Abstract
Social anxiety disorder (SAD) is a highly prevalent and disabling disorder with key behavioral traits of social fearfulness, social avoidance, and submissiveness. Here we argue that hormonal systems play a key role in mediating social anxiety, and so may be important in SAD. Hormonal alterations, often established early in development through the interaction between biological and psychological factors (eg, genetic predisposition x early trauma), predispose to socially fearful, avoidant, and submissive behavior. However, whereas gene variants and histories of trauma persist, hormonal systems can be remodeled over the course of life. Hormones play a key role during the periods of all sensitive developmental windows (ie, prenatal, neonatal, puberty, aging), and are capable of opening up new developmental windows in adulthood. Indeed, the developmental plasticity of our social brain, and thus of social behavior in adulthood, critically depends on steroid hormones such as testosterone and peptide hormones such as oxytocin. These steroid and peptide hormones in interaction with social experiences may have potential for reprogramming the socially anxious brain. Certainly, single administrations of oxytocin and testosterone in humans reduce socially fearful, avoidant, and submissive behavior. Such work may ultimately lead to new approaches to the treatment of SAD.
Keywords: social anxiety disorder, SAD, hormonal system, hormone, oxytocin, testosterone
Abstract
El trastorno de ansiedad social (TAS) es una patología altamente prevalente e incapatitante con características conductuales fundamentales de temor social, evitación social y sumisión. En este artículo se argumenta que el sistema hormonal juega un papel clave en la ansiedad social y esto puede ser importante en el TAS. Las alteraciones hormonales, a menudo establecidas precozmente durante el desarrollo a través de la interacción entre los factores biológicos y psicológicos (por ej. predisposición genética y trauma precoz), predisponen al temor social, la evitación y la conducta sumisa. Sin embargo, mientras persisten las variantes genéticas y las historias de traumas, los sistemas hormonales pueden ser remodelados a lo largo de la vida. Las hormonas juegan un papel clave durante todos los períodos de ventanas sensibles del desarrollo (es detir, prenatal, neonatal, puberal y envejetimiento) y son capaces de abrir nuevas ventanas del desarrollo en la adultez. De hecho, la plastitidad del desarrollo de nuestro cerebro social y por lo tanto de la conducta social en la adultez, depende de manera fundamental de las hormonas esteroidales como la testosterona y de péptides hormonales como la oxitotina. Estos esteroides y péptidos hormonales en interacción con las experiencias sociales pueden tener el potential para reprogramar el cerebro socialmente ansioso. Por tierto, las administraciones únicas de oxitotina y testosterona en humanos reducen el temor social y las conductas de evitación y sumisión. Este trabajo en último término puede conducir a nuevos enfoques para el tratamiento del TAS.
Abstract
La prévalence de l'anxiété sociale, trouble handicapant, est très élevée et ses caractéristiques essentielles sont des traits comportementaux comme la peur en société, l'évitement des situations sociales et la soumission. Nous soutenons dans cet article que le système hormonal joue un rôle central dans la transmission de l'anxiété sociale et peut donc être important dans ce trouble. Les modifications hormonales, souvent précoces dans le développement en raison des interactions entre les facteurs biologiques et psychologiques (par exemple, prédisposition génétique x traumatisme précoce), prédisposent à un comportement social de peur, d'évitement et de soumission. Cependant, alors que les variantes géniques et les antécédents de traumatisme demeurent inchangés, les systèmes hormonaux peuvent être remodelés au cours de la vie. Les hormones jouent un rôle central pendant les phases sensibles du développement (par exemple, période prénatale, néonatale, puberté, vieillissement) et peuvent ouvrir de nouvelles phases de développement chez l'adulte. Effectivement, la plasticité de notre cerveau social au cours du développement et donc de notre comportement social à l'âge adulte dépend essentiellement des hormones stéroïdes comme la testostérone et des hormones peptidiques comme l'ocytotine. Ces hormones peptidiques et stéroïdes interagissant avec les expériences sociales pourraient reprogrammer le cerveau socialement anxieux. L'administration ponctuelle d'ocytocine et de testostérone chez l'homme diminue de façon certaine un comportement social de peur, d'évitement et de soumission. Un tel travail peut aboutir à de nouvelles approches thérapeutiques de l'anxiété sociale.
Introduction
Humans are one of nature's social species. Like other primates, we have evolved to display a range of affiliative and social emotions and behaviors. While affiliative experiences may be rewarding, social situations and hierarchies may also be associated with negative affects such as anxiety. Given the centrality of affiliative and social concerns in human life, the high prevalence of social anxiety is not surprising.1 Furthermore, it is not surprising that social anxiety disorder (SAD) is accompanied by significant distress and functional impairment.2
Behavioral inhibition, characterized by socially fearful and avoidant behavior, is both a feature of SAD and a key predictor for the development of this condition.3 Both in daily life and in experimental work, such social fear and avoidance in SAD manifests in terms of blushing, as well as avoidant or submissive responses to the eye gazes of others. Blushing and gaze aversion in SAD are arguably a pathological manifestation of an evolutionarily evolved submissive response that appears in primate dominance encounters.4 While rodents dominate by means of aggression, in primates subordinates avert their gaze when challenged by the threat stare of the dominant animal, so allowing the social hierarchy to be regulated nonaggressively.5-7
In SAD, however, such social adaptations seem to have gone awry and socially fearful, avoidant, and submissive behaviors are generalized to all of human social interaction. Animal research has established a pivotal role for the neuropeptide oxytocin (OXT) and the steroid testosterone (T) in the development and adaptive preservation of social hierarchies.8,9 Such work, as well as placebo-controlled studies with single administrations of OXT and T in humans, leads to the hypothesis that imbalances in OXT and T systems may contribute to the pathogenesis of SAD.
Oxytocin and testosterone
In many species, including humans, the peptide hormone OXT and the steroid hormone T play a key role in the development and execution of social-emotional behavior, both in males and in females. Depending on the relevant social context and environment, these social hormones act via, and interact with, all the main neurotransmitter systems (that is, the serotoninergic, noradrenergic, and dopaminergic systems). The other key social hormone systems, the estrogen and the vasopressin systems, depend critically on T, as the most important estrogen, estradiol, is a metabolite of T, and vasopressin depends on T for its gene expression.
Since OXT depends on estradiol for its gene expression, all of our social behavior is ultimately- based upon T, a hormone that is ironically associated with antisocial behavior in some folk psychologies.10 Although OXT and T possess opposite behavioral properties, for example in the domain of cognitive empathy,11,12 they are certainly not antagonistic hormones in their effects on brain and behavior. On the contrary, the OXT and T systems are intrinsically intertwined, jointly critical for the execution of sexual behavior, and have important and seemingly complementary anxiolytic/fear-reducing properties.
OXT and T act, directly or indirectly, via other hormone or neurotransmitter systems, on all social-affective brain systems including the orbitofrontal cortex (OFC), anterior cingulate cortex (ACQ, amygdala, ventral striatum, hypothalamus, and brain stem. However, depending on biological predisposition, early adversity (for example, abuse or neglect) can disorganize the expression of social-emotional behaviors by creating imbalances in hormonal systems, and especially in the OXT and T systems. These hormonal imbalances have negative effects on the social-emotional brain and can produce socially fearful, avoidant, and submissive behaviors, with insensitivity for social rewards.
Early-life social experiences shape the expression of adult social behaviors via neuropeptide and steroid systems, and especially the OXT and T systems, which among others organize the expression of fear and aggression, and related approach and avoidance behaviors. Notably, changes in the production and secretion of OXT and T in response to social events, and changes in the social brain's sensitivity to these hormones, are fundamental mechanisms by which social experience affects later social behaviors. Furthermore, the social-emotional behaviors governed by the OXT and T systems seem to play a major role in SAD.
There is some evidence that OXT and T levels measured in plasma or saliva are lowered in SAD.13,14 However, saliva and plasma measures only coarsely- reflect brain OXT and T, as OXT does not cross the bloodbrain barrier, and T is produced not only by the gonads and adrenals, but also is a neurosteroid in the brain that is not measurable peripherally.15 What makes OXT and T of specific interest to SAD are placebo-controlled OXT and T administration studies in humans, which suggest that these hormones can alleviate the core behavioral hallmarks of SAD (social tearfulness, avoidance, submissiveness), and increase sensitivity to social rewards, as described in more detail below.
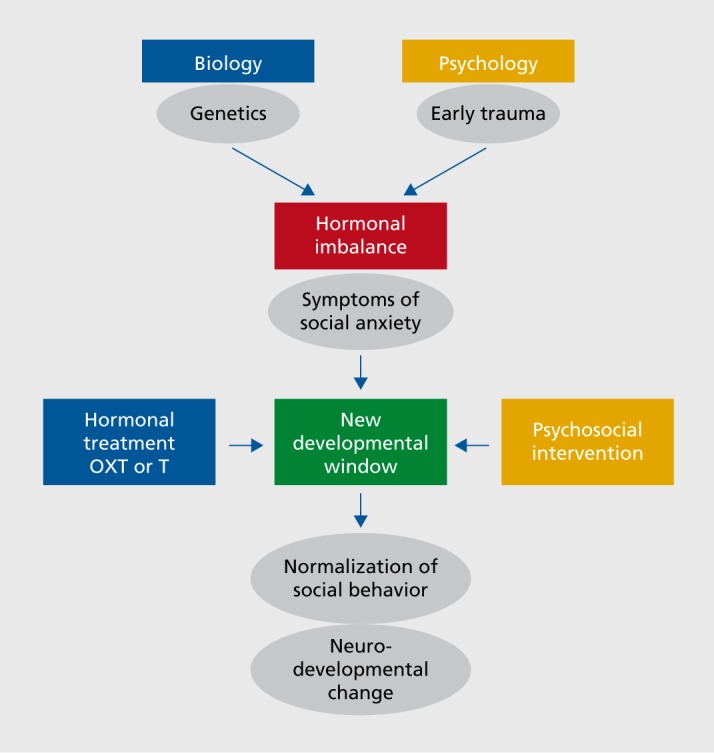
The robust evidence for acute behavioral plasticity in the OXT and T systems, with transient development of new social behaviors in adulthood in response to single administrations of OXT and T, potentially opens up significant therapeutic opportunities, particularly in light of one of the important scientific discoveries of recent decades, that of adult neuroplasticity. Adult neuroplasticity refers to structural changes in brain regions and rewiring of brain pathways in adulthood, and social experiences. OXT and T not only develop and execute our social-emotional behavior,16 but they also are key players in adult neuroplasticity.17-19 Importantly, OXT and T are able to open up new developmental windows in adulthood, and on these windows social experiences (and thus psychosocial interventions) can potentially act to bring lasting change (Figure 1).
Figure 1. The interaction between genetics and early trauma leads to hormonal changes, which in turn influence social anxiety symptoms. Here we hypothesize that hormonal treatments and psychosocial ineteiventions, alone or in combination, may be able to be employed, perhaps in a personalized way, to reduce such symptoms. OXT, oxytocin; T, testosterone.
Effects of oxytocin and testosterone on fear and anxiety
In rodent research, the anxiolytic or (social) fearreducing properties of OXT and T have been well established.20,21 T elevations in rats increase social exploration, while decreasing anxiety, punishment, and avoidance.22-24 Compared with intact male rats and/or gonad ectomized rats with replacement hormone, gonadectomized rats show more anxiety, fear, and freezing behavior on a large variety of tasks.25-28 Finally, other testosterone deficits also result in enhanced fear responses.28
Importantly, anxiolytic/fear-reducing effects of OXT are often observed in social situations in rodents. Single doses of OXT have also been shown to reduce predator fear.29,30 Notably, after being defeated in dominance interactions, rats display social avoidance, but not after administration of OXT31. OXT acts on a localized population of OXT receptors in the amygdala to reduce fear behaviors.32,33
OXT also modulates activity within the hypothalamic-pituitary-adrenal (HPA) axis,34 augmenting HPA-axis activation immediately after stress exposure. This arguably facilitates system adaptation, and subsequently enhances the suppression of the IIPA axis, which helps in the re-establishment of system homeostasis. T also has multiple controls over the IIPA axis; T can inhibit the HPA axis at the hypothalamic, pituitary, and adrenal levels. Thus, OXT and T not only have numerous anxiolytic/fear reducing actions, especially in the social domain,35,36 but also have powerful control over HPA function and activity, particularly during stress.
The evidence for anxiolytic and fear-reducing effects of OXT and T in humans is based upon recent studies with single intranasal administrations of OXT and single sublingual administrations of T.37 Using a placebo-controlled single testosterone administration method developed for human females with an uniquely established quantity and time course of effect (the Tuiten method38), the fear and stress-reducing properties of the steroid hormone testosterone in humans have been demonstrated repeatedly by van Honk and colleagues.39-41
Early human data on the relation between testosterone, fear, and anxiety predominantly involved questionnaires that index the conscious appraisal of anxious mood. However, testosterone does not acutely act on such emotions, but only on genuine fear behaviors. Indeed, in the absence of any effects on anxiety, van Honk et al39 showed significant reductions in vigilant responses to masked facial fear after testosterone administration. Hermans et al40 measured baseline startle and fear-potentiated startle in a threat-of-shock paradigm, and showed that testosterone administration acutely reduced the fearpotentiated startle, but did not affect baseline startle. Finally, Hermans et al41 showed reductions in skin conductance and startle modulation in response to stress-inducing negative and threatening pictures in anxiety-prone participants.
Facial anger: social avoidance and social approach
Angry facial expressions are thought to have evolved in primates with a key function being social threat signaling in dominance encounters.42 In face-to-face challenges between primates, an enduring angry gaze signals dominance, while eye or gaze aversion signals submission.6,7 High levels of testosterone relate to social dominance in numerous species including humans. Indeed, using an adapted emotional Stroop task it was shown that vigilant responses (enduring gazes) to angry facial expressions are positively related to testosterone levels in both males and females.43
In these findings saliva measures were used, and so causality is not demonstrated. However, with a placebo controlled testosterone administration design (the Tuiten method), it was shown that T induces cardiac acceleration to exclusively angry faces in healthy women, indicating that the hormone plays a causal role in encourages dominance behavior.44 Furthermore, using the same method and testing subjects in a social approach-avoidance task, Enter et al45 showed reductions in avoidant responses to facial anger, suggesting that T counteracts submissive responses of eye and gaze aversion to facial anger. This finding is relevant to SAD, because socially anxious subjects are strongly avoidant to facial anger in the same social approach-avoidance task.46 Terburg et al47 developed an eye-tracking paradigm to measure true gaze aversion and staring endurance in unconscious face-to-face confrontations, with backwardly masked angry faces. Backward masking is a phenomenon wherein the presentation of a target stimulus (here the angry face) is immediately followed (in this case 30 milliseconds), by a masking stimulus with the same visual information but scrambled, which results in a failure to consciously perceive the target stimulus.
With this paradigm it was shown that on the dominance-submissive spectrum, relatively high dominance traits predict a prolonged gaze to masked facial anger (enduring gaze), while relatively low dominance traits predict gaze aversion. Crucially, Terburg et al48 next used acute T administration and showed that individual response patterns shifted from gaze aversion to staring endurance. That is, T induced prolonged gaze to unseen angry faces. T thus seems to lead to social dominance in humans (and likely also in other mammals and in reptiles) implicitly and nonconsciously.
On the other hand, with their social approach-avoidance task, which operates at more explicit, conscious levels of processing, Radke and coworkers49 not only showed that avoidant response to angry facial expressions are positively related to social anxiety levels, but also that T reduced this socially avoidant behavior. Taken together, corresponding to the effects on baseline anxiety and cue-specific fear, T and OXT seem to influence dominance-submissive behaviors at different levels of processing. T operates on the most implicit nonconscious level of processing while OXT also acts on automatic behaviors but only at higher, more explicit, levels of information processing.
Furthermore, it has repeatedly been shown that OXT improves or increases the processing of happy facial expressions. These facial expressions of happiness are social reward cues, and invite social interaction.50-52 These data are consistent with findings that intranasal OXT generally leads to increased attention to the eyes of others both in experimental and realistic conditions.53,54
Interestingly, T and OXT may act in opposite ways in the case of facial happiness, as the happy face is also an appeasement signal. T may lead to social approaches in the context of competition eg, for dominance, but not in other situations. Indeed, evidence suggests that T negatively influences the processing of happy facial expressions.55,56 In contrast, OXT seems to make social interactions in general more rewarding, and the hormone therefore enhances the processing of facial happiness. In sum, OXT may increase attention to, and memory for, positive facial expressions, because the hormone increases the rewarding properties of social interactions. T especially induces vigilant attention and responsivity to angry facial expressions, because the angry face functions as a dominance signal in face-to-face interactions, thus social dominance is at stake and the hormone is on its guard.
Toward a personalized approach
Overall, OXT reduces background anxiety, and improves the recognition of, and attention to, positive facial expression. OXT also increases attention to the eye region of the face, a sign of interest in social others, and of the willingness to socialize. Thus, the social peptide OXT seem to hold properties that make social interaction more rewarding.57 The social steroid T on the other hand reduces cue-specific and social fear, and submissiveness and facilitates vigilance and social approach but exclusively in competitive conditions: to compete, approach and defend status in social interactions. Anxiety, social fear and avoidance, and impaired social reward processing all are behavioral hallmarks of SAD, but the specific actions of OXT and T may suggest a personalized approach to their use in therapeutic interventions.
That is, individuals suffering from SAD and a lack of motivation to socialize may show a response to OXT, while socially fearful, submissive SAD subjects who are eager to interact may show a response to T. Importantly, as is clear from the earlier discussion, a range of validated behavioral, neuropsychological, and psychophysiological paradigms are available to assess putative behavioral differences in SAD.
It is important to note that the effects of OXT and T may depend upon various personal and situational factors.58,59 This represents another potential opportunity; that is, for employing OXT and T administration in synergy with exposure to social experiences as part of an innovative combined treatment strategy in SAD. To date, however, research with intranasal OXT in SAD is still scarce, and published research in SAD with T administration is nonexistent.
One trial with intranasal OXT adjunctive to exposure therapy in SAD showed improvement in the mental representation of the self.60 Further, two pharmacological neuroimaging studies in patients with SAD receiving intranasal OXT and placebo showed attenuation of amygdala activity to fear, and the modulation of medial frontal hyperactivity to sad faces.61,62 Taken together these preliminary data are promising and provide a foundation for additional research on OXT administration in SAD.
Conclusion
The modern behavioral and psychophysiological paradigms tapping into social fearfulness, social avoidance, submissiveness, and social reward processing discussed above may be particularly useful in further investigations of the assessment and treatment of SAD. Research on OXT and T has suggested that these hormones alter such paradigms in overlapping but distinct ways. Future research on pathogenesis may usefully focus on identifying subtypes of SAD which respond differentially to OXT and T administration. Ultimately, a better understanding of OXT and T, as well as the pathogenesis of SAD may lead to interventional research which optimally integrates psychotherapy with adjunctive hormone administration.
Acknowledgments
The work in this paper was supported by funding of the South African National Research Foundation, Netherlands Society of Scientific Research, and the Medical Research Council of South Africa.
Contributor Information
Jack van Honk, Department of Psychiatry, University of Cape Town, South Africa; Department of Psychology, Utrecht University, the Netherlands; Institute of Infectious Diseases, and Molecular Medicine, University of Cape Town, South Africa.
Peter A. Bos, Department of Psychiatry, University of Cape Town, South Africa; Department of Psychology, Utrecht University, the Netherlands.
David Terburg, Department of Psychiatry, University of Cape Town, South Africa; Department of Psychology, Utrecht University, the Netherlands.
Sarah Heany, Department of Psychiatry, University of Cape Town, South Africa.
Dan J. Stein, Department of Psychiatry, University of Cape Town, South Africa; MRC Unit on Anxiety & Stress Disorders, Cape Town, South Africa.
REFERENCES
- 1.Stein MB., Stein DJ. Social anxiety disorder. Lancet. 2008;371(9618):1115–1125. doi: 10.1016/S0140-6736(08)60488-2. [DOI] [PubMed] [Google Scholar]
- 2.Stein DJ., Ruscio AM., Lee S., et al Subtyping social anxiety disorder in developed and developing countries. Depress Anxiety. 2010;27(4):390–403. doi: 10.1002/da.20639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fox NA., Henderson HA., Marshall PJ., Nichols KE., Ghera MM. Behavioral inhibition: linking biology and behavior within a developmental framework. Annu Rev Psychol. 2005;56:235–262. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- 4.Stein DJ., Bouwer C. Blushing and social phobia: a neuroethological speculation. Med Hypotheses. 1997;49(1):101–108. doi: 10.1016/s0306-9877(97)90260-7. [DOI] [PubMed] [Google Scholar]
- 5.Gilbert P. Evolution and social anxiety. The role of attraction, social competition, and social hierarchies. Psychiatr Clin North Am . 2001;24(4):723–751. doi: 10.1016/s0193-953x(05)70260-4. [DOI] [PubMed] [Google Scholar]
- 6.Van Honk J., Schutter DJLG. Testosterone reduces conscious detection of signals serving social correction: implications for antisocial behavior. Psychol Sci. 2007;18(8):663–667. doi: 10.1111/j.1467-9280.2007.01955.x. [DOI] [PubMed] [Google Scholar]
- 7.Mazur A., Booth A. Testosterone and dominance in men. Behav Brain Sci. 1998;21(3):353–397. [PubMed] [Google Scholar]
- 8.Timmer M., Cordero Ml., Sevelinges Y., Sandi C. Evidence for a role of oxytocin receptors in the long-term establishment of dominance hierarchies. Neuropsychopharmacology. 2011;36(11):2349–2356. doi: 10.1038/npp.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gesquiere LR., Learn NH., Simao MCM., Onyango PO., Alberts SC., Altmann J. Life at the top: rank and stress in wild male baboons. Science. 2011;333(6040):357–360. doi: 10.1126/science.1207120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Honk J., Terburg D., Bos PA. Further notes on testosterone as a social hormone. Trends Cogn Sci. 2011;15(7):291–292. doi: 10.1016/j.tics.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Van Honk J., Schutter DJ., Bos PA., Kruijt A-W., Lentjes EG., Baron-Cohen S. Testosterone administration impairs cognitive empathy in women depending on second-to-fourth digit ratio. Proc Natl Acad Sci USA. 2011;108(8):3448–3452. doi: 10.1073/pnas.1011891108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Domes G., Heinrichs M., Michel A., Berger C., Herpertz SC. Oxytocin improves “mind-reading” in humans. Biol Psychiatry. 2007;61(6):731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 13.Hoge EA., Pollack MH., Kaufman RE., Zak PJ., Simon NM. Oxytocin levels in social anxiety disorder. CNS Neurosci Ther. 2008;14(3):165–170. doi: 10.1111/j.1755-5949.2008.00051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giltay EJ., Enter D., Zitman FG., et al Salivary testosterone: associations with depression, anxiety disorders, and antidepressant use in a large cohort study. J Psychosom Res. 2012;72(3):205–213. doi: 10.1016/j.jpsychores.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 15.Remage-Healey L., Maidment NT., Schlinger BA. Forebrain steroid levels fluctuate rapidly during social interactions. Nat Neurosci. 2008;11(11):1327–1334. doi: 10.1038/nn.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cushing BS., Kramer KM. Mechanisms underlying epigenetic effects of early social experience: the role of neuropeptides and steroids. Neurosci Biobehav Rev. 2005;29(7):1089–1105. doi: 10.1016/j.neubiorev.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Liston C., Gan W-B. Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo. Proc Natl Acad Sci USA. 2011;108(38):16074–16079. doi: 10.1073/pnas.1110444108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galea LAM., Uban KA., Epp JR., et al Endocrine regulation of cognition and neuroplasticity: our pursuit to unveil the complex interaction between hormones, the brain, and behaviour. Can J Exp Psychol. 2008;62(4):247–260. doi: 10.1037/a0014501. [DOI] [PubMed] [Google Scholar]
- 19.Hatton Gl. Emerging concepts of structure-function dynamics in adult brain: the hypothalamo-neurohypophysial system. Prog Neurobiol. 1990;34(6):437–504. doi: 10.1016/0301-0082(90)90017-b. [DOI] [PubMed] [Google Scholar]
- 20.Rotzinger S., Lovejoy DA., Tan LA. Behavioral effects of neuropeptides in rodent models of depression and anxiety. Peptides. 2010;31(4):736–756. doi: 10.1016/j.peptides.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 21.Toufexis DJ., Myers KM., Davis M. The effect of gonadal hormones and gender on anxiety and emotional learning. Horm Behav. 2006;50(4):539–549. doi: 10.1016/j.yhbeh.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 22.Bitran D., Kellogg CK., Hilvers RJ. Treatment with an anabolic-androgenic steroid affects anxiety-related behavior and alters the sensitivity of cortical GABAa receptors in the rat. Horm Behav. 1993;27:568–583. doi: 10.1006/hbeh.1993.1041. [DOI] [PubMed] [Google Scholar]
- 23.Bing O., Heilig M., Kakoulidis P., Sundblad C., Wiklund L., Eriksson E. High doses of testosterone increase anticonflict behaviour in rat. Eur Neuropsychopharmacol. 1998;8(4):321–323. doi: 10.1016/s0924-977x(97)00095-3. [DOI] [PubMed] [Google Scholar]
- 24.Aikey JL., Nyby JG., Anmuth DM., James PJ. Testosterone rapidly reduces anxiety in male house mice (Mus musculus). Horm Behav. 2002;42(4):448–460. doi: 10.1006/hbeh.2002.1838. [DOI] [PubMed] [Google Scholar]
- 25.Frye CA., Seliga AM. Testosterone increases analgesia, anxiolysis, and cognitive performance of male rats. Cogn Affect Behav Neurosci. 2001;1(4):371–381. doi: 10.3758/cabn.1.4.371. [DOI] [PubMed] [Google Scholar]
- 26.Frye CA., Edinger KL. Testosterone's metabolism in the hippocampus may mediate its anti-anxiety effects in male rats. Pharmacol Biochem Behav. 2004;78(3):473–481. doi: 10.1016/j.pbb.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 27.Toufexis D., Davis C., Hammond A., Davis M. Sex differences in hormonal modulation of anxiety measured with light-enhanced startle: possible role for arginine vasopressin in the male. J Neurosci. 2005;25(39):9010–9016. doi: 10.1523/JNEUROSCI.0127-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.King JA., De Oliveira WL., Patel N. Deficits in testosterone facilitate enhanced fear response. Psychoneuroendocrinology. 2005;30(4):333–340. doi: 10.1016/j.psyneuen.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Febo M., Shields J., Ferris CF., King JA. Oxytocin modulates unconditioned fear response in lactating dams: an fMRI study. Brain Res. 2009;1302:183–193. doi: 10.1016/j.brainres.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braida D., Donzelli A., Martucci R., et al Neurohypophyseal hormones manipulation modulate social and anxiety-related behavior in zebrafish. Psychopharmacology (Berl). 2012;220:319–330. doi: 10.1007/s00213-011-2482-2. [DOI] [PubMed] [Google Scholar]
- 31.Lukas M., Toth I., Reber SO., Slattery DA., Veenema AH., Neumann ID. The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology. 2011;36:2159–2168. doi: 10.1038/npp.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Viviani D., Charlet A., van den Burg E., et al Oxytocin selectively gates fear responses through distinct outputs from the central amygdala. Science. 2011;333(6038):104–107. doi: 10.1126/science.1201043. [DOI] [PubMed] [Google Scholar]
- 33.Knobloch HS., Charlet A., Hoffmann LC., et al Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73(3):553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 34.Cohen H., Kaplan Z., Kozlovsky N., Gidron Y., Matar MA., Zohar J. Hippocampal microinfusion of oxytocin attenuates the behavioural response to stress by means of dynamic interplay with the glucocorticoid-catecholamine responses. J Neuroendocrinol. 2010;22(8):889–904. doi: 10.1111/j.1365-2826.2010.02003.x. [DOI] [PubMed] [Google Scholar]
- 35.Boissy A., Bouissou MF. Effects of androgen treatment on behavioral and physiological responses of heifers to fear-eliciting situations. Horm Behav. 1994;28(1):66–83. doi: 10.1006/hbeh.1994.1006. [DOI] [PubMed] [Google Scholar]
- 36.Vandenheede M., Bouissou MF. Effect of androgen treatment on fear reactions in ewes. Horm Behav. 1993;27(4):435–448. doi: 10.1006/hbeh.1993.1032. [DOI] [PubMed] [Google Scholar]
- 37.Bos PA., Panksepp J., Bluthé R-M., van Honk J. Acute effects of steroid hormones and neuropeptides on human social-emotional behavior: a review of single administration studies. Front Neuroendocrinol. 2012;33(1):17–35. doi: 10.1016/j.yfrne.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Tuiten A., Van Honk J., Koppeschaar H., Bernaards C., Thijssen J., Verbaten R. Time course of effects of testosterone administration on sexual arousal in women. Arch Gen Psychiatry. 2000;57(2):149–153; discussion 155-156. doi: 10.1001/archpsyc.57.2.149. [DOI] [PubMed] [Google Scholar]
- 39.Van Honk J., Peper JS., Schutter DJLG. Testosterone reduces unconscious fear but not consciously experienced anxiety: implications for the disorders of fear and anxiety. Biol Psychiatry. 2005;58(3):218–225. doi: 10.1016/j.biopsych.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 40.Hermans EJ., Putman P., Baas JM., Koppeschaar HP., van Honk J. A single administration of testosterone reduces fear-potentiated startle in humans. Biol Psychiatry. 2006;59(9):872–874. doi: 10.1016/j.biopsych.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 41.Hermans EJ., Putman P., Baas JM., Geeks NM., Kenemans JL., van Honk J. Exogenous testosterone attenuates the integrated central stress response in healthy young women. Psychoneuroendocrinology. 2007;32(8-10):105–261. doi: 10.1016/j.psyneuen.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 42.Ohman A. Face the beast and fear the face: animal and social fears as prototypes for evolutionary analyses of emotion. Psychophysiology . 1986;23(2):123–145. doi: 10.1111/j.1469-8986.1986.tb00608.x. [DOI] [PubMed] [Google Scholar]
- 43.Van Honk J., Tuiten A., Verbaten R., et al Correlations among salivary testosterone, mood, and selective attention to threat in humans. Horm Behav. 1999;36(1):17–24. doi: 10.1006/hbeh.1999.1521. [DOI] [PubMed] [Google Scholar]
- 44.Van Honk J., Tuiten A., Hermans E., et al A single administration of testosterone induces cardiac accelerative responses to angry faces in healthy young women. Behav Neurosci. 2001;115(1):238–242. doi: 10.1037/0735-7044.115.1.238. [DOI] [PubMed] [Google Scholar]
- 45.Enter D., Spinhoven P., Roelofs K. Alleviating social avoidance: effects of single dose testosterone administration on approach-avoidance action. Horm Behav. 2014;65(4):351–354. doi: 10.1016/j.yhbeh.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 46.Roelofs K., Putman P., Schouten S., Lange W-G., Volman I., Rinck M. Gaze direction differentially affects avoidance tendencies to happy and angry faces in socially anxious individuals. Behav Res Ther. 2010;48(4):290–294. doi: 10.1016/j.brat.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 47.Terburg D., Hooiveld N., Aarts H., Kenemans JL., van Honk J. Eye tracking unconscious face-to-face confrontations: dominance motives prolong gaze to masked angry faces. Psychol Sci. 2011;22(3):314–319. doi: 10.1177/0956797611398492. [DOI] [PubMed] [Google Scholar]
- 48.Terburg D., Aarts H., van Honk J. Testosterone affects gaze aversion from angry faces outside of conscious awareness. Psychol Sci. 2012;23(5):459–463. doi: 10.1177/0956797611433336. [DOI] [PubMed] [Google Scholar]
- 49.Radke S., Roelofs K., de Bruijn ERA. Acting on anger: social anxiety modulates approach-avoidance tendencies after oxytocin administration. Psychol Sci. 2013;24(8):1573–1578. doi: 10.1177/0956797612472682. [DOI] [PubMed] [Google Scholar]
- 50.Marsh AA., Yu HH., Pine DS., Blair RJR. Oxytocin improves specific recognition of positive facial expressions. Psychopharmacology (Berl). 2010;209(3):225–232. doi: 10.1007/s00213-010-1780-4. [DOI] [PubMed] [Google Scholar]
- 51.Syal S., Ipser J., Terburg D., et al Improved memory for reward cues following acute buprenorphine administration in humans. Psychoneuroendocrinology. 2015;53:10–15. doi: 10.1016/j.psyneuen.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 52.Domes G., Sibold M., Schulze L., Lischke A., Herpertz SC., Heinrichs M. Intranasal oxytocin increases covert attention to positive social cues. Psychol Med. 2013;43(8):1747–1753. doi: 10.1017/S0033291712002565. [DOI] [PubMed] [Google Scholar]
- 53.Guastella AJ., Mitchell PB., Dadds MR. Oxytocin increases gaze to the eye region of human faces. Biol Psychiatry. 2008;63(1):3–5. doi: 10.1016/j.biopsych.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 54.Auyeung B., Lombardo MV., Heinrichs M., et al Oxytocin increases eye contact during a real-time, naturalistic social interaction in males with and without autism. Transl Psychiatry. 2015;5:e507. doi: 10.1038/tp.2014.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cashdan E. Hormones, sex, and status in women. Horm Behav. 1995;29(3):354–366. doi: 10.1006/hbeh.1995.1025. [DOI] [PubMed] [Google Scholar]
- 56.Dabbs JM. Testosterone, smiling, and facial appearance. J Nonverbal Behav. 1997;21(1):45–55. [Google Scholar]
- 57.Ramos L., Hicks C., Caminer A., Goodwin J., McGregor IS. Oxytocin and MDMA ('Ecstasy') enhance social reward in rats. Psychopharmacology (Berl). 2015;232(14):2631–2641. doi: 10.1007/s00213-015-3899-9. [DOI] [PubMed] [Google Scholar]
- 58.Bos Pa., van Honk J., Ramsey NF., Stein DJ., Hermans EJ. Testosterone administration in women increases amygdala responses to fearful and happy faces. Psychoneuroendocrinology. 2013;38(6):808–817. doi: 10.1016/j.psyneuen.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 59.Bartz JA., Zaki J., Bolger N., Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends Cogn Sci. 2011;15(7):301–309. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 60.Guastella AJ., Howard AL., Dadds MR., Mitchell P., Carson DS. A randomized controlled trial of intranasal oxytocin as an adjunct to exposure therapy for social anxiety disorder. Psychoneuroendocrinology. 2009;34(6):917–923. doi: 10.1016/j.psyneuen.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 61.Labuschagne I., Phan KL., Wood A., et al Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacology. 2010;35(12):2403–2413. doi: 10.1038/npp.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Labuschagne I., Phan KL., Wood A., et al Medial frontal hyperactivity to sad faces in generalized social anxiety disorder and modulation by oxytocin. Int J Neuropsychopharmacol. 2011:1–14. doi: 10.1017/S1461145711001489. [DOI] [PubMed] [Google Scholar]


