Abstract
Background
The aim of this study was to explore the correlations between the different parameters of the cervical sagittal balance in magnetic resonance images (MRI) and evaluate the criteria for their clinical application in disc-degenerative diseases.
Material/Methods
We conducted a retrospective review of the MRIs of 125 adult outpatients with disc-degenerative diseases of the cervical spine; the images were obtained between May and July 2014 at our institute. The control group comprised 50 volunteers whose MRIs were also obtained. The parameters measured in the MRIs were: neck tilt (NT), T1 slope (T1S), thoracic inlet angle (TIA), and Cobb’s angle (C2–7). The correlation between the various parameters was analyzed using the Pearson correlation coefficient.
Results
The outpatients group showed moderate correlation between TIA and T1S, a significant correlation between TIA and NT, a weak correlation between T1S and Cobb’s angle, and a weakly negative correlation between T1S and NT. Further, the TIA showed no significant difference between the outpatient group and the control group, as per the sample t test.
Conclusions
Our findings indicate that TIA, T1S, and NT could be used as indices for the evaluation of cervical sagittal balance and that the TIA could be used as a reference to assess the cervical compensation. Restoration of the NT and T1S should be considered as a goal of surgical treatment during the preoperative planning in patients with disc-degenerative diseases.
MeSH Keywords: Magnetic Resonance Imaging, Neck Pain, Spine
Background
A well balanced sagittal alignment provides biomechanical stability and efficiently maintains the integrity of the musculoskeletal system [1,2]. On the other hand, sagittal malalignment can lead to disc degeneration and associated clinical symptoms. Spinal sagittal imbalance has been shown to be positively correlated with the occurrence of clinical symptoms [3]. In addition, spinal malalignment can explain the clinical symptoms and guide the treatment, particularly individualized perioperative treatment [4–9]. Historically, surgical management is aimed at achieving complete decompression and relieving spinal cord compression and clinical symptoms [5,10]. Head-spine-pelvic balance is now used as an indicator of the symptom severity during preoperative assessment and prognostic evaluation [11]. Most of the conventional parameters pertain to the lumbar–pelvic balance, while those pertaining to cervical alignment are scarce. In order to maintain the spinal sagittal balance and biomechanical stability of the normal spine, the cervical spine alignment is adjusted according to the changes in thoracic kyphosis [2]. However, the correlation between the parameters of the cervical and thoracic spinal parameters is weaker than that between the lumbar and pelvic spinal parameters [3,11,12]. In recent years, thoracic inlet angle (TIA), neck tilt (NT), and T1 slope (T1S) have been extensively investigated as parameters for the quantification of the balance in the head-cervical-thoracic region. In this study, we sought to determine the correlation between TIA, TIS, NT, and Cobb’s Angle in magnetic resonance images (MRI) of patients with disc-degenerative diseases and to compare these values with those obtained for normal individuals.
Material and Methods
The study was designed as a retrospective review of the MRIs of 125 consecutive outpatients with disc-degenerative diseases (C3–C7), obtained at the China-Japan Union Hospital, between May and July 2014. The study protocol was approved by the ethics committee of China-Japan Union Hospital of Jilin University.
The inclusion criteria were defined as presence of neck pain with or without neurologic symptoms (i.e., radiculopathy or myelopathy). The severity of disc degeneration was determined according to the degree of cervical disc herniation. The exclusion criteria were: trauma, rheumatoid arthritis, spinal tumors, history of cervical spine surgery, and evidence of the ossification of the posterior longitudinal ligament and/or the ligamentum flavum. The male-to-female ratio in this group was 56: 69, and the mean age of the patients was 50.3±9.19 years (age range, 26–69 years).
Another group of 50 asymptomatic volunteers was also recruited to serve as the control group, and their MRIs were obtained in a similar fashion. The mean age in this group was 44.76±13.72 years (age range, 21–69 years), and the male-to-female ratio was 19:31.
Ten patients each in both groups were aged between the 3rd decade and 6th decade. The MRIs were obtained by 2 independent observers, with the patients lying in the supine position and facing upwards. All images were obtained using the same imaging system (Siemens 3.0T, AG, Germany). The scan was performed from the level of the external auditory canal to the 6th thoracic vertebra. The value of each parameter, including NT, T1S, TIA and Cobb’s angle, was independently measured twice by both spinal surgeons using the software Centricity Enterprise Web V3.0. The mean of the measurements was taken as the final measurement, and the Pearson correlation coefficient was determined. The statistical analysis was performed using the software IBM Corp. Release 2012 IBM SPSS Statistics for Windows, Version 21.0 (IBM Corp. Armonk, NY, USA).
The measurements were made on the mid-sagittal slice. NT was defined as the angle between a vertical line drawn on the tip of the sternum and a line connecting the center of upper endplate of T1 to the tip of the sternum. T1S is the angle formed between a horizontal line and the upper endplate of T1. Further, TIA is defined as the angle formed between a vertical line at the center of the upper endplate of T1 and a line connecting the center of the upper endplate of the T1 to the sternum tip (Figure 1). Thus, TIA is a sum of T1S and NT, similar to PI, which is the sum of SS and PT [12]. Further, sagittal lordosis was measured as the angle between the horizontal line on the lower endplate of C2 and a horizontal line on the lower endplate of C7.
Figure 1.
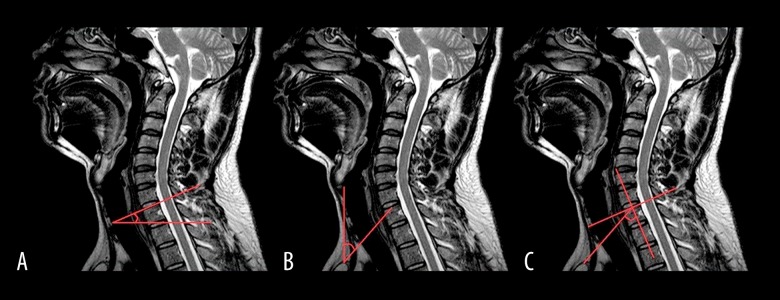
(A) T1S is the angle between a horizontal line and the superior end plate of the first thoracic vertebral. (B) NT is the angle between a vertical line at the sternum tip and a line connecting the center of the T1 upper end plate to the sternum tip. (C) TIA is defined as the angle between the vertical line of the center of the T1 upper end plate and a line connecting the center of the superior end plate of T1 to the sternum tip.
Results
In the outpatient group, the mean T1S was 22.56°±6.88° (range, 4.60° to 39.00°); mean NT, 52.94°±8.92° (range, 33.30° to 78.10°); mean TIA, 75.70°±9.40° (range, 43.60° to 101.60°); and mean Cobb’s angle, 10.64°±8.38° (range, 1.10° to 47.00°) (Table 1). The correlation coefficient for TIA and T1S was 0.40 (p value <0.01), implying a moderate correlation between these parameters. Further, TIA was significantly correlated with NT, with the correlation coefficient being 0.74 (p value, <0.01). However, both TIA and T1S showed a weak correlation with Cobb’s angle (0.22 with p value <0.05 and 0.40 with p value <0.01, respectively). Further, the correlation coefficient between TIS and NT was negative (−0.27, p value <0.01) (Table 2).
Table 1.
The mean value and range of parameters in the outpatient group.
| Mean | Range | Standard deviation | |
|---|---|---|---|
| T1S (degree) | 22.56 | 4.60–39.00 | 6.88 |
| NT (degree) | 52.94 | 33.30–78.10 | 8.92 |
| TIA (degree) | 75.70 | 43.60–101.60 | 9.40 |
| Cobb’s angle (degree) | 10.64 | 1.10–47.00 | 8.38 |
T1S – T1 slope; NT – neck tilt; TIA – thoracic inlet angle.
Table 2.
Pearson correlation coefficients and p value in the outpatient group.
| Age | T1S | NT | TIA | Cobb’s angle | |
|---|---|---|---|---|---|
| Age | – | 0.167 | 0.190* | 0.274* | 0.138 |
| T1S | – | – | −0.267** | 0.403** | 0.399** |
| NT | – | – | – | 0.739** | −0.110 |
| TIA | – | – | – | – | 0.221* |
T1S – T1 slope; NT – neck tilt; TIA – thoracic inlet angle.
Correlation significant at 0.01 (2-tailed);
correlation significant at 0.05 (2-tailed).
In the asymptomatic group, the mean T1S was 29.31°±6.04° (range, 15.7° to 40.9°); mean NT, 45.21°±9.05° (range, 22.7° to 62.5°); mean TIA, 74.45°±10.17° (range, 52.00° to 92.8°); mean Cobb’s angle, 13.55°±7.99° (range, 1.60° to 34.5°) (Table 3). TIA was significantly correlated with NT and Cobb’s angle (p<0.01), but weakly correlated with T1S. Further, the correlation between T1S and Cobb’s angle was weak (Table 4).
Table 3.
Mean value and range of parameters in the asymptomatic group.
| Mean | Range | Standard deviation | |
|---|---|---|---|
| T1S (degree) | 29.31 | 15.70–40.90 | 6.04 |
| NT (degree) | 45.21 | 22.70–62.50 | 9.05 |
| TIA (degree) | 74.45 | 52.00–92.80 | 10.17 |
| Cobb’s angle (degree) | 13.55 | 1.60–34.50 | 7.99 |
T1S – T1 slope; NT – neck tilt; TIA – thoracic inlet angle.
Table 4.
Pearson correlation coefficient and p value in the asymptomatic group.
| Age | T1S | NT | TIA | Cobb’s angle | |
|---|---|---|---|---|---|
| Age | – | −0.127 | 0.347* | 0.238 | 0.285* |
| T1S | – | – | −0.119 | 0.487** | 0.402** |
| NT | – | – | – | 0.805** | 0.228 |
| TIA | – | – | – | – | 0.431** |
T1S – T1 slope; NT – neck tilt; TIA – thoracic inlet angle.
Correlation significant at 0.01 (2-tailed);
correlation significant at 0.05 (2-tailed).
Additionally, the outpatients group was divided into different subgroups numbered according to the number of segments with disc protrusion, e.g., Group 1, with protrusion in 1 segment. Each of these subgroups was compared with the asymptomatic group using the independent-sample t test (Table 5). The value of TIA did not differ significantly between the outpatient group and the asymptomatic group, whereas those of T1S, NT, and Cobb’s angle showed significant differences. The values of NT and T1S differed markedly among groups 1, 2, and 3, which had similar sample sizes (Table 5).
Table 5.
Results of independent samples t-test.
| Independent samples | Parameter | Mean differences | t | df | sig |
|---|---|---|---|---|---|
| Group 1 & Asymptomatic | T1S | −6.23 | −5.08 | 108 | 0.000 |
| NT | 8.95 | 5.46 | 108 | 0.000 | |
| TIA | 3.21 | 1.83 | 108 | 0.071 | |
| Cobb’s angle | −1.85 | −1.13 | 108 | 0.262 | |
| Group 2 & Asymptomatic | T1S | −6.34 | −4.49 | 89 | 0.000 |
| NT | 6.65 | 3.31 | 89 | 0.001 | |
| TIA | 0.41 | 0.18 | 89 | 0.858 | |
| Cobb’s angle | −2.96 | −1.74 | 89 | 0.085 | |
| Group 3 & Asymptomatic | T1S | −8.40 | −5.20 | 67 | 0.000 |
| NT | 6.80 | 2.84 | 67 | 0.006 | |
| TIA | −1.53 | −0.59 | 67 | 0.557 | |
| Cobb’s angle | −7.19 | −4.50 | 67 | 0.000 | |
| Group 4 & Asymptomatic | T1S | −10.37 | −3.54 | 53 | 0.010 |
| NT | 5.29 | 1.24 | 53 | 0.222 | |
| TIA | −4.91 | −1.04 | 53 | 0.304 | |
| Cobb’s angle | 0.95 | 0.25 | 53 | 0.803 | |
| Outpatient & Asymptomatic | T1S | −6.76 | −6.07 | 173 | 0.000 |
| NT | 7.72 | 5.15 | 173 | 0.000 | |
| TIA | 1.25 | 0.78 | 173 | 0.440 | |
| Cobb’s angle | −2.91 | −2.11 | 173 | 0.037 |
Group 1 n=60, Group 2 n=41, Group 3 n=19, Group 4 n=5, Outpatient Group n=125, Asymptomatic Group n=50.
Discussion
In this study, we compared the values of the parameters for the assessment of cervical spine alignment obtained using MRI acquired for patients with disc-degenerative diseases and those acquired for normal subjects.
Although measurements of parameters related to cervical alignment obtained for asymptomatic individuals without a history of spinal surgery are available in literature, most of these measurements were obtained using radiographs acquired in the standing position [11–14]. The contour of the sternum tip is difficult to detect in plain radiographs, which may compromise the accuracy of the measurements. Further, no significant difference has been reported between the measurements of the parameters obtained using cervical computerized tomography scan images acquired in the supine position and those obtained using radiographs acquired in the erect position [14]. Thus, we made the measurements using MRI acquired in the supine position, similar to previous studies in Asian populations.
In the past, Cobb’s angle was considered a gold standard for the assessment of cervical alignment because of its reliability and easy applicability [15]. However, the complexity of the cervical anatomy and biomechanics limited the accuracy of measurements made using Cobb’s methods. Subsequently, it was shown that the Cobb’s angles at C1–7 and C2–7 overestimated and underestimated, respectively, cervical lordosis [16]. Previously, some studies have shown deficiencies in the measurements of the range of cervical lordosis using Cobb’s method [15–17]. Therefore, no normal range and measuring method have been established. With the technological advancements and improvement in our understanding of the properties of the spine, the parameters of regional balance were examined and those related to the cervical alignment attracted considerable attention. Berthonnaud et al. conducted a retrospective review of the lateral radiographs of 160 asymptomatic adults. On analyzing the parameters, he found that a change in a single region would cause a change in the spinal sagittal balance of the adjacent segment. In order to maintain the upright posture, cervical lordosis changes with a change in thoracic kyphosis. However, the correlation coefficient between cervical lordosis and thoracic kyphosis is weaker than that between the pelvis and lumbar alignments [2]. C0–2 had high mobility and strong compensation. Although the head directly influences the stability of this segment, the lower cervical alignment plays a key role in the biomechanics of the spine. Hardacker et al. reported that the lower cervical segment had a significant correlation with the total cervical lordosis, despite the fact that the physical lordosis is lesser at the lower cervical levels. Since the thoracic spine is the skeletal basement of the cervical vertebrae, it is important that the relationship between the 2 segments be properly examined, as is the case with the lumbar and pelvic segments of the spine [3]. Many studies have confirmed the feasibility of TIA for assessing cervical sagittal balance, similar to PI that is used for the assessment of lumbar lordosis. In a retrospective study of standing lateral radiographs of 77 asymptomatic volunteers, Sang-Hun Lee et al. found that T1S correlated significantly with sagittal balance and was, in fact, one of the key factors influencing the latter. They stated that in order to maintain the balance of the cervical spine, T1S would increase if the cervical lordosis increased and that the value of T1S is affected by posture. Therefore, they stressed the importance of using TIA as a constant parameter in the evaluation of cervical balance [13]. In 2012, Sang-Hun Lee et al. conducted a prospective study of lateral radiographs. In that study, the mean value of TIA was 69.5°±8.6 and it was significantly correlated with T1S. They concluded that TIA could be considered as a guide parameter to evaluate the cervical sagittal balance because TIA was not affected by the change of posture. By determining that the normal NT in the upright position was preserved at around 44° and considering that the NT maintained the physiological needs and the direct line of sight, they concluded that TIA would increase when T1S increased, since TIA=T1S+NT [12].
Analysis of the MRI of the asymptomatic group showed that TIA had a strong positive correlation with NT and Cobb’s angle. Further, the value of TIA would change to a certain degree with an increase in the number of degenerative segments, but not significantly. The mean TIA measured in this study was 75.70°±9.40°, which is similar to the values reported by Hyo Sub Jun (75.09°±8.1°) [14] and Ji Hoon Park (70.50°±10.66°) [18] based on measurements obtained using computerized tomography scans. The TIA did not differ significantly in the outpatients group and the asymptomatic group. Further, a decrease in the value of T1S was associated with an increase in the value of NT. The T1S, NT, and Cobb’s angle differed markedly in the 2 groups. Because of the small size of Group 4, there was no statistical significance of the differences noted for this group. Groups 1, 2, and 3, however, differed significantly in T1S and NT, as compared to the control group. With regard to the asymptomatic group, Groups 1 and 2 showed no difference in Cobb’s angle. In general, with an increase in the number of cervical disc protrusions and the severity of degeneration, the values of the parameters tended to decrease. However, the TIA remained within a certain range, and did not differ in the outpatients and the asymptomatic groups. Compared to the lumbar spine, the cervical spine is exposed to a lower level of biological force and its compensatory ability is greater. Cervical vertebral dislocation caused by degenerative disease is rare, but when severe, cervical degenerative diseases can cause bone changes such as osteophyte formation or neck deformity and also lead to difficulty in maintaining a horizontal line of vision. These changes can then lead to changes in the degree of thoracic kyphosis and lumbar lordosis in order to maintain the quality of life. In this study, we found that although TIA did not show any obvious differences in the outpatients group and the asymptomatic group, it does respond to compensatory changes. This indicates that the cervical spine is in a stage of offset, whereby alignment deformity of the cervical spine, such as the swan-neck deformity, does not occur if the TIA is maintained at around 75° in the supine position. The first thoracic vertebra, which is a bony unite connecting the cervical and thoracic spines, plays a key role at the base of the cervical vertebrae. The T1S at the junction of the cervical and thoracic spines reacts to the tilting of the basement of the neck. It is an important process in maintaining the physiological curvature of the cervical spine. NT changes in order to maintain a horizontal vision line. Geometrically, TIA is the sum of T1S and NT. During the process of offset, the neck undergoes gradual hypsokinesis and directly increases NT and indirectly reduces T1S. Meanwhile, the TIA changes within a range of compensatory value. In the cervical disc-degenerative diseases, TIA does not change markedly and could be used as a reference parameter during surgery. When analyzing the data from the MRI obtained in the supine position, the surgeon should consider the restoration of NT and T1S, particularly NT. This is possible because the same data are required for surgery with the anterior approach, which is widely performed for cervical anterior compression. Before the operation, a surgeon can plan the surgery in such a manner that T1S is maintained or increased appropriately, thereby decreasing the NT indirectly with an acceptable range of the TIA; this would minimize the changes of long-term complications of the deformity and ensure an improved postoperative outcome [19].
This study has a few limitations. First, it focused only on imaging findings, rather than the clinical symptoms, and did not assess the outcome of the treatment. Few published reports have been discussed in our paper because of the lack of the research on the parameters of cervical alignment, such as TIA, NT, and T1S, and because the comparison of the parameters in patients with cervical disc-degeneration disease and normal, asymptomatic subjects has not been reported. In future, we intend to evaluate the parameters in patients of different ages undergoing treatment for cervical spondylosis. Further, we intend to assess the reliability of the TIA in explaining symptoms, establishing the goals of treatment, and evaluating prognosis.
Conclusions
The TIA, T1S, and NT could be used as tools for assessing the cervical sagittal balance. TIA could be used as a reference and constant parameter, similar to PI, to assess cervical compensation since TIA in the outpatients group was similar to that in the asymptomatic control group in the supine position. NT and T1S, however, differed remarkably. Therefore, surgical treatment of patients with disc-degenerative diseases should include restoration of NT and T1S as a therapeutic goal during the preoperative planning. Our study showed that TIA, T1S, and NT were predictable indices that can assist preoperative planning.
Footnotes
Source of support: None
Conflicts of interest
None.
References
- 1.Vaz G, Roussouly P, Berthonnaud E, et al. Sagittal morphology and equilibrium of pelvis and spine. Eur Spine J. 2002;11:80–87. doi: 10.1007/s005860000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berthonnaud E, Dimnet J, Roussouly P, et al. Analysis of the sagittal balance of the spine and pelvis using shape and orientation parameters. J Spinal Disord Tech. 2005;18:40–47. doi: 10.1097/01.bsd.0000117542.88865.77. [DOI] [PubMed] [Google Scholar]
- 3.Hardacker JW, Shuford RF, Capicotto PN, et al. Radiographic standing cervical segmental alignment in adult volunteers without neck symptoms. Spine. 1997;22:1472–80. doi: 10.1097/00007632-199707010-00009. [DOI] [PubMed] [Google Scholar]
- 4.Ferrara LA. The biomechanics of cervical spondylosis. Adv Orthop. 2012;2012:493–605. doi: 10.1155/2012/493605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ames CP, Smith JS, Scheer JK, et al. Impact of spinopelvic alignment on decision making in deformity surgery in adults. J Neurosurg Spine. 2012;16:547–64. doi: 10.3171/2012.2.SPINE11320. [DOI] [PubMed] [Google Scholar]
- 6.Glassman SD, Berven S, Bridwell K, et al. Correlation of radiographic parameters and clinical symptoms in adult scoliosis. Spine (Phila Pa 1976) 2005;30(6):682–88. doi: 10.1097/01.brs.0000155425.04536.f7. [DOI] [PubMed] [Google Scholar]
- 7.Kuntz C, Levin LS, Ondra SL, et al. Neutral upright sagittal spinal alignment from theocciput to the pelvis in asymptomatic adults: a review and resynthesis of the literature. J Neurosurg Spine. 2007;6:104–12. doi: 10.3171/spi.2007.6.2.104. [DOI] [PubMed] [Google Scholar]
- 8.Boseker EH, Moe JH, Winter RB, et al. Determination of the “normal” thoracic kyphosis: a roentgenographic study of 121 normal children. J Pediatr Orthop. 2000;20:796–98. doi: 10.1097/00004694-200011000-00019. [DOI] [PubMed] [Google Scholar]
- 9.Misterska E, Jankowski R, Głowacki J, et al. Kinesiophobia in pre-operative patients with cervical discopathy and coexisting degenerative changes in relation to pain-related variables, psychological state and sports activity. Med Sci Monit. 2015;21:181–94. doi: 10.12659/MSM.891045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knott PT, Mardjetko SM, Techy F. The use of the T1 sagittal angle in predicting overall sagittal balance of the spine. Spine J. 2010;10:994–98. doi: 10.1016/j.spinee.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 11.Justin K, Scheer, Jessica A, Tang, et al. Cervical spine alignment, sagittal deformity, and clinical Implications. J Neurosurg Spine. 2013;19:141–59. doi: 10.3171/2013.4.SPINE12838. [DOI] [PubMed] [Google Scholar]
- 12.Lee SH, Kim KT, Seo EM, et al. The Influence of thoracic inlet alignment on the craniocervical sagittal balance in asymptomatic adults. J Spinal Disord Tech. 2012;25:E41–47. doi: 10.1097/BSD.0b013e3182396301. [DOI] [PubMed] [Google Scholar]
- 13.Lee SH, Son ES, Seo EM, et al. Factors determining cervical spine sagittal balance in asymptomatic adults: correlation with spinopelvic balance and thoracic inlet alignment. Spine J. 2013 doi: 10.1016/j.spinee.2013.06.059. S1529-9430(13)00745-6. [DOI] [PubMed] [Google Scholar]
- 14.Jun HS, Chang IB, Song JH, et al. Is it possible to evaluate the parameters of cervical sagittal alignment on cervical computed tomographic scans? Spine (Phila Pa 1976) 2014;39(10):E630–36. doi: 10.1097/BRS.0000000000000281. [DOI] [PubMed] [Google Scholar]
- 15.Harrison DE, Harrison DD, Cailliet R, et al. Radiographic analysis of lumbar lordosis: centroid, Cobb, TRALL, and Harrison posterior tangent methods. Spine. 2001;26(11):E235–42. doi: 10.1097/00007632-200106010-00003. [DOI] [PubMed] [Google Scholar]
- 16.Harrison DE, Harrison DD, Cailliet R, et al. Cobb method or Harrison posterior tangent method: which to choose for lateral cervical radiographic analysis. Spine (Phila Pa 1976) 2000;25:2072–78. doi: 10.1097/00007632-200008150-00011. [DOI] [PubMed] [Google Scholar]
- 17.Yochum T, Rowe L. Essentials of skeletal radiology. 2nd ed. Baltimore: Williams & Wilkins; 1996. [Google Scholar]
- 18.Park JH, Cho CB, Song JH, et al. T1 slope and cervical sagittal alignment on cervical CT radiographs of asymptomatic persons. J Korean Neurosurg Soc. 2013;53(6):356–59. doi: 10.3340/jkns.2013.53.6.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jang JS, Lee SH, Min JH, et al. Surgical treatment of failed back surgery syndrome due to sagittal imbalance. Spine (Phila Pa 1976) 2007;32:3081–87. doi: 10.1097/BRS.0b013e31815cde71. [DOI] [PubMed] [Google Scholar]


