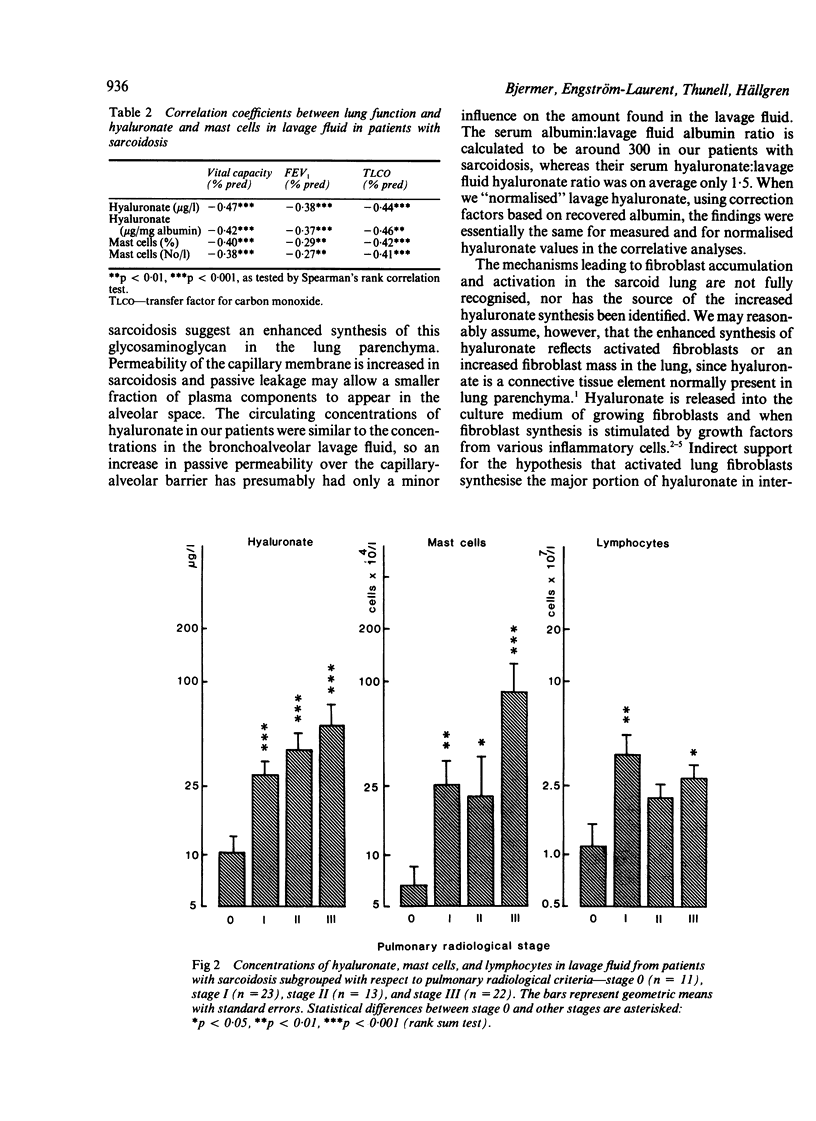
Hyaluronate (hyaluronic acid), a potential marker for activated pulmonary fibroblasts, appears in increased concentrations in bronchoalveolar lavage fluid from patients with sarcoidosis. The mechanisms underlying fibroblast proliferation are largely unknown but activated alveolar T lymphocytes and macrophages probably play a part; the mast cell is also important for fibroblast proliferation. This study was designed to determine whether there is any association between pulmonary mast cells in lavage fluid, which are known to be increased in patients with sarcoidosis, and signs of pulmonary fibroblast activation. A strong correlation was found between lavage fluid hyaluronate and recovered mast cells (r = 0.72, p less than 0.001). Moreover, mast cell and hyaluronate estimations correlated inversely with lung volume and transfer factor for carbon monoxide, and both indices increased with advancing radiological sarcoid stage. Macrophage and granulocyte counts were normal in lavage fluid from patients with sarcoidosis and were not related to lavage fluid hyaluronate or laboratory signs of the disease in the lungs. Lymphocytes were recovered in increased numbers (p less than 0.001) and were related to the lavage fluid mast cells and hyaluronate. It is concluded that in sarcoidosis release of hyaluronate into the airways is related to the degree of lung disease and to the local inflammatory reaction in the lung as defined by increased numbers of mast cells and lymphocytes in lavage fluid. The findings may reflect a link between the immune system, activation of mast cells, and a pulmonary fibroblast proliferation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins F. M., Friedman M. M., Subba Rao P. V., Metcalfe D. D. Interactions between mast cells, fibroblasts and connective tissue components. Int Arch Allergy Appl Immunol. 1985;77(1-2):96–102. doi: 10.1159/000233760. [DOI] [PubMed] [Google Scholar]
- Atkins F. M., Metcalfe D. D. Degradation of the heparin matrix of mast cell granules by cultured fibroblasts. J Immunol. 1983 Sep;131(3):1420–1425. [PubMed] [Google Scholar]
- Bitterman P. B., Rennard S. I., Hunninghake G. W., Crystal R. G. Human alveolar macrophage growth factor for fibroblasts. Regulation and partial characterization. J Clin Invest. 1982 Oct;70(4):806–822. doi: 10.1172/JCI110677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjermer L., Bäck O., Roos G., Thunell M. Mast cells and lysozyme positive macrophages in bronchoalveolar lavage from patients with sarcoidosis. Valuable prognostic and activity marking parameters of disease? Acta Med Scand. 1986;220(2):161–166. doi: 10.1111/j.0954-6820.1986.tb02744.x. [DOI] [PubMed] [Google Scholar]
- Dy M. Increase in histamine production and mast cell proliferation during allograft rejection. Transplant Proc. 1982 Sep;14(3):575–577. [PubMed] [Google Scholar]
- Engström-Laurent A., Feltelius N., Hällgren R., Wasteson A. Raised serum hyaluronate levels in scleroderma: an effect of growth factor induced activation of connective tissue cells? Ann Rheum Dis. 1985 Sep;44(9):614–620. doi: 10.1136/ard.44.9.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engström-Laurent A., Laurent U. B., Lilja K., Laurent T. C. Concentration of sodium hyaluronate in serum. Scand J Clin Lab Invest. 1985 Oct;45(6):497–504. doi: 10.3109/00365518509155249. [DOI] [PubMed] [Google Scholar]
- Goto T., Befus D., Low R., Bienenstock J. Mast cell heterogeneity and hyperplasia in bleomycin-induced pulmonary fibrosis of rats. Am Rev Respir Dis. 1984 Nov;130(5):797–802. doi: 10.1164/arrd.1984.130.5.797. [DOI] [PubMed] [Google Scholar]
- Haig D. M., McKee T. A., Jarrett E. E., Woodbury R., Miller H. R. Generation of mucosal mast cells is stimulated in vitro by factors derived from T cells of helminth-infected rats. Nature. 1982 Nov 11;300(5888):188–190. doi: 10.1038/300188a0. [DOI] [PubMed] [Google Scholar]
- Hamerman D., Wood D. D. Interleukin 1 enhances synovial cell hyaluronate synthesis. Proc Soc Exp Biol Med. 1984 Oct;177(1):205–210. doi: 10.3181/00379727-177-1-rc1. [DOI] [PubMed] [Google Scholar]
- Haslam P. L., Dewar A., Butchers P., Primett Z. S., Newman-Taylor A., Turner-Warwick M. Mast cells, atypical lymphocytes, and neutrophils in bronchoalveolar lavage in extrinsic allergic alveolitis. Comparison with other interstitial lung diseases. Am Rev Respir Dis. 1987 Jan;135(1):35–47. doi: 10.1164/arrd.1987.135.1.35. [DOI] [PubMed] [Google Scholar]
- Hibbs M. S., Postlethwaite A. E., Mainardi C. L., Seyer J. M., Kang A. H. Alterations in collagen production in mixed mononuclear leukocyte-fibroblast cultures. J Exp Med. 1983 Jan 1;157(1):47–59. doi: 10.1084/jem.157.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W. Release of interleukin-1 by alveolar macrophages of patients with active pulmonary sarcoidosis. Am Rev Respir Dis. 1984 Apr;129(4):569–572. [PubMed] [Google Scholar]
- Hällgren R., Eklund A., Engström-Laurent A., Schmekel B. Hyaluronate in bronchoalveolar lavage fluid: a new marker in sarcoidosis reflecting pulmonary disease. Br Med J (Clin Res Ed) 1985 Jun 15;290(6484):1778–1781. doi: 10.1136/bmj.290.6484.1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawanami O., Ferrans V. J., Fulmer J. D., Crystal R. G. Ultrastructure of pulmonary mast cells in patients with fibrotic lung disorders. Lab Invest. 1979 Jun;40(6):717–734. [PubMed] [Google Scholar]
- Kitamura Y., Shimada M., Go S., Matsuda H., Hatanaka K., Seki M. Distribution of mast-cell precursors in hematopoeitic and lymphopoietic tissues of mice. J Exp Med. 1979 Sep 19;150(3):482–490. doi: 10.1084/jem.150.3.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl U., Pertoft H., Seljelid R. Uptake and degradation of mast-cell granules by mouse peritoneal macrophages. Biochem J. 1979 Jul 15;182(1):189–193. doi: 10.1042/bj1820189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykke A. W., Schonell M. E., Stewart B. W. Atypical mast cell degranulation and focal hydropic degeneration of venular endothelium in diffuse fibrosing alveolitis. Experientia. 1979 Nov 15;35(11):1492–1493. doi: 10.1007/BF01962804. [DOI] [PubMed] [Google Scholar]
- NIH conference. Pulmonary sarcoidosis: a disease characterized and perpetuated by activated lung T-lymphocytes. Ann Intern Med. 1981 Jan;94(1):73–94. doi: 10.7326/0003-4819-94-1-73. [DOI] [PubMed] [Google Scholar]
- Norrby K. Intradermal mast-cell secretion causing cutaneous mitogenesis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1983;42(3):263–269. doi: 10.1007/BF02890389. [DOI] [PubMed] [Google Scholar]
- Norrby K. Mast cell histamine, a local mitogen acting via H2-receptors in nearby tissue cells. Virchows Arch B Cell Pathol Incl Mol Pathol. 1980;34(1):13–20. doi: 10.1007/BF02892403. [DOI] [PubMed] [Google Scholar]
- Norrby K. On the 48/80-induced secretion of tissue mast cells and its mitogenic effect on nearby cells in the intact rat. Virchows Arch B Cell Pathol Incl Mol Pathol. 1981;38(1):57–65. doi: 10.1007/BF02892802. [DOI] [PubMed] [Google Scholar]
- Persinger M. A., Lepage P., Simard J. P., Parker G. H. Mast cell numbers in incisional wounds in rat skin as a function of distance, time and treatment. Br J Dermatol. 1983 Feb;108(2):179–187. doi: 10.1111/j.1365-2133.1983.tb00060.x. [DOI] [PubMed] [Google Scholar]
- Postlethwaite A. E., Smith G. N., Mainardi C. L., Seyer J. M., Kang A. H. Lymphocyte modulation of fibroblast function in vitro: stimulation and inhibition of collagen production by different effector molecules. J Immunol. 1984 May;132(5):2470–2477. [PubMed] [Google Scholar]
- Russel J. D., Russell S. B., Trupin K. M. The effect of histamine on the growth of cultured fibroblasts isolated from normal and keloid tissue. J Cell Physiol. 1977 Dec;93(3):389–393. doi: 10.1002/jcp.1040930310. [DOI] [PubMed] [Google Scholar]
- Sisson J. C., Castor C. W., Klavons J. A. Connective tissue activation. XVIII. Stimulation of hyaluronic acid synthetase activity. J Lab Clin Med. 1980 Aug;96(2):189–197. [PubMed] [Google Scholar]
- Strobel S., Miller H. R., Ferguson A. Human intestinal mucosal mast cells: evaluation of fixation and staining techniques. J Clin Pathol. 1981 Aug;34(8):851–858. doi: 10.1136/jcp.34.8.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl S. M., Gately C. L. Modulation of fibroblast growth by a lymphokine of human T cell continuous T cell line origin. J Immunol. 1983 Mar;130(3):1226–1230. [PubMed] [Google Scholar]
- Watanabe S., Watanabe K., Oishi T., Aiba M., Kageyama K. Mast cells in the rat alveolar septa undergoing fibrosis after ionizing irradiation. Ultrastructural and histochemical studies. Lab Invest. 1974 Nov;31(5):555–567. [PubMed] [Google Scholar]
- Wusteman F. S. Glycosaminoglycans of bovine lung parenchyma and pleura. Experientia. 1972 Aug 15;28(8):887–888. doi: 10.1007/BF01924923. [DOI] [PubMed] [Google Scholar]
- Yaron M., Yaron I., Wiletzki C., Zor U. Interrelationship between stimulation of prostaglandin E and hyaluronate production by poly (I) . poly (C) and interferon in synovial fibroblast culture. Arthritis Rheum. 1978 Jul-Aug;21(6):694–698. doi: 10.1002/art.1780210614. [DOI] [PubMed] [Google Scholar]



