Abstract
Physiological and adaptive separation anxiety (SA) is intimately connected with the evolutionary emergence of new brain structures specific of paleomammalians, the growth of neomammalian—and later hominid—brain and skull size, and the appearance of bipedalism. All these evolutionary milestones have contributed to expanding the behavioral repertoire and plasticity of prehuman and human beings, at the cost of more prolonged dependency of the infant and of the child on parental care. Separation anxiety disorder (SAD) can be seen as an exaggerated/inappropriate manifestation of SA that constitutes a gateway to poorer mental and physical health. By blending epidemiological, genetic-epidemiological, endophenotypic, and animal laboratory approaches, it is possible to delineate some of the mechanisms that link childhood-adolescence SA and SAD to health problems later in life. Causal mechanisms include gene-environment interplays and likely differential regulation of genes and functional net-works that simultaneously affect multiple behavioral and physical phenotypes after exposure to early-life adversity, including parental separation/loss.
Keywords: separation anxiety, CO2 hypersensitivity, panic disorder, gene-environment interaction, animal mode, DOhAD
Abstract
La ansiedad de separación (AS) fisiológica y adaptativa está íntimamente relacionada con el surgimiento durante la evolución de nuevas estructuras cerebrales específicas de los paleomamíferos, el aumento del tamaño del cerebro y del cráneo de los neomamíferos y más tarde de los homínidos, y la aparición de la bipedestación. Todos estos hitos evolucionistas han contribuido a la expansión del repertorio conductual y a la plasticidad de los seres prehumanos y humanos, a costa de una dependencia más prolongada del lactante y del niño respecto al cuidado parental. El trastorno por ansiedad de separación (TAS) se puede considerar como una manifestación exagerada/inapropiada de la AS que constituye una puerta de entrada a una peor salud mental y física. Al combinar aproximaciones epidemiológicas, genético-epidemiológicas, endofenotípicas y de animales de laboratorio es posible bosquejar algunos de los mecanismos que relacionan la AS de la niñez-adolescencia y el TAS con problemas de salud en años posteriores. Los mecanismos causales incluyen interjuegos entre genes y ambiente y una probable regulación diferencial de genes y redes funcionales que afectan simultáneamente múltiples fenotipos conductuales y físicos después de la exposición a la adversidad temprana en la vida, incluyendo la separación y la pérdida parental.
Abstract
L'anxiété de séparation (AS) physiologique et adaptée est intimement liée à l'émergence évolutive des nouvelles structures cérébrales spécifiques des paléomammifères, à la croissance du cerveau néomammifère, et plus tard hominidé, à la taille du crâne et à l'apparition de la bipédie. Tous ces jalons évolutifs ont contribué à élargir la plasticité et le répertoire comportementaux des êtres préhumains et humains, au prix d'une dépendance plus longue du nourrisson et de l'enfant au soin parental. Le trouble anxiété de séparation (TAS) peut être considéré comme une manifestation exagérée/inappropriée de l'AS, constituant une passerelle vers une dégradation de la santé mentale et physique. En associant les approches épidémiologiques, génético-épidémiologiques, endophénotypiques et des laboratoires animaux, il est possible de définir certains des mécanismes qui relient l'AS de l'enfance et de l'adolescence et les TAS aux problèmes de santé survenant plus tard dans la vie. Des interactions gène-environnement et probablement une régulation différentielle des gènes et des réseaux fonctionnels font partie du mécanisme causal, touchant simultanément de nombreux phénotypes comportementaux et physiques après une exposition à l'adversité dans la petite enfance, comme la séparation/perte d'un parent.
“ With maternal love, life makes a promise at dawn that it can never hold [Avec l'amour maternel, la vie vous fait à l'aube une promesse qu'elle ne tient jamais]”
Romain Gary, 1960
Separation anxiety: a tentative paleoneurobiological history
Separation anxiety (SA) is indissolubly connected with several essential anatomic and functional milestones that marked the emergence of mammals from mammal-like reptiles. These include the advent of the brain's neopallium during the process of phylogenesis and the evolution into bipedalism, followed by the formidable success story of human adaptation on planet Earth. Interpretations of anthropometric data and tools from our ancestors allow us to infer the way in which brain growth—and the related, augmented plasticity of behavior contributing to extending the infant's dependence on parental care—bring about some novel and specific elements of the mother-child bond and attachment, and also to reinforce and expand the regulatory element of SA.
As with any newly emerging behavior that becomes consistently and adaptively maintained within a species (or rather within a class, as SA is present in virtually all mammals), at least two thoughts relevant to evolution arise. First, a simple consideration: it takes new “hardware” to produce a new behavior. Second, the prolonged dependence on parental care that characterizes our species is probably a cost of the selective advantage of having larger, and thus more plastic, brains.
Brain and body size trade-offs in human phylogenesis
It takes a proportionately large brain to yield a sophisticated, plastic array of behavioral, emotional, and cognitive functions. A suitable ratio of brain-to-body dimensions is, however, necessary to support comparatively large brains, both mechanically and metabolically.
The advent of greater brain cellular mass—like that associated with the emergence of the neopallium—allowed paleomammals to attain greater brain plasticity and extend the array of learning capacities, which are in turn basic ingredients of individual variation in behavior. All forms of learning, however, require time for practicing, as practice implies errors, and correction and consolidation of newly acquired skills. In a growing child this generates dependence on parental care in order to ensure safety, nourishment, and protection. It can thus be expected that the wider the array and plasticity of behavioral repertoire in a species, the longer the time needed for learning and practicing, and the more protracted the dependence on parental care.
Within this frame of reference, mankind constitutes the epitome of a series of evolutionary trade-offs between brain mass, offspring body size (especially relative to skull dimensions), the diameter of maternal pelvic inlet and birth canal, and offspring dependence on parental (mostly maternal) care.1 With their limited range of stereotyped behavior, birds—which need to be light for flying and cannot afford relatively large, and thus heavy, brains—are an example of an opposite evolutionarytrade-off tendency: smaller brains are light to fly away with, but allow for only limited behavioral repertoires.
While brain size at birth is only a precursor of the adult brain size (the human brain expands by a factor of 3.3 from birth to adulthood, compared with a 2.5 factor in our closest cousins, the chimpanzees2), the distinctively human achievement of large adult brains takes place primarily through higher growth rates early in ontogeny, rather than through a markedly extended period of growth.2
Inevitably, the evolutionary history of a best-fitting equation between brain size, brain plasticity, and behavioral sophistication in our species needed to accommodate several constraints, one of which—the diameter of the pelvic inlet and birth canal—is paramount in a bipedal species like ours. Man is actually the only extant mammal that permanently walks on two legs, with skeletal hallmarks of bipedalism being already recognizable in the 4-million-year-old Australopithecus anamensis,3 and significant brain expansion beginning with the Homo genus, some 2.5 million years ago,4 parallel to the appearance of tool utilization. With the advent of bipedalism, anatomy underwent significant departures from the pattern of apes and other nonhuman primates. Bipedalism required a relatively narrow pelvis that could simultaneously accommodate balance and the possibility of running for safety, and still permit bearing infants with increasingly large brains and skulls. Among prehumans these disparate needs entailed the necessity for optimal adjustments between the transverse diameter of the maternal inlet, the infant's longer antero-posterior cranial dimension, and the need for the infant to rotate during labor in order to have the head enter the inlet facing transversely.5 By the coincidence of all these factors, humans (and probably prehumans) became the only species characterized by the need for assistance at birth.5 Neonatal brain size of about 400 cm3 has been identified as a feature of the last common ancestor of Neanderthals and Homo sapiens, probably representing the best evolutionary compromise between pressure and brain mass growth, and the upper physiological and obstetrical limit that can be attained in Homo, irrespective of the course of postnatal brain expansion.6 Another implication of hominid and human fetal brain growth is the need for substantial maternal resources investment7 to sustain brain progression during gestation and after delivery, together with the implication of a more prolonged relationship of interdependence between infant and mother. The progressive extension of a period of dependence from maternal care that can be attributed to an increasingly complex, and thus immature, brain at birth, probably prepared the ground for the development and maintenance of SA as an element of reciprocal regulation of the infant-mother bond, and a moderator between the child's cycles of exploration of the environment, learning, and safe return to the mother.1
New hardware for new functions: the emergence of the neo mammalian brain and the separation call
It has been noted that three elements of behavior mark the transition from reptiles to early mammals: nursing, play, and the separation call.8 This latter behavior, intended to maintain mother-offspring contact, has been suggested to be the most ancient form of mammal communication, and together with nursing and play, probably became possible by the emergence of the distinctively mammalian brain structures of the cingulate cortex and the so-called thalamo-cingulate division.8 Since the mid-1950s, murine lesion studies have shown the importance of this complex for nursing9 and playing, a fundamental element for learning and socializing in mammals.10 In many primates the separation call is a regular message to “check” that the offspring-mother distance is acceptable, again with the rostral cingulate gyrus being implicated.8 In man, the separation call can take on different nuances and communicate hunger, pain, and other needs, and primordial “limbic affective vocalizations” may have constituted a springboard for the evolution of successive neocortical propositional speech.8
Through the advent of the separation call, a clearer, more interactive mother-infant bond became possible, and formed the basis for an exquisitely human variant of separation anxiety, the one that, by strengthening the mother-infant bond, created the basis for another distinctively human realization: the family as stable unit of highly individualized relationships and the smallest component of societies. Once more, it is fascinating to notice the correspondence between anatomical changes, new behavioral displays, and adaptation: based on fossil analyses, it has been argued that the mammalianlike reptiles were most probably deaf and mute.8 If they were also cannibalistic, as are most of today's lizards, it would have been highly maladaptive for their offspring to call attention to themselves.
SA and SAD in contemporary human societies
Epidemiology, physiological course, and the relationships between SA and SAD
Physiological SA typically becomes manifest in man between 6 and 12 months of age and remains clearly observable until approximately the age of 3 years, to steadily abate afterwards, without notable sex-related differences.11 Although SA is most typically observable in the mother-child interaction and within nuclear family interactions, it may involve a relatively extended array of figures of attachment, and is typically expected to contribute to moulding attachment and separation patterns later in life.11 Children are able to form several figures of attachment. However, as far as SA is concerned, the construction of a clearly identifiable bond with a small number of individuals seems to constitute the optimal format, as suggested by higher incidence of non-autonomous attachment representations and less competent coping with imagined separations among adolescents raised in a communal kibbutz setting, compared with peers raised in more traditional familial settings.12
The SA-related diagnostic category of SAD has features that connect to both normal and abnormal development. Consistent with prior diagnostic formulations, the DSM-V13 recognizes SAD as an exaggerated and/or age inappropriate manifestation of physiological SA: this supports a degree of continuity between adaptation and psychopathology, the differences being a matter of intensity of the manifestations, and appropriateness for age and context. While the DSM-V diagnosis of SAD requires—as is typical for a polythetic approach—the presence of a minimum of 3 among 8 symptoms, three manifestations: (i) overt distress related to separation; (ii) reluctance to sleep separated from a major attachment figure; (iii) fear of being alone or without an attachment figure, seem to best discriminate children with higher vs lower SA, according to recent item-response analyses.14
It is also generally recognized that children can show exacerbations/remissions of anxiety about separation, so that self-limiting phases of heightened SA manifestations do not necessarily warrant a diagnosis of SAD,13 and may represent transient, and even possibly adaptive, responses to changing environments. Due to the continuity between physiological (ie, transient and self-limiting) SA and clinical SAD, and the relationships between childhood SAD and later psychopathology (panic disorder and agoraphobia most prominently, see next section), early identification of children with a profile of high SA who are more likely to go on to develop full-blown SAD is of paramount importance.
The prevalence of SAD is estimated at 4% in population samples15 and 7.6% in pediatric clinical samples.16 While large-scale epidemiological studies have reported 6 years as the age of onset for typical SAD,17 recent pediatric primary care surveys indicate that SAD is quite common among preschool children.18 This spurred our recent efforts to characterize the longitudinal trajectories of SA among general population children from age 1.5 years to age 6 years, and the study of the factors associated with higher and more persistent SA symptoms in the preschool years.
In the representative, population-based cohort of the Quebec Longitudinal Study of Child Development19 (QLSCD) we20 found four well-distinguishable trajectories, including a prevailing, physiological Low-Persistent group (60.2%), and, at the opposite end of the distribution, a High-Increasing (6.9%) group. The High-Increasing group showed a remarkably stable, elevated SA profile throughout the preschool years, and predicted teacher-assessed SA profiles at age 6 years. The High-Increasing group was also distinctively associated with higher maternal depression, maternal smoking during pregnancy, and parental unemployment. Except for the High-Increasing group, the other three trajectories showed very substantial symptom reduction by age 6 years. While internalizing and externalizing problems often co-occur in children,21,22 the High-Increasing group showed no significant association with externalizing problems,20 suggesting that high and persistent SA tends to present as a predominantly internalizing picture. In keeping with the general indication that development tends to evolve into adaptation, these data20 show that the majority of children with a high SA profile at age 1.5 years will progressively recover by age 4 to 5 years. However, high SA that persists over time from age 1.5 years onwards deserves special attention, as it may predict SAD. Another clinical implication of our findings is that the early identification of children at risk for SAD should not rely solely on early assessment, but also adopt a trajectory approach, ie, seek evidence for consistently high SA profile, as assessed by repeated assessments over time.
Do separation events precipitate SA? Are separation events purely “environmental”?
An interesting aspect pertains to the role of actual vs feared separation events in the etiology of SA and SAD. Do separation events (eg, parental separation/divorce/death, protracted illnesses) put children at heightened risk for SA and SAD? Our epidemiological longitudinal study20 of SA showed only a transient association (in the 1.5- to 2.5-year window of measurement) between parental divorce and SA trajectories, suggesting only transitory effects on children's evolution of SA. However, longitudinal data on the association between parental separation/divorce and offspring SA after age 6 are lacking, and it would be conceivable for such an association to become significant when parental separation/divorce strikes at a later age, when children's cognitive abilities are more developed, and allow for a deeper understanding of the impact and implications of such events. Moreover, the occurrence and impact of childhood parental loss (through parental separation/ divorce, or death) on offspring psychopathology is further complicated by the difficulties in teasing apart the cross-sectional (age at impact) from the longitudinal (duration, including the pre-separation/divorce period, which is arguably marked by parental conflict) components of this adversity.
Possible risk factors such as parental conflict are typically intended as mediators that act between negative familial atmosphere and children's psychopathology. However, the rearing environment is influenced in part by parental heritable behavioral characteristics that may lead simultaneously to familial disruption and psychological difficulties in the offspring.23 This connects to the general concept that, while adversities convey a risk that occurs in the physical world, their causal architecture is often partially genetic. This implies the need for thorough understanding of risk processes, including the delineation and quantification of separate effects, such as gene-environment correlation (rGE) and gene-environment interaction (GxE).
Further etiological questions pertain to the relationships between childhood parental loss SAD and the future development of panic disorder (see next section).
Etiological architecture of SA and SAD, and their neurobiology
The bearing of SAD on later individual risk for psychopathology (and to some extent, to familial-genetic aggregations of mental illness) has been debated over time. A specific longitudinal relationship between SAD in childhood, and the later appearance of panic disorder (PD)-agoraphobia (AGO) in the same individual was first suggested by Klein24 and Gittelman-Klein25 on the basis of clinical observation. The best available source of information available on this topic is probably offered by meta-analytic data26 from 25 independent studies and some 15 000 subjects worldwide, showing a specificity of the link between childhood SAD and the later development of PD/PD-AGO in the same individuals by an odds ratio (OR) of 3.45 [2.4-5.0]. This confirmed earlier findings27-29 that SAD, together with a family history of PD-AGO, predicts earlier onset of PD-AGO. The same meta-analysis26 failed to find a significant relationship of SAD to the development of later depression, or substance use, while the bearing of the development of PD-AGO on anxiety disorders other than SAD combined together had a lower OR of 2.19.26 This again supports a specific relationship between SAD and PD. Since childhood SAD and adolescence/early adulthood PD are two phenomenologically diverging disorders, this makes a case for heterotypic continuity.
A further, powerful approach to clarifying the neurobiological basis of SA/SAD, its relationships to earlylife adversities (including separation and loss), and its bearing on other mental—and possibly physical—illnesses posits on the endophenotype30 approach. Childhood SAD shares with adult PD the endophenotypic trait of exaggerated emotional and respiratory reactivity to CO2.31,32
By genetically informed strategies, we showed that: (i) a significant proportion of genetic factors are proper to the response to CO2 stimulation, and do not overlap with the genetic influences of pretest baseline, general trait anxiety33; (ii) childhood SAD, CO2 hypersensitivity, and PD in adulthood co-occur in the same individuals, largely due to a common set of genes34,35; (iii) the early-life adversity of childhood parental loss (CPL), contributes to explaining these reciprocal relationships35; (iv) family-wide environmental influences are important influences for SAD.36 Moreover, common and stressful life events: (v) heighten CO2 sensitivity37; and (vi) enhance the influence of genetic factors on post-CO2 anxiety,38 a form of GxE.39,40 Interestingly, GxE effects on CO2 sensitivity are explained by events that strike within childhood-adolescence, not adulthood.38 Based on this latter finding, and the notion that hyperventilation and increased arousal in response to heightened CO2 are common to all mammals, we modeled early life adversities in a repeated cross-fostering (RCF) paradigm of early interference of mother-pups interplay, and studied its consequences on responses to CO2 stimulation41 (See the following section).
Corroborations of our findings, including the shared genetic diathesis between PD and SAD42 and the links between CO2 hypersensitivity, SAD, and familiality of PD,32 have come from other research groups and independent samples. Some reported associations between SAD, specific risk factors, and adult psychopathology appear counterintuitive, however. It is still unclear whether and how real vs feared separation/loss act in influencing SA/SAD, and to what extent these elements affect the later development of PD. Research on clinical and community-based samples of adult subjects35,43 concur in finding that retrospectively assessed childhood SAD predicts PD in early adulthood,26 and childhood parental loss has been associated with heightened risk for PD.44 However, the correlations between separation/loss events and retrospective assessments of childhood SAD were reported as being close to zero.35,43 There may thus be heterogeneous, or independent, pathways that lead from childhood adversity to the SAD-PD developmental continuum: one can conceive a first pathway whereby actual early separation/loss events predispose later development of PD without inducing SAD, and on the opposite end a pathway whereby children fear separation/loss excessively, and therefore meet the criteria for SAD only to develop PD when they enter late adolescence/early adulthood. While this doublepathway hypothesis constitutes an oversimplification, our recent longitudinal data on SA trajectories20 are compatible with this view.
SAD in the era of DOhAD: early life adversity, animal models, and gene-environment interplays
While a clear causal relationship between early life adversity and SAD is yet to be proven in human naturalistic cohorts and awaits to be better understood, we have shown that a specific category of early life adversity, namely childhood parental loss (in the form of parental separation/divorce or death), is significantly associated with SAD, PD, and their related endophenotype of heightened CO2 sensitivity. This suggests that neural systems involved in two different phenotypes—emotionality and respiration physiology—are to some extent simultaneously affected by a specific type of early life adversity.
In mice, by cross-fostering newborn pups from their biological mother to other lactating dams, we induced both heightened ultravocalization (the murine equivalent of the separation call) during pups' isolation, and exaggerated hyperventilation during 6% CO2 breathing.45,46 The abnormal CO2 sensitivity induced by RCF is stable from childhood into adulthood,45 and in late adulthood is associated with reduced sensitivity- to natural rewards and increased susceptibility to adverse events.47 The RCF findings cannot be ascribed to altered maternal care, since the amount of nursing, and licking/grooming behavior received by RCF and control pups was indistinguishable.45 By partitioning the variance of the respiratory responses provided by genetically related and unrelated cross-fostered and control mice, we found that RCF is associated with a significant increase in genetic variance and almost doubling of heritability of the respiratory responses45 to CO2. This indicates the presence of GxE effects evoked by the early life adversity of interference with mother-pup bond, in close similarity with human results.38
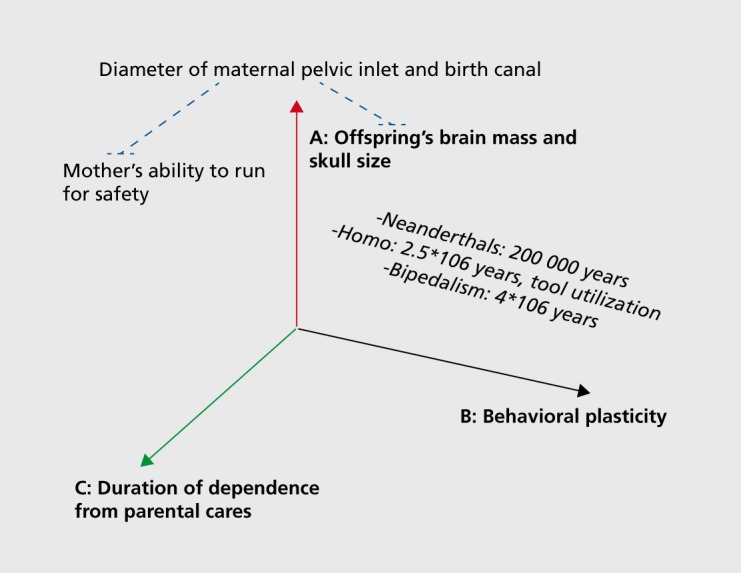
While quantitative GxE effects are essentially an algebraic finding and require painstaking and complex statistical control for biases, their ultimate relevance is to be searched for in biological meaning and mechanisms, the essential implication of GxE being that the genetic predisposition of an individual to develop a condition can manifest differentially- according to environmental circumstances. Most prominently, GxE effects can inform us on how early life experiences and exposures to adverse environments become biologically embedded, and can exert lifelong influences on several complex phenotypes simultaneously.48,49 This same insight is at the basis of the rapidly growing interest in the developmental origins of health and disease (DOHaD), and is consonant with the fact that childhood SAD has recently been identified as a possible gateway to both physical and mental health problems later in life.15 In addition to rendering the artificial distinction between physical and mental health definitively obsolete, the bulk of these data call for real-world, comprehensive approaches to complex disorders that simultaneously tackle behavioral and physical manifestations of illness. Can we speculate on a possible example that applies to the data discussed in this paper? Figure 1.
Figure 1. Interplays among three major evolutionary trends. Three related evolutionary trends in human evolution: the relative increase in brain mass (A) predicts greater and more sophisticated behavioral plasticity (B), which in turn predicts longer dependence on parental care (C) due to prolonged learning needs. The diameter of maternal pelvic inlet (and width of birth canal) constitute a constraint (blue dotted lines) to: (i) prenatal growth of offspring brain; and (ii) maternal agility, including the vital ability to run for safety. The estimated time of appearance of bipedalism, tool utilization, and Neanderthal man are also depicted. See main text for further details.
As mentioned, children with SAD and adults with PD show exaggerated emotional and respiratory responses to CO2-enriched air mixtures; since CO2 provokes rapid acidification of blood, these anxiety disorders may share a distinctive sensitivity to acidification of brain pH.50-52 A recent, promising avenue towards the understanding of CO2 hypersensitivity in animals and man has been developed by focusing on the possible role of the acid-sensing ion channels (ASICs) that are implicated in anxiety, pH sensing, and nociception.53,54
Investigations on the possible effects of early life adversities on the ASIC functions could add significantly to the comprehension of the links among early separation events, CO2 hypersensitivity, and anxiety disorders in childhood and early adulthood. The viability of such approach, which would encompass pleiotropic effects of early adversities on anxiety and nociception simultaneously, is indeed supported by some data. Early life adversities predict augmented visceral pain in man,55 and both rats and mice exposed to early adversities including maternal separation show diminished nociceptive threshold/hyperalgesia.56,57 In a timely tendency, DNA methyiation has been suggested as a core mechanism to explain altered nociception in response to adversity.58 The co-occurrence of anxiety and pathological pain syndromes (exaggerated/aberrant nociception) in the same subjects provides another possible support to our example, as much as it constitutes a poorly understood case for comorbidity. Chronic pain affects ≥20% of adults and it is often comorbid with mood/anxiety disorders.59,60 Pain and psychiatric comorbidity, however, have typically been studied in clinical samples of depressed patients, which constitute a possible source of biased estimates. Indeed, better-designed, large-scale general population epidemiological surveys59 found associations between chronic pain syndromes (including migraine, back pain, and arthritis) and common anxietydisorders (panic, generalized anxiety) with odds ratios (up to 2.5-3.2) that outnumber those found between depression and pain (average odds ratios: 2.1).
Very-large-scale parental separation events in contemporary human societies, together with their possible implications on children and adolescents' health, evoke further questions. It has been estimated that some 61 million Chinese children are being exposed to prolonged parental separation (>3 months at a time, according to the All-China Women's Federation, a Communist Party advocacy group: http://www.womenofchina.cn) as a consequence of their mothers' and fathers' migration form rural villages to industrial settlements in their search for better wages. These children (38% aged between 0 and 6 years), now widely known as the “left-behind,” report seeing their father and/or mother only seldom (75%: once a year), suffer from significantly more accidental injuries, and 42% of them report “having no one talking to them when they feel bad.” A staggering figure of 2.1 million of these children are reported to live alone, and 80% of their parents report feeling inadequate for having left their children behind. Of course, a relatively distal “macrovariable” of this type encompasses several likely other potential risk factors, such as lower socioeconomic status, or diminished external control and discipline, and may extend past separation, to include neglect or even abuse. While it remains true that children are able to build multiple figures of attachment, in keeping with the data and speculations presented in this paper, several61,62 but not all63 investigations on the Chinese “left behind” children show deleterious consequences on a wide array of indices of both physical and mental health.
Conclusions
Both SA and SAD are profoundly related to human unique evolutionary, biological, and cultural history: without taking these elements into account their comprehension cannot be fully accomplished. A relatively wide array of research approaches and findings indicate that early life exposure to adversities and genetic elements of risk interplay in influencing the risk for, and manifestation of, SAD. An emerging set of data converges in indicating that SAD can constitute a possible gateway towards future mental and physical health problems, so that early identification and follow-up of children with unusually high and persistent profiles of SA during the preschool years may constitute a valuable preventive strategy.
REFERENCES
- 1.Pfeiffer JE. The Emergence of Man. New York, NY: Harper & Row; 1969 [Google Scholar]
- 2.DeSilva J., Lesnik J. Chimpanzee neonatal brain size: Implications for brain growth in Homo erectus. J Hum Evol. 2006;51:207–212. doi: 10.1016/j.jhevol.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Leakey MG., Feibel CS., McDougall I., Walker A. New four-million year-old hominid species from Kanapoi and Allia Bay, Kenya. Nature. 1995;376:565–571. doi: 10.1038/376565a0. [DOI] [PubMed] [Google Scholar]
- 4.Schick K., Toth N. Making Silent Stones Speak: Human Evolution and the Dawn of Technology. New York, NY: Touchstone, Simon and Schuster; 1993 [Google Scholar]
- 5.Rosenberg K., Trevathan W. Birth, obstetrics and human evolution. Br J Obstet Gynaecol. 2002;109:1199–1206. doi: 10.1046/j.1471-0528.2002.00010.x. [DOI] [PubMed] [Google Scholar]
- 6.Ponce de Leon MS., Golovanova L., Doronichev V., et al Neanderthal brain size at birth provides insights into the evolution of human life history. Proc Natl Acad Sci U S A. 2008;105:13764–13768. doi: 10.1073/pnas.0803917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin RD. Human brain evolution in an ecological context. 52nd James Arthur Lecture. American Museum of Natural History, New York; 1983 [Google Scholar]
- 8.MacLean PD. Brain evolution relating to family, play, and the separation call. Arch Gen Psychiatry. 1985;42:405–417. doi: 10.1001/archpsyc.1985.01790270095011. [DOI] [PubMed] [Google Scholar]
- 9.Stamm JS. The function of the median cerebral cortex in maternal behavior of rats. J Comp Physiol Psychol. 1955;48:347–356. doi: 10.1037/h0042977. [DOI] [PubMed] [Google Scholar]
- 10.MacLean PD. Mind of Three Minds: Educating the Triune Brain. he 77th Annual Yearbook of the NSSE, Part II. Chicago, IL: National Society for the Study of Education; 1978. [Google Scholar]
- 11.Ainsworth MS., Blehar MC., Waters E., Wall S. Patterns of Attachment: a Psychological Study of the Strange Situation. Hillsdale, NJ: Erlbaum; 1978. [Google Scholar]
- 12.Scharf MA. A “natural experiment” in childrearing ecologies and adolescents“ attachment and separation representations. Child Dev. 2001;72:236–251. doi: 10.1111/1467-8624.00276. [DOI] [PubMed] [Google Scholar]
- 13.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 14.Cooper-Vince CE., Emmert-Aronson BO., Pincus DB., Comer JS. The diagnostic utility of separation anxiety disorder symptoms: an item response theory analysis. J Abnorm Child Psychol. 2014;42:417–428. doi: 10.1007/s10802-013-9788-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.15 Copeland WE., Angold A., Shanahan L., Costello EJ. Longitudinal patterns of anxiety from childhood to adulthood: the Great Smoky Mountains Study. J Am Acad Child Adolesc Psychiatry. 2014;53:21–33. doi: 10.1016/j.jaac.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ginsburg GS., Becker EM., Keeton CP., et al Naturalistic follow-up of youths treated for pediatric anxiety disorders. JAMA Psychiatry. 2014;71:310–318. doi: 10.1001/jamapsychiatry.2013.4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shear K., Jin R., Ruscio AM., Walters EE., Kessler RC. Prevalence and correlates of estimated DSM-IV child and adult separation anxiety disorder in the national comorbidity survey replication. Am J Psychiatry. 2006;163:1074–1083. doi: 10.1176/appi.ajp.163.6.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.18 Franz L., Angold A., Copeland W., Costello EJ., Towe-Goodman N., Egger H. Preschool anxiety disorders in pediatric primary care: prevalence and comorbidity. J Am Acad Child Adolesc Psychiatry. 2013;52:1294–1303. doi: 10.1016/j.jaac.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petitclerc A., Boivin M., Dionne G., Zoccolillo M., Tremblay RE. Disregard for rules: the early development and predictors of a specific dimension of disruptive behavior disorders. J Child Psychol Psychiatry. 2009;50:1477–1484. doi: 10.1111/j.1469-7610.2009.02118.x. [DOI] [PubMed] [Google Scholar]
- 20.Battaglia M., Touchette E., Garon-Carrier E., et al Distinct trajectories of separation anxiety in the preschool years: Persistence at school entry and early-life associated factors. J Child Psychol Psychiatry. In press. doi: 10.1111/jcpp.12424. [DOI] [PubMed] [Google Scholar]
- 21.Spatola CAM., Fagnani C., Pesenti-Gritti P., Ogliari A. Stazi MA, Battaglia M. A general population twin study of the CBCL/6-18 DSM-oriented categories. J Am Acad Child Adolesc Psychiatry. 2007;46: 619–627. doi: 10.1097/CHI.0b013e3180335b12. [DOI] [PubMed] [Google Scholar]
- 22.Pesenti-Gritti P., Spatola CAM., Fagnani C., Ogliari A., Stazi MA., Battaglia M. The co-occurrence between internalizing and externalizing behaviors: a general population twin study. Eur Child Adolesc Psychiatry. 2008;17:82–92. doi: 10.1007/s00787-007-0639-7. [DOI] [PubMed] [Google Scholar]
- 23.Rutter M. Environmentally mediated risks for psychopathology: Research strategies and findings. J Am Acad Child Adolesc Psychiatry. 2005;44:3–18. doi: 10.1097/01.chi.0000145374.45992.c9. [DOI] [PubMed] [Google Scholar]
- 24.Klein DF. False suffocation alarms, spontaneous panics, and related conditions. An integrative hypothesis. Arch Gen Psychiatry. 1993;50:306–317. doi: 10.1001/archpsyc.1993.01820160076009. [DOI] [PubMed] [Google Scholar]
- 25.Klein RG. Is panic disorder associated with childhood separation anxiety disorder? Clin Neuropharmacol. 1995;18(suppl 2):S7–S14. [Google Scholar]
- 26.Kossowsky J., Pfaltz MC., Schneider S., Taeymans J., Locher C., Gaab J. The separation anxiety hypothesis of panic disorder revisited: a metaanalysis. Am J Psychiatry. 2013;170:768–781. doi: 10.1176/appi.ajp.2012.12070893. [DOI] [PubMed] [Google Scholar]
- 27.Battaglia M., Bertella S., Politi E., et al Age at onset of panic disorder: influence of familial liability to the disease and of childhood separation anxiety disorder. Am J Psychiatry. 1995;152:1362–1364. doi: 10.1176/ajp.152.9.1362. [DOI] [PubMed] [Google Scholar]
- 28.Battaglia M., Bertella S., Bajo S., Binaghi F., Bellodi L. Anticipation of age at onset in panic disorder. Am J Psychiatry. 1998;155:590–595. doi: 10.1176/ajp.155.5.590. [DOI] [PubMed] [Google Scholar]
- 29.Smeraldi E., Orsini A., Gasperini M., et al Gruppo Italiano Disturbi d'Ansia. Familial analysis of panic disorder and agoraphobia. J Affect Disord. 1989;17:1–8. doi: 10.1016/0165-0327(89)90017-7. [DOI] [PubMed] [Google Scholar]
- 30.Gottesman II., Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 31.Battaglia M., Bertella S., Ogliari A., Bellodi L., Smeraldi E. Modulation by muscarinic antagonists of the response to carbon dioxide challenge in panic disorder. Arch Gen Psychiatry. 2001;58:114–119. doi: 10.1001/archpsyc.58.2.114. [DOI] [PubMed] [Google Scholar]
- 32.Roberson-Nay R., Klein DF., Klein RG., et al Carbon dioxide hypersensitivity in separation-anxious offspring of parents with panic disorder. Biol Psychiatry. 2010;67:1171–1177. doi: 10.1016/j.biopsych.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberson-Nay R., Moruzzi S., Ogliari A., et al Evidence for distinct genetic effects associated with response to 35% CO2. Depress Anxiety. 2013;30:259–266. doi: 10.1002/da.22038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Battaglia M., Pesenti-Gritti P., Spatola CA., Ogliari A., Tambs K. A twin study of the common vulnerability between heightened sensitivity to hypercapnia and panic disorder. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:586–593. doi: 10.1002/ajmg.b.30647. [DOI] [PubMed] [Google Scholar]
- 35.Battaglia M., Pesenti-Gritti P., Medland SE., Ogliari A., Tambs K., Spatola CA. A genetically informed study of the association between childhood separation anxiety, sensitivity to CO2, panic disorder, and the effect of childhood parental loss. Arch Gen Psychiatry. 2009;66:64–71. doi: 10.1001/archgenpsychiatry.2008.513. [DOI] [PubMed] [Google Scholar]
- 36.Scaini S., Ogliari A., Eley TC., Zavos HM., Battaglia M. Genetic and environmental contributions to separation anxiety: a meta-analytic approach to twin data. Depress Anxiety. 201 2;29:754–761. doi: 10.1002/da.21941. [DOI] [PubMed] [Google Scholar]
- 37.Ogliari A., Tambs K., Harris JR., et al The relationships between adverse events, early antecedents, and carbon dioxide reactivity as an intermediate phenotype of panic disorder: a general population study. Psychother Psychosom. 2010;79:48–55. doi: 10.1159/000259417. [DOI] [PubMed] [Google Scholar]
- 38.Spatola CA., Scaini S., Pesenti-Gritti P., et al Gene-environment interactions in panic disorder and CO sensitivity: Effects of events occurring early in life. Am J Med Genet B Neuropsychiatr Genet. 2011;156:79–88. doi: 10.1002/ajmg.b.31144. [DOI] [PubMed] [Google Scholar]
- 39.Battaglia M. Challenges in the appraisal and application of geneenvironment interdependence. Eur J Dev Psychol. 2012;9:419–425. [Google Scholar]
- 40.Battaglia M. Gene-environment interaction in panic disorder and posttraumatic stress disorder. Can J Psychiatry. 2013;58:69–75. doi: 10.1177/070674371305800202. [DOI] [PubMed] [Google Scholar]
- 41.Battaglia M., Ogliari A., D'Amato FR., Kinkead R. Early-life risk factors for panic and separation anxiety disorder: insights and outstanding questions arising from human and animal studies of CO2 sensitivity. Neurosci BiobehavRev. 2014;46:455–464. doi: 10.1016/j.neubiorev.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 42.Roberson-Nay R., Eaves LJ., Hettema JM., Kendler KS., Silberg JL. Childhood separation anxiety disorder and adult onset panic attacks share a common genetic diathesis. Depress Anxiety. 2012;29:320–327. doi: 10.1002/da.21931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bandelow B., Alvarez Tichauer G., Spath C., Broocks A., Hajak G., Bleich S., Ruther E. Separation anxiety and actual separation experiences during childhood in patients with panic disorder. Can J Psychiatry. 2001;46:948–952. doi: 10.1177/070674370104601007. [DOI] [PubMed] [Google Scholar]
- 44.Kessler RC. Psychiatric epidemiology: selected recent advances and future directions. Bull World Health Organ. 2000;78:464–474. [PMC free article] [PubMed] [Google Scholar]
- 45.D'Amato FR., Zanettini C., Lampis V., et al Unstable maternal environment, separation anxiety, and heightened CO2 sensitivity induced by gene-by-environment interplay. PLoS One. 2011;6 doi: 10.1371/journal.pone.0018637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luchetti A., Oddi D., Lampis V., et al Early handling and repeated crossfostering have opposite effect on mouse emotionality. Front Behav Neurosci. 2015;9:93.doi:10.3389/fnbeh.201 5.00093.. doi: 10.3389/fnbeh.2015.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ventura R., Coccurello R., Andolina D., et al Postnatal aversive experience impairs sensitivity to natural rewards and increases susceptibility to negative events in adult life. Cereb Cortex. 2013;23:1606–1717. doi: 10.1093/cercor/bhs145. [DOI] [PubMed] [Google Scholar]
- 48.Shonkoff JP., Boyce WT., McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA. 2009;301:2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- 49.McEwen BS., Gianaros PJ. Stress- and allostasis-induced brain plasticity. Annu Rev Med. 2011;62:431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Esquivel G., Schruers KR., Maddock RJ., Colasanti A., Griez EJ. Acids in the brain: a factor in panic? J Psychopharmacol. 2010;24:639–647. doi: 10.1177/0269881109104847. [DOI] [PubMed] [Google Scholar]
- 51.Magnotta VA., Heo HY., Dlouhy BJ., et al Detecting activity-evoked pH changes in human brain. Proc Natl Acad Sci USA. 2012;109:8270–8273. doi: 10.1073/pnas.1205902109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maddock RJ., Buonocore MH., Miller AR., Yoon JH., Soosman SK., Unruh AM. Abnormal activity-dependent brain lactate and glutamate+glutamine responses in panic disorder. Biol Psychiatry. 2013;73:1111–1119. doi: 10.1016/j.biopsych.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ziemann AE., Allen JE., Dahdaleh NS., et al The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell. 2009;139:1012–1021. doi: 10.1016/j.cell.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wemmie JA., Taugher RJ., Kreple CJ. Acid-sensing ion channels in pain and disease. Nat Rev Neurosci. 2013;14:461–471. doi: 10.1038/nrn3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chaloner A., Greenwood-VanMeerveld B. Early life adversity as a risk factor for visceral pain in later life: importance of sex differences. Front Neurosci. 2013;7 doi: 10.3389/fnins.2013.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coutinho SV., Plotsky PM., Sablad M., et al Neonatal maternal separation alters stress-induced responses to viscerosomatic nociceptive stimuli in rat. Am J Physiol Gastrointest Liver Physiol. 2002;282:G307–G316. doi: 10.1152/ajpgi.00240.2001. [DOI] [PubMed] [Google Scholar]
- 57.Dickinson AL., Leach MC., Flecknell PA. Influence of early neonatal experience on nociceptive responses and analgesic effects in rats. Lab Anim. 2009;43:11–16. doi: 10.1258/la.2007.007078. [DOI] [PubMed] [Google Scholar]
- 58.Tran L., Chaloner A., Sawalha AH., Greenwood Van-Meerveld B. Importance of epigenetic mechanisms in visceral pain induced by chronic water avoidance stress. Psychoneuroendocrinology. 2013;38:898–906. doi: 10.1016/j.psyneuen.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 59.McWilliams LA., Goodwin RD., Cox BJ. Depression and anxiety associated with three pain conditions: results from a nationally representative sample. Pain. 2004;111:77–83. doi: 10.1016/j.pain.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 60.Battaglia M., Bernardeschi L., Politi E., Bertella S., Bellodi L. Comorbidity of panic and somatization disorder: a genetic-epidemiological approach. Compr Psychiatry. 1995;36:411–420. doi: 10.1016/s0010-440x(95)90248-1. [DOI] [PubMed] [Google Scholar]
- 61.Gao Y., Li LP., Kim JH., Congdon N., Lau J., Griffiths S. The impact of parental migration on health status and health behaviours among left behind adolescent school children in China. BMC Public Health. 2010;10:56. doi: 10.1186/1471-2458-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cheng J., Sun YH. Depression and anxiety among left-behind children in China: a systematic review. Child Care Health Dev. 2015;41:515–523. doi: 10.1111/cch.12221. [DOI] [PubMed] [Google Scholar]
- 63.Wu YL., Ding XX., Li YF., Wang WJ., Yang HY., Sun YH. Children in rural China enjoyed a significant increase in quality of life from 2009 to 2011. Acta Paediatr. 2015;104(8):849–854. doi: 10.1111/apa.13008. [DOI] [PubMed] [Google Scholar]


