Abstract
The anxiety disorders include generalized anxiety disorder, specific phobia, social phobia, agoraphobia, and panic disorder. In addition to the specific symptoms of these disorders, there may be a common experience of anxiety and even dysphoria across the conditions, and of course recourse to the same drug or choice of drugs for treatment. This overlap probably occurs because of universal dimensions of distress or negative affectivity, a shared genetic predisposition, and a common neurobiology Evidence of shared genes is still based mainly on twin studies, but the shared neurobiology can be investigated directly by the investigation of emotional or cognitive bias either behaviorally or using functional brain imaging. This intermediate phenotype can then provide a substrate for understanding and developing medicines and psychological treatments.
Keywords: anxiety, depression, compulsive disorder, OCD
Abstract
Los trastornos de ansiedad incluyen el trastorno de ansiedad generalizada, la fobia específica, la fobia social, la agorafobia y el trastorno de pánico. Además de los síntomas específicos de estos trastornos, en estas condiciones puede existir una experiencia común de ansiedad e incluso disforia y, por supuesto, el hecho de recurrir al mismo fármaco o a la elección de medicamentos para el tratamiento. Esta sobreposición se debe probablemente a las dimensiones universales del distrés o afectividad negativa, a una predisposición genética compartida y a una neurobiología común. Todavía la evidencia de genes compartidos se basa principalmente en estudios en gemelos, pero la neurobiología compartida puede ser investigada directamente por medio de la investigación de sesgos emocionales o cognitivos ya sea a través del comportamiento o empleando imágenes cerebrales funcionales. Este fenotipo intermedio puede proporcionar entonces un sustrato para la comprensión y el desarrollo de medicamentos y terapias psicológicas.
Abstract
L'anxiété généralisée, la phobie spécifique, la phobie sociale, l'agoraphobie et le trouble panique font partie des troubles anxieux. En plus des symptômes propres à ces troubles, il peut cependant exister un vécu commun d'anxiété et même de dysphorie entre ces pathologies, nécessitant bien sûr un recours au même médicament ou au même choix de médicament pour le traitement. Les dimensions universelles de la souffrance ou de l'affectivité négative, une prédisposition génétique partagée et une neurobiologie commune sont probablement à l'origine de ce chevauchement. Les données des gènes partagés sont toujours fondées sur des études de jumeaux, mais la neurobiologie partagée peut être étudiée directement par la recherche de biais émotionnels ou cognitifs soit de façon comportementale ou en utilisant l'imagerie cérébrale fonctionnelle. Ce phénotype intermédiaire peut alors fournir un substrat pour comprendre et développer des médicaments et des traitements psychologiques.
Introduction
DSM-5 provides a somewhat divisive starting point for looking at the overlap between major depression, anxiety disorder, and obsessive-compulsive disorder (OCD). This is because it proposes a separation between anxiety disorders and OCD by placing them in separate chapters of the North American “diagnostic bible.”1 In previous versions, they had been united as anxiety disorders. The obsessive-compulsive disorders include OCD itself, body dysmorphic disorder, hoarding disorder, trichotillomania, and excoriation disorder. The anxiety disorders include generalized anxiety disorder (GAD), specific phobia, social phobia, agoraphobia, and panic disorder. These two major groups are in turn separated from the trauma and stressor-related disorders, and of course, mood disorder.
The diagnostic tradition in medicine has always been divided between those who have been called the “lumpers” and those called the “splitters”; in other words, experts driven by their recognition of the similarities between diagnoses and those driven by the differences. Since diagnosis and classification more generally are a preliminary to more profound understanding of disease, neither is wholly right (nor wholly wrong) and, as the DSM-5 version illustrates, the emphasis can change. Therefore, a diagnosis of major depression, an anxiety disorder, or OCD may make perfect sense in terms of the primary symptoms of which the patient complains, and on which a differentiated diagnosis is based, yet there may well be a common experience of anxiety and even dysphoria across the conditions, and of course recourse to the same drug or choice of drugs for treatment. In addressing why this overlap occurs, a common neurobiology seems the most obvious explanation.
Lumping: the evidence
DSM-5 itself advocates consideration of shared neural substrate, family traits, genetic risk factors, specific environmental risk factors, biomarkers, shared temperament, abnormalities of emotional processing, symptom similarity, course of illness, high comorbidity, and shared treatment response for confirming relationships between diseases. In fact, this use of more numerous and more global factors leads to the lumping idea of internalizing disorders on the one hand (into which all the disorders here fall) and externalizing disorders on the other (characterized by aggression, anger outbursts, law-breaking, or hyperactivity). The introduction of this more dimensional approach to diagnosis in DSM-5 also takes note of the likely advantages for bridging to neurobiology.
To demonstrate that there is an overlap between depression, anxiety disorder, and OCD that is likely to rest on shared brain mechanisms, it will be important to consider evidence for shared genes, shared brain mechanisms, and shared treatment effects. However, the starting point is the obvious simple overlap of morbid phenomena in the acute presentation of the different disorders and the common co-occurrence of full syndromes in the same individuals diagnosed impartially using DSM criteria. The common phenomenology is typically the experience of fear and anxiety across a very wide range of psychiatric diagnoses. Formally, the comorbidity of one diagnosis with another beyond chance is the necessary confirmation of a close phenomenological relationship between them. Thus, the various anxiety disorders are highly comorbid with each other. For instance, using lifetime diagnoses in the US population data, 74.1% of those with agoraphobia, 68.7% of those with simple phobia, and 56.9% of those with social phobia also met criteria for another anxiety disorder.2 In general, OCD cases are more likely to show lifetime incidence of other anxiety disorders than vice versa, because of their greater severity and rarity.3 Depression is a comorbidity common to all. Thus, the mood disorders are strongly comorbid with the anxiety disorders, and vice versa. For example, in analyses of lifetime DSM-III-R diagnoses in US population sample data, 58% of individuals with major depression also met criteria for a comorbid anxiety disorder4; the comorbidity rate was only slightly reduced to 51.2% when 12-month diagnoses were used. Conversely, most individuals with diagnosed anxiety disorders also met criteria for major depression, although comorbidity rates varied widely across disorders.
More recent, community-based estimates of the lifetime morbid risk/12-month prevalence ranked by frequency were: major depressive episode: 29.9%/8.6%; specific phobia: 18.4/12.1%; social phobia: 13.0/7.4%; post-traumatic stress disorder: 10.1/3.7%; generalized anxiety disorder: 9.0/2.0%; separation anxiety disorder: 8.7/1.2%; panic disorder: 6.8%/2.4%; bipolar disorder: 4.1/1.8%; agoraphobia: 3.7/1.7%; obsessive-compulsive disorder: 2.7/1. 2.5 From the developmental perspective, the anxiety-mood disorders with the earlier median ages of onset are phobias and separation anxiety disorder (ages 15 to 17) and those with the latest are panic disorder, major depression, and generalized anxiety disorder (ages 23 to 30). Comorbidity between anxiety disorders and depressive disorder are common in community samples in various countries where comparable studies have been conducted.6 In summary, the fact of an overlap between different anxiety diagnoses and themselves as well as with depression diagnoses is beyond dispute.
Much of the evidence to this point is based on categorical groups of individuals with disorders as defined in DSM terms. Of all the psychiatric disorders, the anxiety disorders have always been supposed to be common because they reflect experience that is not much removed from normality. Thus, any model based on pathology should also be testable in the reported subjective dimensional experience of healthy populations. This turns out to be the case. A well-known model of normal emotion proposes a “Big Two” dimensional solution; that is dimensions of Negative Affect and Positive Affect7,8 in which Negative Affect is a general dimension of subjective distress. Hence, it subsumes fear, anger, sadness, guilt, and disgust. An individual who reports feeling sad is also likely to report substantial levels of anger, guilt, fear, etc. This dimension would inevitably predict and so potentially explain major overlaps in the reported experience of someone with anxiety or depression.
The general Positive Affect dimension predicts that an individual who reports feeling happy and joyful will also report feeling interested, excited, confident, and alert. It is more related (negatively) to sadness than to fear. Thus, anhedonia appears as a potentially defining feature of mood disorder as distinct from anxiety, whereas the general subjective distress dimension is feature shared between anxiety disorders and depression. Clark and Watson subsequently proposed a third component, somatic tension and hyperarousal (eg, shortness of breath, dizziness) as unique to anxiety.9 Therefore, simple subjective symptoms present in a healthy population tend to confirm both a general factor of psychopathology that is expressed across disorders (distress/negative affectivity) and putative specific traits mapping to depression on the one hand and anxiety on the other. The presence of these factors, unconfounded by help seeking and other factors that contribute to clinical samples, is helpful in understanding why anxiety disorders are likely both to lump and to split. Furthermore it supports the idea that anxiety disorders in particular are simply extreme expressions of traits present across the whole population.
Inheritance of traits and disorders
Since many behavioral traits and psychiatric disorders are heritable, it follows that the structure of these traits or disorders in populations should follow rules of genetic inheritance like other complex traits like height or weight. Twin studies continue to provide the critical observational design: any genetic condition will be more present in identical (ie, monozygotic or MZ) twins than nonidentical twins (dizygotic or DZ). Indeed, such genetic data in a large study of female twins was the first surprisingly strong evidence for genetic overlap between major depression and GAD.10
For family studies of the anxiety disorders of interest here, odds ratios predicting association of illness in first-degree relatives with the illness status of the proband (ie, whether the disorder was present or absent) were homogeneous across studies for all disorders and ranged from 4 to 6, depending on the disorder.11 This extensive overlap may well be associated with a common risk factor such as neuroticism, which has been shown to predict the onset of the anxiety disorders, OCD, and major depression. Neuroticism, a concept originally attributable to Hans Eysenck's studies of personality structure, is about 40% heritable and has been an independent focus for genetic analysis. The contribution through genetic mechanisms of such a globally identifiable factor, which may be summed up to be the trait for anxious worrying, is of great interest. It may represent the major “internalizing factor” that plausibly underlies this wide range of emotional disorders and the ultimate target for all lumpers. Despite its apparent importance, its status is often attacked as being simply a dilute measure of symptoms and its neurobiology has attracted surprisingly limited attention from investigators (see below).
Splitting: the evidence
While lumping and splitting can be presented as profound alternatives, some etiological features, like neuroticism, may necessarily lump, while others may necessarily split. Furthermore, the structural analysis of symptoms suggests that while some are global as described, others are more specific to individual disorders. Indeed, they define what we mean by specificity. For example, Mineka et al12 proposed an integrative hierarchical model of the anxiety disorders. In this model, each individual syndrome was hypothesized to contain both a common and a unique component. The shared component represented broad individual differences in general distress and negative affectivity. As already discussed it will behave as a pervasive higher-order factor (the lumping factor one might say) that is common to both the anxiety and mood disorders. Hence, it will be primarily responsible for the comorbidity issues that were highlighted earlier. However, in addition, each disorder also includes unique features that differentiate it from all of the others. Thus, anxious arousal assumes a limited role as a specific element in syndromes such as panic disorder; trauma history and flashbacks will define post-traumatic stress disorder (PTSD), and obsessions and compulsions define OCD.
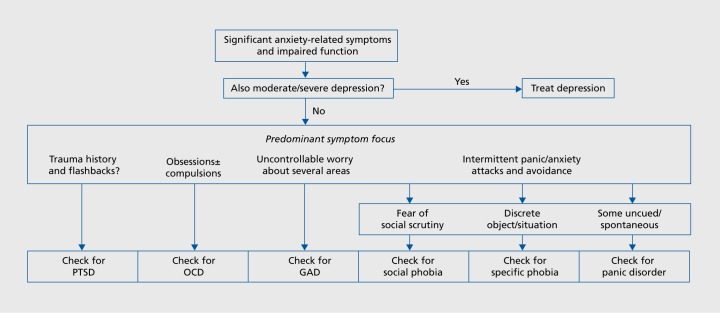
This approach to modeling the disorders can provide a simple practical model for diagnostic evaluation via the general and specific components as shown in Figure 1, from the guidelines of the British Association for Psychopharmacology.13 This proposes initial inquiry to establish anxiety symptoms and the presence or absence of depression. If depression is present its treatment is recommended as primary. If it is not, then specific anxiety diagnoses can be made on the basis of defining specific features, so trauma/flashbacks suggest PTSD as above; obsessions and or compulsions, OCD; worry and rumination, GAD, and so on, as shown.
Figure 1. Suggested scheme for exploring a suspected anxiety disorder. PTSD, post-traumatic stress disorder; OCD, obsessive-compulsive disorder; GAD, generalized anxiety disorder From ref 13: Baldwin DS, Anderson IM, Nutt DJ, et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol. 2014;28(5):403-339. Copyright © British Association for Psychopharmacology 2014.
Why disorders split
In the case of the disorders here, the age of exposure to exacerbating or precipitating stressors, or their nature, might contribute to the determination of a different phenotype from a common genetic background. Indeed, there is some agreement in the existing literature about why an individual at genetic risk for major depressive disorder (MDD)/GAD gets one or the other phenotype. This is based on observed differences in the nature of the environmental stresses that provoke a diagnosable episode. Thus, loss and humiliation events more often precede MDD and danger events more often precede GAD.14
A more detailed consideration of inheritance of anxiety disorders in male and female twin pairs has suggested further division of genetic factors for agoraphobia at one extreme, the specific phobias at the other, and social phobia intermediate between them. The remaining associations between the disorders are largely explained by a unique environmental factor shared across the disorders and, to a lesser extent, a common shared environmental factor. In the most parsimonious model, there appears to be an agoraphobia-specific genetic factor and unique environmental effects (triggers) for each disorder. So, individual genetic factors derive from sets of genes that increase risk for generalized-agoraphobic anxiety on the one hand and specific phobias on the other. Risk across all of the anxiety diagnoses appears to be further increased by life experiences either shared with other family members or unique to the individual. The impact of these life experiences will depend on the disorder. Added to this may be a set of unique environmental factors that increase the liability for one anxiety disorder independent of any other. This is a complicated story, and it is limited by uncertainties of how far subjective reports and recall in a diagnostic interview may shape the findings.15 However, the state of the art is now consistent enough to support the model and suggest the ways in which larger genetic studies may inform our understanding of the molecular underpinnings of emotional behavior.
Molecular genetics
The hard finding of recent years is that success in molecular genetic studies of psychiatric disorder has only come in highly heritable conditions with enormous samples. For the anxiety disorders, there is some suggestion that neuroticism or other broad risk dimensions may provide a tractable target, but we remain a long way from confidence that genetic biomarkers will be of practical value in understanding mechanisms. In the case of neuroticism, there was an early start with the apparent success of candidate gene analysis based on the genetic variation 5-HTTLPR on the serotonin transporter gene SLC6A4 and an apparent link to individual differences in neuroticism. The implicated polymorphism influences mRNA expression so carriers of the short variant (s-allele) appear to show higher neuroticism and also lower mRNA expression of this gene.16 The link with serotonin and the role of selective serotonin reuptake inhibitors (SSRIs) in treating anxiety and depression gave this finding a compelling suggestion of face validity. It could be said that it was a finding that the field yearned to be found correct. Unfortunately, the subsequent findings with respect to 5-HTTLPR and personality traits are heterogenous and have led to a sequence of meta-analyses of the accumulating data, which have led to opposing conclusions. However, there is no doubt that the actual effect size of any association is much smaller than originally believed, if it is real at all.17
In the development of this story, the 5-HTTLPR gene was also implicated as mediating an important gene-environment interaction. It was claimed that the s-allele was associated with a substantially greater probability of depressive illness in response to adverse life events. This study is instructive in a number of ways. It was, if possible, an even more attractive finding than the link with neuroticism and appeared to illustrate both the importance of heritability and a gene x environment interaction. It has been massively cited in consequence.18 However, it has also come to exemplify the problems for genetic analysis of behavioral traits and psychiatric disorder more generally.19 Crucially, efforts to replicate the finding and critical analysis of the accumulating findings have led to the conclusion that if the effect is real it is again of much smaller size than originally proposed. The idea that it was a major gene effect that could lead to the development of animal models relevant to anxiety and mood disorder seems in retrospect to have been far too over-optimistic. Instead, it illustrates the general inference that a single common genetic variant when associated with a complex behavioural phenotype will contribute a trivial fraction of phenotypic variance (typically <0.1%).
More recently, genome-wide association studies (GWAS) have permitted testing of about 2 million genetic loci at a time and they have confirmed that the effects of common genetic variants on psychiatric disorders are very small and so require very large sample sizes to be detected. While this has led to success in reliably identifying numerous loci associated with severe disorders such as schizophrenia,20 the sample sizes have not yet grown sufficiently large to yield meaningful results for depression and anxiety. However, it is already clear that there is substantial common variance between the loci identified for schizophrenia and those for bipolar disorder and major depression. The preliminary analyses suggest that the key pathways implicated by these associations relate to synaptic function, immune and neuronal/neurotrophic pathways and histone methylation.21
The neurobiology of the amygdala
Notwithstanding the challenge of understanding its genetic foundations, the neurobiology of anxiety (and depression) is of considerable interest. If we are looking for a structure or mechanisms that may provide the experimental focus for understanding the general mechanisms identified from psychopathology, then few look further than the amygdala and its connections. The amygdala is located bilaterally within the medial temporal lobe of higher animals. It forms a complex extended structure with multiple sub-nuclei. In the rat, the nuclei are divided into three main groups: the basolateral complex, which includes the lateral nucleus, the basal nucleus, and accessory basal nucleus; the cortical nucleus, which includes the lateral olfactory tract; and the centromedial nucleus. The connections give important support to theories of its key functional role. Thus, there is input from all sensory systems. In the case of olfactory, somatosensory, gustatory, and visceral centres the afferent input is from primary sensory structures to the lateral basal and central nuclei. Rather differently auditory and visual information appears to originate in association rather than primary sensory cortex, implying more organized informational content. The amygdala's outgoing pathways project to cortex, hypothalamus, and brain stem; they are potentially targeted to relevant behavioral and neuroendocrine systems. The central anatomical position of the amygdala within the so-called limbic system or emotional brain was widely accepted by the mid 20th century, before functional studies had investigated how it functioned in this role. It continues to occupy an important position for function, even now when confidence in the usefulness of a limbic/ emotional/archaic brain system has greatly diminished with the recognition of a key cognitive role for the hippocampus.
The function of the amygdala in animals is understood from its established role in fear conditioning. Classical conditioning is a type of learning in which an emotionally neutral conditioned stimulus (CS), often a tone, is presented in advance but predictive of an aversive unconditioned stimulus (US), typically an electric shock to the foot of the animal. After one or more pairings, the previously emotionally neutral stimulus (CS) elicits a constellation of species-specific conditioned responses (CRs) that are taken to be characteristic of fear, such as freezing or escape behavior, autonomic responses (elevated heart rate and blood pressure), potentiated acoustic startle to aversive acoustic stimuli, and increased neuroendocrine responses (release of stress hormones). Fear conditioning can be seen as an important adaptive way in which new threats are quickly learned and behavioral responses activated for self-protection. The emergence of a completely neutral stimulus as a potent CS also suggests ways in which the system might fail and give rise to anxiety disorder. Thus a false association of innocuous chance stimuli with threat might be misleadingly incorporated into exaggerated behavioral psychopathology (and explain pathological anxiety). It was an early suggestion that individuals at risk of anxiety disorders would either condition more easily than controls or extinguish fear responses more slowly. In fact only in the last decade has fear extinction in particular come to be widely studied as a translational model for neuroscience.
Numerous studies, employing lesions or electrophysiological recordings, have demonstrated the amygdala's central place in classical conditioning. Thus, lesions to the amygdala impair the acquisition and expression of conditioned fear in rats. The basolateral complex of the amygdala is a potential substrate for the complex sensory convergence from both cortical and subcortical areas required for OS-US association during fear conditioning. In fact, its cells appear to encode the signal by long-term potentiation of EPSPs evoked in the basolateral complex. On the output side, the central nucleus of the amygdala may act as the common pathway to hypothalamus for the generation of fear-conditioned responses.
In man, damage to the amygdala, or areas of the temporal lobe that include the amygdala can be studied occasionally in suitable single cases. One patient with a rare congenital disease that results in the bilateral degeneration of the amygdala was exposed to live snakes and spiders, toured a “haunted” house, and was shown emotionally harrowing films. There was no experience of fear reported, and when she underwent fear conditioning with either visual or auditory CSs and a loud noise as the US, she showed no evidence of fear conditioning (as measured by galvanic skin response). However, her recall of events associated with the fear conditioning procedure was intact. These data support the hypothesis that the amygdaloid complex plays a key role in the acquisition of fear conditioning (whereas the hippocampus is important in remembering the conditioning context).22
Experimental neuropsychology has been transformed, along with the rest human/cognitive neuroscience, by noninvasive brain imaging. With respect to emotional learning, early fMRI studies sought to determine the extent to which rodent models of the amygdala were valid in the human brain. Using a simple differential fear-conditioning paradigm in healthy humans (a blue square as the CS and a mild shock as the US), amygdala activation increased in response to the CS+ (CS that is paired with the US) as compared with the CS- (CS that is not paired with the shock).23 Subsequent fMRI studies using subliminal presentations of fearful faces as stimuli also showed significant amygdala activation in healthy humans.24 These observations provided unequivocal evidence that amygdala function was conserved across species, and validated the use of fMRI for studying fear learning in humans. Accordingly, the amygdala and its connectivity provide a key target for understanding the anxiety disorders and their treatment.
Neuroimaging and gene function
Neuroimaging has also seemed to offer a further advantage: access to measures of brain function that might be intermediate to and more sensitive than illness phenotypes to genetic analysis. The early observation of a possible link between genetic variation of the 5-HTTLPR gene and neuroticism suggested the hypothesis that there might be a more detectable effect of polymorphism in this gene and amygdala function. This has proved controversial and again highlights general problems for the field. Thus, a recent meta-analysis has indicated that there is a statistically significant but small effect of 5-HTTLPR on amygdala activity.25 However, perhaps more striking was the between-study heterogeneity and the evidence for “excess statistical significance.” In summary, all the individual published studies have been considerably underpowered to detect the size of effect that is likely to be present, which is smaller than originally thought. In addition, the retreat to a very small or no effect for genetic variation exactly parallels what was summarized previously for this gene and its association with neuroticism. Therefore the claimed advantage of intermediate phenotypes may also be wrong. Measures of systems level neurocognition with fMRI may be no more or less helpful than the behavioral phenotypes like neuroticism or DSM diagnosis for genetic analysis.
Neurobiology and psychopathology
A complete summary of this literature is beyond the scope of the present article. However, there are important general themes that reflect a growing consensus. Anxiety disorder is closely associated with increased processing of threat-related stimuli and particularly increased attention to threats in the environment. Broadly speaking, the mechanisms behind this behavior have been described in terms of increased sensitivity on the one hand and impaired discrimination on the other. Thus the content specificity of threat-related attention bias in anxiety disorder has been investigated in a recent meta-analysis.26 The results indicated greater attention bias toward disorder-congruent relative to disorder-incongruent threat stimuli (d = 0.28, P < 0.0001). The effect appeared to be independent of age, type of anxiety disorder, visual attention tasks, or type of disorder-incongruent stimuli. Volunteers with a high level of neuroticism (high-N) showed increased processing of negative and/or decreased processing of positive information in emotional categorization and memory, facial expression recognition, and emotion-potentiated startle (EPS), in the absence of global memory or executive deficits. By contrast, there was no evidence for effects of neuroticism on attentional bias (as measured with the dot-probe task), over-general autobiographical memory, or awakening Cortisol levels.27
Neuroimaging is being used to dissect mechanisms in more detail. Again, the common emerging theme is of common effects and disorder-specific details in the response to a range of experimental paradigms. For example, greater differential right amygdala activation when matching fearful and happy facial expressions could be associated with greater negative affectivity across three different anxiety disorder groups (GAD, panic, and social anxiety disorder) compared with controls. However, the panic disorder group showed increased posterior insula activation.28 Other data support the existence of a common abnormality in anxiety and depression in the ventral cingulate and the amygdala, but with disorderspecific compensation during implicit regulation of emotional processing apparently through engagement of cognitive control circuitry in the depressed group.29 The complexity of the likely mechanisms involved in different disorders remains challenging.
Emotional processing as the target for drugs and psychological treatments
Most treatment studies assume a primary effect on symptoms. It is an important new conceptual step to try to move beyond symptoms and address the cognitive mechanisms that may be disturbed in psychopathology. This echoes the approach to psychopathology advocated by research domain criteria (RDoC). It also provides one of the first potential examples of the use of biomarkers to monitor treatment in psychiatry. There is now a substantial descriptive knowledge, at the level of behavior, of how drugs for depression and anxiety affect automatic processing or negative biases independent of the effects of depression and anxiety per se. Such biases can be estimated in a variety of ways: for example, the attention to threat, the perception of social expressions on the faces of volunteers or the access to self-referring adjectives in recall memory tasks as described above. The most consistent observed effect has been to increase positive bias in self-referent memory. Thus, for example, treatment with citalopram or reboxetine has a similar effect demonstrable in healthy volunteers taking either drug for 1 week. Obviously this positive effect on emotional processing contrasts with the prevailing negative bias in depressed patients. Could one be the basis for the correction of the other? And could the effect be as immediate as the effect seen in healthy volunteers in the absence of depressed mood? Surprisingly, a single dose of reboxetine was subsequently shown to correct and indeed to normalize the unconscious negative bias in patients being treated for major depression.30 There was of course no immediate effect on symptoms which as usual resolved slowly in these patients. However, very importantly the correction of negative bias on day 1 predicted the subsequent change in symptoms at 6 weeks. The status of emotional processing as a biomarker has the potential to predict individual patient responsiveness to established treatments (as a clinical tool) or drug efficacy in clinical trials of novel compounds.
These findings were initially made purely on the basis of behavior, but have now been confirmed in studies employing brain imaging. Thus, both anxious and depressed patients show increased activity in the amygdala which could be related to their hypersensitivity to negative facial information in particular. The amygdala's functional role described above would predict wide involvement in all kinds of emotional disorders, and this seems to be confirmed by imaging studies of psychopathology to date. It may even be appropriate to think of it providing the substrate for the general negative affectivity experience that seems to unite mood and anxiety disorders. It would therefore be predicted to be a likely locus for drug action, especially. Indeed, we showed almost a decade ago that the SSRI paroxetine reduced responsiveness of the amygdala to fearful faces in healthy controls31: the effect was a very early one like the effects of comparable drugs on behavioral measures of emotional processing. Therefore our hypothesis was that the impact of treatment in anxiety or depression might be to correct prevailing overactivity. Such normalization was initially shown to take place, but over a course of treatment so was not necessarily an early effect. But in fact it has now been shown that the effect precedes a change in symptoms, at least in depression. Patients with major depression were studied after treatment for 7 days with either escitalopram or placebo.32 The sensitivity to fearful faces in the escitalopram-treated patients was markedly attenuated. This normalization of the responses in patients was comparable to the levels seen in healthy volunteers.
Just as changes in behavior early in treatment predicted subsequent clinical improvement the change in amygdala activity also predicted symptomatic changes at 6 weeks.32 Such results with brain imaging increase the confidence with which we can claim an important action of SSRIs and other drugs for depression is to decrease the amygdala responses to aversive stimuli. How this automatic effect is translated into clinical improvement, which it appears to be, remains an interesting and challenging question. One possibility is that the change in emotional bias allows a relearning of normal and emotional responsiveness in the flow of everyday life. To prove that this is actually the key mechanism through which the drugs work is challenging and currently unachieved.
An analogous effort to understand the impact of psychological treatments has only just begun. Interestingly, in patients with panic disorder, a single session of cognitive behavior therapy (CBT) produced immediate correction of negative emotional bias in some of the tests shown to be sensitive to SSRIs. This experimental effect on implicit responding was not accompanied by an immediate effect on subjective symptoms, but again the early changes predicted subsequent response.33
The findings to date highlight the potential for convergent methods and convergent actions in understanding drug and psychological treatments. Neurocognitive tests relevant to psychopathology are the key requirement. An experimental approach can then shed light on actions and interactions. In an early example of this kind of experiment, we assessed the effects of combining an SSRI with a cognitive intervention on measures of affective processing bias and resilience to external stress as tested by measuring the increase in negative symptoms induced by a negative mood induction in healthy subjects. Those who received both citalopram and active cognitive bias training task showed a smaller alterations in emotional memory and categorization bias than did those who received either active intervention singly. The change in negative bias produced by citalopram predicted resistance to the negative mood induction. Thus coadministration of an SSRI and a cognitive training intervention reduced the effectiveness of either treatment alone in relation to anxiety- and depression-relevant emotional processing and citalopram was more effective in increasing resilience to negative mood induction. This approach illustrates the potential for refining how and when we combine different modalities of treatment. It could also help tailor more specific approaches to individual disorders than is currently possible. However, such studies can inform but not replace clinical trial data, which provide the basis for confidence of clinical benefit for the range of treatments available for the anxiety disorders.
Treatment effects: medicines
The role of medicines in the treatment of the anxiety disorders has recently been comprehensively reviewed as an expert guideline.13 The paragraphs below briefly summarize the conclusions, together with additional information on agomelatine, findings for which are relatively recent. More detail for the other drug options is available in the original publication, in particular the references to supporting evidence. In general, the actions of medicines in anxiety disorders have not demonstrated important differential effects between disorders.
Reuptake inhibitors: serotonergic and serotonergic/ noradrenergic
SSRIs exhibit a “broad-spectrum” efficacy in both short-term and long-term treatment in patients with major depression, anxiety disorders, or OCD. They can be presumed to have effect on the general distress dimension described above. Their use is limited by tolerability especially in the longer term, and in particular by the impact on sexual function.
The serotonergic/noradrenergic agents duloxetine and venlafaxine are effective in short-term and long-term treatment of major depression and GAD, and venlafaxine is also effective in the acute treatment and prevention of relapse in panic disorder. Duloxetine and venlafaxine may be less well tolerated than the SSRIs.
Older reuptake inhibitors (tricyclic antidepressants or TCAs) are efficacious in some anxiety disorders, but are associated with a greater burden of adverse effects than the more selective drugs.
Agomelatine
Agomelatine has efficacy in acute treatment and prevention of relapse in GAD34 (for which it not yet licensed) as well as better-known equivalent actions in major depression35: agomelatine's freedom from actions on serotonergic function means that discontinuation symptoms and sexual dysfunction are much less likely than with most other choices for drug treatment.36 Agomelatine's mechanisms of action (Ml, M2 melatonergic agonist, 5-HT2c antagonist) is distinctive. If it turns out, like serotonergic drugs, to act across the general distress dimension, then this has interesting implications because the common benefit seems unlikely to rely on convergent actions on serotonin. Instead it raises the possibility that drug action is better understood at a higher-level mechanism of action. One possibility is by actions on emotional memory processing. Thus, in healthy volunteers after 1 week dosing with 25 mg of agomelatine, the recall of positive words was increased compared with a group taking placebo.37 This is a common effect of drugs for unipolar depression and seems likely to occur because of changed automatic bias within a complex neuronal network.
Monoamine oxidase inhibitors
The irreversible monoamine oxidase inhibitor (MAOI) phenelzine has efficacy in panic disorder and social phobia as well as major depression, when given at adequate doses. Moclobemide, a reversible inhibitor of monoamine oxidase A has efficacy in social phobia and perhaps some value in panic disorder: despite the reversibility of its action, which distinguishes it from other older compounds, the reduced need for dietary restrictions holds only for lower daily doses; it is advisable to avoid tyramine-containing foods at higher doses.
γ-Aminobutyric acid partial allosteric modulators
Drugs acting at the γ-aminobutyric acid (GABA) receptor, often described generically as benzodiazepines, have a common action to facilitate GABA function. A number have proven efficacy in the treatment of patients with panic disorder, GAD and social anxiety disorder, despite causing troublesome sedation and cognitive impairment in both short-term and long-term treatment (with tolerance and dependence a risk in vulnerable patients). It is uncertain whether they are effective in relieving depressive symptoms in patients with anxiety disorders but there is no evidence of efficacy for major depression. They are an example of a class of medicines that lack efficacy across the distress dimension, and hence may be limited to specific actions across the fear spectrum. However, decisive evidence for differential effects are limited.
Pregabalin
Pregabalin is a glutamate voltage calcium channel blocker with proven efficacy in both acute treatment and prevention of relapse in GAD and social anxiety disorder. Long-term treatment is accompanied by weight gain in approximately 20% of patients. Discontinuation symptoms after abrupt withdrawal of pregabalin have been reported, as has its abuse in individuals with a history of other substance abuse.
Dopamine and serotonin receptor antagonists
Drugs for psychosis have often been prescribed to patients with anxiety disorders, although the best controlled evidence for benefit is only recent and restricted to acute treatment and prevention of relapse with quetiapine in GAD, and for the augmentation of SSRI treatment in patients with OCD.
Treatment effects: psychological interventions
Psychological treatments have been described for most anxiety disorders (GAD, social anxiety disorder, PTSD, OCD). A systematic review of 21 studies in patients with depression or anxiety disorders suggest that guided self-help has similar effectiveness to face-to-face psychotherapy38 and in a further 31 randomized controlled trials in anxiety disorders self-help interventions were more effective than being placed on a waiting list (essentially nocebo) but less effective than therapist-administered treatments.39 The key development in self-help approaches will be Internet-based psychological treatments. Their place is not yet established but a systematic review of 52 studies in depression or anxiety disorder suggested that they held promise, notably for mild/moderate depression.40 Nevertheless, the highest effect is against waiting list, which is an unsatisfactory standard. The great advantage of computerization will be the potential for systematic comparison of the active elements of any psychotherapy by excellent matching of alternative treatments and large numbers of participants: this is simply not possible with traditional therapy based on very high-level cognitive constructs and a folk psychology emphasis on therapist behavior.
It is uncertain whether combining psychological treatments with pharmacological treatments is associated with greater long-term benefit than with either treatment alone. There would appear to be ways to look for drug/training interactions in experimental medicine models, as explained above. A more rational combination of drug and psychological treatment appears to have the potential to improve outcomes.
Conclusions
The anxiety disorders display both common and unique features. The common features may well be based on a shared genetic and biological foundation that is linked to the normal dimensions of experience we can summarize as distress and negative affectivity. Anti-anxiety treatments like the SSRIs appear to act across the anxiety and depressive disorders, and it is tempting to suppose that the serotonin system and its connections, especially in the amygdala, provide a common nonspecific substrate both for disturbance and treatment effects. The unique features of the different disorders may be important targets for complete treatment. In the case of depression effects on positive mood (the reversal of anhedonia) is attracting increasing interest. In the case of the anxiety disorders, their unique features may provide an important potential focus for psychological approaches, although the mechanisms of psychological treatments are still poorly understood. Our improving understanding could facilitate the development of better treatment strategies, especially in combining medicines and psychotherapy.
Disclosures: In the last 3 years the author has held grants from Servier, received honoraria for speaking or chairing educational meeting from Abbvie, AstraZeneca, GSK, Lundbeck, Medscape, and Servier, and advised AstraZeneca, Cephalon/Teva, Lundbeck, Merck, Otsuka, PWital, Servier, Shire, Sunovion, and Takeda. He holds shares in Plvital and has acted as an expert witness for Lilly.
REFERENCES
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association. 2013;53 [Google Scholar]
- 2.Magee WJ., Eaton WW., Wittchen HU., McGonagle KA., Kessler RC. Agoraphobia, simple phobia, and social phobia in the National Comorbidity Survey. Arch Gen Psychiatry. 53(2):159–168. doi: 10.1001/archpsyc.1996.01830020077009. [DOI] [PubMed] [Google Scholar]
- 3.Crino RD., Andrews G. Obsessive-compulsive disorder and axis I comorbidity. J Anxiety Disord. 1996;10(1):37–46. [Google Scholar]
- 4.Kessler RC., Nelson CB., McGonagle KA., Liu J., Swartz M., Blazer DG. Comorbidity of DSM-III-R major depressive disorder in the general population: results from the US National Comorbidity Survey. Br J Psychiatry Suppl. 1996;(30):17–30. [PubMed] [Google Scholar]
- 5.Kessler RC., Petukhova M., Sampson NA., Zaslavsky AM., Wittchen HU. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Meth Psychiatr Res. 2012;21(3):169–184. doi: 10.1002/mpr.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hofmeijer-Sevink MK., Batelaan NM., van Megen HJ., et al Clinical relevance of comorbidity in anxiety disorders: a report from the Netherlands Study of Depression and Anxiety (NESDA). J Affect Disord. 2012;137(1-3):106–112. doi: 10.1016/j.jad.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Watson D., Tellegen A. Toward a consensual structure of mood. Psychol Bull. 1985;98(2):219–235. doi: 10.1037//0033-2909.98.2.219. [DOI] [PubMed] [Google Scholar]
- 8.Watson D., Wiese D., Vaidya J., Tellegen A. The two general activation systems of affect: Structural findings, evolutionary considerations, and psychobiological evidence. J Personal Soc Psychol. 1999;76(5):820–838. [Google Scholar]
- 9.Clark LA., Watson D. Tripartite model of anxiety and depression: psychometric evidence and taxonomic implications. J Abnorm Psychol. 1991;100(3):316–336. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- 10.Kendler KS., Prescott CA., Myers J., Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry. 2003;60(9):929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- 11.Hettema JM., Neale MC., Kendler KS. A review and meta-analysis of the genetic epidemiology of anxiety disorders. Am J Psychiatry. 2001;158(10):1568–1578. doi: 10.1176/appi.ajp.158.10.1568. [DOI] [PubMed] [Google Scholar]
- 12.Mineka S., Watson DW., Clark LA. Psychopathology: comorbidity of anxiety and unipolar mood disorders. Ann Rev Psychol. 1998;49:377–412. doi: 10.1146/annurev.psych.49.1.377. [DOI] [PubMed] [Google Scholar]
- 13.Baldwin DS., Anderson IM., Nutt DJ., et al Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol. 2014;28(5):403–339. doi: 10.1177/0269881114525674. [DOI] [PubMed] [Google Scholar]
- 14.Kendler KS., Hettema JM., Butera F., Gardner CO., Prescott CA. Life event dimensions of loss, humiliation, entrapment, and danger in the prediction of onsets of major depression and generalized anxiety. Arch Gen Psychiatry. 2003;60(8):789–796. doi: 10.1001/archpsyc.60.8.789. [DOI] [PubMed] [Google Scholar]
- 15.Hettema JM., Prescott CA., Myers JM., Neale MC., Kendler KS. The structure of genetic and environmental risk factors for anxiety disorders in men and women. Arch Gen Psychiatry. 2005;62(2):182–189. doi: 10.1001/archpsyc.62.2.182. [DOI] [PubMed] [Google Scholar]
- 16.Lesch KP., Bengel D., Heils A., et al Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274(5292):1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 17.Munafo MR., Freimer NB., Ng W., et al 5-HTTLPR genotype and anxiety-related personality traits: a meta-analysis and new data. Am J Med Genet B Neuropsychiatr Genet. 2009;150B(2):271–281. doi: 10.1002/ajmg.b.30808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caspi A., Sugden K., Moffitt TE., et al Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 19.Munafo MR., Flint J. How reliable are scientific studies? Br J Psychiatry. 2010;197(4):257–258. doi: 10.1192/bjp.bp.109.069849. [DOI] [PubMed] [Google Scholar]
- 20.Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Network and Pathway Analysis Subgroup of Psychiatric Genomics Consortium. Psychiatric genome-wide association study analyses implicate neuronal, immune and histone pathways. Nat Neurosci. 2015;18(2):199–209. doi: 10.1038/nn.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feinstein JS., Adolphs R., Damasio A., Tranel D. The human amygdala and the induction and experience of fear. Curr Biol. 2011;21(1):34–38. doi: 10.1016/j.cub.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LaBar KS., Gatenby JC., Gore JC., LeDoux JE., Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20(5):937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- 24.Whalen PJ., Rauch SL., Etcoff NL., Mclnerney SC., Lee MB., Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci. 1998;18(1):411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphy SE., Norbury R., Godlewska BR., et al The effect of the serotonin transporter polymorphism (5-HTTLPR) on amygdala function: a metaanalysis. Mol Psychiatry. 2013;18(4):512–520. doi: 10.1038/mp.2012.19. [DOI] [PubMed] [Google Scholar]
- 26.Pergamin-Hight L., Naim R., Bakermans-Kranenburg MJ., van IMH., Bar-Haim Y. Content specificity of attention bias to threat in anxiety disorders: a meta-analysis. Clin Psychol Rev. 2015;35:10–18. doi: 10.1016/j.cpr.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Chan SW., Goodwin GM., Harmer CJ. Highly neurotic never-depressed students have negative biases in information processing. Psychol Med. 2007;37(9):1281–1291. doi: 10.1017/S0033291707000669. [DOI] [PubMed] [Google Scholar]
- 28.Fonzo GA., Ramsawh HJ., Flagan TM., et al Common and disorder-specific neural responses to emotional faces in generalised anxiety, social anxiety and panic disorders. Br J Psychiatry. 2015;206(3):206–215. doi: 10.1192/bjp.bp.114.149880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Etkin A., Schatzberg AF. Common abnormalities and disorder-specific compensation during implicit regulation of emotional processing in generalized anxiety and major depressive disorders. Am J Psychiatry. 2011;168(9):968–978. doi: 10.1176/appi.ajp.2011.10091290. [DOI] [PubMed] [Google Scholar]
- 30.Harmer CJ., O'Sullivan U., Favaron E., et al Effect of acute antidepressant administration on negative affective bias in depressed patients. Am J Psychiatry. 2009;166(10):1178–1184. doi: 10.1176/appi.ajp.2009.09020149. [DOI] [PubMed] [Google Scholar]
- 31.Harmer CJ., Mackay CE., Reid CB., Cowen PJ., Goodwin GM. Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biol Psychiatry. 2006;59(9):816–820. doi: 10.1016/j.biopsych.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 32.Godlewska B., Browning M., Norbury R., Cowen P., Harmer C. Early changes in neural responses to emotional information predict clinical response to antidepressant treatment in depression. Int J Neuropsychopharmacol. 2014;17:82. [Google Scholar]
- 33.Reinecke A., Waldenmaier L., Cooper MJ., Harmer CJ. Changes in automatic threat processing precede and predict clinical changes with exposure-based cognitive-behavior therapy for panic disorder. Biol Psychiatry. 2013;73(11):1064–1070. doi: 10.1016/j.biopsych.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 34.Stein DJ., Ahokas AA., de Bodinat C. Efficacy of agomelatine in generalized anxiety disorder: a randomized, double-blind, placebo-controlled study. J Clin Psychopharmacol. 2008;28(5):561–566. doi: 10.1097/JCP.0b013e318184ff5b. [DOI] [PubMed] [Google Scholar]
- 35.Taylor D., Sparshatt A., Varma S., Olofinjana O. Antidepressant efficacy of agomelatine: meta-analysis of published and unpublished studies. BMJ. 2014;348:g1888. doi: 10.1136/bmj.g1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stein DJ., Ahokas A., Albarran C., Olivier V., Allgulander C. Agomelatine prevents relapse in generalized anxiety disorder: a 6-month randomized, double-blind, placebo-controlled discontinuation study. J Clin Psychiatry. 2012;73(7):1002–1008. doi: 10.4088/JCP.11m07493. [DOI] [PubMed] [Google Scholar]
- 37.Harmer CJ., de Bodinat C., Dawson GR., et al Agomelatine facilitates positive versus negative affective processing in healthy volunteer models. J Psychopharmacol. 2011;25(9):1159–1167. doi: 10.1177/0269881110376689. [DOI] [PubMed] [Google Scholar]
- 38.Cuijpers P., Donker T., van Straten A., Li J., Andersson G. Is guided self-help as effective as face-to-face psychotherapy for depression and anxiety disorders? A systematic review and meta-analysis of comparative outcome studies. Psychol Med. 2010;40(12):1943–1957. doi: 10.1017/S0033291710000772. [DOI] [PubMed] [Google Scholar]
- 39.Lewis C., Pearce J., Bisson Jl. Efficacy, cost-effectiveness and acceptability of self-help interventions for anxiety disorders: systematic review. Br J Psychiatry. 2012;200(1):15–21. doi: 10.1192/bjp.bp.110.084756. [DOI] [PubMed] [Google Scholar]
- 40.Arnberg FK., Linton SJ., Hultcrantz M., Heintz E., Jonsson U. Internet-delivered psychological treatments for mood and anxiety disorders: a systematic review of their efficacy, safety, and cost-effectiveness. PLoS One. 2014;9(5):e98118. doi: 10.1371/journal.pone.0098118. [DOI] [PMC free article] [PubMed] [Google Scholar]