Abstract
Background
Oxaliplatin is a crucial chemotherapy drug that plays an important role in colorectal cancer and oral cancer treatment. However, the molecular mechanism of oxaliplatin in killing tongue squamous cell cancer cells is still unknown. This paper investigates the mechanism of by which oxaliplatin regulates tongue squamous cell carcinoma Tca8113 cell survival and death.
Material/Methods
Tca8113 was treated with 1 μmol/L oxaliplatin for 24 h. Tca8113 cell proliferation and apoptosis were determined by MTT method and flow cytometry, respectively. Western blot was applied to detect receptor-interacting protein kinase 1 (RIP1) level. Tca8113 was transfected with siRNA RIP1 and then treated with 1 μmol/L oxaliplatin, and the cell apoptosis was detected.
Results
We found that 1 μmol/L oxaliplatin could inhibit Tca8113 cell growth (cell survival rate was 19.3%), reduce mitochondrial membrane potential (reduce 82.3%) and phosphatidylserine eversion (positive rate was 62.7%), and activate caspase-3 (increased 2.6 times). We also found that 1 μmol/L oxaliplatin treatment could increase RIP1 expression in Tca8113 cells. Cell apoptosis rate increased after siRNA RIP1 and 1 μmol/L oxaliplatin treatment (apoptosis rate was 90.2%).
Conclusions
Down-regulating RIP1 promotes oxaliplatin induced Tca8113 cells apoptosis.
MeSH Keywords: 6-Cyano-7-nitroquinoxaline-2,3-dione; Inhibitor of Apoptosis Proteins; Nuclear Receptor Co-Repressor 1
Background
Oxaliplatin is a crucial chemotherapy drug that plays an important role in colorectal cancer and oral cancer treatment. However, the molecular mechanism of oxaliplatin in killing tongue squamous cancer cells is still unknown [1,2]. Previous studies demonstrated that oxaliplatin could kill cancer cells through induction of apoptosis of tongue squamous cells [1,2]. However, the mechanism by which oxaliplatin exert its function on the induction of apoptosis is still unknown as is the molecule involved in this process.
The main target of oxaliplatin is protein kinase, such as Ras, Raf, and receptor-interacting protein kinase 1 (RIP1), which is an important protein kinase in apoptosis [3], necroptosis [4], autophagy [5], and NF-κB signaling pathway [6]. Research has shown that knockdown RIP1 level can enhance oxaliplatin-induced apoptosis of oral cancer cell KB [6] and gastric cancer cells [7]. Further studies showed that RIP1 could activate caspase, leading to caspase-dependent apoptosis [3,6]. However, the molecular mechanism of oxaliplatin in killing tongue squamous cell cancer is still unknown. This study investigated the mechanism of oxaliplatin in regulating tongue squamous cell carcinoma Tca8113 survival and death.
Material and Methods
Reagents
We purchased 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT), Escort™ Transfection Reagent, and RIP1 primary antibody from Sigma. Tetramethylrhodamine ethylester (TMRE), FITC-Annexin-V, caspase-3, and caspase-8 activity detection kits were bought from Beyotime Biotechnology Co., LTD (Haimen, China). RIP1 siRNA was designed and synthesized by Genepharma (Shanghai, China). The sequence was: RIP1 siRNA 5′-GGGATTTTCCGGGAATTT-3′, negative control 5′-AAATATTCCGAATAATTT-3′. DMEM medium and fetal bovine serum were from Dingguo Biological Technology Co., LTD (Beijing, China).
Cell culture
Tongue squamous cell carcinoma Tca8113 was bought from the American Type Culture collection (ATCC). The cells were maintained in DMEM medium (containing 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin) at 37°C and 5% CO2. Cells in different groups were treated by 1 μmol/L oxaliplatin or DMSO for 24 h.
Transfection
RIP1 siRNA at 50 nM was mixed with transfection reagent at room temperature for 20 min, then they were added to the cells and incubated for 24 h for the following experiments [8].
MTT assay
Cell survival and growth was determined by MTT method [9]. Cells at 10 000 cells/well in 96-well plate were cultured for 24 h, and then MTT solution was added to each well and cultured at 37°C and 5% CO2 for 4 h. After adding 160 μl DMSO for 8 min, the absorbance (OD) value of each well was detected by microplate reader at a wavelength of 490 nm to calculate the cell proliferation rate.
Flow cytometry
TMRE and FITC-Annexin-V were used to detect Tca8113 cell apoptosis [10,11]. We resuspended 80 000 cells in 250 μl Annexin V-FITC binding buffer, and 5 μl Annexin V-FITC reaction liquid was added and incubated at room temperature away from light for 20 min. The cells were detected on a flow cytometry instrument with the exciting and absorbed wavelength as 488 nm and 625 nm, respectively. Mitochondrial membrane potential detection was as follows: the cells were incubated in 10 μmol/L TMRE at room temperature away from light for 20 min and measured by flow cytometry.
Western blot
The cells were digested with lysis buffer as previously reported [12]. Total protein was separated by denaturing 10% SDS – polyacrylamide gel electrophoresis. Detection was performed with chemiluminescence and calculated with Quantity One.
Caspase-3 activity detection
Caspase-3 activity was tested by use of a kit, following the manual [13]. Cell lysis was added to the cells and incubated for 10 min at 4°C. After adding the substrate for 5 min at room temperature, the plate was read on a microplate reader at 560 nm wavelength.
Statistical analysis
All statistical analyses were performed using SPSS18.0 software (Chicago, IL). Numerical data are presented as means and standard deviation (±SD). Differences between groups were analyzed using one-way ANOVA. P<0.05 was considered as a significant difference.
Results
Oxaliplatin inhibits Tca8113 cell proliferation
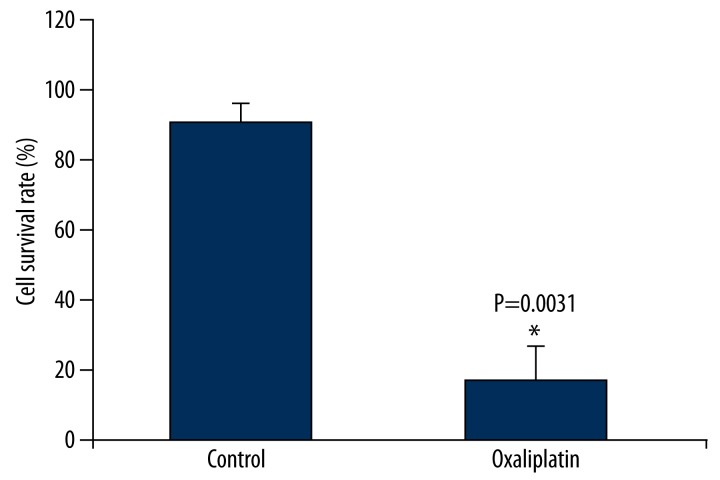
We first tested oxaliplatin’s impact on tongue squamous cell carcinoma Tca8113 cells proliferation. We used 3 different concentrations – 0.1 μmol/L, 1 μmol/L, and 10 μmol/L – and found no obvious apoptosis under the concentration of 0.1 μmol/L and 100% apoptosis under 10 μmol/L. Therefore, we chose the concentration of 1 μmol/L. As shown in Figure 1, oxaliplatin treatment significantly inhibited Tca8113 cells growth. Tca8113 cells survival rate decreased to 19.3% after 1 μmol/L oxaliplatin treatment. Oxaliplatin could restrain Tca8113 cells growth.
Figure 1.
Oxaliplatin inhibits Tca8113 cell proliferation. After treatment of Tac8113 cells by oxaliplatin, MTT assay was used to evaluate the effect of oxaliplatin on the proliferation of Tac8113 cells.
Oxaliplatin induces Tca8113 cell membrane potential reduction
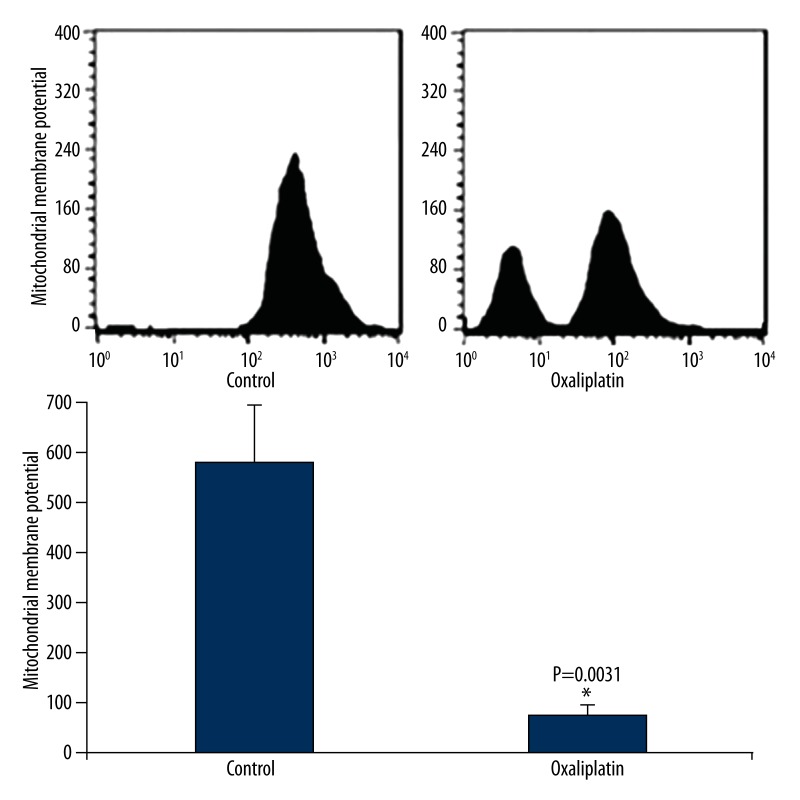
We then detected oxaliplatin’s effect on Tca8113 cell mitochondrial membrane potential. As shown in Figure 2, oxaliplatin treatment significantly decreased Tca8113 cells mitochondrial membrane potential by 82.3%. The results suggest that oxaliplatin may cause apoptosis in tongue squamous cancer Tca8113 cells.
Figure 2.
Oxaliplatin induces Tca8113 cell membrane potential reduction. Tac8113 cells were treated with oxaliplatin and flow cytometry was used to evaluate the effect of oxaliplatin on the mitochondria membrane potential in Tac8113 cells.
Oxaliplatin induces Tca8113 cell apoptosis
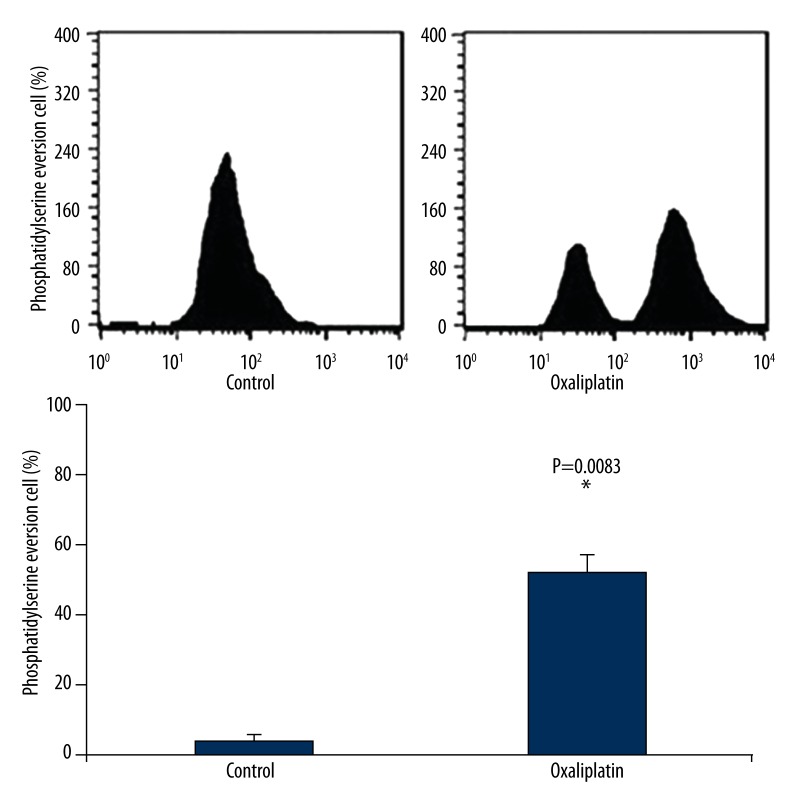
As shown in Figure 3, oxaliplatin could cause phosphatidylserine eversion in Tca8113 cells, with the positive rate as 62.7%, revealing that oxaliplatin may cause Tca8113 cells apoptosis.
Figure 3.
Oxaliplatin induces Tca8113 cell phosphatidylserine exposure. After treatment of Tac8113 cells by oxaliplatin, flow cytometry was used to evaluate the effect of oxaliplatin on phosphatidylserine exposure in Tac8113 cells.
Oxaliplatin activates Caspase-3 in Tca8113 cells
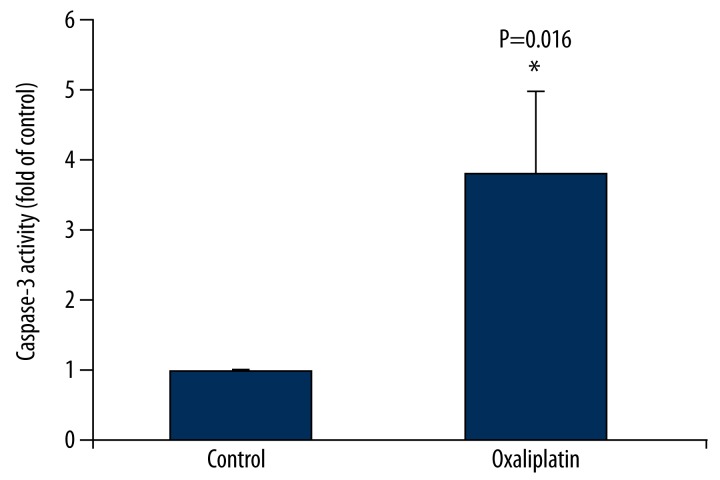
As shown in Figure 4, Tca8113 cells underwent caspase-3 activation under the effect of oxaliplatin, and the caspase activity increased by 2.6 times. However, under the same condition, caspase-8 was not activated. The results suggest that oxaliplatin can cause tongue squamous cancer cells Tca8113 cells apoptosis.
Figure 4.
Oxaliplatin activates Caspase-3 in Tca8113 cells. Tac8113 cells were treated with oxaliplatin and ELISA was used to evaluate the effect of oxaliplatin on caspase-3 activation in Tac8113 cells.
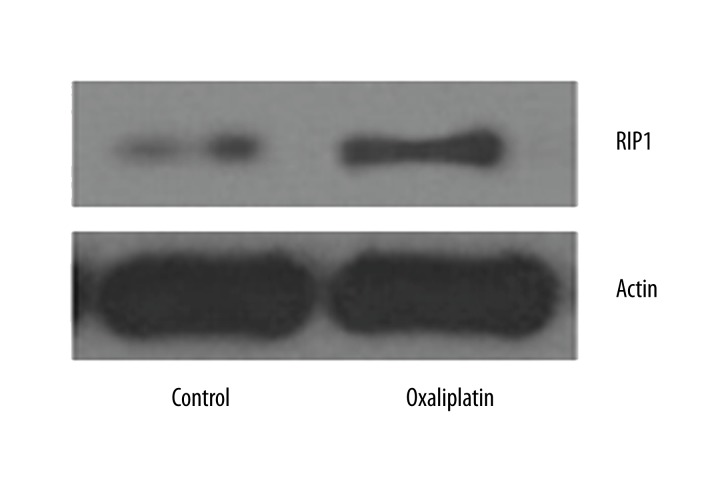
Oxaliplatin up-regulates RIP1 expression in Tca8113 cells
Western blot was applied to detect of oxaliplatin effect on RIP1 expression in Tca8113 cells. As shown in Figure 5, RIP1 expression increased after oxaliplatin treatment.
Figure 5.
Oxaliplatin up-regulates RIP1 expression in Tca8113 cells. Western blot was used to evaluate the effect of oxaliplatin on the expression of RIP1 in Tca8113 cells after treatment with oxaliplatin.
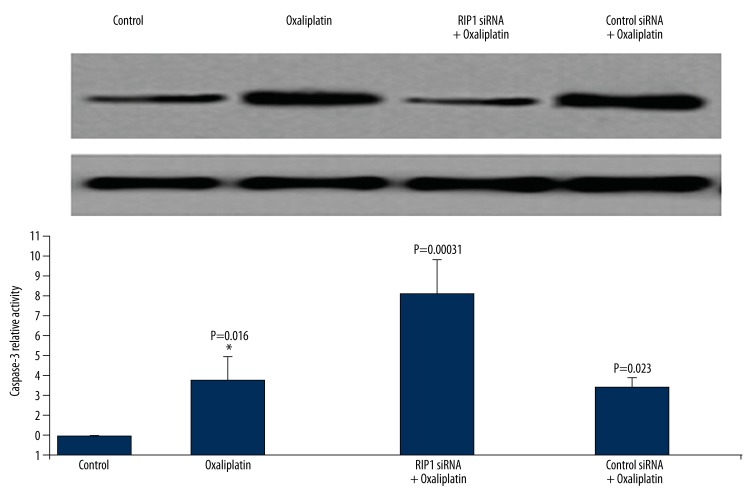
Down-regulating RIP1 promotes oxaliplatin induced Tca8113 cell apoptosis
As shown in Figure 6, RIP1 expression level decreased after siRNA transfection protein kinase 1 (RIP1) of siRNA, while oxaliplatin treatment further induced caspase-3 activation. The cell apoptosis rate (phosphatidylserine eversion rate) was 90.2%. The results indicate that down-regulating RIP1 could promote oxaliplatin-induced Tca8113 cells apoptosis.
Figure 6.
Down-regulating RIP1 promotes oxaliplatin-induced Tca8113 cell apoptosis. Tac8113 cells were transfected by siRNA RIP1 or siRNA control followed by treatment with oxaliplatin. Western blot was used to evaluate the effect of oxaliplatin on RIP1 expression and apoptosis of Tca8113 cells.
Discussion
Oxaliplatin is an important anti-cancer drug in clinical use [1,2]. Studies have shown that the RIP1siRNA could enhance the oral cancer cell KB apoptosis induced by oxaliplatin [6]. We investigated oxaliplatin’s effect on tongue squamous cancer cell Tca8113 growth and apoptosis.
First, we investigated oxaliplatin’s effect on Tca8113 survival. The results showed that oxaliplatin significantly inhibited Tca8113 cells growth, which is consistent with previous reports. Then, we studied the role of oxaliplatin in inducing Tca8113 cells apoptosis. Flow cytometry and Caspase-3 activity detection results revealed that oxaliplatin may cause Tca8113 cells mitochondrial membrane potential reduction, phosphatidylserine eversion, and Caspase-3 activation. It suggested that oxaliplatin can induce Tca8113 cell apoptosis, which is consistent with previous results [14,15]. These results suggest that oxaliplatin might exert its anti-cancer effect through induction of apoptosis of Tca8113 cells, which needs to be confirmed in further in vivo studies. On the other hand, multiple results exhibited that Tca8113 showed more sensitivity to oxaliplatin compared with oral cancer cell KB. This may be because different cells show different sensitivity to the same chemotherapy drugs [16–18].
The main target of oxaliplatin is protein kinase, such as Ras, Raf, and receptor-interacting protein kinase 1 (RIP1). Receptor-interacting protein kinase 1 (RIP1) is an important protein kinase in apoptosis [3], necroptosis [4], autophagy [5], and NF-κB signaling pathway [6]. Research has shown that knockdown RIP1 level can enhance oxaliplatin-induced oral cancer cell KB apoptosis [6]. Further studies showed that RIP1 could activate caspase, leading to caspase-dependent apoptosis [3,6]. Therefore, in this study, we chose RIP1 as the target. We speculate that oxaliplatin might induce cell apoptosis through affecting caspase-3 activation, either directly or indirectly.
There are 2 types of apoptosis: death receptor-mediated external signaling pathway [19,20] and mitochondria-mediated signaling pathway [21,22]. We investigated the specific pathway during oxaliplatin-induced Tca8113 cells apoptosis. Death receptor-mediated external signaling pathway and mitochondrial-mediated internal signaling pathway induced different caspases. The former mainly causes caspase-8 activation, while the later mainly activates caspase-3/7. Our results show that oxaliplatin could activate caspase-3 but not caspase-8, revealing that oxaliplatin-induced Tca8113 cells apoptosis is mainly through the mitochondrial internal signaling pathway. This is consistent with previous research [19,21]. These results were consistent with previous studies, suggesting that mitochondria-induced cell apoptosis might be another pathway for intracellular cell apoptosis.
RIP1 plays an important role in apoptosis [3], necroptosis [4], autophagy [5], and NF-κB signaling pathway [6]. RIP1 siRNA can enhance oxaliplatin-induced oral cancer cell KB apoptosis [3]. We used RIP1 siRNA knockdown RIP1 expression in Tca8113 cells, and treated them by oxaliplatin. We found that Tca8113 cell apoptosis rate increased, suggesting that down-regulating RIP1 expression can promote Tca8113 sensitivity to oxaliplatin.
Our results suggest that further investigation of oxaliplatin should focus on the following aspects: firstly, collecting clinical tongue squamous cell carcinoma specimens in different stages and testing their apoptosis level and RIP1 expression; secondly, we could further study the target and mechanism of oxaliplatin by different oxaliplatin modifications and similar drugs; thirdly, RIP1 knockout mice could be used to verify the results obtained in the present study; and lastly, we could investigate the effect of RIP1 and oxaliplatin in inducing cancer cell apoptosis in vivo in a tongue squamous cell animal model.
Conclusions
In conclusion, our study demonstrated that down-regulating RIP1 promotes oxaliplatin-induced Tca8113 cell apoptosis.
Footnotes
Source of support: Departmental sources
References
- 1.Vandamme M, Pauwels W, Bleecker J. A case of delayed oxaliplatin-induced pseudo-obstruction: an atypical presentation of oxaliplatin neurotoxicity. Acta Clin Belg. 2015;70(3):207–10. doi: 10.1179/2295333714Y.0000000110. [DOI] [PubMed] [Google Scholar]
- 2.Aroldi F, Prochilo T, Bertocchi P, Zaniboni A. Oxaliplatin-induced hypersensitivity reaction: underlying mechanisms and management. J Chemother. 2015;27:63–66. doi: 10.1179/1973947814Y.0000000204. [DOI] [PubMed] [Google Scholar]
- 3.Yao Z, Zhang P, Guo H, et al. RIP1 modulates death receptor mediated apoptosis and autophagy in macrophages. Mol Oncol. 2015;9(4):806–17. doi: 10.1016/j.molonc.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park EJ, Min KJ, Lee TJ, et al. beta-Lapachone induces programmed necrosis through the RIP1-PARP-AIF-dependent pathway in human hepatocellular carcinoma SK-Hep1 cells. Cell Death Dis. 2014;5:e1230. doi: 10.1038/cddis.2014.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kikuchi M, Kuroki S, Kayama M, et al. Protease activity of procaspase-8 is essential for cell survival by inhibiting both apoptotic and nonapoptotic cell death dependent on receptor-interacting protein kinase 1 (RIP1) and RIP3. J Biol Chem. 2012;287:41165–73. doi: 10.1074/jbc.M112.419747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu J, Huang Y, Li Y, et al. Small interfering RNA-mediated RIP1 knockdown enhances L-OHP sensitivity of human oral squamous carcinoma cells. Nan Fang Yi Ke Da Xue Xue Bao. 2013;33:1004–7. [in Chinese] [PubMed] [Google Scholar]
- 7.Wu P, Zhu X, Jin W, et al. Oxaliplatin triggers necrosis as well as apoptosis in gastric cancer SGC-7901 cells. Biochem Biophys Res Commun. 2015;460:183–90. doi: 10.1016/j.bbrc.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Henrich CJ, Brooks AD, Erickson KL, et al. Withanolide E sensitizes renal carcinoma cells to TRAIL-induced apoptosis by increasing cFLIP degradation. Cell Death Dis. 2015;6:e1666. doi: 10.1038/cddis.2015.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ashkenazi A. Targeting the extrinsic apoptotic pathway in cancer: lessons learned and future directions. J Clin Invest. 2015;125:487–89. doi: 10.1172/JCI80420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh K, Poteryakhina A, Zheltukhin A, et al. NLRX1 acts as tumor suppressor by regulating TNF-alpha induced apoptosis and metabolism in cancer cells. Biochim Biophys Acta. 2015;1853:1073–86. doi: 10.1016/j.bbamcr.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 11.Kim JS, Oh D, Yim MJ, et al. Berberine induces FasL-related apoptosis through p38 activation in KB human oral cancer cells. Oncol Rep. 2015;33:1775–82. doi: 10.3892/or.2015.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chou YC, Chang MY, Wang MJ, et al. PEITC induces apoptosis of Human Brain Glioblastoma GBM8401 Cells through the extrinsic- and intrinsic -signaling pathways. Neurochem Int. 2015;81:32–40. doi: 10.1016/j.neuint.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Sun L, Fan H, Yang L, et al. Tyrosol prevents ischemia/reperfusion-induced cardiac injury in H9c2 cells: involvement of ROS, Hsp70, JNK and ERK, and apoptosis. Molecules. 2015;20:3758–75. doi: 10.3390/molecules20033758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang HJ, Wang M, Wang L, et al. NF-kappaB regulates caspase-4 expression and sensitizes neuroblastoma cells to Fas-induced apoptosis. PLoS One. 2015;10:e0117953. doi: 10.1371/journal.pone.0117953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rogalska A, Marczak A. Epothilone B induces human ovarian cancer OV-90 cell apoptosis via external pathway. Environ Toxicol Pharmacol. 2015;39:700–12. doi: 10.1016/j.etap.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 16.Liu YK, Chen KH, Leu YL, et al. Ethanol extracts of Cinnamomum kanehirai Hayata leaves induce apoptosis in human hepatoma cell through caspase-3 cascade. Onco Targets Ther. 2015;8:99–109. doi: 10.2147/OTT.S68765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen TL, Zhu GL, He XL, et al. Short-term pretreatment with atorvastatin attenuates left ventricular dysfunction, reduces infarct size and apoptosis in acute myocardial infarction rats. Int J Clin Exp Med. 2014;7:4799–808. [PMC free article] [PubMed] [Google Scholar]
- 18.Lutz A, Sanwald J, Thomas M, et al. Interleukin-1beta enhances FasL-induced caspase-3/-7 activity without increasing apoptosis in primary mouse hepatocytes. PLoS One. 2014;9:e115603. doi: 10.1371/journal.pone.0115603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo H, Omoto S, Harris PA, et al. Herpes simplex virus suppresses necroptosis in human cells. Cell Host Microbe. 2015;17:243–51. doi: 10.1016/j.chom.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen G, Cheng X, Zhao M, et al. RIP1-dependent Bid cleavage mediates TNFalpha-induced but Caspase-3-independent cell death in L929 fibroblastoma cells. Apoptosis. 2015;20:92–109. doi: 10.1007/s10495-014-1058-0. [DOI] [PubMed] [Google Scholar]
- 21.Zhang W, Zhao L, Liu J, et al. Cisplatin induces platelet apoptosis through the ERK signaling pathway. Thromb Res. 2012;130:81–91. doi: 10.1016/j.thromres.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Zhang W, Liu J, Sun R, et al. Calpain activator dibucaine induces platelet apoptosis. Int J Mol Sci. 2011;12:2125–37. doi: 10.3390/ijms12042125. [DOI] [PMC free article] [PubMed] [Google Scholar]