Abstract
Vitamin D deficiency is associated with increased incidence and severity of various immune-mediated diseases. Active vitamin D (1α,25-dihydroxyvitamin D3; 1,25(OH)2D3) up-regulates CD4+ T-cell expression of the purine ectonucleotidase CD39, a molecule that is associated with the generation of anti-inflammatory adenosine. Here we aimed to investigate the direct impact of 1,25(OH)2D3 on expression of the downstream ecto-5′-nucleotidase CD73 by human CD4 T cells, and components of the transforming growth factor-β (TGF-β) pathway, which have been implicated in the modulation of CD73 by murine T cells. At 10−8 to 10−7 m, 1,25(OH)2D3 significantly increased expression of CD73 on peripheral human CD4+ T cells. Although 1,25(OH)2D3 did not affect the mRNA expression of latent TGF-β1, 1,25(OH)2D3 did up-regulate expression of TGF-β-associated molecules [latency-associated peptide (LAP), glycophorin A repetitions predominant (GARP), GP96, neuropilin-1, thrombospondin-1 and αv integrin] which is likely to have contributed to the observed enhancement in TGF-β bioactivity. CD73 was highly co-expressed with LAP and GARP following 1,25(OH)2D3 treatment, but unexpectedly, each of these cell surface molecules was expressed primarily on CD4+ Foxp3– T cells, rather than CD4+ Foxp3+ T cells. Notably, neutralization of TGF-β significantly impaired 1,25(OH)2D3-mediated induction of CD73. Collectively, we show that 1,25(OH)2D3 enhances expression of CD73 on CD4+ Foxp3– T cells in a process that is at least partially TGF-β-dependent. These data reveal an additional contributing mechanism by which vitamin D may be protective in immune-mediated disease.
Keywords: 1α,25-dihydroxyvitamin D3; CD73; Foxp3; transforming growth factor-β
Introduction
Vitamin D insufficiency has been negatively associated with multiple immune-mediated diseases which is believed to be attributed to its numerous immunomodulatory properties (recently reviewed in refs 1,2). We and others have shown that the frequency of Foxp3+ regulatory T cells can be increased by either high concentrations (10−6 m) of the active form of vitamin D (1α,25-dihydroxyvitamin D3; 1,25(OH)2D3), or lower doses (10−7 to −8 m) in the presence of high T-cell stimulation.3,4 Between 10−7 and 10−8 m 1,25(OH)2D3 can also increase the frequency of a population of regulatory CD4+ IL-10+ Foxp3– T cells.3 Importantly these experimental data are supported by in vivo evidence whereby serum vitamin D status is positively associated with the frequency of Foxp3+ T cells in the periphery and airways, as well as levels of IL-10 in the airways.3,5–7 The downstream effects of 1,25(OH)2D3 are significantly influenced by the cytokine milieu, such that the combination of 1,25(OH)2D3 and transforming growth factor-β1 (TGF-β1) induces Foxp3 expression to a greater extent than either compound alone.8
Regulatory T cells employ various immunoregulatory mechanisms including the secretion of immunosuppressive cytokines such as interleukin-10 (IL-10) and TGF-β, as well as modulating dendritic cell behaviour and granzyme deployment.9 Of particular relevance here, cells can take up extracellular adenosine by two main mechanisms, either through specific adenosine transporters or through binding to one of four G-protein-coupled P1 purinergic receptors (A1, A2A, A2B and A3). Binding to the purinergic receptors can result in suppression of T-cell receptor signalling and induction of various immunomodulatory effects as reviewed elsewhere.10–12 In contrast, ATP is sensed by P2X and P2Y purinergic receptors and generally promotes a pro-inflammatory environment.13 The cell surface ecto-enzymes CD39 (ectonucleoside triphosphate dephosphorylase) and CD73 (ecto-5′-nucleotidase) regulate levels of ATP and adenosine, and therefore inflammation, by dephosphorylating ATP into ADP and AMP (CD39), and then into adenosine (CD73).10,11 We have previously shown that 1,25(OH)2D3 can modulate this pathway by increasing expression of CD39 on peripheral blood human CD4+ T cells, which contributes to dampening IL-17A secretion.14
A multifaceted cytokine, TGF-β is involved in processes such as differentiation of T helper type 17 cells and Foxp3+ regulatory T cells, proliferation of CD4+ T cells and innate immune cell chemotaxis.15 Humans express three TGF-β isoforms (TGFβ1–3), all of which are synthesized in a latent form bound to latency-associated peptide (LAP) either alone or with latent TGF-β binding protein. Latent TGF-β can be expressed on the cell surface by the transmembrane orphan toll-like receptor glycophorin A repetitions predominant (GARP), which itself requires the chaperone heat-shock protein GP96 for correct conformational folding.16 The active form of TGF-β is only released following cleavage of LAP or conformational remodelling by a range of molecules including neuropilin-1, thrombospondin-1 and various αv integrins.17,18 The active moiety can then bind to a heterodimer of type I and II TGF-β receptors to mediate downstream effects.15,19
Cross-talk between the TGF-β and adenosine pathways is indicated by the fact that treating mouse CD4+ T cells with TGF-β increases expression of CD73, and in CD73 knockout mice, experimental induction of TGF-β mRNA was blocked.20,21 Furthermore, the ability of CD4+ CD73+ T cells to delay the development of diabetes in a mouse model was impaired by anti-TGF-β, indicating a key protective role for this cytokine.22
1,25(OH)2D3 is known to enhance the frequency of human CD4+ CD39+ T cells; however, whether it controls the expression of the related ecto-5′-nucleotidase CD73 is unknown. We further investigated the role of TGF-β in this process given existing evidence for cross-talk between the purine and TGF-β pathways in mice.
Materials and methods
Cell isolation and culture
Ethical approval was granted by Guy's Hospital Ethics Committee (09/H0804/77) and full written informed consent was obtained from all donors. CD4+ cells were isolated from peripheral venous blood as previously described using Dynabeads (Invitrogen, Paisley, UK).3 Then, 1 × 106 cells/ml were cultured in RPMI-1640 containing 10% fetal calf serum, 2 mm l-glutamine and 50 μg/ml gentamycin, and stimulated with plate-bound anti-CD3 (1 μg/ml; OKT-3) and 50 IU/ml recombinant human IL-2 (Eurocetus, Harefield, UK) in the presence or absence of 1,25(OH)2D3 (BIOMOL Research Labs, Exeter, UK). For cultures going beyond 7 days, cells were counted and re-stimulated at 1 × 106 cells/ml on day 7 with fresh medium containing drugs. Where relevant, anti-TGF-β1–3 or an isotype control (5 μg/ml; R&D Systems, Abingdon, UK) was added to the cultures on days 0 and 7. Viability did not significantly differ between culture conditions (data not shown).
Quantitative RT-PCR
RNA was extracted from cell pellets using the RNeasy Mini kit (Qiagen, Crawley, UK) according to the manufacturer's instructions and quantified using a Nanodrop ND-1000 spectrophotometer (ThermoScientific, Wilmington, NC). RNA (250 ng) was reverse transcribed into cDNA then quantitative RT-PCR was performed in triplicate using an Applied Biosystems 7900 HT system and FAM labelled assay-on-Demand reagent sets (cd73 – Hs00159686_m1; tgfβ1 – Hs00998133_m1; lrrc32 – Hs00194136_m1; gp96 – Hs00427665_g1; nrp-1 – Hs00826128_m; tsp-1 – Hs00962908_m1; αv integrin – Hs00233808_m1). Quantitative RT-PCR were multiplexed using VIC-labelled 18s probe (Hs99999901_s1) as an endogenous control and analysed using SDS software version 2·1 (Applied Biosystems, Foster City, CA) in accordance with the 2−(ΔΔCt) method.
Flow cytometry
The following antibodies were used for cell surface phenotyping – CD73 (AD2; eBiosciences, Hatfield, UK), GARP and LAP (7B11 and TW4-2F8, respectively; Biolegend, London, UK). Where relevant, cells were further stained for intranuclear Foxp3 (PCH101; eBiosciences) using the eBiosciences Foxp3 staining kit as per the manufacturer's instructions. Unstained cells, isotype controls, single cell stains and fluorescence minus one controls were employed. Dead cells (7-aminoactinomycin D positive; Sigma-Aldrich, Gillingham, UK) were gated out.
ATP assay
After 7 days of culture alone or in the presence of 10−7 m 1,25(OH)2D3, 25,000 CD4+ T cells were seeded in 100 μl culture medium with 500 μm ATP. Consumption of ATP was assessed 90 minutes later using a CellTiter-Glo Luminescent Cell Viability Assay (Promega, Southampton, UK) in accordance with the manufacturer's instructions. A standard curve was generated immediately before running the assay by dissolving ATP disodium salt hydrate (Sigma-Aldrich) in cell culture medium (100 pm to 100 mm).
Suppression assay
CD4+ T cells were cultured with 10−7 m 1,25(OH)2D3 for 7 days as described, then live cells (7-aminoactinomycin-D-negative) were sorted based on CD73 expression using a BD FACSAria (BD Biosciences, Oxford, UK). CD73– or CD73+ cells were subsequently cultured at a ratio of 0·25: 1 with freshly isolated autologous CellTrace violet-labelled CD4+ T cells for a further 7 days. Cell proliferation was assessed by the loss in fluorescence intensity of CellTrace Violet on an NxT attune (Life Technologies, Paisley, UK).
TGF-β bioassay
Bioactivity of TGF-β was assessed using mink lung epithelial cells transfected with a construct containing the 5′ end of the human plasminogen activator inhibitor-1 gene fused to the firefly luciferase reporter gene (kindly donated by Prof. Daniel B Rifkin; New York University).23 Then, 3 × 105 mink lung epithelial cells/ml were incubated for 14 hr at 37°C in 5% CO2 with cell culture supernatants. Culture media were aspirated, cells were lysed and then luciferase activity (which corresponds to TGF-β activity) was determined using a Biotium Firefly Luciferase Assay Kit (Promega, Southampton, UK) in accordance with the manufacturer's instructions. Serum-free RPMI-1640 was used for these experiments due to high levels of TGF-β found in fetal calf serum.
Data analysis
Flow cytometry data were analysed using FlowJo (Treestar Inc., Ashland, OR; version 10) and cumulative data analysis was performed in Graphpad Prism version 6·00 for Windows (Graphpad Software Inc., San Diego, USA). Data were assessed for Gaussian distribution and the appropriate statistical test was then performed as described in the figure legends. Data are shown as mean ± standard error of mean.
Results
1,25(OH)2D3 up-regulates CD4+ T-cell expression of CD73
It has previously been shown that human CD4+ T cells cultured in the presence of 10−7 m 1,25(OH)2D3 express elevated levels of CD39.14 Here this observation is extended to reveal that expression of downstream ecto-5′-nucleotidase CD73 is also significantly up-regulated at the gene (Fig. 1a) and protein level (% CD73+ cells and mean fluorescence intensity; Fig. 1b and c) following 7 or 14 days culture with 1,25(OH)2D3. Furthermore, a trend towards lower ATP levels 90 min after spiking cells with 500 μm ATP was observed in 1,25(OH)2D3-treated CD4+ T cells (P = 0·06; see Supplementary material, Fig. S1A). CD73+ cells were also shown to be regulatory because they suppressed the proliferation of autologous CellTrace Violet-labelled CD4+ T cells (ratio of 0·25: 1) to a greater extent than CD73– cells (lower percentage of cells divided (P < 0·05) and division index (P = 0·09); see Supplementary material, Figure S1B,C).
Figure 1.
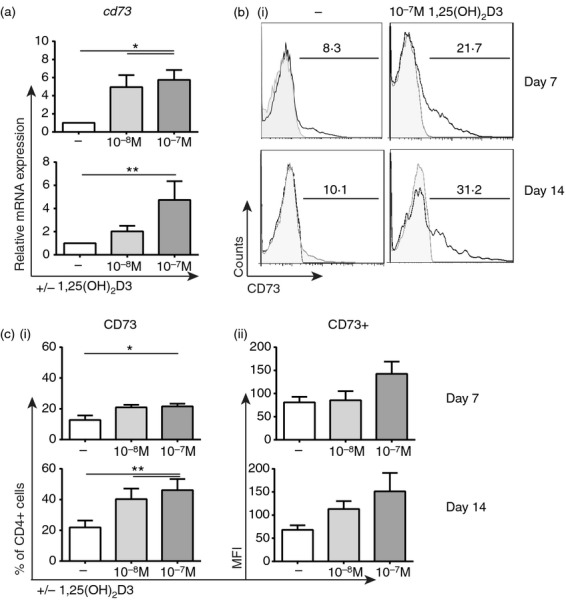
1α,25-dihydroxyvitamin D3 (1,25(OH)2D3) up-regulates CD73 expression on CD4+ T cells. CD4+ T cells stimulated with anti-CD3 and interleukin-2 (IL-2) for 7 or 14 days alone (−) or in the presence of the indicated concentration of 1,25(OH)2D3 (10−x m). (a) Relative gene expression of cd73 as determined by quantitative RT-PCR at days 7 (n = 8) and 14 (n = 6). (b) Representative histograms of CD73 expression (bold line) compared with isotype control (grey shaded). (c) Cumulative data of the percentage of CD4+ CD73+ T cells (c i) and CD73 mean fluorescence intensity (c ii) at days 7 (top; n = 8) and 14 (bottom, n = 6). Data assessed by repeated measures one-way analysis of variance with Tukey's multiple comparison test. *P ≤ 0·05; **P ≤ 0·01.
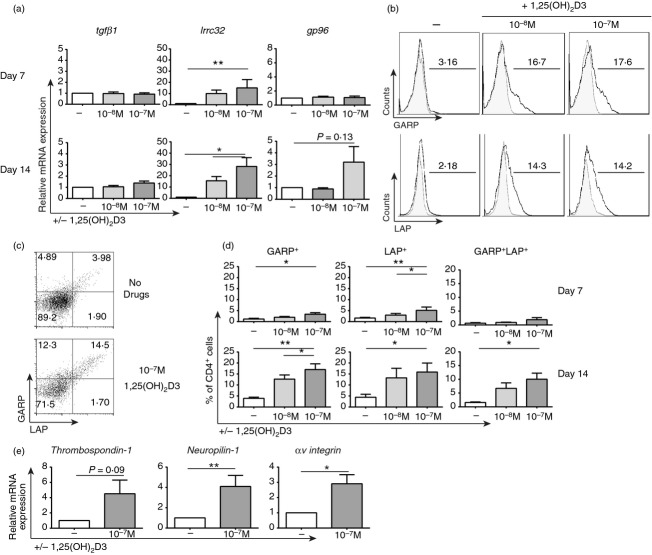
1,25(OH)2D3 up-regulates expression of TGFβ-associated molecules
To investigate whether enhancement of CD73 expression was TGF-β dependent, we first assessed the impact of 1,25(OH)2D3 on components of the TGF-β pathway. After 7 and 14 days in culture alone or in the presence of the indicated concentration of 1,25(OH)2D3, CD4+ T-cell expression of latent tgfβ2 and tgfβ3 was typically undetectable by quantitative RT-PCR (data not shown). Although latent tgfβ1 was highly expressed at the mRNA level, its expression was not modulated by 1,25(OH)2D3 treatment (Fig. 2a). In contrast, lrrc32 (gene for GARP) mRNA was significantly up-regulated by 1,25(OH)2D3 with a trend (P = 0·13) towards increased expression of gp96, a chaperone for GARP, by day 14. At the protein level, 1,25(OH)2D3 treatment significantly increased overall expression of GARP and LAP as well as the percentage of cells co-expressing both of these molecules (Fig. 2b–d).
Figure 2.
1α,25-dihydroxyvitamin D3 (1,25(OH)2D3) up-regulates expression of transforming growth factor-β (TGF-β) -associated molecules. CD4+ T cells stimulated (anti-CD3/IL-2) for 7 or 14 days alone (−) or in the presence of the indicated concentration of 1,25(OH)2D3 (10−x m). (a) Relative gene expression of tgfβ1, lrrc32 and gp96 at days 7 (top) and 14 (bottom) as determined by quantitative RT-PCR [n = 7; repeated measures one-way analysis of variance (anova) with Tukey's multiple comparison test]. Representative histograms (b) and dot plots (c) of glycophorin A repetitions predominant (GARP) and latency-associated peptide (LAP) expression at day 14. (d) Cumulative data showing the frequency of GARP+ (left), LAP+ (middle) and LAP+GARP+ (right) CD4+ T cells at day 7 (top) and day 14 (bottom) (n = 7; repeated measures one-way anova with Tukey's multiple comparison test). (e) Relative gene expression of thrombospondin-1, neuropilin-1 and αv integrin at day 14 as determined by quantitative RT-PCR (n = 8; paired t-test). *P ≤ 0·05; **P ≤ 0·01.
In order to generate active TGF-β, LAP must be cleaved. Within the immune cell compartment, this is reported to be primarily carried out by the cell surface expressed molecules thrombospondin-1, neuropilin-1 and αv integrin,17,18 each of which were also up-regulated by 1,25(OH)2D3 treatment at the mRNA level, most notably after 14 days in culture (P = 0·09, P < 0·05 and P < 0·05, respectively; Fig. 2e, and see Supplementary material, Fig. S2).
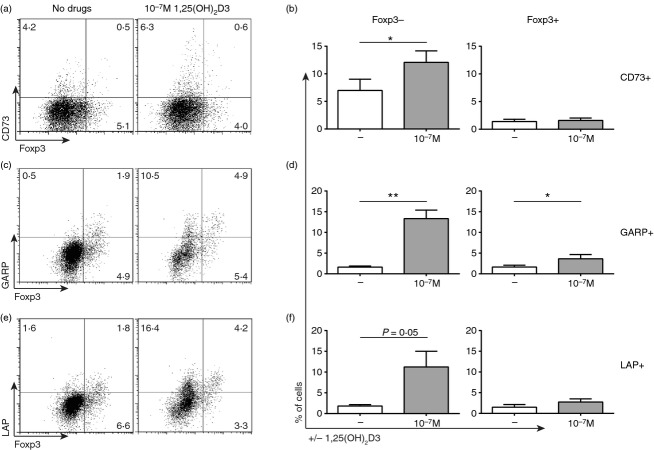
1,25(OH)2D3-mediated up-regulation of CD73, LAP and GARP occurs primarily on CD4+ Foxp3– T cells
Foxp3 is conventionally seen as the lineage defining transcription factor of natural and induced regulatory T cells, but Foxp3– suppressor cells do exist.24 As the purinergic and TGF-β pathways have been linked to the function of regulatory T cells,9 the interaction between intranuclear Foxp3 expression and surface expression of CD73, LAP and GARP was assessed. In agreement with previous findings, no significant increase in Foxp3 expression was observed in the presence of 10−7 m 1,25(OH)2D3 (Fig. 3).3 Furthermore, 1,25(OH)2D3-mediated up-regulation of CD73 (Fig. 3a, b), GARP (Fig. 3c, d) and LAP (Fig. 3e, f) was principally seen on CD4+ Foxp3– T cells as opposed to CD4+ Foxp3+ cells.
Figure 3.
1α,25-dihydroxyvitamin D3 (1,25(OH)2D3)-mediated up-regulation of CD73, latency-associated peptide (LAP) and glycophorin A repetitions predominant (GARP) occurs primarily on Foxp3– CD4+ T cells. CD4+ T cells stimulated [anti-CD3/interleukin-2 (IL-2)] alone (−) or in the presence of 10−7 m 1,25(OH)2D3 for 14 days. Surface staining for CD73, GARP and LAP was performed before intranuclear Foxp3 staining. Representative dot plots showing expression of Foxp3 and CD73 (a), GARP (c) or LAP (e). Cumulative data of the percentage of cells expressing CD73 (b; n = 6), GARP (d; n = 6) or LAP (f; n = 6) on either Foxp3– or Foxp3+ CD4+ T cells. Data assessed by paired t-test. *P ≤ 0·05; **P ≤ 0·01.
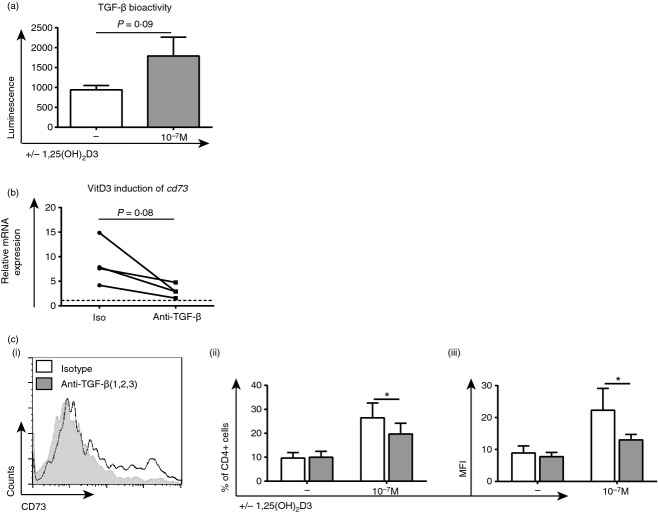
Cross-talk between CD73 and TGF-β in 1,25(OH)2D3-treated CD4+ T cells
Mouse studies have shown that cross-talk can occur between the adenosine and TGF-β pathways,20,22 but whether this is true in humans and any impact of 1,25(OH)2D3 remains to be ascertained. Indeed, cell surface staining showed a significant increase in the percentage of cells co-expressing CD73 with TGF-β-associated molecules LAP, GARP and neuropilin -1 following 1,25(OH)2D3 treatment (Fig. 4).
Figure 4.
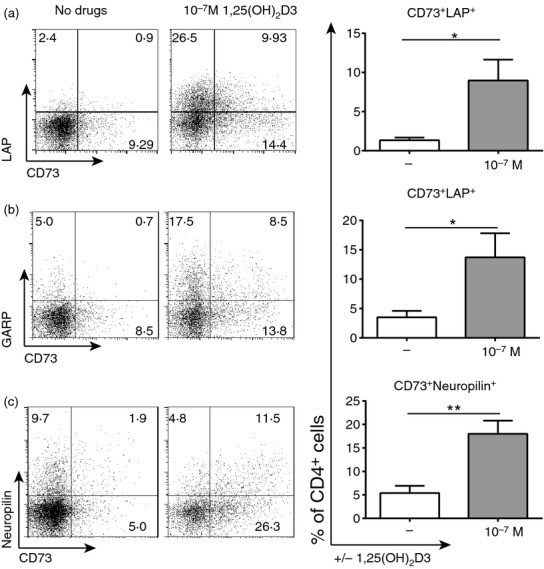
CD73 is co-expressed with latency-associated peptide (LAP) and glycophorin A repetitions predominant (GARP) following 1α,25-dihydroxyvitamin D3 (1,25(OH)2D3) treatment. CD4+ T cells stimulated [anti-CD3/interleukin-2 (IL-2)] alone (−) or in the presence of 10−7 m 1,25(OH)2D3 for 14 days. Shown are representative dot plots and cumulative data of the percentage of cells co-expressing CD73 with LAP (a; n = 7), GARP (b; n = 6) or neuropilin-1 (c; n = 5). Data assessed by paired t-test. *P ≤ 0·05; **P ≤ 0·01.
Furthermore, TGF-β bioactivity was elevated in supernatants harvested after 7 days of culture with 1,25(OH)2D3 (P = 0·09), as determined using mink lung epithelial cells and a luciferase assay (Fig. 5a). These observations were extended by culturing CD4+ T cells in the presence of a neutralizing TGF-β antibody or the relevant isotype control. Anti-TGF-β impaired the 1,25(OH)2D3-mediated induction of CD73 at the mRNA level (P = 0·08; Fig. 5a) and in both the percentage of CD4+ CD73+ T cells and the mean fluorescence intensity of CD73 (P < 0·05; Fig. 5b).
Figure 5.
1α,25-dihydroxyvitamin D3 (1,25(OH)2D3)-mediated up-regulation of CD73 is partially inhibited by anti-transforming growth factor-β (TGF-β). CD4+ T cells stimulated [anti-CD3/interleukin-2 (IL-2)] alone (−) or in the presence of 10−7 m 1,25(OH)2D3. (a) Day 7 supernatants were harvested and TGF-β bioactivity was assessed using transfected mink lung epithelial cells and a luminescence assay; levels of luminescence were proportional to TGF-β bioactivity (n = 7; paired t-test). (b, c) Cells were cultured with anti-TGF-β (grey) or an isotype control for 14 days. (b) 1,25(OH)2D3-mediated induction of cd73 gene expression as determined by quantitative RT-PCR (n = 4; two-tailed paired Wilcoxon test). (c i) representative histograms of CD73 expression and cumulative data of the percentage of CD4+ CD73+ T cells (c ii) and CD73 mean fluorescence intensity (c iii) (n = 5; data assessed by two-way analysis of variance with Sidak's multiple comparison test). *P ≤ 0·05.
Discussion
The frequency of regulatory T-cell populations is known to be enhanced by vitamin D both in vitro and in vivo. However, how these cells suppress in different environments and what markers identify them is not clear.3,4 The focus of this work was to assess the immunomodulatory effects of 1,25(OH)2D3 on the induction of two complex immunoregulatory molecules, CD73 and TGF-β.
In the present study a significant up-regulation of CD73 expression in CD4+ T cells by 1,25(OH)2D3 was observed, complimenting the previously published finding that 1,25(OH)2D3 enhances surface expression of CD39.14 Heightened expression of these two ecto-nucleotidases most likely contributes to the observed faster consumption of ATP by 1,25(OH)2D3-treated cells (Fig. S1A) and to the enhanced generation of downstream adenosine.25 Studies have shown that CD73 is critical in mediating many immunosuppressive properties of cells in mice and man26,27 and that adenosine can impair T-cell proliferation in humans.28,29 The latter data correlate with the anti-proliferative properties of CD73+ cells that are reported both here and elsewhere.30 As well as the traditional CD39 and CD73 pathway for ATP catabolism, a CD39-independent pathway involving CD38 and CD73 has been documented in human T cells.31 As CD38 is known to be highly up-regulated on T cells in response to 1,25(OH)2D3,3,32 we hypothesize that 1,25(OH)2D3 is further enhancing ATP breakdown and adenosine generation by acting via the CD39-dependent and -independent pathways.
Since 10−7 m 1,25(OH)2D3 has previously been shown to increase the frequency of CD4+ Foxp3+ cells in the presence of TGF-β,8 the relationship between 1,25(OH)2D3 and TGF-β was investigated further. 1,25(OH)2D3 increased expression of LAP (component of latent TGF-β), GARP (latent TGF-β receptor) and GP96 (latent TGF-β chaperone), which together result in cells that have an enhanced capacity to both generate and present latent TGF-β. These data are in line with a study in patients with multiple sclerosis in whom vitamin D supplementation resulted in increased levels of LAP in the serum relative to those in the placebo group.33 Although a whole host of molecules and environmental conditions such as heat and extreme pH have been implicated in activating TGF-β by cleaving LAP, αv integrins, neuropilin-1 and thrombospondin-1 are the primary mediators within the immune cell compartment;18 here expression of each of these molecules, at least at the mRNA level, was shown to be higher in 1,25(OH)2D3-treated cells, which most likely contributed to the enhanced TGF-β bioactivity observed.
In addition to its anti-inflammatory role, TGF-β is involved in tissue remodelling and therefore can have potentially negative consequences in diseases such as asthma.34 However, 1,25(OH)2D3 has also been shown to inhibit the proliferation of airway smooth muscle cells.35 Moreover, in utero vitamin D deficiency in mice leads to increased airway smooth muscle mass and airway resistance36 and in children with severe asthma, lower levels of vitamin D were associated with increased airway smooth muscle mass.37,38 As with many in vitro studies, the data presented here are observational. Actual levels of molecules as well as their expression relative to other mediators in vivo are critical; for example, lung remodelling in mice has been shown to result from an imbalance between TGF-β1 and bone morphogenic protein 7 (BMP-7).39 Whether TGF-β acts in a regulatory or tissue remodelling manner in various disease contexts is most likely dependent on the cytokine milieu as a whole and so warrants studying in vivo.
A major limitation in the study of regulatory T cells is a lack of specific cell surface markers, particularly in humans.40,41 Foxp3 is a widely accepted marker of many regulatory T-cell populations, but is intracellular so cells must be permeabilized in order to perform staining, which limits the isolation of pure and live cells. Since CD73, GARP and LAP are putative markers of regulatory T cells,42–44 their relationship with Foxp3 was examined in our culture conditions. CD73 was expressed primarily on CD4+ Foxp3– T cells and 1,25(OH)2D3-mediated induction of CD73, GARP and LAP was only clearly evident on CD4+ Foxp3– T cells. In support of this, Foxp3 expression has been found to be independent of GARP expression in cells from HIV-positive patients.45 Although not absolute markers, these data support the idea that CD73, GARP and LAP help to identify Foxp3– regulatory cells.
Previous studies have shown that cross-talk between the TGF-β and adenosine pathways exists in mice,20–22 and here we confirm this in humans too. A significant proportion of CD4+ T cells co-express CD73 with GARP, LAP and neuropilin-1 following 1,25(OH)2D3 treatment and by impairing TGF-β activity, the induction of CD73 by 1,25(OH)2D3 was reduced.
A high concentration of 1,25(OH)2D3 (10−8/−7 m/10–100 nm) was used throughout this work for optimal in vitro responses, although trends were seen at 10−9 m (data not shown). Previous in vitro studies have shown that monocyte-derived DCs, alveolar macrophages and bronchial epithelial cells express the enzyme 25-hydroxyvitamin D3-1α-hydroxylase and can consequently produce up to 5 × 10−9 m functional 1,25(OH)2D3 from 25(OH)D.46–49 Although the levels of 1,25(OH)2D3 produced depend upon the concentration of 25(OH)D,50 there is evidence that systemic levels of 1,25(OH)2D3 may not reflect an individual's vitamin D status;51 these findings highlight the importance of local conversion of 25(OH)D within immune tissues producing currently undefined levels of 1,25(OH)2D3.
In summary, we show that 1,25(OH)2D3 can enhance expression of CD73 on CD4+ Foxp3– T cells in part by up-regulating autocrine TGF-β bioactivity. These data help to further elucidate the mechanisms by which vitamin D may skew the immune system towards a more regulatory phenotype and consequently protect against various immune-mediated diseases. Further work to probe the functional consequences of these observations is warranted both in vitro and in vivo.
Acknowledgments
EHM, ESC and CHM designed the study and wrote the manuscript. EHM, ESC, YHC and DFR performed experiments. EHM is supported by an MRC and Asthma UK PhD Studentship. ESC was funded by a MRC British Thoracic Society/Morriston Davies Trust Capacity Building PhD Studentship and an MRC Centenary Fellowship. The authors would like to acknowledge the much appreciated purine expertise from Lynette Fairbanks and the generous donation of mink lung epithelial cells from Professor Daniel B Rifkin. The research was supported by the National Institute for Health Research (NIHR) Clinical Research Facility at Guy's & St Thomas’ NHS Foundation Trust and NIHR Biomedical Research Centre based at Guy's & St Thomas’ NHS Foundation Trust and King's College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department for Health.
Glossary
- 1,25(OH)2D3
1α,25-dihydroxyvitamin D3
Disclosures
The authors declare no competing financial interests.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. CD4+ CD73+ T cells suppress the proliferation of autologous CD4+ T cells to a greater extent than CD4+ CD73– T cells.
Figure S2. The effect of 1α,25-dihydroxyvitamin D3 (1,25(OH)2D3) treatment on the mRNA expression of transforming growth factor-β-associated molecules.
References
- Mann EH, Chambers ES, Pfeffer PE, Hawrylowicz CM. Immunoregulatory mechanisms of vitamin D relevant to respiratory health and asthma. Ann N Y Acad Sci. 2014;1317:57–69. doi: 10.1111/nyas.12410. [DOI] [PubMed] [Google Scholar]
- Cantorna MT, Snyder L, Lin YD, Yang L. Vitamin D and 1,25(OH)2D regulation of T cells. Nutrients. 2015;7:3011–21. doi: 10.3390/nu7043011. , Switzerland. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry Z, Chambers ES, Xystrakis E, et al. The role of 1α,25-dihydroxyvitamin D3 and cytokines in the promotion of distinct Foxp3+ and IL-10+ CD4+ T cells. Eur J Immunol. 2012;42:2697–708. doi: 10.1002/eji.201242370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery LE, Burke F, Mura M, et al. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J Immunol. 2009;183:5458–67. doi: 10.4049/jimmunol.0803217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry Z, Xystrakis E, Richards DF, et al. Ligation of TLR9 induced on human IL-10-secreting Tregs by 1α,25-dihydroxyvitamin D3 abrogates regulatory function. J Clin Invest. 2009;119:387–98. doi: 10.1172/JCI32354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers ES, Nanzer AM, Richards DF, et al. Serum 25-dihydroxyvitamin D levels correlate with CD4+Foxp3+ T-cell numbers in moderate/severe asthma. J Allergy Clin Immunol. 2012;130:542–4. doi: 10.1016/j.jaci.2012.04.022. [DOI] [PubMed] [Google Scholar]
- Gupta A, Dimeloe S, Richards DF, et al. Defective IL-10 expression and in vitro steroid-induced IL-17A in paediatric severe therapy-resistant asthma. Thorax. 2013;69:508–15. doi: 10.1136/thoraxjnl-2013-203421. [DOI] [PubMed] [Google Scholar]
- Chambers ES, Suwannasaen D, Mann EH, Urry Z, Richards DF, Lertmemongkolchai G, Hawrylowicz CM. 1α,25-dihydroxyvitamin D3 in combination with transforming growth factor-β increases the frequency of Foxp3+ regulatory T cells through preferential expansion and usage of interleukin-2. Immunology. 2014;143:52–60. doi: 10.1111/imm.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–32. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regateiro FS, Cobbold SP, Waldmann H. CD73 and adenosine generation in the creation of regulatory microenvironments. Clin Exp Immunol. 2013;171:1–7. doi: 10.1111/j.1365-2249.2012.04623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idzko M, Ferrari D, Eltzschig HK. Nucleotide signalling during inflammation. Nature. 2014;509:310–7. doi: 10.1038/nature13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta A, Sitkovsky M. Extracellular adenosine-mediated modulation of regulatory T cells. Front Immunol. 2014;5:304. doi: 10.3389/fimmu.2014.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bours MJ, Swennen EL, Di Virgilio F, Cronstein BN, Dagnelie PC. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther. 2006;112:358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Nanzer AM, Chambers ES, Ryanna K, et al. Enhanced production of IL-17A in patients with severe asthma is inhibited by 1α,25-dihydroxyvitamin D3 in a glucocorticoid-independent fashion. J Allergy Clin Immunol. 2013;132:297–304. doi: 10.1016/j.jaci.2013.03.037. [DOI] [PubMed] [Google Scholar]
- Tran DQ. TGF-β: the sword, the wand, and the shield of FOXP3+ regulatory T cells. J Mol Cell Biol. 2012;4:29–37. doi: 10.1093/jmcb/mjr033. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wu BX, Metelli A, et al. GP96 is a GARP chaperone and controls regulatory T cell functions. J Clin Invest. 2015;125:859–69. doi: 10.1172/JCI79014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger JS, Sheppard D. Cross talk among TGF-β signaling pathways, integrins, and the extracellular matrix. Cold Spring Harb Perspect Biol. 2011;3:a005017. doi: 10.1101/cshperspect.a005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovics JA, Araya J, Cambier S, et al. Interleukin-1β induces increased transcriptional activation of the transforming growth factor-β-activating integrin subunit β8 through altering chromatin architecture. J Biol Chem. 2011;286:36864–74. doi: 10.1074/jbc.M111.276790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirado-Rodriguez B, Ortega E, Segura-Medina P, Huerta-Yepez S. TGF-β: an important mediator of allergic disease and a molecule with dual activity in cancer development. J Immunol Res. 2014;2014:318481. doi: 10.1155/2014/318481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regateiro FS, Howie D, Nolan KF, Agorogiannis EI, Greaves DR, Cobbold SP, Waldmann H. Generation of anti-inflammatory adenosine by leukocytes is regulated by TGF-β. Eur J Immunol. 2011;41:2955–65. doi: 10.1002/eji.201141512. [DOI] [PubMed] [Google Scholar]
- Fernández P, Perez-Aso M, Smith G, et al. Extracellular generation of adenosine by the ectonucleotidases CD39 and CD73 promotes dermal fibrosis. Am J Pathol. 2013;183:1740–6. doi: 10.1016/j.ajpath.2013.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai N, Wong FS, Wen L. TLR9 deficiency promotes CD73 expression in T cells and diabetes protection in nonobese diabetic mice. J Immunol. 2013;191:2926–37. doi: 10.4049/jimmunol.1300547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe M, Harpel JG, Metz CN, Nunes I, Loskutoff DJ, Rifkin DB. An assay for transforming growth factor-β using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal Biochem. 1994;216:276–84. doi: 10.1006/abio.1994.1042. [DOI] [PubMed] [Google Scholar]
- Roncarolo MG, Gregori S, Bacchetta R, Battaglia M. Tr1 cells and the counter-regulation of immunity: natural mechanisms and therapeutic applications. Curr Top Microbiol Immunol. 2014;380:39–68. doi: 10.1007/978-3-662-43492-5_3. [DOI] [PubMed] [Google Scholar]
- Schuler PJ, Saze Z, Hong CS, et al. Human CD4+ CD39+ regulatory T cells produce adenosine upon co-expression of surface CD73 or contact with CD73+ exosomes or CD73+ cells. Clin Exp Immunol. 2014;177:531–43. doi: 10.1111/cei.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Thyagarajan K, Kesarwani P, et al. Reducing CD73 expression by IL1β-programmed Th17 cells improves immunotherapeutic control of tumors. Cancer Res. 2014;74:6048–59. doi: 10.1158/0008-5472.CAN-14-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth LA, Ratnasothy K, Tsang JY, Boardman D, Warley A, Lechler R, Lombardi G. CD73 expression on extracellular vesicles derived from CD4+ CD25+ Foxp3+ T cells contributes to their regulatory function. Eur J Immunol. 2013;43:2430–40. doi: 10.1002/eji.201242909. [DOI] [PubMed] [Google Scholar]
- Häusler SF, Del Barrio IM, Diessner J, Stein RG, Strohschein J, Hönig A, Dietl J, Wischhusen J. Anti-CD39 and anti-CD73 antibodies A1 and 7G2 improve targeted therapy in ovarian cancer by blocking adenosine-dependent immune evasion. Am J Transl Res. 2014;6:129–39. [PMC free article] [PubMed] [Google Scholar]
- Häusler SF, Montalbán del Barrio I, Strohschein J, et al. Ectonucleotidases CD39 and CD73 on OvCA cells are potent adenosine-generating enzymes responsible for adenosine receptor 2A-dependent suppression of T cell function and NK cell cytotoxicity. Cancer Immunol Immunother. 2011;60:1405–18. doi: 10.1007/s00262-011-1040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler PJ, Macatangay BJ, Saze Z, et al. CD4+CD73+ T cells are associated with lower T-cell activation and C reactive protein levels and are depleted in HIV-1 infection regardless of viral suppression. AIDS. 2013;27:1545–55. doi: 10.1097/QAD.0b013e328360c7f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horenstein AL, Chillemi A, Zaccarello G, Bruzzone S, Quarona V, Zito A, Serra S, Malavasi F. A CD38/CD203a/CD73 ectoenzymatic pathway independent of CD39 drives a novel adenosinergic loop in human T lymphocytes. Oncoimmunology. 2013;2:e26246. doi: 10.4161/onci.26246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckler JD, Stoeckler HA, Kouttab N, Maizel AL. 1α,25-Dihydroxyvitamin D3 modulates CD38 expression on human lymphocytes. J Immunol. 1996;157:4908–17. [PubMed] [Google Scholar]
- Åivo J, Hänninen A, Ilonen J, Soilu-Hänninen M. Vitamin D3 administration to MS patients leads to increased serum levels of latency activated peptide (LAP) of TGF-β. J Neuroimmunol. 2015;280:12–5. doi: 10.1016/j.jneuroim.2015.01.005. [DOI] [PubMed] [Google Scholar]
- Bottoms SE, Howell JE, Reinhardt AK, Evans IC, McAnulty RJ. Tgf-β isoform specific regulation of airway inflammation and remodelling in a murine model of asthma. PLoS ONE. 2010;5:e9674. doi: 10.1371/journal.pone.0009674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damera G, Fogle HW, Lim P, et al. Vitamin D inhibits growth of human airway smooth muscle cells through growth factor-induced phosphorylation of retinoblastoma protein and checkpoint kinase 1. Br J Pharmacol. 2009;158:1429–41. doi: 10.1111/j.1476-5381.2009.00428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foong RE, Bosco A, Jones AC, Gout A, Gorman S, Hart PH, Zosky GR. In utero vitamin D deficiency increases airway smooth muscle mass and impairs lung function. Am J Respir Cell Mol Biol. 2015 doi: 10.1165/rcmb.2014-0356OC. http://www.ncbi.nlm.nih.gov/pubmed/25867172 ) [DOI] [PubMed] [Google Scholar]
- Gupta A, Sjoukes A, Richards D, Banya W, Hawrylowicz C, Bush A, Saglani S. Relationship between serum vitamin D, disease severity, and airway remodeling in children with asthma. Am J Respir Crit Care Med. 2011;184:1342–9. doi: 10.1164/rccm.201107-1239OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berraies A, Hamzaoui K, Hamzaoui A. Link between vitamin D and airway remodeling. J Asthma Allergy. 2014;7:23–30. doi: 10.2147/JAA.S46944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumm CL, Halcsik E, Landgraf RG, Camara NO, Sogayar MC, Jancar S. Lung remodeling in a mouse model of asthma involves a balance between TGF-β1 and BMP-7. PLoS ONE. 2014;9:e95959. doi: 10.1371/journal.pone.0095959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Chen M, Liu Y, Guo Z, He X, Brand D, Zheng SG. Advances in distinguishing natural from induced Foxp3+ regulatory T cells. Int J Clin Exp Pathol. 2013;6:116–23. [PMC free article] [PubMed] [Google Scholar]
- Dhamne C, Chung Y, Alousi AM, Cooper LJ, Tran DQ. Peripheral and thymic foxp3+ regulatory T cells in search of origin, distinction, and function. Front Immunol. 2013;4:253. doi: 10.3389/fimmu.2013.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia M, Roncarolo MG. The Tregs’ world according to GARP. Eur J Immunol. 2009;39:3296–300. doi: 10.1002/eji.200940117. [DOI] [PubMed] [Google Scholar]
- Mandapathil M, Lang S, Gorelik E, Whiteside TL. Isolation of functional human regulatory T cells (Treg) from the peripheral blood based on the CD39 expression. J Immunol Methods. 2009;346:55–63. doi: 10.1016/j.jim.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam MS, Kurtz CC, Rowlett RM, et al. CD73 is expressed by human regulatory T helper cells and suppresses proinflammatory cytokine production and Helicobacter felis-induced gastritis in mice. J Infect Dis. 2009;199:494–504. doi: 10.1086/596205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Kozhaya L, Mercer F, Khaitan A, Fujii H, Unutmaz D. Expression of GARP selectively identifies activated human FOXP3+ regulatory T cells. Proc Natl Acad Sci U S A. 2009;106:13439–44. doi: 10.1073/pnas.0901965106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsche J, Mondal K, Ehrnsperger A, Andreesen R, Kreutz M. Regulation of 25-hydroxyvitamin D3-1α-hydroxylase and production of 1α,25-dihydroxyvitamin D3 by human dendritic cells. Blood. 2003;102:3314–6. doi: 10.1182/blood-2002-11-3521. [DOI] [PubMed] [Google Scholar]
- Sigmundsdottir H, Pan J, Debes GF, Alt C, Habtezion A, Soler D, Butcher EC. DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nat Immunol. 2007;8:285–93. doi: 10.1038/ni1433. [DOI] [PubMed] [Google Scholar]
- Hansdottir S, Monick MM, Hinde SL, Lovan N, Look DC, Hunninghake GW. Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J Immunol. 2008;181:7090–9. doi: 10.4049/jimmunol.181.10.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewison M, Burke F, Evans KN, Lammas DA, Sansom DM, Liu P, Modlin RL, Adams JS. Extra-renal 25-hydroxyvitamin D3-1α-hydroxylase in human health and disease. J Steroid Biochem Mol Biol. 2007;103:316–21. doi: 10.1016/j.jsbmb.2006.12.078. [DOI] [PubMed] [Google Scholar]
- Jeffery LE, Wood AM, Qureshi OS, et al. Availability of 25-hydroxyvitamin D(3) to APCs controls the balance between regulatory and inflammatory T cell responses. J Immunol. 2012;189:5155–64. doi: 10.4049/jimmunol.1200786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. CD4+ CD73+ T cells suppress the proliferation of autologous CD4+ T cells to a greater extent than CD4+ CD73– T cells.
Figure S2. The effect of 1α,25-dihydroxyvitamin D3 (1,25(OH)2D3) treatment on the mRNA expression of transforming growth factor-β-associated molecules.