Abstract
Gr-1+ CD11b+ myeloid-derived suppressor cells (MDSCs) accumulate in tumor-bearing animals and play a critical negative role during tumor immunotherapy. Strategies for inhibition of MDSCs are expected to improve cancer immunotherapy. Polysaccharide Agaricus blazei Murill (pAbM) has been found to have anti-cancer activity, but the underlying mechanism of this is poorly understood. Here, pAbM directly activated the purified MDSCs through inducing the expression of interleukin-6 (IL-6), IL-12, tumour necrosis factor and inducible nitric oxide synthase (iNOS), CD86, MHC II, and pSTAT1 of it, and only affected natural killer and T cells in the presence of Gr-1+ CD11b+ monocytic MDSCs. On further analysis, we demonstrated that pAbM could selectively block the Toll-like receptor 2 (TLR2) signal of Gr-1+ CD11b+ MDSCs and increased their M1-type macrophage characteristics, such as producing IL-12, lowering expression of Arginase 1 and increasing expression of iNOS. Extensive study showed that Gr-1+ CD11b+ MDSCs by pAbM treatment had less ability to convert the CD4+ CD25− cells into CD4+ CD25+ phenotype. Moreover, result from selective depletion of specific cell populations in xenograft mice model suggested that the anti-tumour effect of pAbM was dependent on Gr-1+ CD11b+ monocytes, nether CD8+ T cells nor CD4+ T cells. In addition to, pAbM did not inhibit tumour growth in TLR2–/– mice. All together, these results suggested that pAbM, a natural product commonly used for cancer treatment, was a specific TLR2 agonist and had potent anti-tumour effects through the opposite of the suppressive function of Gr-1+ CD11b+ MDSCs.
Keywords: myeloid derived suppressor cells, polysaccharide Agaricus blazei Murill, Toll-like receptor-2
Introduction
Inflammation and cancer are connected, as cancers arise at chronic inflammatory sites,1,2 and inflammatory cells participate in the processes of tumour progression such as evasion of anti-tumour immunity. Myeloid-derived suppressor cells (MDSCs), a heterogeneous group of host immune cells comprised of immature macrophages and granulocytes with immunosuppressive functions,3,4 are a major factor that limits the effectiveness of cancer immunotherapy, and are an appealing target for therapeutic intervention.5,6 Tumour growth and metastasis can be suppressed by decreasing the number of MDSCs in tumours.7,8 However, tumour-derived factors can induce the expansion and accumulation of MDSCs in the bone marrow, spleen and blood and at the site of the tumour and inhibit the differentiation and maturation of these cells, which endows the MDSCs with potent, immunosuppressive functions.9 A significant increase in MDSCs is observed in tumour-bearing mice, as well as in a variety of cancers in humans.8–10 More importantly, depletion of MDSCs has also been explored to improve the efficacy of tumour immunotherapy.11 Strategies for the elimination or inhibition of MDSCs are a new development in tumour therapy.
Accumulating evidence suggests that MDSCs have many characteristics similar to those of tumour-associated macrophages (TAMs). Both MDSCs and TAMs have suppressive functions on T cells in the tumour environment, as well as inducing the expression of inducible nitric oxide synthase (iNOS) and Arginase 1 (Arg1). In the tumour microenvironment, TAMs have two distinctive subpopulations: classical, or M1, macrophages are characterized by the expression of high amounts of iNOS and tumour necrosis factor-α (TNF-α), whereas alternatively activated M2 macrophages typically produce Arg1 and interleukin-10 (IL-10).12 At the tumour site, TAMs are predominantly M2-like macrophages; they suppress general anti-tumour immune responses, promote tumour neoangiogenesis, and facilitate tumour metastasis and dissemination.13 In contrast, M1 macrophages exhibit a tumoricidal effect.13,14 The Tsuyoshi Takami group showed that Gr-1+ CD11b+ MDSCs in tumours had pleiotropic characteristics of both M1 and M2 monocytes/macrophages.12–14
Agaricus blazei Murill, a native Brazilian edible mushroom, has been used as a health food or a non-prescription remedy in traditional medicines for cancer, diabetes, hyperlipidaemia, arteriosclerosis and chronic hepatitis in Brazil.15 Polysaccharide A. blazei (pAbM) is one of the most commonly used mushroom extracts, and has been effectively used to treat and prevent cancer.15,16 Oral intake of extracts of A. blazei has been reported to improve the quality of life of patients with cancer, especially after chemotherapy.15 Polysaccharide A. blazei is suggested to be an immune modulator in many studies, can influence the expression of IL-12 in peripheral blood mononuclear cells after oral administration,15 stimulate T-cell proliferation,17 and improve the function of CD4+ T cells in gut-associated lymphoid tissue.17,18 The receptor/pathways by which pAbM stimulates immune cells remain unknown.
Toll-like receptors (TLRs) have recently emerged as key receptors responsible for recognizing specific conserved components of microbes and can trigger macrophage activation and cytokine production, so effectively bridging innate and adaptive immunity.19 However, it has been known for a long time that microbial compounds can be used as efficient adjuvants in anti-tumour vaccine formulations, and numerous animal tumour models clearly indicate the potency of different TLR agonists on anti-tumour immune responses.20 In this regard, it is demonstrated that TLRs are expressed on a variety of immune cells, including T and B lymphocytes, neutrophils, monocytes and natural killer (NK) cells.21 In light of those findings, we hypothesized that the immunomodulatory function of TLRs on immune cells may underlie their anti-tumour activity. Here is it shown that pAbM efficiently modified the tumour microenvironment, in a TLR2 dependent manner, polarized MDSCs toward an M1 phenotype and promoted anti-tumour activity in mouse model of breast cancer.
Materials and methods
Animals
Toll-like receptor 2-deficient (TLR2–/–) mice were originally obtained from Jackson Laboratory. Wild-type C57BL/6 mice were obtained from the Experimental Animal Centre of the Chinese Academy of Sciences (Shanghai, China) for studies approved by the Animal Care and Use Committee of Tongji Medical College (Wuhan, China). Mice were maintained under strict inbreeding conditions.
Reagents
Reagents used included fetal bovine serum (Gemini Bioproducts, West Sacramento, CA); and RPMI-1640, PBS, penicillin–streptomycin and l-glutamine (Invitrogen Life Technologies, Carlsbad, CA). Fluorochrome-conjugated monoclonal antibodies (mAbs) targeting CD3, CD4, CD8, CD11b, Gr-1, NK1.1, MHC-II, CD86, Foxp3, CD25, Ly6C, isotype controls and iNOS were purchased from (eBiosciences, San Diego, CA); Arg1, TLR2 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Dectin-1 and CR3 were purchased from (R&D Systems, Minneapolis, MN), phosphorylated signal transducer and activator of transcription 3 (pSTAT3) and pSTAT1 antibodies were purchased from Cell Signaling Technology (Beverly, MA), and [3H]thymidine from PerkinElmer (Waltham, MA). The pAbM (see Supplementary material, Fig. S1) was kindly offered by Dr Chang (National Taiwan University, Taipei, Taiwan, PRC). It was dissolved in PBS at a stock concentration of 10 mg/ml and aliquots of 100 ml were stored at −80°. The frozen aliquots were thawed immediately before use.
MDSC purification
Spleens and bone marrow of mice with high tumour burden were harvested. Following the removal of red blood cells, bone marrow cells and splenocytes were fractionated by centrifugation on a Percoll density gradient (GE Healthcare, Piscataway, NJ).22 The cell layer between 50 and 60% Percoll was collected and washed twice with PBS. CD11b+ cells were positively selected from this fraction, which included predominantly the Gr-1+ populations using anti-CD11b-phycoerythrin and anti-phycoerythrin microbeads (Miltenyi Biotec, Auburn, CA). Cells were sorted to > 94% purity before use in subsequent experiments. MDSCs were sorted on a FACSAria II sorter (BD Biosciences, San Jose, CA).
Monocytes were identified for quantification
The reference population for gating was Gr-1+, thereby excluding granulocytes. Absolute monocyte numbers were quantified as previously described.23 In brief, after organ harvest, single-cell suspensions were obtained from bone marrow. Total viable cell numbers were determined using Trypan Blue. Absolute monocyte numbers were calculated as number of total viable cells multiplied by the percentage of Gr-1+ CD11b+ Ly6C+monocytes of all live cells.
pAbM activation of splenocytes in vitro
The proliferation of splenocytes from mice after in vitro pAbM treatment was evaluated using a [3H]thymidine incorporation assay in 96-well plates as previously described.24 In brief, splenocytes were cultured in 96-well plates (200 000 cells per well) in RPMI-1640 and incubated with pAbM (0–200 µg/ml) for 96 hr. [3H]thymidine (1 µCi per well) was included for the last 16 hr of culture. For time–course treatment, splenocytes were stimulated with pAbM (100 µg/ml) for 24, 48, 72, or 96 hr. Cytokine levels in the culture supernatant were measured using a mouse T helper type 1 (Th1)/Th2 cytokine kit according to the manufacturer's instructions (Meso Scale Discovery, Rockville, MD). To measure the percentage of each immune subset (T cells, NK cells and B cells), pAbM or control PBS-treated splenocytes were stained with fluorochrome-conjugated anti-CD3, CD11b+, NK1.1 and CD19 antibodies using standard procedures as previously described.22 To measure the expression of TLR2 on each immune subset, splenocytes were stained with anti-TLR2-phycoerythrin mAb (eBiosciences) following the manufacturer's instructions and the cells were co-stained with mAbs against CD3, NK1.1, CD19 and CD11b+. The expression of TLR2 on mouse mammary carcinoma (MMC) tumour cells was measured using a similar method.
Flow cytometry
Cells were incubated with fluorescence-labelled antibody for flow cytometric analysis. Parameters were acquired on a FACSCalibur flow cytometer (BD Biosciences) and analysed using the FlowJo software (TreeStar, Ashland, OR).
Immunofluorescence
Purification of MDSCs from MMC tumour-bearing mice treated with or without pAbM were sectioned and stained with anti-F4/80 (Santa Cruz Biotechnology), anti-iNOS and anti-Arg1 (BD Biosciences) followed by Cy3-conjugated anti-mouse, Cy2-conjugated donkey anti-rat and Cy5-conjugated donkey anti-rabbit (Jackson Immunoresearch, West Grove, PA) secondary antibodies, respectively. Stained slides were visualized using a Leica DM RA2 fluorescence microscope (Leica Microsystems, Wetzlar, Germany).
MDSC suppression assay
The suppressive activity of CD11b+ monocytes was assessed using an assay based on peptide-mediated proliferation of T-cell receptor transgenic T cells as described previously.22 In brief, 1 × 105 splenocytes from OT-II mice were cultured in the presence of ovalbumin peptides (1 μg/ml) and serial dilutions of 2000 rad irradiated CD11b+ monocytes in 96-well microplates. CD4 ovalbumin peptide (323–339: ISQAV-HAAHAEINEAGR) was purchased from Washington Biotechnology, Inc. (Baltimore, MD). Cells were pulsed with [3H]thymidine during the last 16 hr of a 3-day culture.
Regulatory T cell conversion assay
Splenocytes (4 × 106) from OT-II mice were cultured in the presence of OVA peptides (1 μg/ml) and 1 × 106 2000-rad-irradiated CD11b+ monocytes in 12-well microplates. After 5 days, cells were harvested and stained with fluorochrome-conjugated anti-CD4, anti-CD25, anti-Foxp3 or isotype control antibodies before flow cytometric analysis.
ELISA
Cytokines in cell culture supernatants from untreated or treated cells were measured using ELISA kits with the following purified coating and biotinylated detection antibodies: anti-IL-12, anti-IL-6, anti-TNF, anti-interferon-γ, anti-transforming growth factor-β (TGF-β), and anti-IL-10 (R&D Systems).
Treatment of tumour-bearing mice with oral pAbM
The anti-tumour effect of pAbM was evaluated in mice in the implanted tumour setting, and in TLR2–/– and wild-type C57BL/6 mice with implanted tumours. For implanted tumours in the mice, 1 × 106 MMC cells were injected into the mice subcutaneously. Treatment with pAbM was started 2 weeks after the implantation when the implanted tumour just became palpable (average size 50 mm3). Mice were randomly assigned to be treated with pAbM (2 mg per mouse; equivalent to 100 mg/kg, three times per week for 4 weeks) or control PBS (n = 5 per group). For implanted tumours in C57BL/6 and TLR2–/– mice, the tumour was started by subcutaneous injection of 40 000 TC-1 cervical cancer cells (C57BL/6 background). The pAbM or control PBS treatment was started 10 days after the injection when tumours just became palpable. The pAbM was dissolved in PBS and given via oral gavage in a 200 ml volume. Mice in the control group received oral gavage of PBS of the same volume. Tumours were measured every other day with vernier calipers and tumour volume was calculated as the product of length × width × height × 0·5236. In vivo data are presented as mean ± SEM. In some experiments, mice were depleted of specific lymphocytes (CD4+, CD8+ T cells, or MDSCs) during pAbM treatment (2 mg per mouse; equivalent to 100 mg/kg, three times per week for 4 weeks).
Statistical analysis
Statistical analysis was performed using GraphPad (GraphPad Software, San Diego, CA). Data were analysed using the two-tailed Student's t-test or analysis of variance. A value of P < 0·05 was considered statistically significant.
Results
Gr-1+ CD11b+ monocytes in tumour-bearing mice exhibit M2 characteristics
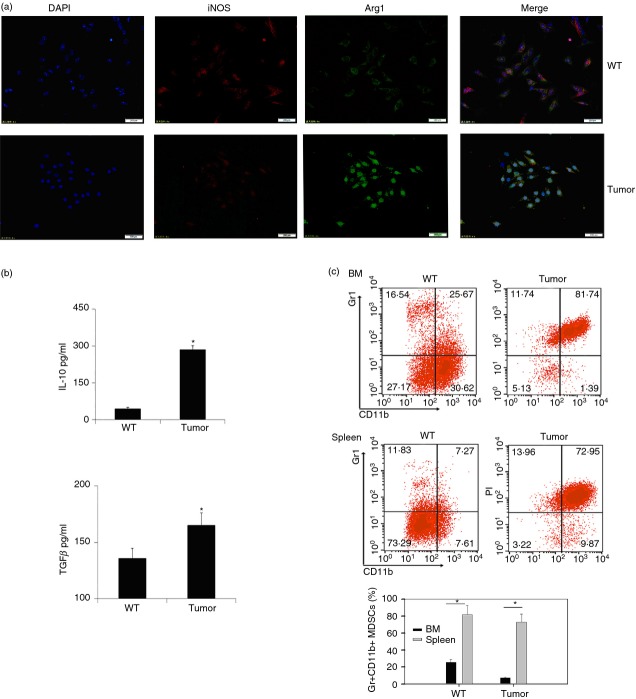
In order to examine the characteristics of Gr-1+ CD11b+ MDSCs in tumour-bearing mice, we first inoculated 1 × 106 MMC cells into C57BL/6 mice subcutaneously. Fourteen to 20 days later, Gr-1+ CD11b+ cells were purified from the spleen of wild-type or tumour-bearing mice and embedded into the optimum cutting temperature compound. Frozen pathology slides were stained with antibodies against iNOS and Arg1. Observing under the fluorescence microscope, we found that expression of Arg1 was abundant and iNOS was much less in Gr-1+ CD11b+ cells of tumour-bearing mice (Fig.1a). At the same time, we also found that some cytokines such as TGF-β and IL-10 were significantly increased in MDSCs of tumour-bearing mice (Fig.1b). These findings suggested that Gr-1+ CD11b+ MDSCs in tumour-bearing mice displayed M2-like characteristics. Single cell suspensions of spleen and bone marrow from normal or tumour-bearing mice were stained with anti-Gr-1 and anti-CD11b fluorescence antibodies, followed by FACS analysis. The results show that the percentages of Gr-1+ CD11b+ cells were increased in both spleen and bone marrow in tumour-bearing mice compared with tumour-free mice (Fig.1c). Furthermore, absolute monocytes were identified for quantification and sorting as Gr-1+ CD11b+ Ly6C+ cells of bone marrow from tumour-bearing mice (see Supplementary material, Fig. S2). It was demonstrated that Ly-6C+ Gr-1+ CD11b+ monocytic MDSCs have a stronger suppressive effect on T cells than granulocytic MDSCs.23
Figure 1.
Gr-1+ CD11b+ monocytes exhibited an M2 phenotype in tumour-bearing mice. (a) Gr-1+ CD11b+ myeloid-derived suppressor cells (MDSCs) purified from wild-type (WT) and tumour-bearing mice were stained with anti-inducible nitric oxide synthase (iNOS; red) and anti-Arginase 1 (Arg1; green) fluorescent antibody and analysed by immunofluorescence microscopy. Data are representative of three independent experiments. (b) Concentration of interleukin-10 (IL-10) and transforming growth factor-β (TGF-β) of Gr-1+ CD11b+ monocytes in spleen of normal C57BL/6 mice (indicated as WT) or tumour-bearing mice (indicated as tumour) were detected by ELISA. Data are representative of three independent experiments. *P < 0·05, Student's t-test. (c) Percentages of Gr-1+ CD11b+ monocytes in bone marrow and spleen were assayed by flow cytometry. Cells were prepared on a Percoll density gradient as described in the Materials and methods, and stained with anti-Gr1 and anti-CD11b antibodies. Percentages of Gr-1+ CD11b+ MDSCs were stained as described in (c) (upper) for flow cytometric analysis. *P < 0·05, Student's t-test. Data are representative of at least three independent experiments (b, c).
TLR-2 is the target receptor of pAbM in Gr-1+ CD11b+ monocytes
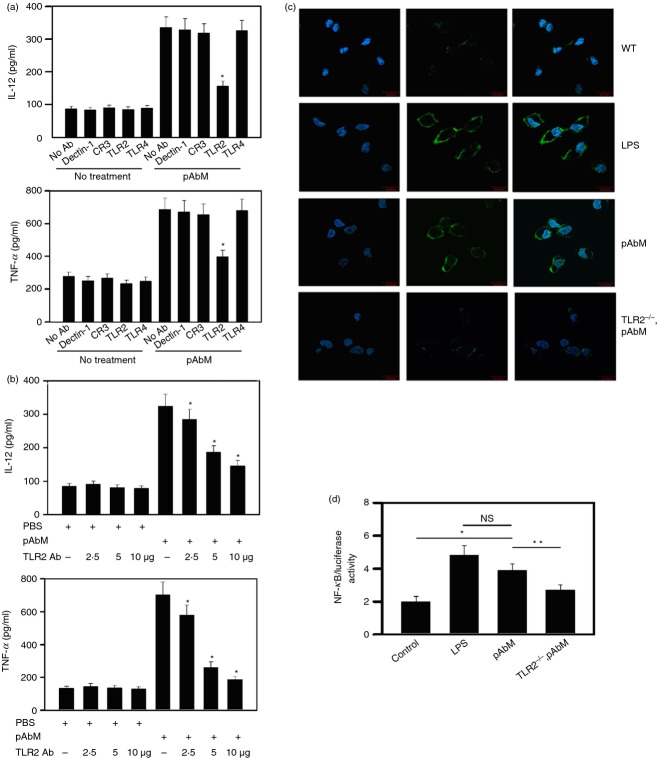
Polysaccharide A. blazei Murill is a mushroom extract that induces IL-12 production from peripheral blood mononuclear cells.18 To confirm the immunostimulatory properties of pAbM, we used an ELISA to analyse the concentrations of major immunoactivity cytokines IL-12 and TNF-α in a culture medium of Gr-1+ CD11b+ monocytes after pAbM treatment. All of these cytokines were increased over two-fold (see Supplementary material, Fig. S3), which suggested that pAbM may be an immunoactivity agent to activated Gr-1+ CD11b+ monocytes. Next, to identify the target receptor of pAbM, we focused on β-glucan receptors (Dectin-1 and CR3) and TLR2 and TLR4, which are implicated in the recognition of various bacterial cell wall components and are naturally derived heteroglycans and proteoglycans.25 Gr-1+ CD11b+ monocytes from bone-marrow-derived monocytes were pre-treated with blocking mAbs in the absence or presence of pAbM in vitro, followed by the cytokine production analysis. Only pre-treatment of the TLR2 blocking antibody significantly abrogated expression of the cytokines by pAbM-stimulated cells (Fig.2a). The cytokine-inhibiting behaviour of the TLR2 blocking antibody also varied based on dosage manner (Fig.2b). In addition, results of fluorescence microscopy also showed that the expression of TLR2 was dramatically increased in Gr-1+ CD11b+ monocytes after lipopolysaccharide (LPS) or pAbM treatment compared with untreated cells or TLR2–/– Gr-1+ CD11b+ monocytes upon pAbM stimulation (Fig.2c). Escherichia coli LPS, an endotoxin that acts as a strong stimulator for TLR2 activation (see Supplementary material, Fig. S4), was used as a positive control.26 It is known that nuclear factor-κB (NF-κB) plays a crucial role in TLR2-induced cytokine expression.25 We verified whether pAbM drove TLR2 to influence NF-κB activation. Gr-1+ CD11b+ monocytes transfected with NF-κB luciferase reporter were treated with pAbM, or LPS, for 24 hr. As shown in Fig.2(d), the NF-κB-driven luciferase activity was much higher in cells treated with pAbM or LPS than in untreated control group or TLR2–/– Gr-1+ CD11b+ monocytes upon pAbM stimulation. These data suggested that pAbM selectively activated TLR2 to increase cytokine expression in monocytes.
Figure 2.
The identification of receptors targeted by polysaccharide Agaricus blazei Murill (pAbM) on Gr-1+ CD11b+ monocytes. (a) Gr-1+ CD11b+ monocytes (5 × 105 cells) were pre-treated with monoclonal blocking antibodies (5 μg) specific to Dectin-1, CR3, Toll-like receptor 2 (TLR2) and TLR4 and then incubated with pAbM at a concentration of 100 μg/ml. After 24 hr, the conditioned medium was harvested, and the concentrations of interleukin-12 (IL-12) and tumour necrosis factor-α (TNF-α) were assayed by ELISA. *P < 0·05 from no antibody control, analysis of variance (anova). (b) Gr-1+ CD11b+ monocytes (5 × 105 cells) were pre-treated with blocking antibodies against TLR2 (2, 5 and 10 μg) and then incubated with pAbM at a concentration of 100 μg/ml. The conditioned medium was harvested after 24 hr and analysed for the concentrations of IL-12 and TNF-α using ELISA. *P < 0·05 from PBS control, anova. (c) Gr-1+ CD11b+ monocytes (5 × 105 cells) were untreated and treated with pAbM, LPS, or TLR2–/– Gr-1+ CD11b+ monocytes treated with pAbM. Cells were stained with DAPI (blue), anti-TLR2 (green). Experiments were repeated twice with similar results. (d) Gr-1+ CD11b+ monocytes or TLR2–/– Gr-1+ CD11b+ monocytes were transiently transfected with nuclear factor-κB luciferase reporter plasmids followed by stimulation with pAbM and the positive control, lipopolysaccharide (LPS), for 24 hr, and the luciferase activity was then measured. *P < 0·05; **P < 0·01, anova. The results are expressed as the mean ± SE from three independent experiments (a, b, d).
Skewed Gr-1+ CD11b+ monocytes from M2 to M1 type and enhanced differentiation by pAbM in tumour-bearing mice via TLR2 signalling
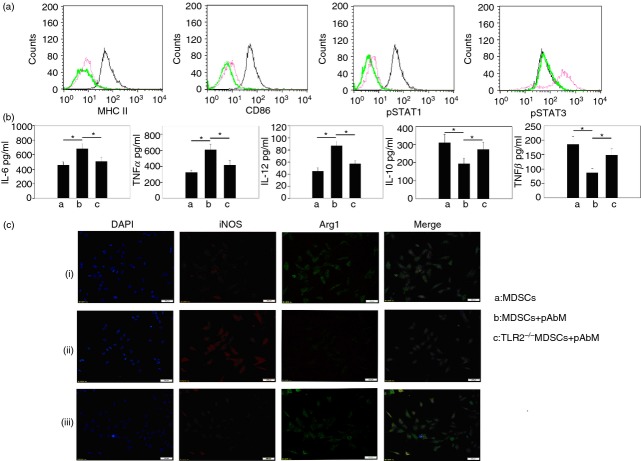
The differentiation of MDSCs into mature myeloid cells results in a reduction in their suppressive function.27,28 As shown in Fig.3(a), Gr-1+ CD11b+ MDSCs of tumour-bearing mice in the pAbM group expressed higher pSTAT1, MHC-II and CD86, lower pSTAT3, which gives evidence for pAbM promoting the maturation of Gr-1+ CD11b+ MDSCs. The expression of IL-6, IL-12 and TNF-α was significantly increased and the expression of TGF-β and IL-10 was significantly decreased in pAbM-treated Gr-1+ CD11b+ monocytes compared with untreated cells or TLR2−/− Gr-1+ CD11b+ monocytes upon pAbM stimulation (Fig.3b). Furthermore, pAbM significantly decreased the expression of Arg1 and increased the expression of iNOS in Gr-1+ CD11b+ monocytes, but not in TLR2−/− Gr-1+ CD11b+ monocytes (Fig.3c). These findings suggested that polysaccharide A. blazei impelled the conversion of Gr-1+ CD11b+ monocytes from M2-type to M1-type in tumour-bearing mice via the TLR2 signal.
Figure 3.
Expression of some representative molecules of M1/M2 type cells in polysaccharide Agaricus blazei Murill (pAbM) -treated Gr-1+ CD11b+ monocytes. (a) Expression of pSTAT1, CD86, MHCII, pSTAT3, and in untreated (red line) and pAbM (100 μg/ml) -treated (black line) Gr-1+ CD11b+ monocytes. Green line indicated as isotype antibody control. Data are representative of three independent experiments. (b, c) pAbM (100 μg/ml) treated Gr-1+ CD11b+ monocytes or TLR2–/– Gr-1+ CD11b+ monocytes and untreated Gr-1+ CD11b+ monocytes purified from tumour-bearing mice, after incubation, (b) concentration of interleukin-6 (IL-6), tumour necrosis factor-α (TNF-α), IL-12, IL-10, and transforming growth factor-β (TGF-β) by ELISA. The results are expressed as the mean ± SE from three independent experiments. *P < 0·05, analysis of variance. The results are expressed as the mean ± SE from three independent experiments. (c) Cells were analysed by immunofluorescence microscopy, conducted using anti-inducible nitric oxide synthase (iNOS; red) and anti-Arginase 1 (Arg1; green) staining. Data are representative of three independent experiments.
Type I inflammatory response induced by pAbM is dependent on TLR2 activation
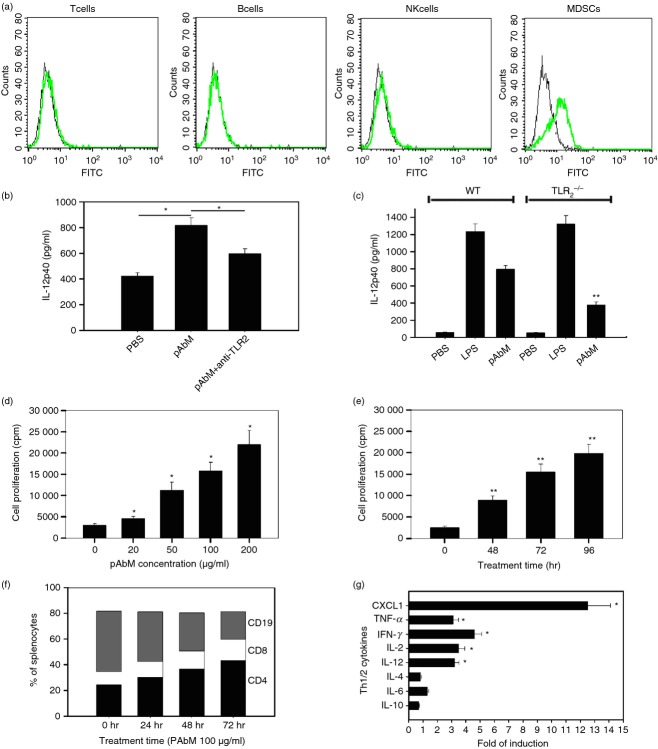
It has been shown that fungal pathogens can activate TLRs,29 so we questioned whether pAbM, which is a fungal product, might induce type I immunity through the TLR signal. The different subsets of splenocytes from mice after pAbM treatment were evaluated. The expression of TLR2 was detectable in CD19+ B cells and was strong in CD11b+ monocytes, but was undetectable or at very low levels in CD3+ T cells and NK1.1+ NK cells (Fig.4a). In addition, TLR2 was not expressed on tumour cells from mice (data not shown). Pre-incubation with an anti-TLR2 mAb inhibited IL-12p40 production induced by pAbM in Gr-1+ CD11b+ monocytes (P < 0·01; Fig.4b). Gr-1+ CD11b+ monocytes from TLR2−/− mice stimulated by pAbM secreted significantly less IL-12p40 compared with Gr-1+ CD11b+ monocytes from wild-type mice (379 ± 32 pg/ml versus 796 ± 41 pg/ml; P < 0·001; Fig.4c). Similarly, pAbM treatment (10–200 μg/ml, 48–96 hr) significantly stimulated the proliferation of splenocytes in a dose-dependent (Fig.4d) and time-dependent manner (Fig.4e). Treatment with pAbM increased the percentages of CD4+ (24·5 ± 1·5% in control versus 44·1 ± 3·7% after 72 hr pAbM treatment at 100 μg/ml; P < 0·01) and CD8+ T cells (10·1 ± 0·6% versus 16·2 ± 1·3%; P < 0·01) among total splenocytes and reduced the percentage of B cells (47·1 ± 0·9% versus 23·3 ± 1·9%; P < 0·01; Fig.4f). Treatment with pAbM (10–200 μg/ml, 48–96 hr) also induced secretion of Th1 cytokines in a dose- and time-dependent manner. After 96 hr of pAbM treatment (100 μg/ml), the level of interferon-γ was increased by 4·03 ± 0·51-fold (P < 0·01 from control). TNF-α was increased by 3·21 ± 0·34-fold (P < 0·01 from control). IL-2 was increased by 3·40 ± 0·16-fold (P < 0·05 from control). The levels of IL-4 and IL-5 were no different from controls (P < 0·22 and 0·11), respectively; (Fig.4g).
Figure 4.
Polysaccharide Agaricus blazei Murill (pAbM) stimulates type I inflammatory response and secretion of T helper type 1 (Th1) cytokines. (a) Histograms showing the expression of Toll-like receptor 2 (TLR2) in T cells, B cells, natural killer cells, and Gr-1+ CD11b+ monocytes from mice. The filled histogram represents cells stained with anti-TLR2-FITC (green); the unfilled histogram represents cells stained with an isotype (black) control antibody. (b) Interleukin-12p40 (IL-12p40) levels (mean ± SEM) in Gr-1+ CD11b+ monocytes isolated from mice pre-treated with/or without anti-TLR2 (10 μg/µl) for 1 hr before pAbM treatment (100 μg/ml for 48 hr). *P < 0·05, analysis of variance (anova). (c) The columns represent average IL-12p40 levels (mean ± SEM) in PBS-, pAbM-, or lipopolysaccharide (LPS) -treated Gr-1+ CD11b+ monocytes from wild-type (WT) or TLR2–/– mice. **P < 0·01 compared with pAbM treatment group in WT, anova. (d) Dose of pAbM on the horizontal axis and cell proliferation in cpm on the vertical axis. *Values significantly different from control, P < 0·01, anova. The bars represent the mean ± SEM of triplicate wells in each treatment group. (e) Time course of pAbM-stimulated splenocyte proliferation. The bars represent the mean ± SEM of triplicate wells in each treatment group. **Significantly different from control, P < 0·01, anova. (f) Percentages of CD4+ (black bar), CD8+ (white bar), and CD19+ cells (grey bar) among total splenocytes at various treatment times (x-axis). (g) The columns represent the fold of induction in pAbM-treated samples over untreated controls for each cytokine by ELISA. Mean ± SEM of duplicate wells is shown in each treatment group, *P < 0·05, anova. Data are representative of at least three independent experiments (a–g).
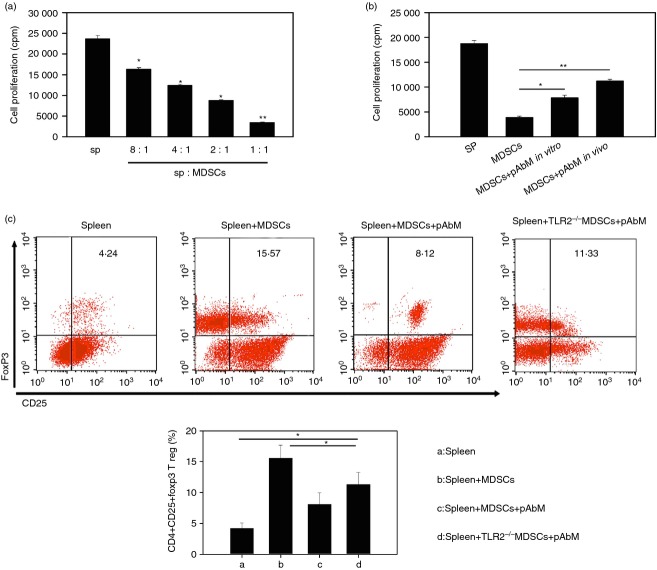
Treatment with pAbM leads to a loss of suppressive function of Gr-1+ CD11b+ monocytes
To examine the suppressive function of Gr-1+ CD11b+ monocytes after pAbM treatment in vivo, we inoculated 1 × 106 MMC cells into mice subcutaneously, followed by administration pAbM (50 μg/mouse) into these mice 1 week later as described in the Materials and methods. After 14–20 days, Gr-1+ CD11b+ monocytes were purified and co-cultured with purified OT-II CD4+ T cells stimulated with peptide pulsed irradiated splenocytes. Gr-1+ CD11b+ monocytes, purified from untreated mice, inhibited the proliferation of OT-II CD4+ T cells in a dose-dependent manner (Fig.5a). However, once in the presence of pAbM (100 μg/ml), Gr-1+ CD11b+ monocytes lost some degree of suppressive activity on the proliferation of OT-II CD4+ T cells, either in vivo or in vitro (Fig.5b). Furthermore, one of the mechanisms of MDSCs exerting their suppressive function is converting conventional CD4+ T cells into CD4+ CD25+ Foxp3+ regulatory T cells.20 To explore the possibility of the effect of pAbM on Gr-1+ CD11b+ monocytes inducing regulatory T cell conversion, we co-cultured spleen cells with irradiated Gr-1+ CD11b+ monocytes from tumour-bearing mice. After 5 days, intracellular staining for Foxp3 and surface staining for CD25 were conducted followed by FACS analysis gated on CD4+ cells. As shown in Fig.5(c), the percentage of Foxp3+ CD25+ cells were significantly increased in the co-cultured system. The percentage of CD4+ CD25+ Foxp3+ cells was significantly decreased in the pAbM treatment group compared with the untreated group (Fig.5c). However, splenocytes from TLR2−/− mice did not significantly decrease the percentage of CD4+ CD25+ Foxp3+ cells upon pAbM stimulation (Fig.5c). These results suggested that pAbM treatment in vivo or in vitro inhibited the suppressive function of Gr-1+ CD11b+ monocytes.
Figure 5.
Polysaccharide Agaricus blazei Murill (pAbM) leads to a loss of suppressive function of Gr-1+ CD11b+ monocytes dependent on Toll-like receptor 2 (TLR2) activation. (a) Proliferation of splenocytes from OTII mice stimulated with OTII peptide in the presence of different ratios of Gr-1+ CD11b+ monocytes (myeloid-derived suppressor cells; MDSCs) purified from normal mouse bone marrow (BM). *P < 0·05; **P < 0·01, values significantly different from control, analysis of variance (anova). The bars represent the mean ± SEM of triplicate wells in each treatment group. (b) Proliferation of OTII splenocytes stimulated with OTII peptide in the presence or absence of Gr-1+ CD11b+ monocytes (MDSCs) purified from pAbM-treated (indicated as pAbM in vivo) or untreated mice (indicated as MDSCs), or from untreated mice but adding pAbM into the culture system (indicated as MDSC+ pAbM in vitro). *P < 0·05; **P < 0·01, values significantly different from control, anova. The bars represent the mean ± SEM of triplicate wells in each treatment group. (c) The spleen cells were cultured with and without Gr-1+ CD11b+ monocytes (indicated as spleen+ MDSC), in the presence or absence of pAbM (100 ng/ml) (indicated as spleen and spleen+ MDSC+ pAbM) from tumour-bearing mice or TLR2–/– tumour-bearing mice for 5 days, cells were stained with anti-CD4, anti-CD25, anti-Foxp3 antibodies. Data shown here were gated on CD4+. *P < 0·05, anova. The results are expressed as the mean ± SEM from three independent experiments.
Anti-tumour effect of pAbM is dependent on skewing Gr-1+ CD11b+ monocytes from M2 to M1 type in mice and is mediated by TLR2
To determine the role of different immune subsets in the anti-tumour effect of pAbM, we selectively depleted CD4+ CD8+ T cells or B cells during pAbM treatment. As shown in Fig.6(a), selective depletion of CD8+ T cells and Gr-1+ CD11b+ monocytes, but not CD4+ T cells, significantly inhibited the anti-tumour effect of pAbM in mice with implanted breast tumours. Depletion of both Gr-1+ CD11b+ monocytes and CD8+ T cells during pAbM treatment resulted in larger tumours than depleting one of the two (data not shown). To investigate whether the anti-tumour effect of pAbM was mediated by TLR2 activation, we implanted the same amount of TC-1 tumour cells into TLR2 knockout mice and wild-type C57BL/6 mice. Then we treated the tumour bearing TLR2–/– mice or wild-type mice with oral pAbM or control PBS. As shown in Fig.6(b), pAbM significantly inhibited tumour growth in wild-type mice (P < 0·001 between the PBS and pAbM groups), but not in TLR2–/– mice (P = 0·3 between PBS and pAbM groups). Moreover, after a 4-week treatment, the expression of IL-2, IL-12 and TNF-α was significantly increased and the expression of TGF-β and IL-10 was significantly decreased in the pAbM-treated groups compared with untreated groups or TLR2–/– mice upon pAbM stimulation (Fig.6c). Together these results indicated that TLR2 was critical in the anti-tumour effect of pAbM as it can convert the suppressive function of Gr-1+ CD11b+ MDSCs.
Figure 6.
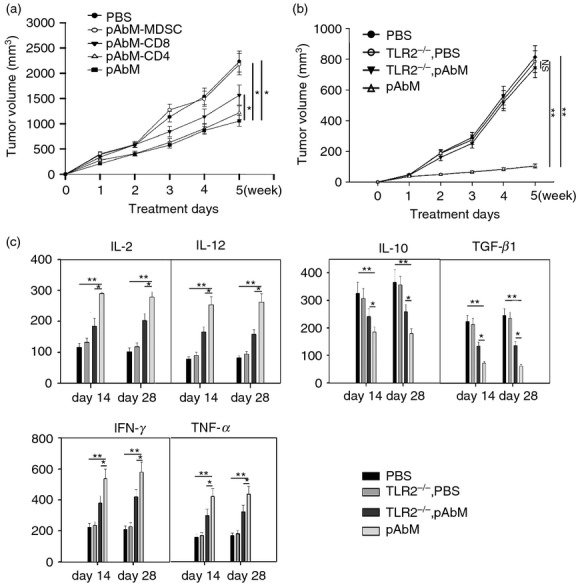
The anti-tumour effect of PAbM is dependent on CD8+ T cells and Gr-1+ CD11b+ monocytes and is mediated by Toll-like receptor 2 (TLR2). (a) The results shown are the growth curves of implanted mouse mammary carcinoma (MMC) tumours in C57BL/6 mice having received PBS (•), polysaccharide Agaricus blazei Murill (pAbM) (▪), pAbM with CD4 depletion (▽), pAbM with CD8 depletion (▾), or pAbM with Gr-1+ CD11b+ monocyte depletion (○). Data represent five mice in each treatment group. *P < 0·05, analysis of variance (anova). (b) The results shown are the growth curves of implanted TC-1 tumours in wild-type (WT) C57BL/6 mice receiving PBS (•), WT mice receiving PSK (▵), TLR2−/− mice receiving PBS (○), and TLR2−/− mice receiving PSK (▾). Data represent five mice in each treatment group. **P < 0·01, anova. (c) Concentration of interleukin-2 (IL-2), IL-12, interferon-γ, tumour necrosis factor-α (TNF-α), IL-10, and transforming growth factor-β (TGF-β) (mean ± SEM) Gr-1+ CD11b+ monocytes from WT or TLR2−/− mice on days 14 and 28 in PBS or pAbM-treated groups after tumour inoculation were assayed by ELISA. **P < 0·001 compared with pAbM treatment group in WT, *P < 0·05 compared with pAbM treatment group in TLR2−/−, anova. Similar results were observed in three independent experiments.
Discussion
Myeloid-derived suppressor cells are a heterogeneous population of cells, and have many similar characteristics with TAMs; they both have suppressive functions on T cells in the tumour microenvironment.6,30 Several therapeutic strategies targeting tumour-induced MDSCs are currently being explored experimentally, including the depletion of MDSCs with anti-Gr-1 mAb31 or promotion of MDSC differentiation with all-trans retinoic acid.32 However, the feasibility and possible adverse effects of these strategies translated from research into the clinic require further evaluation and additional therapeutic strategies need to be explored. In this study, we showed that pAbM was a potent and selective TLR2 agonist, could inhibit tumour growth and effectively reduce the suppressive function of tumour-induced Gr-1+ CD11b+ monocytic MDSCs on host T-cell proliferation and effector function (Fig.4). In experiments in vivo and in vitro, pAbM directly attenuated the immunosuppressive functions of monocytic MDSCs by signalling through TLR2 receptors (Fig.6).
Toll-like receptors are highly conserved from Drosophila to humans and share structural and functional similarities. They respond to the presence of pathogen-associated molecules, especially the sugar components, or glycoconjugates, of a diverse group of microbes.33,34 These molecules mediate the cytokine production necessary for the development of effective immunity. TLR2 causes an increase in the transcription factor NF-κB and the production of cytokines, including IL-12 and TNF-α, which enhance immunity in host defence and cytotoxicity. Therefore, TLR2 was considered to be a good target for immune therapy in malignant diseases to elicit innate immunity and facilitate Th1 type immune responses.34,35 In our study, pAbM was demonstrated to be a potent agonists of TLR2 (Fig.2). The expression of TLR2 on T cells is shown to be up-regulated following T-cell activation and can act as a co-stimulatory receptor.36 Recently, it has been found that this stimulation via TLR2 is more responsible for proliferation and survival of CD8+ T cells than for that of CD4+ T cells.36,37 Functionally, pAbM treatment induces type I T cells potentially through its effect on increased cytotoxic T lymphocyte proliferation and the expression of various effector molecules on T cells.37 Moreover, the study shows that TLR2 is minimally expressed on T cells but highly expressed on Gr-1+ CD11b+ monocyte MDSCs (Fig.4a). On the basis of the stimulation with TLR2 ligands in pAbM, we found that Gr-1+ CD11b+ monocyte MDSCs expressed higher levels of IL-6, IL-12, TNF, iNOS, CD86, MHCII and pSTAT1 after pAbM treatment in vitro (Fig.3a,b). By staining the sections of Gr-1+ CD11b+ MDSCs purified from tumour-bearing mice, we also found that iNOS was much higher in pAbM-treated MDSCs compared with non-treated MDSCs, but Arg1 was significantly decreased in pAbM-treated MDSCs. According to these observations, it is possible that pAbM could directly function on Gr-1+ CD11b+ monocyte MDSCs and skew them to become M1-type cells in tumour-bearing mice and in an in vitro culture system. Recent literature suggests that TLR2 can abolish the suppressive capacity of regulatory T cells or make effector T cells resistant to the suppression of regulatory T cells.36,37 We reasoned that pAbM might have this anti-tumour effect on the down-regulation of regulatory T cell function indirectly, by inhibiting MDSCs.
Reviewing our present findings, we performed experiments into the depletion of immune cells. The results showed that the anti-tumour effect of pAbM depends largely on monocytic MDSCs. The pAbM may inhibit tumour growth in at least two ways, one was to increase the iNOS production and inhibit the Arg1 production of Gr-1+ CD11b+ monocyte MDSCs (Fig.3c), the other was to inhibit the conversion from CD4+ CD25− T cells to CD4+ Foxp3+ CD25+ regulatory T cells (Fig.5).
In fact, several other mechanisms have been implicated in MDSCs mediating suppression of T-cell function. Reactive oxygen species (ROS) are important factors that contribute to the suppressive activity of MDSCs. Increasing production of ROS is one of the main characteristics of MDSCs from both tumour-bearing mice and patients with cancer. Inhibition of ROS production by MDSCs isolated from tumour-bearing mice and patients with cancer can abrogate the suppressive effect of these cells in vitro. It has also emerged that peroxynitrite is another crucial mediator of MDSC-mediated suppression of T-cell function.6 Whether PAbM could impair the suppressive function of MDSCs in other ways is still unclear. In addition, PAbM strongly suppresses the growth of various tumours in vitro and in vivo; for example, it acts as an enhancer to sensitize Dox-mediated tumour apoptosis by the inhibition of NF-κB activity.38
It was noted that pAbM-induced IL-12 production from peripheral blood mononuclear cells was not completely abrogated in TLR2–/– mice. Hence, it is possible that receptors other than TLR2 may also be stimulated by pAbM and may have contributed to IL-12 production in the knockout mice. It has been reported that C-type lectins, such as dectin-1 and dectin-2, are involved in recognition of some fungal pathogens.39,40 Whether PAbM could also activate these lectin receptors remains to be investigated.
Acknowledgments
This work was supported by the National Science Foundation of China (nos. 81373433), National Science Foundation of Hubei province (nos. 2012FFB01906), and Education Department of Hubei province for Young Talents Foundation (nos. Q20132605).
Disclosure
The authors have no conflicts of interest.
Statement
Manuscripts describing studies involving animals comply with China national guidelines governing the use of experimental animals and the procedures have been approved by the appropriate regulatory body.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Chemical structure of polysaccharide Agaricus blazei Murill.
Figure S2. Fluorescence-activated cell sorting plots showing the gating strategy for identification and isolation of monocytes myeloid-derived suppressor cells.
Figure S3. Gr-1+ CD11b+ monocytes (5 × 105 cells) were untreated and treated with polysaccharide Agaricus blazei Murill (100 µg/ml).
Figure S4. Expression of Toll-like receptor 2 (TLR2) in Gr-1+ CD11b+ monocytes, and in untreated (red line) and lipopolysaccharide (100 ng/ml) treated (green line) Gr-1+ CD11b+ monocytes.
References
- Chen W, Ma T, Shen XN, Xia XF, Xu GD, Bai XL, Liang TB. Macrophage-induced tumor angiogenesis is regulated by the TSC2-mTOR pathway. Cancer Res. 2012;72:1363–72. doi: 10.1158/0008-5472.CAN-11-2684. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Liu YY, Sun LC, Wei JJ, et al. Tumor cell-released TLR4 ligands stimulate Gr-1+ CD11b+ F4/80+ cells to induce apoptosis of activated T cells. J Immunol. 2010;185:2773–82. doi: 10.4049/jimmunol.1000772. [DOI] [PubMed] [Google Scholar]
- Talmadge JE. Pathways mediating the expansion and immunosuppressive activity of myeloid-derived suppressor cells and their relevance to cancer therapy. Clin Cancer Res. 2007;13:5243–8. doi: 10.1158/1078-0432.CCR-07-0182. [DOI] [PubMed] [Google Scholar]
- Shojaei F, Wu X, Malik AK, et al. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+ Gr1+ myeloid cells. Nat Biotechnol. 2007;25:911–20. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Zhou H, Krueger J, et al. Targeting tumor-associated macrophages as a novel strategy against breast cancer. J Clin Investig. 2006;116:2132–41. doi: 10.1172/JCI27648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh H, Wang D, Daikoku T, Sun H, Dey SK, Dubois RN. CXCR2-expressing myeloid-derived suppressor cells are essential to promote colitis-associated tumorigenesis. Cancer Cell. 2013;24:631–44. doi: 10.1016/j.ccr.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko JS, Zea AH, Rini BI, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15:2148–57. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer immunology, immunotherapy: CII. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Kapanadze T, Gamrekelashvili J, Manns MP, Korangy F, Greten TF. Anti-Gr-1 antibody depletion fails to eliminate hepatic myeloid-derived suppressor cells in tumor-bearing mice. J Leukoc Biol. 2012;92:1199–206. doi: 10.1189/jlb.0212059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemura N, Saio M, Suwa T, et al. Tumor-infiltrating myeloid-derived suppressor cells are pleiotropic-inflamed monocytes/macrophages that bear M1- and M2-type characteristics. J Leukoc Biol. 2008;83:1136–44. doi: 10.1189/jlb.0907611. [DOI] [PubMed] [Google Scholar]
- Wang YC, He F, Feng F, et al. Notch signaling determines the M1 versus M2 polarization of macrophages in antitumor immune responses. Cancer Res. 2010;70:4840–9. doi: 10.1158/0008-5472.CAN-10-0269. [DOI] [PubMed] [Google Scholar]
- Ma G, Pan PY, Eisenstein S, Divino CM, Lowell CA, Takai T, Chen SH. Paired immunoglobin-like receptor-B regulates the suppressive function and fate of myeloid-derived suppressor cells. Immunity. 2011;34:385–95. doi: 10.1016/j.immuni.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzaga ML, Bezerra DP, Alves AP, et al. In vivo growth-inhibition of Sarcoma 180 by an α-(1–>4)-glucan-β-(1–>6)-glucan-protein complex polysaccharide obtained from Agaricus blazei Murill. J Nat Med. 2009;63:32–40. doi: 10.1007/s11418-008-0286-4. [DOI] [PubMed] [Google Scholar]
- Tang NY, Yang JS, Lin JP, et al. Effects of Agaricus blazei Murill extract on immune responses in normal BALB/c mice. In vivo. 2009;23:761–6. [PubMed] [Google Scholar]
- Mizuno M, Morimoto M, Minato K, Tsuchida H. Polysaccharides from Agaricus blazei stimulate lymphocyte T-cell subsets in mice. Biosci Biotechnol Biochem. 1998;62:434–7. doi: 10.1271/bbb.62.434. [DOI] [PubMed] [Google Scholar]
- Yuminamochi E, Koike T, Takeda K, Horiuchi I, Okumura K. Interleukin-12- and interferon-γ-mediated natural killer cell activation by Agaricus blazei Murill. Immunology. 2007;121:197–206. doi: 10.1111/j.1365-2567.2006.02558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole C, Thomas S, Filak H, Henson PM, Lenz LL. Nitric oxide increases susceptibility of Toll-like receptor-activated macrophages to spreading Listeria monocytogenes. Immunity. 2012;36:807–20. doi: 10.1016/j.immuni.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepanski MJ, Czystowska M, Szajnik M, et al. Triggering of Toll-like receptor 4 expressed on human head and neck squamous cell carcinoma promotes tumor development and protects the tumor from immune attack. Cancer Res. 2009;69:3105–13. doi: 10.1158/0008-5472.CAN-08-3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laresgoiti-Servitje E. A leading role for the immune system in the pathophysiology of preeclampsia. J Leukoc Biol. 2013;94:247–57. doi: 10.1189/jlb.1112603. [DOI] [PubMed] [Google Scholar]
- Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, Divino CM, Chen SH. Gr-1+ CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–31. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P, Van Ginderachter JA. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111:4233–44. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- Knutson KL, Lu H, Stone B, et al. Immunoediting of cancers may lead to epithelial to mesenchymal transition. J Immunol. 2006;177:1526–33. doi: 10.4049/jimmunol.177.3.1526. [DOI] [PubMed] [Google Scholar]
- Wang CL, Lu CY, Pi CC, Zhuang YJ, Chu CL, Liu WH, Chen CJ. Extracellular polysaccharides produced by Ganoderma formosanum stimulate macrophage activation via multiple pattern-recognition receptors. BMC Complement Altern Med. 2012;12:119. doi: 10.1186/1472-6882-12-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondadi PK, Pevez J, Hanninen ML, Rossi M. Sialylation of Helicobacter bizzozeronii lipopolysaccharides modulates Toll-like receptor (TLR) 2 mediated response. Vet Res. 2015;21:4. doi: 10.1186/s13567-014-0133-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoglmeier C, Bauer H, Norenberg D, et al. CpG blocks immunosuppression by myeloid-derived suppressor cells in tumor-bearing mice. Clin Cancer Res. 2011;17:1765–75. doi: 10.1158/1078-0432.CCR-10-2672. [DOI] [PubMed] [Google Scholar]
- Kusmartsev S, Gabrilovich DI. Immature myeloid cells and cancer associated immune suppression. Cancer Immunol Immunother. 2002;51:293–8. doi: 10.1007/s00262-002-0280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea MG, Gow NA, Munro CA, et al. Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J Clin Investig. 2006;116:1642–50. doi: 10.1172/JCI27114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delano MJ, Scumpia PO, Weinstein JS, et al. MyD88-dependent expansion of an immature GR-1+ CD11b+ population induces T cell suppression and Th2 polarization in sepsis. J Exp Med. 2007;204:1463–74. doi: 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko HJ, Kim YJ, Kim YS, Chang WS, Ko SY, Chang SY, Sakaguchi S, Kang CY. A combination of chemoimmunotherapies can efficiently break self-tolerance and induce antitumor immunity in a tolerogenic murine tumor model. Cancer Res. 2007;67:7477–86. doi: 10.1158/0008-5472.CAN-06-4639. [DOI] [PubMed] [Google Scholar]
- Nefedova Y, Fishman M, Sherman S, Wang X, Beg AA, Gabrilovich DI. Mechanism of all-trans retinoic acid effect on tumor-associated myeloid-derived suppressor cells. Cancer Res. 2007;67:11021–8. doi: 10.1158/0008-5472.CAN-07-2593. [DOI] [PubMed] [Google Scholar]
- Arpaia N, Barton GM. Toll-like receptors: key players in antiviral immunity. Curr Opin Virol. 2011;1:447–54. doi: 10.1016/j.coviro.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai CC, Lin CR, Tsai HY, et al. The immunologically active oligosaccharides isolated from wheatgrass modulate monocytes via Toll-like receptor-2 signaling. J Biol Chem. 2013;288:17689–97. doi: 10.1074/jbc.M112.448381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelsadik A, Trad A. Toll-like receptors on the fork roads between innate and adaptive immunity. Hum Immunol. 2011;72:1188–93. doi: 10.1016/j.humimm.2011.08.015. [DOI] [PubMed] [Google Scholar]
- Komai-Koma M, Jones L, Ogg GS, Xu D, Liew FY. TLR2 is expressed on activated T cells as a costimulatory receptor. Proc Natl Acad Sci USA. 2004;101:3029–34. doi: 10.1073/pnas.0400171101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asprodites N, Zheng L, Geng D, Velasco-Gonzalez C, Sanchez-Perez L, Davila E. Engagement of Toll-like receptor-2 on cytotoxic T-lymphocytes occurs in vivo and augments antitumor activity. FASEB J. 2008;22:3628–37. doi: 10.1096/fj.08-108274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Hong EK. Agaricus blazei Murill enhances doxorubicin induced apoptosis in human hepatocellular carcinoma cells by NF-κB mediated increase of intracellular doxorubicin accumulation. Int J Oncol. 2011;38:401–8. doi: 10.3892/ijo.2010.852. [DOI] [PubMed] [Google Scholar]
- Saijo S, Fujikado N, Furuta T, et al. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat Immunol. 2007;8:39–46. doi: 10.1038/ni1425. [DOI] [PubMed] [Google Scholar]
- Saijo S, Ikeda S, Yamabe K, et al. Dectin-2 recognition of α-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity. 2010;32:681–91. doi: 10.1016/j.immuni.2010.05.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Chemical structure of polysaccharide Agaricus blazei Murill.
Figure S2. Fluorescence-activated cell sorting plots showing the gating strategy for identification and isolation of monocytes myeloid-derived suppressor cells.
Figure S3. Gr-1+ CD11b+ monocytes (5 × 105 cells) were untreated and treated with polysaccharide Agaricus blazei Murill (100 µg/ml).
Figure S4. Expression of Toll-like receptor 2 (TLR2) in Gr-1+ CD11b+ monocytes, and in untreated (red line) and lipopolysaccharide (100 ng/ml) treated (green line) Gr-1+ CD11b+ monocytes.