Abstract
Development of inflammatory diseases, such as metabolic syndrome and cancer, is prevalent in individuals that encounter continuous disruption of their internal clock. Further, daily oscillations in susceptibility to infection as well as a multitude of other immunological processes have been described. Much progress has been made and various mechanisms have been proposed to explain circadian variations in immunity; yet much is still unknown. Understanding the crosstalk between the circadian and the immune systems will allow us to manipulate clock outputs to prevent and treat inflammatory diseases in individuals at risk. This review briefly summarizes current knowledge about circadian rhythms and their role in the immune system and highlights progress and challenges in chrono-immunological research.
Keywords: circadian, cytokines, immunity, metabolism, sleep
Introduction
The circuitry of the mammalian circadian clock
Circadian rhythms are daily oscillations in behaviour and physiology that prepare organisms to better react to and anticipate changes in the environment that are a consequence of the earth's rotation. These cellular clocks are conserved from cyanobacteria to mammals and are accordingly found in almost every cell in multicellular organisms that evolved from unicellular progenitors.
Higher life forms, as they faced the daunting task of sustaining synchrony of their peripheral clocks, started to use exogenous signals – termed zeitgeber, the German word for ‘time giver’1 – that could be sensed by the organism to entrain and align circadian rhythms. Under free running conditions – an environment that lacks zeitgeber signals – robust circadian outputs are sustained, whereas diurnal outputs are lost as they are a direct effect of exogenous signals. Environmental conditions can additionally obscure circadian outputs, which is described in a concept called masking.2 Outputs can thereby be phase-shifted directly by masking stimuli without clock entrainment; yet in the absence of the masking stimulus, the originally entrained rhythm is restored. Although research to date has focused on light, it is not the only zeitgeber. A variety of signals, such as temperature, food and social cues3–6 among others, have been described as zeitgebers with varying potential. Under normal, physiological circumstances all zeitgebers are tightly correlated. For example, the light cycle, outdoor temperature and food availability tend to fluctuate in a predictable way according to the time of day. In Zebrafish, peripheral clocks, for example in the liver and heart, are directly light entrained and changes in light patterns result in rapid phase shifts of core clock proteins.7 Equally, locomotor activity in blinded birds remains an immediate function of light.8 In other organisms, such as mammals, extra-retinal cells have lost the ability to directly interpret photic cues. Instead, mechanical destruction of mammalian brain regions identified a light-regulated circadian master pacemaker in the suprachiasmatic nucleus (SCN), which regulates subordinate peripheral clocks.9,10 Light, as the primary environmental stimulus, correlates with the earth's rotation and is sensed by photosensitive cells in the retina and regulates the SCN in the hypothalamic region of the brain via the retino–hypothalamic tract. The SCN subsequently synchronizes peripheral clocks via mediators including hormones and neuronal signals, primarily using the hypothalamic–pituitary–adrenal axis and the autonomic nervous system. This model of a hierarchical organization of the body clock, with the brain clock at the top, has recently been refined. Specific ablation of the cellular clock in the SCN in mice on a light/dark regimen did not result in loss of circadian behaviour as expected. When mice were placed in constant darkness, the amplitude of clock genes in the periphery diminished over time,11 suggesting a dual role for the SCN in processing light signals. Besides the conventional master clock, which is dependent on functional cellular clocks, there appears to be a clock-independent, light-responsive element. In the absence of both SCN signals, peripheral clock rhythmicity can be temporarily sustained, but gradual weakening of the amplitude eventually results in loss of synchrony, similar to what is seen in peripheral clocks in vitro.12
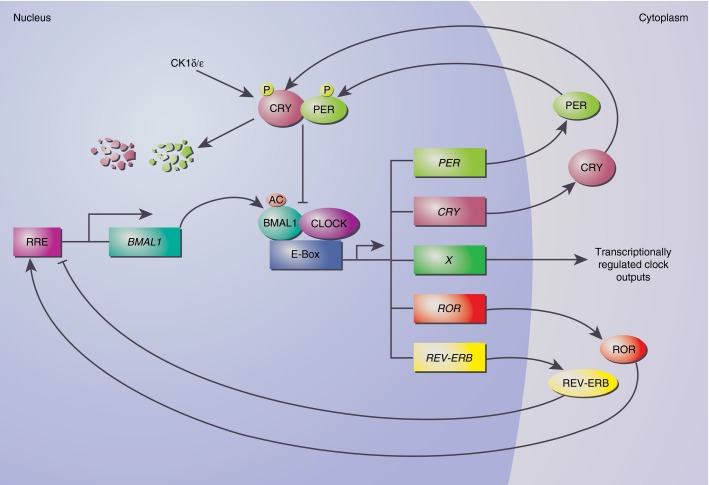
The core molecular clock, in both the periphery and the SCN, is comprised of two main transcriptional–translational feedback loops, which counter-regulate each other to generate a circadian cycle of gene expression. Brain and Muscle ARNT-like 1 (BMAL1) and Circadian Locomotor Output Cycles Kaput (CLOCK) proteins heterodimerize13 and bind to E-boxes (CACGTG)14 to induce transcription of target genes15 along with a set of regulatory proteins including PERIOD (PER1, 2 and 3), CRYPTOCHROME (CRY1 and 2), REV-ERB (REV-ERBα and β) and RAR-Related Orphan Receptor (RORα, β and γ), which feed back to regulate their own transcription. REV-ERBs and RORs bind to ROR-responsive elements (RREs)16,17 to regulate BMAL1 transcription, whereas PER and CRY dimerize to inhibit the BMAL1–CLOCK dimer (Fig.1). CRY recruitment is facilitated via CLOCK-mediated acetylation of BMAL1, whereas PER and CRY phosphorylation and degradation is controlled by the casein kinases I δ and ε (CKIδ and ε) to restrict their negative feedback potential.18,19 These feedback loops are the fundamental building blocks of the cellular clock; however, many more genes are directly regulated by the clock machinery, resulting in rhythmic expression of clock-controlled genes via E-boxes, D-boxes and RREs, which are also responsible for phase delays of circadian genes (E-boxes at the transition into the active phase, D-boxes during activity and RREs at rest).20,21 Primary and secondary clock-regulated genes can also regulate the level of transcription and post-transcriptional modification of central clock genes, thereby reinforcing their integrity. Eight per cent of the transcriptome in murine peritoneal macrophages has been shown to be cycling,12 which is probably an under-estimate of the actual amount of circadian outputs, as post-translational modifications can also result in circadian activity of genes whose transcription is not cyclic.
Figure 1.
The molecular clock. BMAL1 (Brain and Muscle ARNT-like 1) heterodimerizes with CLOCK (Circadian Locomotor Output Cycles Kaput) to initiate transcription of target genes via E-boxes. PER (PERIOD) and CRY (CRYPTOCHROME) proteins dimerize and inhibit their own expression by disrupting the acetylated (AC) BMAL1:CLOCK dimer. REV-ERB (nuclear receptor subfamily 1, group D) and ROR (RAR-Related Orphan Receptor) bind to ROR responsive elements (RRE) to regulate BMAL1 transcription. PER and CRY phosphorylation (P) and degradation is governed by casein kinases I δ and ε (CKIδ/ε).
Recently, interest in circadian rhythms and the effect that this might pose on the immune system has grown and much progress has been made in unravelling the mechanisms of the clock and its outputs. Nevertheless, many circadian phenotypes remain to be understood. Even though it is challenging to decipher the importance of a distinct zeitgeber on a specific phenotype, roles for zeitgebers other than light that may impact the immune system are starting to be appreciated.
Daily oscillations in immunity
‘Sleep will heal’: immunological memory and immune activity during sleep
The sleep/wake cycle is one of the most prominent manifestations of the circadian rhythm and communicates tightly with the immune system in a bi-directional manner. Although a direct role was only shown for interleukin-1β (IL-1β), tumour necrosis factor-α (TNF-α), interferon-γ22 and IL-6,23 pro-inflammatory cytokines are generally classified as somnogenic (reviewed in ref. 24), and basal plasma levels of these cytokines appear higher during the rest phase. Infection-associated sleepiness,25 in conjunction with increased duration and intensity of slow-wave sleep and decreased rapid eye movement sleep (reviewed in ref. 26), have been attributed to increased pro-inflammatory cytokine plasma levels. In contrast, anti-inflammatory cytokines, such as IL-4 and IL-10, are induced after awakening27 and exhibit inhibitory effects on sleep.28 In support of this, IL-1- and TNF-receptor deficient mice display decreased baseline sleep and lack an increase in slow-wave sleep and decrease in rapid eye movement sleep following stimulation with IL-1 or TNF, respectively.29,30 Sleep also directly regulates oscillations of an assortment of cytokines and cytokine receptors, such as IL-231 and soluble IL-6 receptor,32 that are abolished in sustained wakefulness. Equally, a collection of immuoregulatory hormones such as renin, growth hormone and prolactin are dependent on sleep, and sleep deprivation is sufficient to abrogate daily oscillations.32 Other rhythmic hormones, like melatonin and cortisol, however, do not appear to be affected by sleep directly. Although the reason why we sleep is still largely elusive and highly investigated, its role in memory formation33 is generally appreciated. Findings of blunted response to vaccination in humans34 and mice35 as a result of sleep deprivation, argue for a similar role of sleep in immunological memory formation, which was later associated with slow-wave sleep. The early rest phase (the time when slow-wave sleep is dominating) is correlated with high levels of pro-inflammatory cytokines, high growth hormone and prolactin levels and low catecholamine and cortisol levels, paralleled by a high abundance of memory cells36 in the bloodstream, providing excellent conditions for immunological memory formation. Local cytotoxic activities in contrast appear to be restricted to times of activity,37 when wounding and acute pathogen exposure are more prominent.
Sleep has tremendous effects on health and well-being, and represents a considerable proportion of our lives. Research has mainly focused on sleep deprivation assays, which hardly control for effects caused by other zeitgebers. It will therefore be of great interest to perform immunological studies on individuals in an environment that only allows for sleep during the natural active phase or exposure of subjects to light during the inactive, and darkness in the active phase.
Rhythmic cytokine production and cytotoxic activities
In 1960, Halberg et al.38 laid the foundation of modern circadian research in immunology by illustrating a diurnal susceptibility pattern in mice challenged with the bacterial endotoxin lipopolysaccharide (LPS), with lethality ranging from 80% towards the end of the rest (light) phase to 20% in the middle of the active (dark) phase. Even though much progress has been made and human pathologies were reported to follow similar daily rhythms, we understand little about the mechanistic basis of this significant finding. Daily variations in the intensity of symptoms in chronic diseases like rheumatoid arthritis39,40 and rhinitis41,42 are highly prevalent and morning stiffness, as it occurs in patients with rheumatoid arthritis, has been correlated with elevated pro-inflammatory cytokine production.43 Systemic pro-inflammatory activity is basally elevated during the rest phase in healthy individuals and anti-inflammatory mediators are sharply induced upon awakening27 (Fig.2). In addition to a possible contribution from mechanical stiffness after rest, morning stiffness in patients with rheumatoid arthritis may result from a hyper-inflammatory phase at night that is sustained into the awakening phase. Joint stiffness only slowly improves as activity commences and anti-inflammatory mediators reach higher systemic levels.
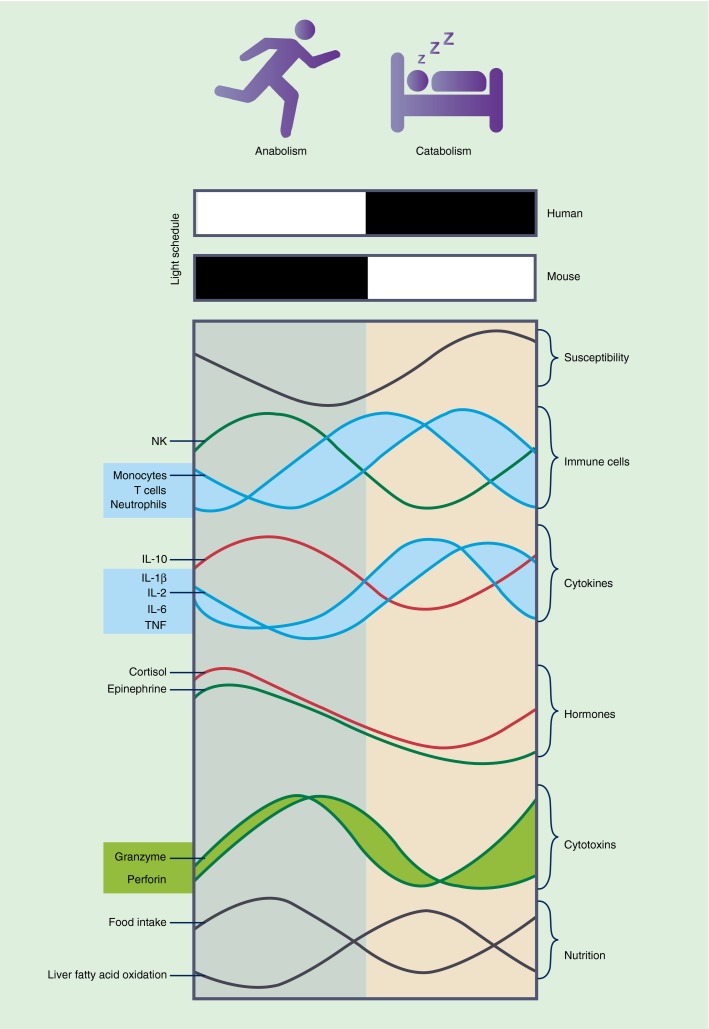
Figure 2.
Circadian oscillations of immune factors. Schematic illustration of rhythmic immunological readouts. Activity in nocturnal mice is restricted to the dark phase (filled bar), which coincides with the rest phase of humans. A range of immunological processes oscillate similarly in mice and humans with regard to rest and activity. Susceptibility to infection is elevated in the late rest phase and during transition to the active phase. Memory formation and systemic inflammatory response (blue curves) are denoted by high blood count of neutrophils, T cells and monocytes and increased pro-inflammatory cytokine production. Anti-inflammatory signals (red curves) such as interleukin-10 and cortisol peak during the active phase when local inflammation (green curves) – as indicated by blood abundance of natural killer cells as well as granzyme and perforin production and epinephrine abundance – is increased. Food intake parallels activity and delivers nutrients to support anabolic metabolism, whereas times of starvation due to inactivity generally promote catabolism, as indicated by increased fatty acid oxidation in the liver.
Whole blood stimulation with LPS in vitro, at night and early in the morning, displayed increased interferon-γ, TNF-α, IL-6, IL-1 and IL-12 production in samples from healthy volunteers compared with other times.44 Similar results were obtained during a human in vivo study, where Escherichia coli LPS injection at night resulted in increased IL-6 and TNF levels compared with noon infection.45 Increased cytokine production and sensitivity46 was also seen in nocturnal organisms such as mice when stimulated during the inactive phase or at the transition into the active phase.47,48
Before functional clocks in immune cells were discovered, inflammatory outputs were correlated with hormone levels to mechanistically investigate the interplay between the clock and immunity. Cortisol and melatonin both regulate immunity and their expression follows a strong diurnal pattern. Cortisol reaches peak serum levels at the beginning of the active phase and suppresses inflammation and cytokine production.44 The role of melatonin in contrast appears rather diverse, as studies showing immunostimulatory effects of melatonin are as abundant as reports claiming an immuno-inhibitory role. Melatonin may therefore act as an immune buffer, stimulating the immune response under basal conditions, while promoting anti-inflammatory pathways in states of inflammation (reviewed in ref. 49).
The characterization of functional, cell autonomous clocks in almost every cell in the human body sparked interest in the mechanistic role of clocks in immune cells. In macrophages, inflammatory outputs such as phagocytosis, cytokine and chemokine mRNA are regulated by the cell autonomous clock and induced at various times of the day.50 Cytotoxic natural killer cell activity is similarly dependent on the cell intrinsic clock, with TNF being rapidly induced at the beginning of the active phase, and granzyme and perforin protein levels peaking later in the active phase.37
Cytokine signalling pathways and the clock appear to reciprocally regulate each other. For example, pro-inflammatory signalling triggered by TNF is intensified in Cry mutants via the regulation of glycogen synthase kinase 3β phosphorylation and subsequent nuclear factor-κB induction.51 Upon engagement of TNF with TNF receptor 1, the negative arm of the molecular clock (Per1/2, Cry1) is induced in a P38–mitogen-activated protein kinase-dependent fashion52 and in the absence of the central clock machinery, nuclear factor-κB-mediated transcription is up-regulated, thereby reinforcing the inflammatory response.53
In addition to the direct influence of the circadian clock cycle on immune cell gene expression and function, a role for core clock components exists independently of a functional clock. Rev-erbα deficiency, for example, abrogates rhythmic cytokine response in macrophages, particularly IL-6 production, while a functional clock is sustained.54 However, silencing of Cry2 also induces IL-6 expression,55 arguing for Rev-erbα not being exclusively responsible for rhythmic IL-6 regulation. Discrimination and attribution of phenotypes to single clock components is not always possible, and can be confounded by the effects of deletion of core clock elements such as BMAL1, which result in a complete collapse of the cellular clock. However, it is clear that the entity of a functional clock and its constituents are critical for homeostatic balance.
The intensity of cytokine production upon stimulation appears to sustain rhythmicity in constant darkness,54,56 conversely, circadian differences in survival in response to LPS could not be observed under these conditions.47 It should be noted that the majority of studies investigating diurnal rhythms in susceptibility to infection were consistent regarding the trough in survival rate during the late rest phase despite the use of different protocols ranging from TNF46 and LPS in BALB/c mice38 to coxsackie B3 virus infection in CD-1 mice.57 Persistence of circadian rhythms without appropriate cues, like light (free running conditions), that are necessary to classify rhythmic oscillations as circadian or diurnal, has not been tested.
Besides cytokine regulation, toll-like receptors such as TLR9 and accordingly TLR9-mediated responsiveness to vaccination, display daily variations with peak values during the active phase.58 Bacterial burden and pro-inflammatory response are further heightened following Salmonella infection at the beginning of the rest phase compared with infections during the late active phase.59 The 70 000 MW ζ-chain associated protein kinase ZAP70 is another immune-regulator that is rhythmically expressed under physiological and inflammatory conditions, which mediates increased T-cell proliferation upon T-cell receptor stimulation in the organism's inactive phase.60
Inflammation is tightly regulated and although we do not fully understand why and how immune function displays such strong diurnal variation, a more systemic, pro-inflammatory response during the inactive phase that allows immunological memory formation, and a generally anti-inflammatory state during the active phase that promotes a local immune response in case of pathogen exposure, for example as a result of wounding, has been described (reviewed in ref. 61) (Fig.2).
Diurnal variations in immune cell trafficking
In line with the concept that diurnal variations promote immunological memory formation at rest and a relatively local immune response during the active period, rhythmic cell counts of immune subsets in the blood are apparent (Fig.2). Total leucocyte numbers in humans are low in the morning hours and peak at midnight.62 This is paralleled by increased abundance of memory and naive T cells in the bloodstream at night, whereas CD8+ effector T cells and natural killer cells peak during the daytime.31,36 Nocturnal animals, such as mice and rats, display a similar pattern when correlated to times of activity63 (e.g. nocturnal mice and rats are active at night, while diurnal humans are at rest).
In humans, epinephrine64 and cortisol65 are rhythmically released and are highly abundant in the early morning hours with reduced levels at night. Epinephrine rapidly recruits natural killer cells and effector T cells in the morning from the marginal pool to the circulation by increasing the expression of β-adrenergic receptors and the chemokine receptor CX3CR1 and a corresponding decrease in adhesion molecules. Conversely, cortisol, with a 3-hr delay, increases the expression of CXCR4 to mediate bone marrow homing, thereby negatively regulating the abundance of circulating naive and memory T cells in the blood.36 The epinephrine/cortisol rhythms are highly robust, and sleep appears to consolidate oscillations in leucocyte abundance by preserving these hormonal rhythms.61 Adrenergic signalling also regulates expression of vascular cell adhesion molecule-166 in the bone marrow microenvironment to induce homing of leucocytes to the bone marrow late in the activity phase, as well as intercellular adhesion molecule-1 and chemokine (C-C Motif) ligand (CCL2) expression on endothelial cells in skeletal muscle to facilitate adhesion and rolling of leucocytes.66 Hence, trafficking to peripheral sites occurs simultaneously, thereby resulting in anti-phasic abundance in the bloodstream.
Whereas most studies correlate immune cell trafficking to glucocorticoids originating from the hypothalamic–pituitary–adrenal system and catecholamines via the sympathetic nervous system,67 some more recent studies implicate involvement of the peripheral clock in immune cells (reviewed in ref. 68). Recruitment of Ly6Chigh monocytes to the bloodstream and inflamed tissue, for example, peaks in the late rest phase and was shown to be dependent on the abundance of myeloid BMAL1, which acts as a transcriptional repressor of CCL2 and CCL8 in monocytes. As BMAL1 reaches a nadir at the transition to the active phase, repression is withdrawn and CCL2 consequently rises to peak levels, allowing monocyte recruitment to the bloodstream and inflamed peripheral tissues.48
The role of chrono-nutrition
Recently, interest in the effects of metabolism on immunity has flourished, and provided a new level of understanding regarding the role of the Warburg effect in immune cell function. Warburg's initial observation69 of a shift from oxidative phosphorylation towards increased levels of glycolysis in tumour cells has been linked to a pro-inflammatory state of immune cells in multiple systems.70 Whether immune cell metabolism can be influenced via nutritional signals is not yet clear.
Obesity is highly prevalent and promotes inflammatory diseases including type 2 diabetes and certain forms of cancer, and control of weight is central in disease prevention. Recently, a critical role for timed food intake in terms of duration and solar time in the development of obesity has been identified. Restriction of food availability to 8 hr during the active phase prevents weight gain compared with ad libitum feeding, although caloric intake is sustained,71 and even food restriction to the inactive phase limits weight gain compared with ad libitum feeding on a high-fat diet.72 Locomotor and feeding activity on high-fat diet are additionally blunted, promoting increased food intake during the inactive phase and subsequent progression of metabolic disease.73
Similar to the light-entrained master regulator in the SCN, a food-entrained master regulator has been proposed to regulate food-entrained oscillators, such as food anticipatory activity (Fig.3). Food anticipatory activity is characterized by elevated hormone levels (e.g. the glucocorticoid corticosterone and ghrelin74) and increased core body temperature, and is sustained under temporal food restriction even in BMAL1-deficient and SCN-lesioned mice.75 The location and the mechanism by which the food-entrained master regulator is entrained by food stimuli and how it subsequently regulates subordinate, peripheral tissues is however unknown.
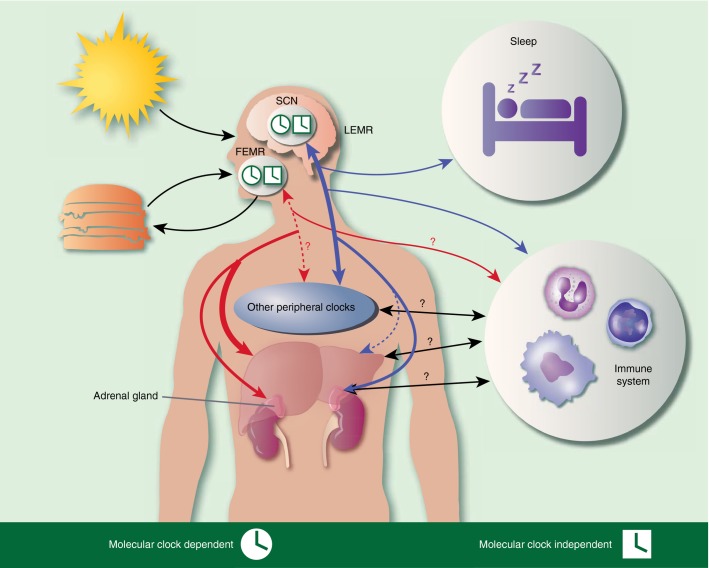
Figure 3.
The body clock. Zeitgebers, such as food and light, entrain master regulators, which communicate with peripheral clocks in a bi-directional fashion to sustain clock alignment. Light stimuli are processed in the light-entrained master regulator (LEMR) in the suprachiasmatic nucleus (SCN), whereas food signals are conveyed to the food-entrained master regulator (FEMR), whose location is not yet known. LEMR regulates sleep and immune response, as well as other peripheral clocks (blue arrows). FEMR strongly controls the liver clock among others (red arrows). The question marks indicate a proposed, but not yet proven, interplay and the black arrows represent general crosstalk. Round clocks indicate cellular clock involvement, whereas rectangular clock symbols reference a system that is independent of the molecular clock.
Based on current knowledge, it appears that food is a strong zeitgeber, with the ability to shift clock gene expression in a variety of peripheral tissues.76–78 Temporal food restriction allows robust entrainment of cellular clocks, and even though misalignment between the master and food entrained clocks has disadvantageous effects on metabolic homeostasis, it appears that complete loss of rhythmicity in food-entrained oscillators, via unrestricted, ad libitum feeding, intensifies metabolic pathology.72 High-fat diet additionally consolidates food intake during the inactive phase, eventually causing loss of food-entrained oscillator rhythmicity. This has significant implications in the prevention and therapy of metabolic diseases and cancer caused by shiftwork and diet-induced obesity, and it will be of particular interest to investigate whether timed food intake may affect weight loss on non-high-fat diet in obese individuals. Shift work, as a form of chronic clock disruption, is associated with increased risk of infectious diseases79 and metabolic syndrome,80 which subsequently promotes diabetes81 and cardiovascular disease. It is not known whether this is due to misaligned clocks or is mainly a result of inappropriate mealtimes and requires further investigation.
It is of great interest to locate and characterize the proposed food-entrained master relay to provide the tools needed to perform detailed studies of the entrainment and sustained rhythmicity of food-entrained oscillators using conditional mutations. However, this is a challenging undertaking considering the sustained rhythmicity in Bmal1-deficient mice and the fact that food deprivation can only be for a relatively short period of time.
Conclusion
Society and industry have dramatically changed our working and eating habits, thereby promoting chronic disruption of biological clocks. Only now are we beginning to understand the adverse effects this may pose to our health. Evolutionary pressure has forced organisms to maximize efficiency and the occupation of distinct niches has consequently resulted in a defined pattern of predator and food abundance, social interactions and pathogen exposure in the course of a day, allowing organisms to anticipate and quickly react to environmental changes. Accordingly, the immune system has evolved to accurately balance immune responses to clear infection while keeping tissue damage to a minimum by restricting maximum immune reactivity and intensity to certain times of the day.
The level of complexity is not surprising, considering how conserved this timing system is, and this, without doubt, is one of the reasons why we are still unable to explain phenotypes that have been characterized decades ago. Modern technology has helped us tremendously to shed light on a variety of aspects and we now know that anti- and pro-inflammatory processes are oscillating and are regulated on several levels ranging from hormonal to cell autonomous. It appears that immune components that are necessary for immunological memory are highly active during the sleep phase, whereas local, cytotoxic immune cell function is induced at the transition into the active phase. Despite our knowledge about the importance of sleep in memory formation and as a regulator of immunity, research often neglects sleep, not least because it is challenging to dissociate this from other zeitgebers.82 The importance of zeitgebers other than light has generally been ignored in the field of chrono-immunology and we are now facing a growing need to evaluate the contribution of various zeitgebers, like food and sleep, rather than solely correlating phenotypes to the solar time. Importantly, we will also need to reconsider the physiological relevance of in vitro studies using clock-deficient cells to examine pathologies relevant in chronically desynchronized individuals, such as shift workers. A significant body of literature has emerged over the previous year reporting sustained rhythmicity in clock-deficient compartments,83,84 underpinning the relevance of systemic signals in tissue function and homeostasis. Loss of molecular clock components can therefore not necessarily be correlated with loss of functional rhythmicity. Usually, in vitro studies do not take those systemic factors into consideration and often attribute readouts exclusively to the lack of particular clock factors or clock break down, although the system might not be affected in vivo. Hence, in vitro studies are often questionable and have to be reviewed critically. It is also of much greater interest to investigate the impact of misaligned clocks on immunological outputs, similar to what is observed in acute and chronic jet lag and night-time eaters, rather than clock deficiency per se.
Our understanding of food intake and nutrition as a player in immunity has been growing in the past years, and we can now start to appreciate the role of metabolism in immunity, yet there is still much to be learned in this area. Recent discoveries about the preventive role of timed food intake in metabolic diseases have paved the way for future investigations focusing on the role of chrono-nutrition in inflammation, but our knowledge so far is based on phenotypic observations as a result of time-restricted feeding in mice.
Despite active investigation of the crosstalk between the clock and the immune systems, we still understand very little about the mechanism by which clock disruption hyper-activates immunity and what effect chronic inflammation might have on the circadian machinery. The importance of chrono-immunological research goes beyond chrono-pharmacological applications, where medication can be optimized by timed drug administration. Better understanding of this area may allow preventive and improved therapeutic approaches for immunological diseases and inflammation-linked disease states including metabolic syndrome.
Acknowledgments
This work was supported by the Wellcome Trust and the intramural research funding from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the U.S. National Institutes of Health. The authors thank the reviewer for helpful comments and Sahaana B Arumugam, Anthony C Cruz, John R Ferdinand, Christian Geiger and Arianne C Richard for critical reading of the manuscript.
Disclosures
The authors declare that they have no competing interests.
References
- Aschoff J. Die 24-Stunden-Periodik der Maus unter konstanten Umgebungsbedingungen. Naturwissenschaften. 1951;38:506–7. [Google Scholar]
- Aschoff J. Exogenous and endogenous components in circadian rhythms. Cold Spring Harb Symp Quant Biol. 1960;25:11–28. doi: 10.1101/sqb.1960.025.01.004. [DOI] [PubMed] [Google Scholar]
- Brown SA, Zumbrunn G, Fleury-Olela F, Preitner N. Rhythms of mammalian body temperature can sustain peripheral circadian clocks. Curr Biol. 2002;12:1574–83. doi: 10.1016/s0960-9822(02)01145-4. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE, Marchant EG. Computational and entrainment models of circadian food-anticipatory activity: evidence from non-24-hr feeding schedules. Behav Neurosci. 1995;109:790–8. [PubMed] [Google Scholar]
- Edmonds SC, Adler NT. Food and light as entrainers of circadian running activity in the rat. Physiol Behav. 1977;18:915–9. doi: 10.1016/0031-9384(77)90201-3. [DOI] [PubMed] [Google Scholar]
- Aschoff J, Fatranska M, Giedke H, Doerr P, Stamm D, Wisser H. Human circadian rhythms in continuous darkness: entrainment by social cues. Science. 1971;171:213–5. doi: 10.1126/science.171.3967.213. [DOI] [PubMed] [Google Scholar]
- Whitmore D, Foulkes NS, Sassone-Corsi P. Light acts directly on organs and cells in culture to set the vertebrate circadian clock. Nature. 2000;404:87–91. doi: 10.1038/35003589. [DOI] [PubMed] [Google Scholar]
- Menaker M. Extraretinal light perception in the sparrow. I. Entrainment of the biological clock. Proc Natl Acad Sci USA. 1968;59:414–21. doi: 10.1073/pnas.59.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42:201–6. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci USA. 1972;69:1583–6. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husse J, Leliavski A, Tsang AH, Oster H, Eichele G. The light–dark cycle controls peripheral rhythmicity in mice with a genetically ablated suprachiasmatic nucleus clock. FASEB J. 2014;28:4950–60. doi: 10.1096/fj.14-256594. [DOI] [PubMed] [Google Scholar]
- Keller M, Mazuch J, Abraham U, Eom GD, Herzog ED, Volk H-DD, et al. A circadian clock in macrophages controls inflammatory immune responses. Proc Natl Acad Sci USA. 2009;106:21407–12. doi: 10.1073/pnas.0906361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, King DP, et al. Role of the CLOCK protein in the mammalian circadian mechanism. Science. 1998;280:1564–9. doi: 10.1126/science.280.5369.1564. [DOI] [PubMed] [Google Scholar]
- Hao H, Allen DL, Hardin PE. A circadian enhancer mediates PER-dependent mRNA cycling in Drosophila melanogaster. Mol Cell Biol. 1997;17:3687–93. doi: 10.1128/mcb.17.7.3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripperger JA, Schibler U. Rhythmic CLOCK-BMAL1 binding to multiple E-box motifs drives circadian Dbp transcription and chromatin transitions. Nat Genet. 2006;38:369–74. doi: 10.1038/ng1738. [DOI] [PubMed] [Google Scholar]
- Harding HP, Lazar MA. The orphan receptor Rev-ErbA α activates transcription via a novel response element. Mol Cell Biol. 1993;13:3113–21. doi: 10.1128/mcb.13.5.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguere V, Tini M, Flock G, Ong E, Evans RM, Otulakowski G. Isoform-specific amino-terminal domains dictate DNA-binding properties of RORα, a novel family of orphan hormone nuclear receptors. Genes Dev. 1994;8:538–53. doi: 10.1101/gad.8.5.538. [DOI] [PubMed] [Google Scholar]
- Keesler GA, Camacho F, Guo Y, Virshup D. Phosphorylation and destabilization of human period I clock protein by human casein kinase Iε. NeuroReport. 2000;11:951–5. doi: 10.1097/00001756-200004070-00011. [DOI] [PubMed] [Google Scholar]
- Camacho F, Cilio M, Guo Y, Virshup DM, Patel K, Khorkova O, et al. Human casein kinase Iδ phosphorylation of human circadian clock proteins period 1 and 2. FEBS Lett. 2001;489:159–65. doi: 10.1016/s0014-5793(00)02434-0. [DOI] [PubMed] [Google Scholar]
- Ukai-Tadenuma M, Kasukawa T, Ueda HR. Proof-by-synthesis of the transcriptional logic of mammalian circadian clocks. Nat Cell Biol. 2008;10:1154–63. doi: 10.1038/ncb1775. [DOI] [PubMed] [Google Scholar]
- Ueda HR, Hayashi S, Chen W, Sano M, Machida M. System-level identification of transcriptional circuits underlying mammalian circadian clocks. Nat Genet. 2005;37:187–92. doi: 10.1038/ng1504. [DOI] [PubMed] [Google Scholar]
- Krueger JM. Somnogenic activity of immune response modifiers. Trends Pharmacol Sci. 1990;11:122–6. doi: 10.1016/0165-6147(90)90198-h. [DOI] [PubMed] [Google Scholar]
- Späth-Schwalbe E, Hansen K, Schmidt F, Schrezenmeier H, Marshall L, Burger K, et al. Acute effects of recombinant human interleukin-6 on endocrine and central nervous sleep functions in healthy men. J Clin Endocrinol Metab. 1998;83:1573–9. doi: 10.1210/jcem.83.5.4795. [DOI] [PubMed] [Google Scholar]
- Imeri L, Opp MR. How (and why) the immune system makes us sleep. Nat Rev Neurosci. 2009;10:199–210. doi: 10.1038/nrn2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth LA, Krueger JM. Alteration of sleep in rabbits by Staphylococcus aureus infection. Infect Immun. 1988;56:1785–91. doi: 10.1128/iai.56.7.1785-1791.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant PA, Trinder J, Curtis N. Sick and tired: does sleep have a vital role in the immune system? Nat Rev Immunol. 2004;4:457–67. doi: 10.1038/nri1369. [DOI] [PubMed] [Google Scholar]
- Krueger JM, Obál F, Fang J, Kubota T. The role of cytokines in physiological sleep regulation. Ann N Y Acad Sci. 2001;933:211–21. doi: 10.1111/j.1749-6632.2001.tb05826.x. [DOI] [PubMed] [Google Scholar]
- Young MRI, Matthews JP. Circadian rhythmometry of serum interleukin-2, interleukin-10, tumor necrosis factor-α, and granulocyte–macrophage colony-stimulating factor in men. Chronobiol Int. 1995;12:19–27. doi: 10.3109/07420529509064496. [DOI] [PubMed] [Google Scholar]
- Fang J, Wang Y, Krueger JM. Effects of interleukin-1β on sleep are mediated by the type I receptor. Am J Physiol. 1998;274(3 Pt 2):R655–60. doi: 10.1152/ajpregu.1998.274.3.R655. [DOI] [PubMed] [Google Scholar]
- Fang J, Wang Y, Krueger JM. Mice lacking the TNF 55 kDa receptor fail to sleep more after TNFα treatment. J Neurosci. 1997;17:5949–55. doi: 10.1523/JNEUROSCI.17-15-05949.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born J, Lange T, Hansen K, Mölle M, Fehm HL. Effects of sleep and circadian rhythm on human circulating immune cells. J Immunol. 1997;158:4454–64. [PubMed] [Google Scholar]
- Dimitrov S, Lange T, Benedict C, Nowell MA, Jones SA. Sleep enhances IL-6 trans-signaling in humans. FASEB J. 2006;20:2174–6. doi: 10.1096/fj.06-5754fje. [DOI] [PubMed] [Google Scholar]
- Born J, Rasch B, Gais S. Sleep to remember. Neuroscientist. 2006;12:410–24. doi: 10.1177/1073858406292647. [DOI] [PubMed] [Google Scholar]
- Lange T, Perras B, Fehm HL, Born J. Sleep enhances the human antibody response to hepatitis A vaccination. Psychosom Med. 2003;65:831–5. doi: 10.1097/01.psy.0000091382.61178.f1. [DOI] [PubMed] [Google Scholar]
- Brown R, Pang G, Husband AJ, King MG. Suppression of immunity to influenza virus infection in the respiratory tract following sleep disturbance. Reg Immunol. 1989;2:321–5. [PubMed] [Google Scholar]
- Dimitrov S, Benedict C, Heutling D, Westermann J, Born J, Lange T. Cortisol and epinephrine control opposing circadian rhythms in T cell subsets. Blood. 2009;113:5134–43. doi: 10.1182/blood-2008-11-190769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arjona A, Sarkar DK. Circadian oscillations of clock genes, cytolytic factors, and cytokines in rat NK cells. J Immunol. 2005;174:7618–24. doi: 10.4049/jimmunol.174.12.7618. [DOI] [PubMed] [Google Scholar]
- Halberg F, Johnson E, Brown B, Bittner J. Susceptibility rhythm to E. coli endotoxin and bioassay. Exp Biol Med. 1960;103:142–4. doi: 10.3181/00379727-103-25439. [DOI] [PubMed] [Google Scholar]
- Kowanko IC, Knapp MS, Pownall R. Domiciliary self-measurement in the rheumatoid arthritis and the demonstration of circadian rhythmicity. Ann Rheum Dis. 1982;41:453–5. doi: 10.1136/ard.41.5.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JT. Morning stiffness in rheumatoid arthritis. Ann Rheum Dis. 1960;19:361–8. doi: 10.1136/ard.19.4.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trousseau A. Clinique medicale de L'Hotel dieu Paris. Paris: Bailliere; 1865. p. 373. , Vol. 2. [Google Scholar]
- Reinberg A, Gervais P, Levi F, Smolensky M, Del Cerro L, Ugolini C. Circadian and circannual rhythms of allergic rhinitis: an epidemiologic study involving chronobiologic methods. J Allergy Clin Immunol. 1988;81:51–62. doi: 10.1016/0091-6749(88)90220-5. [DOI] [PubMed] [Google Scholar]
- Clarke LL, Jessop DS, Hunt LP, Straub RH, Perry MG, Kirwan JR. Alleviation of morning joint stiffness by low-dose prednisone in rheumatoid arthritis is associated with circadian changes in IL-6 and cortisol. Int J Clin Rheumatol. 2011;6:241–9. [Google Scholar]
- Petrovsky N, McNair P, Harrison LC. Diurnal rhythms of pro-inflammatory cytokines: regulation by plasma cortisol and therapeutic implications. Cytokine. 1998;10:307–12. doi: 10.1006/cyto.1997.0289. [DOI] [PubMed] [Google Scholar]
- Alamili M, Bendtzen K, Lykkesfeldt J, Rosenberg J, Gogenur I. Pronounced inflammatory response to endotoxaemia during nighttime: a randomised cross-over trial. PLoS ONE. 2014;9:e87413. doi: 10.1371/journal.pone.0087413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrushesky WJ, Langevin T, Kim Y, Wood PA. Circadian dynamics of tumor necrosis factor α (cachectin) lethality. J Exp Med. 1994;180:1059–65. doi: 10.1084/jem.180.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marpegan L, Leone MJ, Katz ME, Sobrero PM, Bekinstein TA, Golombek DA. Diurnal variation in endotoxin-induced mortality in mice: correlation with proinflammatory factors. Chronobiol Int. 2009;26:1430–42. doi: 10.3109/07420520903408358. [DOI] [PubMed] [Google Scholar]
- Nguyen KD, Fentress SJ, Qiu Y, Yun K, Cox JS, Chawla A. Circadian gene Bmal1 regulates diurnal oscillations of Ly6Chi inflammatory monocytes. Science. 2013;341:1483–8. doi: 10.1126/science.1240636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo-Vico A, Lardone PJ. Melatonin: buffering the immune system. Int J Mol Sci. 2013;14:8636–83. doi: 10.3390/ijms14048638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M, Shimba S, Tezuka M. Characterization of the molecular clock in mouse peritoneal macrophages. Biol Pharm Bull. 2007;30:621–6. doi: 10.1248/bpb.30.621. [DOI] [PubMed] [Google Scholar]
- Lee JH, Sancar A. Regulation of apoptosis by the circadian clock through NF-κB signaling. Proc Natl Acad Sci USA. 2011;108:12036–41. doi: 10.1073/pnas.1108125108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrzilka S, Taraborrelli C, Cavadini G. Clock gene modulation by TNF-α depends on calcium and p38 MAP kinase signaling. J Biol Rhythms. 2009;24:283–94. doi: 10.1177/0748730409336579. [DOI] [PubMed] [Google Scholar]
- Spengler ML, Kuropatwinski KK. Core circadian protein CLOCK is a positive regulator of NF-κB-mediated transcription. Proc Natl Acad Sci USA. 2012;109:E2457–65. doi: 10.1073/pnas.1206274109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs JE, Blaikley J, Beesley S, Matthews L, Simpson KD, Boyce SH, et al. The nuclear receptor REV-ERBα mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc Natl Acad Sci USA. 2012;109:582–7. doi: 10.1073/pnas.1106750109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AE, Zheng T, Stevens RG, Ba Y, Zhang Y. Clock-cancer connection in non-Hodgkin's lymphoma: a genetic association study and pathway analysis of the circadian gene cryptochrome 2. Cancer Res. 2009;69:3605–13. doi: 10.1158/0008-5472.CAN-08-4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams KL, Castanon-Cervantes O, Evans JA, Davidson AJ. Environmental circadian disruption elevates the IL-6 response to lipopolysaccharide in blood. J Biol Rhythms. 2013;28:272–7. doi: 10.1177/0748730413494561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigin RD, Middelkamp JN, Reed C. Circadian rhythmicity in susceptibility of mice to sublethal coxsackie B3 infection. Nature. 1972;240:57–8. doi: 10.1038/newbio240057a0. [DOI] [PubMed] [Google Scholar]
- Silver AC, Arjona A, Walker WE, Fikrig E. The circadian clock controls toll-like receptor 9-mediated innate and adaptive immunity. Immunity. 2012;36:251–61. doi: 10.1016/j.immuni.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellet MM, Deriu E, Liu JZ, Grimaldi B, Blaschitz C, Zeller M, et al. Circadian clock regulates the host response to Salmonella. Proc Natl Acad Sci USA. 2013;110:9897–902. doi: 10.1073/pnas.1120636110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier EE, Rooney J, Dardente H. Circadian variation of the response of T cells to antigen. J Immunol. 2011;187:6291–300. doi: 10.4049/jimmunol.1004030. [DOI] [PubMed] [Google Scholar]
- Lange T, Dimitrov S, Born J. Effects of sleep and circadian rhythm on the human immune system. Ann N Y Acad Sci. 2010;1193:48–59. doi: 10.1111/j.1749-6632.2009.05300.x. [DOI] [PubMed] [Google Scholar]
- Abo T, Kawate T, Itoh K, Kumagai K. Studies on the bioperiodicity of the immune response. I. Circadian rhythms of human T, B, and K cell traffic in the peripheral blood. J Immunol. 1981;126:1360–3. [PubMed] [Google Scholar]
- Pelegrí C, Vilaplana J, Castellote C. Circadian rhythms in surface molecules of rat blood lymphocytes. Am J Physiol Cell Physiol. 2003;284:C67–76. doi: 10.1152/ajpcell.00084.2002. [DOI] [PubMed] [Google Scholar]
- Dodt C, Breckling U, Derad I, Fehm HL, Born J. Plasma epinephrine and norepinephrine concentrations of healthy humans associated with nighttime sleep and morning arousal. Hypertension. 1997;30(1 Pt 1):71–6. doi: 10.1161/01.hyp.30.1.71. [DOI] [PubMed] [Google Scholar]
- Nichols CT, Tyler FH. Diurnal variation in adrenal cortical function. Annu Rev Med. 1967;18:313–24. doi: 10.1146/annurev.me.18.020167.001525. [DOI] [PubMed] [Google Scholar]
- Scheiermann C, Kunisaki Y, Lucas D, Chow A, Jang J-EE, Zhang D, et al. Adrenergic nerves govern circadian leukocyte recruitment to tissues. Immunity. 2012;37:290–301. doi: 10.1016/j.immuni.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronfol Z, Nair M, Zhang Q, Hill EE, Brown MB. Circadian immune measures in healthy volunteers: relationship to hypothalamic-pituitary-adrenal axis hormones and sympathetic neurotransmitters. Psychosom Med. 1997;59:42–50. doi: 10.1097/00006842-199701000-00006. [DOI] [PubMed] [Google Scholar]
- Druzd D, de Juan A, Scheiermann C. Circadian rhythms in leukocyte trafficking. Semin Immunopathol. 2014;36:149–62. doi: 10.1007/s00281-013-0414-4. [DOI] [PubMed] [Google Scholar]
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–14. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- Krawczyk CM, Holowka T, Sun J, Blagih J, Amiel E, DeBerardinis RJ, et al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood. 2010;115:4742–9. doi: 10.1182/blood-2009-10-249540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatori M, Vollmers C, Zarrinpar A, DiTacchio L. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15:848–60. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschos GK, Ibrahim S, Song WL, Kunieda T. Obesity in mice with adipocyte-specific deletion of clock component Arntl. Nat Med. 2012;18:1768–77. doi: 10.1038/nm.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohsaka A, Laposky AD, Ramsey K, Estrada C, Joshu C, Kobayashi Y, et al. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6:414–21. doi: 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Patton DF, Katsuyama ÂM, Pavlovski I, Michalik M, Patterson Z, Parfyonov M, et al. Circadian mechanisms of food anticipatory rhythms in rats fed once or twice daily: clock gene and endocrine correlates. PLoS ONE. 2014;9:e112451. doi: 10.1371/journal.pone.0112451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendergast JS, Nakamura W, Friday RC, Hatanaka F, Takumi T, Yamazaki S. Robust food anticipatory activity in BMAL1-deficient mice. PLoS ONE. 2009;4:e4860. doi: 10.1371/journal.pone.0004860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–61. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291:490–3. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- Hara R, Wan K, Wakamatsu H, Aida R, Moriya T, Akiyama M, et al. Restricted feeding entrains liver clock without participation of the suprachiasmatic nucleus. Genes Cells. 2001;6:269–78. doi: 10.1046/j.1365-2443.2001.00419.x. [DOI] [PubMed] [Google Scholar]
- Mohren DCL, Jansen NWH, Kant IJ. Prevalence of common infections among employees in different work schedules. J Occup Environ Med. 2002;44:1003–11. doi: 10.1097/00043764-200211000-00005. [DOI] [PubMed] [Google Scholar]
- Karlsson B, Knutsson A, Lindahl B. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occup Environ Med. 2001;58:747–52. doi: 10.1136/oem.58.11.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan A, Schernhammer ES, Sun Q, Hu FB. Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women. PLoS Med. 2011;8:e1001141. doi: 10.1371/journal.pmed.1001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilenko KV, Cajochen C, Wirz-Justice A. Is sleep per se a zeitgeber in humans? J Biol Rhythms. 2003;18:170–8. doi: 10.1177/0748730403251732. [DOI] [PubMed] [Google Scholar]
- Hemmers S, Rudensky AY. The cell-intrinsic circadian clock is dispensable for lymphocyte differentiation and function. Cell Rep. 2015;11:1339–49. doi: 10.1016/j.celrep.2015.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ieyasu A, Tajima Y, Shimba S. Clock gene Bmal1 is dispensable for intrinsic properties of murine hematopoietic stem cells. J Negat Results Biomed. 2014;13:4. doi: 10.1186/1477-5751-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]