Abstract
Background:
Inflammatory and procoagulant markers are potential mediators for the cardiovascular risk in hemodialysis patients. Lipoprotein (a) [Lp(a)], is another important risk factor with inflammatory and procoagulant effects.
Materials and methods:
In 78 hemodialysis patients and 40 controls, C-reactive protein (CRP), Interleukin-6 (IL-6), lipoprotein (a) [Lp (a)], fibrinogen, D-dimer, von Wilebrand factor (vWF) and serum albumin were determined.
Results:
CRP, IL-6, Lp(a), fibrinogen, D-dimer and vWF, were significantly higher, and serum albumin was significantly lower in patients compared to controls (24.40 mg/L vs. 6.39 mg/L, p<0.001; 1.92 pg/ml vs. 0.35 pg/ml, 28.05 mg/dL vs.16.25 mg/dL, p<0.001; 3.44 g/L vs. 2.55 g/L, p<0.01; 1.81 µgFEU /ml vs. 0.50 µgFEU /ml, p<0.01; 152.9 % vs. 85.6 %, p<0.001; 32.1 g/L vs. 40.50 g/L, p<0.001). The patients were divided into two groups: 40 patients with CRP levels over than 10 mg/L and 38 with CRP levels in normal range. These parameters showed significant differences between patients with elevated CRP and patients with normal CRP levels. CRP and IL-6 correlated positively with Lp(a), (r = 0.62, p < 0.001; r=0.54, p<0.001), fibrinogen, (r = 0.63, p < 0.001; r = 0.49, p<0.01) D dimer (r = 0.72, p<0.001; r = 0.55, p<0.01), vWF (r = 0.76, p<0.01; r = 0.63, p<0.001) and negatively with serum albumin (r = -0.80, p<0.01; r = -0.60, p<0.001), in patients with elevated CRP, but not in patients with normal CRP levels and controls.
Conclusion:
According to the results hemodialysis patients with increased inflammatory markers, have the elevated Lp(a) and procoagulant markers and the greater risk for atherosclerotic cardiovascular disease.
Keywords: inflammation, Lp(a), hypercoagulability, chronic hemodialysis
1. INTRODUCTION
Cardiovascular disease is an important predictor of mortality in hemodialysis patients (1). Inflammation is considered one of the key factors in accelerating atherosclerosis and endothelial dysfunction.
The uremic state is associated with an altered immune response (2) and intermittent stimulation by endotoxins from the dialysis water supply and artificial vein grafts or bio incompatibility caused the increased inflammatory proteins, such as C-reactive protein (CRP) (3). In addition to inflammatory marker, CRP as a mediator for developing atherosclerosis, directly affect endothelial cells, monocytes-macrophages and smooth muscle cells. (4). CRP is produced by the liver under the control of various proinflammatory cytokines, but mainly by hepatocytes in response to IL-6.
Among the various cytokines, IL-6 was found to be most closely related to mortality in both hemodialysis and pre dialysis patients (5) and to be associated with more causes of inflammation than other cytokines (6).
IL-6 increases the risk of atherosclerosis through metabolic, endothelial and coagulant mechanisms. In hemodialysis patients elevation of IL-6 is associated with carotid atherosclerosis (7).
Inflammation is associated with a prothrombotic tendency even in acute renal disease (8). Among the non-classic cardiovascular risk factors, plasma fibrinogen, D-dimer and von Willebrand factor (vWF), are potential markers of cardiovascular morbidity (9). D-dimer levels are significantly higher in renal insufficiency (10). Renal dysfunction is identified as a state of activated coagulation due to the elevated D-dimer levels, which are not cleared by the kidney (11).
The vWF, a marker of endothelial injury, showed a positive correlation with total mortality in the hemodialysis patients. Chronic endothelial cell activation, but not platelet activation, is related to all-cause mortality in patients in chronic hemodialysis (12).
In this context, renal failure can be viewed as a clinical model of mild, protracted systemic inflammation that is intrinsically associated with endothelial dysfunction and hemostatic activation, which are all important mediators of atherosclerosis and thrombotic events.
The contribution of cardiovascular events to the extraordinary high mortality in end-stage-renal disease has generated some interest in non-traditional atherosclerotic risk factors, such as Lipoprotein (a) [Lp(a)]. High concentrations of Lp(a) are considered a major risk factor for atherosclerosis (13). Lp(a) is frequently elevated in hemodialysis patients (14).
In hemodialysis patients, fibrin clot properties are markedly altered. The structure and function of the fibrin clot are modulated by several environmental and genetic factors. Lp(a) (15) and CRP (16), which along with fibrinogen are significantly elevated have also been reported to reduce clot permeability and susceptibility to lysis (17).
Hypoalbuminemia is associated with cardiovascular disease in general population as well as in hemodialysis patients. It has been found a significant correlation of IL-6 and hypoalbuminemia which is considered as a powerful predictor of mortality (18).
A significant inverse relationship was also found between Lp(a) and albumin (19). Hypoalbuminemia can induce a hypercoagulable and atherogenic state (20).
2. AIM
The aim was to study relationships between inflammation, Lp(a) and hypercoagulability in hemodialysis patients with no evidence of cardiovascular disease.
3. MATERIALS AND METHODS
In this study were included 78 patients, undergoing maintenance hemodialysis treatment in the Clinical Centre in Prishtina, for a period longer than 6 months. Age range was from 24 to 65 year. We selected the patients with no evidence of cardiovascular disease history. Patients were divided in two groups: 40 patients (22 female and 18 male) with elevated CRP levels over than 10 mg/L and 38 patients (18 female and 20 male) with CRP levels in the normal range. 40 healthy people (18 females and 22 males), were a control group.
CRP was measured by the turbidimetric method based in combines of CRP with specific antibody.
IL-6 was measured with Enzyme Linked Immunosorbent Immunoassay (ELISA).
Diazyme’s Lipoprotein (a) assay is based on a latex enhanced immunoturbidimetric method.
D-dimer was determined by the immuno-turbidimetric assay with monoclonal antibodies F(ab)2 fragments to the D-dimer epitope.
Fibrinogen was determined based on Clause’s method by measuring of ratio of fibrin formation.
Won Willebrand factor (vWF) was measured by ELISA and expressed as a percentage of normal value.
Serum albumin was determinate with bromocresol purple.
4. RESULTS
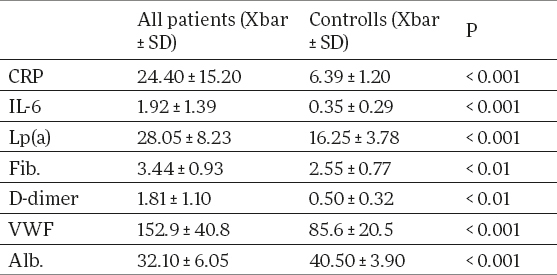
Serum concentration of CRP, IL-6, Lp(a), fibrinogen, D-dimer and vWF in hemodialysis patients were significantly higher than in controls (24.40 ± 15.20mg/L vs 6.39 ± 1.20 mg/L p<0.001, 1.92 ± 1.39 pg/ml vs 0.35 ± 0.29 pg/ml, p < 0.001; 28.05 ± 8.23 mg/dl vs 16.25 ± 3.78 mg/dL, p<0.001; 3.44 ± 0.93 g/L vs 2.55 ± 0.77 g/L, p<0.01; 1.81 ± 1.10 µgFEU /ml vs 0.50 ± 0.32 µgFEU /ml, p<0.01; 152.9 ± 40.8 % vs 85.6 ± 20.5 %, p<0.001 (Table 1), whereas serum albumin was significantly lower (32.10 ± 6.05 g/L vs 40.50 ± 3.90 g/L, p<0.001 (Table 1).
Table 1.
Biochemical parameters (means and SD) in hemodialysis patients and healthy controls
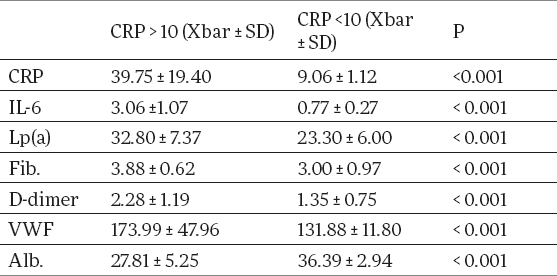
Serum concentration of IL-6, Lp(a), fibrinogen, D-dimer, v WF, were significantly higher and serum albumin was significantly lower in patients with elevated CRP levels than in patients with CRP levels in normal range. (3.06 ± 1.07 pg/ml vs 0.77 ± 0.27 pg/ml, p<0.01; 32.80 ± 7.37 mg/dL vs 23.30 ± 6.0 mg/dL, p<0.001; 3.88 ± 0.62 g/L vs 3.0 ± 0.97 g/L, p<0.01; 2.28 ± 1.19 µgFEU /ml vs 1.35 ± 0.75 µgFEU /ml, p<0.01; 173.99 ± 47.96 % vs 131.88 ± 11.80 %, p<0.001; 27.81 ± 5.25 g/L vs 36.39 ± 2.94 g/L, p<0.001 (Table 2).
Table 2.
Biochemical parameters (means and SD) in hemodialysis patients with elevated CRP and CRP in normal range
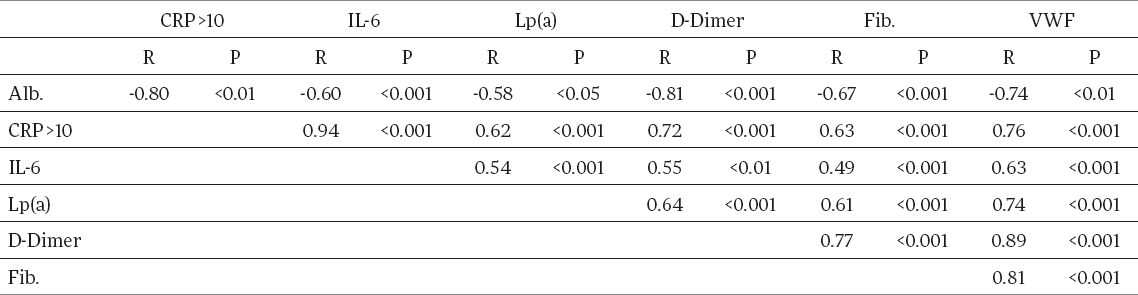
Significant correlation exists between CRP and IL-6 in patients with high CRP levels. (r = 0.94, p<0.001). CRP and IL-6 correlated positively with Lp(a), (r = 0.62, p< 0.001; r=0.54, p<0.001), fibrinogen (r = 0.63, p< 0.001; r = 0.49, p<0.01), D dimer (r = 0.72, p<0.001; r = 0.55, p<0.01), vWF (r=0.76, p<0.01; r=0.63, p<0.001) and negatively with serum albumin (r = -0.80, p<0.01; r = -0.60, p<0.001), in patients with elevated CRP (Table 3). There was no correlation in patients with CRP levels in normal range or in controls.
Table 3.
Correlations of all parameters in haemodialysis patients with elevated CRP (CRP > 10 mg/L)
5. DISCUSSION
Chronic inflammation, predicts all-cause and cardiovascular mortality in hemodialysis patients in short- and long term studies (21), In hemodialysis patients, serum IL-6 level is known to be much higher than in general subjects, and is associated with mortality (7). Much more IL-6 predict outcome of hemodialysis patients.
In this study CRP and IL-6 were significantly higher in patients than in controls (24.40 mg/L vs 6.39 mg/L, p<0.001; 1.92 pg/ml vs 0.35 pg/ml, p < 0.001 (Table 1) and their positive correlation is significant (r = 0.94, p<0.001 (Table 3).
Pathways that might mediate the association between renal dysfunction and cardiovascular risk are the inflammatory and coagulation cascades. Activation of hemostasis is a common feature of uremia and its link with inflammation, has been reported (22).
In this study serum concentration of fibrinogen and D-dimer in patients were significantly higher (3.44 g/L vs 2.55 g/L, p<0.01; 1.81 µgFEU /ml vs 0.50 µgFEU /ml, p<0.01) than in controls.
Atherosclerosis is initiated through endothelial abnormalities and elevated plasma concentration of fibrinogen and putative markers of endothelial dysfunction including vWF (23).
Hemodialysis patients presenting thrombotic complications had significantly elevated vWF levels (24).
We also found significantly higher levels of vWF in patients compared with controls (152.9 % vs 85.6%, p<0.001 (Table 1). Comparing the two groups, D-dimer fibrinogen and vWF levels were higher in patients with elevated CRP than in patients with CRP levels in normal range (2.28 µg FEU /ml vs 1.35 µgFEU /ml, p<0.001; 3.88 g/L vs 3.00 g/L, p<0.001; 173.99% vs 131.88%; p<0.001 (Table 2).
IL-6 is demonstrated to increase the risk of atherosclerosis through metabolic endothelial and coagulant mechanisms, increasing the hepatic release of fibrinogen as well as having procoagulant effects of platelet (25).
We also find positive correlation of IL-6 with fibrinogen, (r = 0.49, p<0.001), D dimer (r = 0.55, p<0.01), and vWF (r = 0.63, p<0.001 (Table 3) in patients with high CRP levels. Based on results inflammatory response coexists with endothelial dysfunction. Increased levels of Lp (a) is an independent risk factor for coronary disease.
Lp(a) mean value was significantly higher in patients than in controls (28.05 mg/dl vs 16.25 mg/dL, p<0.001 (Table 1), Lp(a), occurs at high concentrations largely because of reduced clearance, or as a result of increased hepatic synthesis induced by an acute phase reaction or by proteinuria.
Lp(a) was significantly higher in patients exhibiting elevated CRP than to those with CRP in normal range, (32.80 mg/dL vs 23.30 mg/dL, p<0.001 (Table.2). A close relationship exist between Lp(a) levels and inflammation, as shown by correlations with CRP and IL-6 (r = 0.62, p< 0.001 ; r=0.54, p<0.001 (Table 3).
Both end-stage renal disease and thromboembolic coronary events have been shown to be associated with the formation of dense fibrin clots resistant to fibrinolysis.
There are evidences that Lp(a) reduce clot permeability and susceptibility to lysis (17). A significant positive correlations of Lp(a) were observed with D-dimer (r=0.64, p<0.001), fibrinogen (r = 0.61, p<0.001) and vWF (r = 0.74, p<0.001 (Table 3). Patients with high CRP showed evidence of a higher degree of hyper coagulation than patients with CRP levels in normal range. Thus, hemostatic abnormalities may be affected to other cardiovascular risk factors as Lp(a).
Based in results chronic hemodialysis may be a marker for systemic inflammation, which in turn is associated with high levels of Lp(a) and hypercoagulability.
IL-6 is known to be associated with the severity of hypoalbuminemia in hemodialysis patients (18).
We also find that CRP and IL-6 correlated negatively with serum albumin (r = -0.80, p<0.01; r = -0.60, p<0.001 (Table 3), which proved that hypoalbuminemia in hemodialysis patients is partially a consequence of inflammation.
Inverse relationship existed also between serum albumin and Lp(a) in patients with elevated CRP (r = -0.58, p<0.05 (Table 3). This correlation is a significant indicator for cardiovascular death of hemodialysis patients (26). Albumin levels correlated negatively with D-dimer and vWF in patients with elevated CRP (r = -0.81, p<0.001; r = -0.74, p<0.01 (Table 3). The negative correlation of albumin with D- dimer and vWF confirm that hypoalbuminemia is associated with hypercoagulability.
6. CONCLUSION
A persistent inflammatory response and high levels of Lp(a), in hemodialysis patients, may contribute to coagulation activation and increased cardiovascular risk. These elevations were apparent even among patients with no evidence of clinical or subclinical cardiovascular disease.
REFERENCES
- 1.Samerio Faria M, Ribeiro S, Costa E, et al. Risk Factors for Mortality in Hemodialysis Patients: Two-Year Follow-Up Study. Disease Markers. 2013;35(6):791–798. doi: 10.1155/2013/518945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen G, Haag-Weber M, Horl WH. Immune dysfunction in uremia. Kidney Int. Suppl. 1997;62:S79–S82. [PubMed] [Google Scholar]
- 3.Perez-Garcia R, Rodriguez-Benitez P. Why and how to monitor bacterial contamination of dialysate? Nephrol. Dial. Transplant. 2000;15:760–764. doi: 10.1093/ndt/15.6.760. [DOI] [PubMed] [Google Scholar]
- 4.Van der Sande FM, Kooman JP, Leunissen KM. The predictive value of C-reactive protein in end-stage renal disease: is it clinically significant? Blood Purif. 2006;24:335–341. doi: 10.1159/000092279. [DOI] [PubMed] [Google Scholar]
- 5.Barreto DV, Barreto FC, Liabeuf S, et al. European Uremic Toxin Work Group (EUTox): Plasma interleukin-6 is independently associated with mortality in both hemodialysis and pre-dialysis patients with chronic kidney disease. Kidney Int. 2010;77:550–556. doi: 10.1038/ki.2009.503. [DOI] [PubMed] [Google Scholar]
- 6.Beberashvili I, Sinuani I, Azar A, et al. IL-6 Levels, Nutritional Status, and Mortality in Prevalent Hemodialysis Patients. Clinical Journal of the American Society of Nephrology. 2011;6(9):2253–2263. doi: 10.2215/CJN.01770211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kato A, Odamaki M, Takita T, et al. Association between interleukin-6 and carotid atherosclerosis in hemodialysis patients Kidney International. 2002;61:1143–1152. doi: 10.1046/j.1523-1755.2002.00215.x. [DOI] [PubMed] [Google Scholar]
- 8.Jalal D, Chonchol M, Targher G, et al. Disorders of Hemostasis Associated with Chronic Kidney Diseases. Seminars in Thrombosis and Hemostasis. 2010;36(1):34–40. doi: 10.1055/s-0030-1248722. [DOI] [PubMed] [Google Scholar]
- 9.Kirmizis D, Tsiandoulas A, Pangalou M, et al. Validity of plasma fibrinogen, D-dimer, and the von Willebrand factor as markers of cardiovascular morbidity in patients on chronic hemodialysis. Medical science monitor international medical journal of experimental and clinical research. 2006;12:55–62. [PubMed] [Google Scholar]
- 10.Costa E, Rocha S, Rocha-Pereira P, et al. Cross-talk between inflammation, coagulation/fibrinolysis and vascular access in hemodialysis patients. The Journal of Vascular Access. 2008;9(4):248–253. [PubMed] [Google Scholar]
- 11.Shibata T, Magari Y, Kamberi P, et al. Significance of urinary fibrin/fibrinogen degradation products (FDP) D-dimer measured by a highly sensitive ELISA method with a new monoclonal antibody (D-D E72) in various renal diseases. Clin Nephrol. 1995;44:91–95. [PubMed] [Google Scholar]
- 12.Péquériaux NC, Fijnheer R, Gemen EF, et al. Plasma concentration of von Willebrand factor predicts mortality in patients on chronic renal replacement therapy. Nephrol Dial Transplant. 2012;27(6):2452–2457. doi: 10.1093/ndt/gfr735. [DOI] [PubMed] [Google Scholar]
- 13.Jacobson TA. Lipoprotein(a), Cardiovascular Disease, and Contemporary Management. Mayo Clinic Proceedings. 2013;88(11):1294–1311. doi: 10.1016/j.mayocp.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Longenecker C, Klag M, Marcovina S, et al. High Lipoprotein(a) Levels and Small Apolipoprotein(a) Size Prospectively Predict Cardiovascular Events in Dialysis Patients. Am Soc Nephrol. 2005;16:1794–1802. doi: 10.1681/ASN.2004110922. [DOI] [PubMed] [Google Scholar]
- 15.Undas A, Stepien E, Tracz W, et al. Lipoprotein(a) as a modifier of fibrin clot permeability and susceptibility to lysis. J Thromb Haemost. 2006;4:973–975. doi: 10.1111/j.1538-7836.2006.01903.x. [DOI] [PubMed] [Google Scholar]
- 16.Undas A, Celinska-Lowenhoff M, Lowenhoff T, et al. Statins, fenofibrate, and quinapril increase clot permeability and enhance fibrinolysis in patients with coronary artery disease. J Thromb Haemost. 2006;4:1029–1036. doi: 10.1111/j.1538-7836.2006.01882.x. [DOI] [PubMed] [Google Scholar]
- 17.Undas A, Kolarz M, et al. Altered fibrin clot properties in patients on long-term haemodialysis: relation to cardiovascular mortality. Nephrol Dial Transplant. 2008;23(6):2010–2015. doi: 10.1093/ndt/gfm884. [DOI] [PubMed] [Google Scholar]
- 18.Bologa RM, Levine DM, Parker TS, et al. Interleukin-6 predicts hypoalbuminemia, hypocholesterolemia, and mortality in hemodialysis patients. Am J Kidney Dis. 1998;32:107–114. doi: 10.1053/ajkd.1998.v32.pm9669431. [DOI] [PubMed] [Google Scholar]
- 19.Yang WS, Kim SB, Min W, et al. Atherogenic lipid profile and lipoprotein(a) in relation to serum albumin in haemodialysis patients. Nephrol Dial Transplant. 1995;10:1668–1671. [PubMed] [Google Scholar]
- 20.Aguilera A, Sánchez-Tomero JA, Bajo MA, et al. Malnutrition-inflammation syndrome is associated with endothelial dysfunction in peritoneal dialysis patients. Adv Perit Dial. 2003;19:240–245. [PubMed] [Google Scholar]
- 21.Wanner C, Zimmermann J, et al. Inflammation and cardiovascular risk in dialysis patients. Kidney Int Suppl. 2002;(80):99–102. doi: 10.1046/j.1523-1755.61.s80.18.x. [DOI] [PubMed] [Google Scholar]
- 22.Gamboa L, Pretorius M, Deanna R, et al. Comparative Effects of Angiotensin-Converting Enzyme Inhibition and Angiotensin-Receptor Blockade on Inflammation during Hemodialysis. JASN. 2012;23(2):334–342. doi: 10.1681/ASN.2011030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lip GYH, Foster W, Blann AD. Plasma von Willebrand factor levels and surrogates of atherosclerosis. J Thromb Haemost. 2005;3:659–661. doi: 10.1111/j.1538-7836.2005.01284.x. [DOI] [PubMed] [Google Scholar]
- 24.Sioulis A, Malindretos P, et al. Coagulation factors as biological risk markers of endothelial dysfunction. Association with the thrombotic episodes of chronic hemodialysis patients. Hipocratia. 2009;13(4):237–241. [PMC free article] [PubMed] [Google Scholar]
- 25.Yudkin JS, Kumari M, Humphries SE, et al. Inflammation, obesity, stress and coronary heart disease: Is interleukin-6 the link? Atherosclerosis. 2000;148:209–214. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- 26.Koda Y, Nishi Sh.I, et al. Lipoprotein(a) is a predictor for cardiovascular mortality of hemodialysis patients. Kidney International. 1999;56:S251–S253. doi: 10.1046/j.1523-1755.1999.07167.x. [DOI] [PubMed] [Google Scholar]


