Abstract
Background
Suppressed mitochondrial biogenesis (MB) contributes to acute kidney injury (AKI) after many insults. AKI occurs frequently after liver transplantation (LT) and increases mortality. This study investigated whether disrupted mitochondrial homeostasis plays a role in AKI after LT.
Methods
Livers were explanted from Lewis rats and implanted after 18 h cold storage. Kidney and blood were collected 18 h after LT.
Results
In the kidney, oxidative phosphorylation (OXPHOS) proteins ATP synthase-β and NADH dehydrogenase-3 decreased 44% and 81%, respectively, with marked reduction in associated mRNAs. Renal PGC-1α, the major regulator of MB, decreased 57% with lower mRNA and increased acetylation, indicating inhibited synthesis and suppressed activation. Mitochondrial transcription factor-A, which controls mtDNA replication and transcription, protein and mRNA decreased 66% and 68%, respectively, which was associated with 64% decreases in mtDNA. Mitochondrial fission proteins Drp-1 and Fis-1 and mitochondrial fusion protein mitofusin-1 all decreased markedly. In contrast, PTEN-induced putative kinase 1 and microtubule-associated protein 1A/1B-light chain 3 increased markedly after LT, indicating enhanced mitophagy. Concurrently, 18- and 13-fold increases in neutrophil gelatinase-associated lipocalin and cleaved caspase-3 occurred in renal tissue. Both serum creatinine and blood urea nitrogen increased >2 fold. Mild to moderate histological changes were observed in the kidney, including loss of brush border, vacuolization of tubular cells in the cortex, cast formation and necrosis in some proximal tubular cells. Finally, myeloperoxidase and ED-1 also increased, indicating inflammation.
Conclusion
Suppression of MB, inhibition of mitochondrial fission/fusion and enhancement of mitophagy occur in the kidneys of recipients of liver grafts after long cold storage, which may contribute to the occurrence of AKI and increased mortality after LT.
Introduction
Orthotopic liver transplantation (LT) is the only proven therapy for end-stage liver diseases [1–5]. However, acute renal dysfunction and chronic renal diseases often occur after LT [6–9]. The incidence of perioperative acute kidney injury (AKI) in liver transplant recipients varies significantly, ranging from 17% to 95% [7,9–13]. After LT, 5–30% of recipients have to receive renal replacement therapy due to severe AKI [7,11]. AKI also increases infection, sepsis, and acute rejection and substantially decreases patient survival after LT [11,14–16]. Increasing evidence indicates that AKI also adversely affects long-term patient outcomes [17,18]. Ultimately, acute renal dysfunction in LT recipients prolongs stays in intensive care units and the hospital, and increases re-hospitalization, the need for postoperative dialysis, and the cost of care.
While AKI after LT frequently presents as acute tubular necrosis (ATN, ~70% of AKI) [11,12,14], the mechanisms underlying AKI after LT remain unclear. More severe liver dysfunction and higher MELD scores before transplantation, severe hypotension/hypoperfusion, anesthesia, transfusion of highly packed red blood cells during surgery, and use of calcinurin inhibitors after transplantation may increase the risk of post-transplantation acute renal dysfunction [11,19,20]. Whether the presence of pre-transplantation AKI increases post-transplantation AKI remains controversial [11]. The degree of liver graft dysfunction is a strong and consistent predictor of AKI after LT [11,19,20].
The renal tubular cells have high energy consumption due to active energy-dependent processes such as reabsorption of filtered blood components and secretion of many substances in these cells. Therefore, mitochondrial homeostasis is crucial for proper renal function. Mitochondrial homeostasis is maintained by mitochondrial biogenesis (MB), mitophagy and mitochondrial dynamics, and disrupted mitochondrial homeostasis frequently leads to organ failure [21]. Persistent disruption of mitochondrial homeostasis has been observed in several animal models of AKI [21,22]. MB is a process that generates new mitochondria in response to increased energy demand (e.g. exercise) and mitochondrial stress/damage [23]. Suppression of MB reduces the capability of cells to adapt to stresses and to maintain proper mitochondrial function, increasing injury and/or inhibiting functional recovery and repair processes after injury. In recent years, evidence suggests that inhibited MB and mitochondrial dysfunction play essential roles in AKI caused by many different insults. For example, renal MB suppression occurs after kidney ischemia/reperfusion (I/R), sepsis, folic acid and glycerol treatment, leading to decreased oxidative phosphorylation (OXPHOS) proteins, mitochondrial dysfunction and renal injury [22,24–28]. In contrast, stimulation of MB attenuates AKI [22,24–28]. Mitophagy selectively removes depolarized/damaged mitochondria, thus controlling mitochondrial quality [29,30]. Inhibited mitophagy leads to impairment of mitochondrial function [31]. Mitochondria divide (fission) and fuse (fusion) continuously in healthy cells [32]. Mitochondrial dynamics also play an essential role in mitochondrial quality control, thus affecting cell function and survival [33–35]. Alteration of mitochondrial dynamics occurs in I/R- and glycerol-induced AKI [22]. Whether mitochondrial homeostasis is disrupted in the kidney after LT and its relation to occurrence of post-transplantation AKI remains unknown. Therefore, in the present study we explored renal MB, mitophagy and mitochondrial dynamics after LT.
Materials and Methods
Rat liver transplantation
Inbred male Lewis rats (200–250 g) were used as both donors and recipients in LT experiments. LT was performed under isofluorane anesthesia (2–3%) using the two-cuff technique with the hepatic artery and bile duct re-constructed as described previously [36]. Liver grafts were stored in University of Wisconsin storage solution (Bridge to Life, Ltd., Columbia, SC) at 0–1°C for 18 hours. Anhepatic time was ~17 min, and implantation surgery took ~40 min. For sham operation, ligaments around the liver were dissected, and the abdominal wall was closed 40 min later without transplantation.
Blood creatinine and urea nitrogen
Under pentobarbital anesthesia (50 mg/kg, ip) at 18 h after implantation, blood samples were collected from the inferior vena cava. Serum was obtained by centrifugation. Serum creatinine and blood urea nitrogen (BUN) were determined using analytical kits from Sigma-Aldrich (St. Louis, MO) and Bioassay Systems (Atlanta, GA), respectively, according to the manufacturers’ protocols.
Histology
Kidneys were collected 18 h after implantation under pentobarbital anesthesia and fixed with 4% paraformaldehyde (VWR Inc., West Chester, PA) in 0.1 mM phosphate buffered-saline (Mediatech Inc., Manassas, VA). Tissue blocks were imbedded in paraffin after 48-hour fixation. After hematoxylin-eosin (H&E) staining, kidney sections were analyzed microscopically for pathology (Zeiss Axiovert 100 microscope, Thornwood, NY) using a 20x objective lens [37].
Analysis of mitochondrial DNA (mtDNA) content
mtDNA copy number was assessed by quantitative polymerase chain reaction (qPCR) [38,39]. Total DNA was isolated from renal cortex using the DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA) and mtDNA content was determined as mtDNA-encoded NADH dehydrogenase-1 and normalized against the nuclear-encoded POU class 5 homeobox 1 gene as described previously [38,39].
Detection of the mRNAs of oxidative phosphorylation (OXPHOS) proteins and MB signaling molecules
mRNAs of ATP synthase-β (AS-β), NADH dehydrogenase-3 (ND3), peroxisome proliferator-activated receptor γ co-activator 1α (PGC-1α) and mitochondrial transcription factor A (Tfam) were detected by qPCR as described elsewhere [38,39]. PCR reactions were performed in triplicate with a reaction mixture containing 2x IQTMSYBR Supermix (Bio-Rad), cDNA template, and 0.1 nM of the forward and reverse primers using a CFX96 Real Time-PCR Detection System (Bio-Rad, Hercules, CA). The abundance of mRNA was normalized against hypoxanthine phospho-ribosyl-transferase using the ΔΔCt method.
PGC-1α immunoprecipitation (IP)
PGC-1α activity is inhibited by acetylation [40,41]. PGC-1α acetylation status was assessed by immunoblotting of acetylated lysine residues after IP of PGC-1α from kidney cortex homogenates as described previously [38]. Protein content in the immunoprecipitates was measured using the Bio-Rad reagent (Bio-Rad Laboratory, Hercules, CA), an equal amount of PGC-1α (10 μg) was loaded to each lane, and immunoblotting was performed using specific antibody against acetylated lysine residues (Cell Signaling Technology, Danvers, MA) [38].
Immunoblotting
Proteins of interest in renal cortex homogenates were detected by immunoblotting as described previously [36]. Primary antibodies were against: AS-β, neutrophil gelatinase-associated lipocalin (NGAL), Tfam (GenWay Biotech, San Diego, CA), ED-1 (Serotek, Raleigh, NC), myeloperoxidase (MPO; DAKO, Carpinteria, CA), cleaved caspase-3 (Cell Signaling Technology, Danvers, MA), dynamin-related GTPase protein Drp-1, fissin-1 (Fis-1), mitofusin-1 (Mfn-1), ND3, PGC-1α, PTEN-induced putative kinase 1 (PINK-1) (Santa Cruz Biotech., Santa Cruz, CA) and microtubule-associated protein 1A/1B-light chain 3 (LC3, MBL International, Des Plainers, IL) at concentrations of 1:100 to 1000, and actin (ICN, Costa Mesa, CA) at a concentration of 1:3000. Detection was achieved by chemiluminescence (Pierce Biotechnology, Rockford, IL).
Statistical analysis
Groups were compared using the Student’s-t test. There were 3–4 rats per group for all parameters. Data shown are means ± S.E.M. Differences were considered significant at p<0.05.
Ethics statement
All rats were given humane care in compliance with institutional guidelines using protocols approved by the Institutional Animal Care and Use Committee of Medical University of South Carolina. All survival surgeries were performed under isoflurane anesthesia (2–3%) and all non-survival surgeries were performed under sodium pentobarbital anesthesia (50 mg/kg, i.p.).
Results
Suppressed renal function after LT
Our previous study showed that transplantation of liver grafts after long preservation caused severe graft injury and decreased survival of recipients to ~25% [36]. Here we investigated whether renal function was altered in these recipients. Serum creatinine increased 2.2 fold and BUN increased 2.5 fold 18 h after LT (Fig 1A and 1B). These results demonstrate that renal dysfunction occurs in the recipients after LT.
Fig 1. Suppressed renal function after liver transplantation.
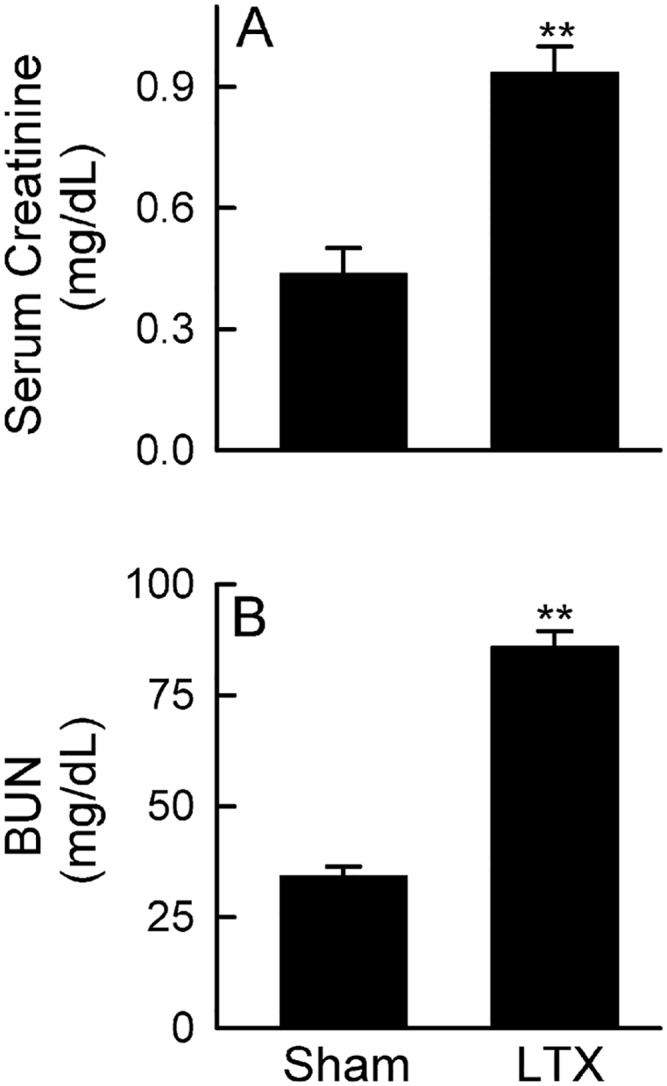
Transplantation was performed as described in “Methods” and blood was collected 18 h after sham-operation (Sham) or liver transplantation (LTX). A: serum creatinine; B: blood urea nitrogen (BUN). Values are mean ± SEM. **p<0.01 vs sham (n = 4/group).
Kidney injury after LT
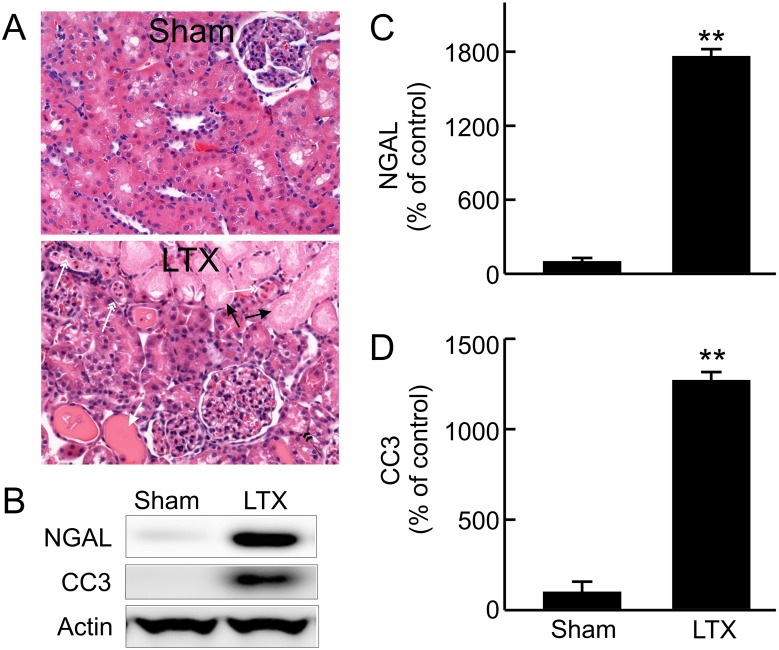
Renal dysfunction could occur in the presence or absence of overt renal injury. We examined whether pathological changes existed in kidneys of liver recipients (Fig 2A). The kidneys of sham-operated rats showed normal histology. Mild to moderate pathological changes were observed in the kidneys of recipients, mainly loss of brush border and vacuolization of tubular cells in the cortex. Cast formation and necrosis appeared in some proximal tubular cells. Infiltration of leukocytes was also observed in renal tissue and in the casts in tubular lumens. Most of these alterations appeared as patches and located in cortex. No overt pathological changes were observed in glomeruli.
Fig 2. Renal injury after liver transplantation.
Transplantation was performed as described in “Methods” and kidneys were collected 18 h after sham-operation (Sham) or liver transplantation (LTX). A, H&E-stained slides; black arrow, necrotic tubules; black double arrow head, loss of brush border; white arrow, hyaline cast formation; white double arrow head, infiltrated leukocytes and other cell debris in tubular lumen. B, representative immunoblot images of neutrophil gelatinase-associated lipocalin (NGAL) and cleaved caspase-3 (CC3); C, quantification of immunoblot images of NGAL by densitometry; D, quantification of immunoblot images of CC3 by densitometry. Values are mean ± SEM. **p<0.01 vs sham (n = 3-4/group).
The molecular markers of kidney injury also increased. NGAL, a marker of AKI [42], was barely detectable in the kidneys of sham-operated rats (Fig 2B and 2C). NGAL increased 18 fold after LT. Cleaved caspase-3 was barely detectable in kidneys from sham-operated rats but increased 13-fold after LT (Fig 2B and 2D), indicating apoptosis.
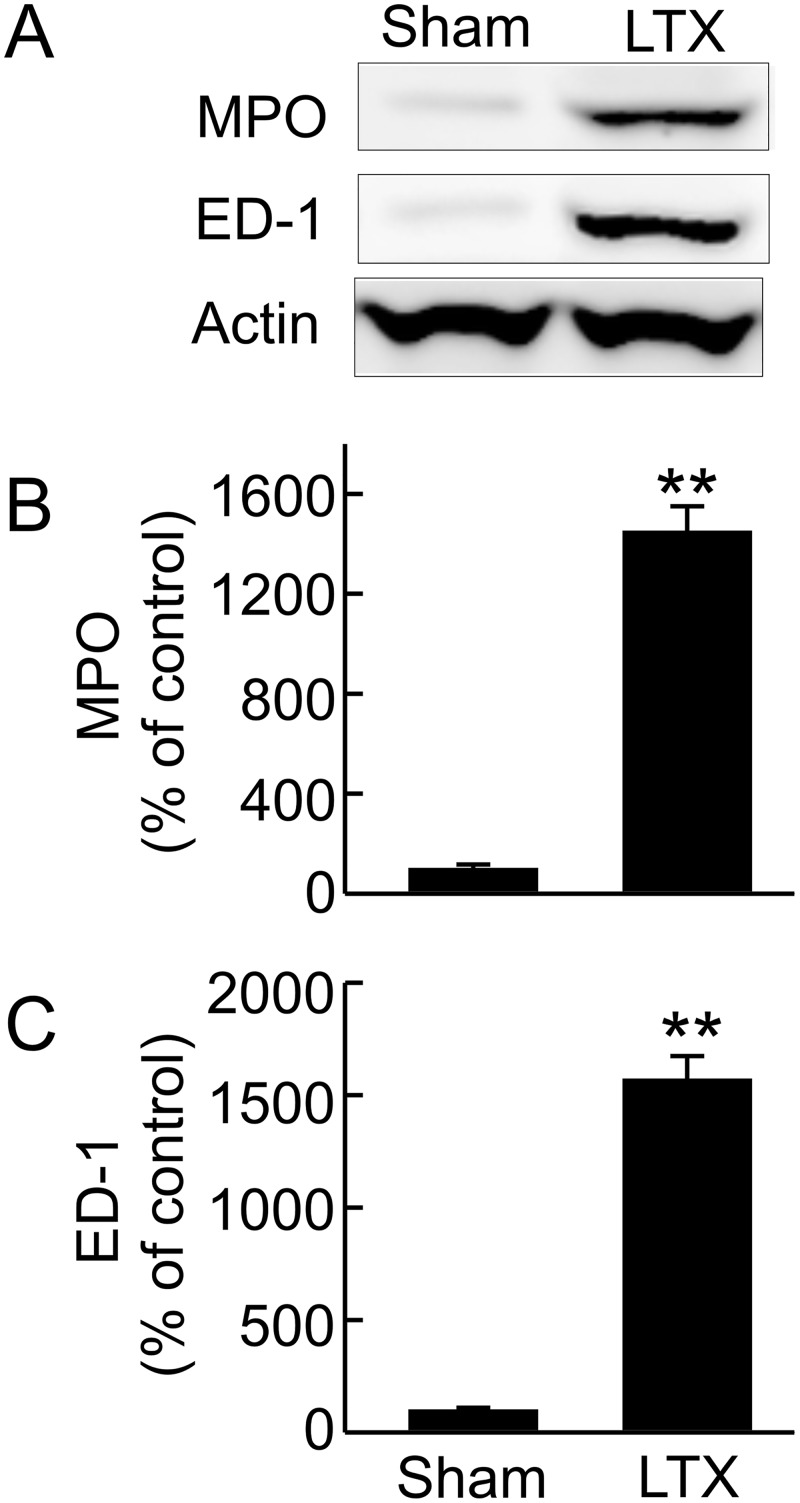
Consistent with histological leukocyte infiltration, MPO, an indicator of neutrophil infiltration, increased 15-fold after LT (Fig 3A and 3B) and ED-1, a marker of monocyte/macrophage infiltration, increased 16-fold after LT (Fig 3A and 3C).
Fig 3. Renal leukocyte infiltration after liver transplantation.
Transplantation was performed as described in “Methods” and kidneys were collected 18 h after sham-operation (Sham) or liver transplantation (LTX). A, representative immunoblot images of myeloperoxidase (MPO) and ED-1; B, quantification of immunoblot images of MPO by densitometry; C, quantification of immunoblot images of ED-1 by densitometry. Values are mean ± SEM. **p<0.01 vs sham (n = 3-4/group).
Decreases in renal mitochondrial OXPHOS proteins and associated mRNAs after LT
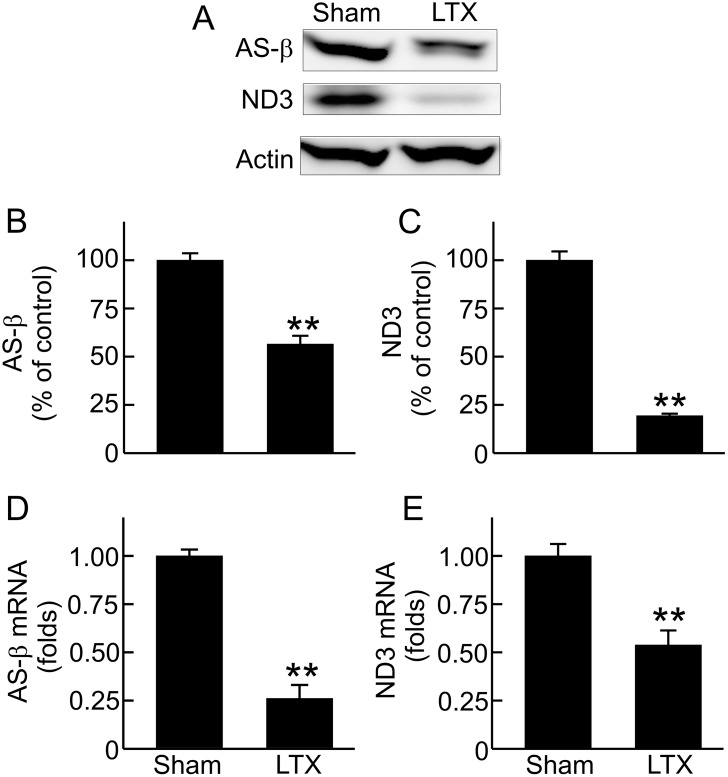
Mitochondrial dysfunction is linked to occurrence of AKI after many acute insults [21,22,24–28]. Decreases in mitochondrial respiratory chain proteins inhibit oxidative phosphorylation and ATP production. To investigate whether mitochondrial homeostasis is disrupted in the kidney after LT, we examined the renal mitochondrial OXPHOS proteins. AS-β, a subunit of mitochondrial respiratory chain Complex V that is encoded by nDNA, decreased 44% compared to sham-operated rats (Fig 4A and 4B). ND3, a mtDNA-encoded mitochondrial OXPHOS protein, decreased 81% (Fig 4A and 4C).
Fig 4. Decreases in renal ATP synthase-β (AS-β) and NADH dehydrogenase-3 (ND3) expression after liver transplantation.
Transplantation was performed as described in “Methods” and kidneys were collected 18 h after sham-operation (Sham) or liver transplantation (LTX). A, representative immunoblot images of AS-β and ND3; B, quantification of immunoblot images of AS-β by densitometry; C, quantification of immunoblot images of ND3; D, detection of AS-β mRNA by qPCR; E, detection of ND3 mRNA by qPCR. Values are mean ± SEM. **p<0.01 vs sham (n = 3-4/group).
We further measured the associated mRNAs of these proteins to determine whether decreases in these proteins after LT are linked to suppressed formation. AS-β and ND3 mRNAs decreased 74% and 46%, respectively, after LT (Fig 4D and 4E). These data reveal that decreases in AS-β and ND3 proteins are due, at least in part, to decreases in their expression.
Decreases in renal PGC-1α protein, mRNA and activation after LT
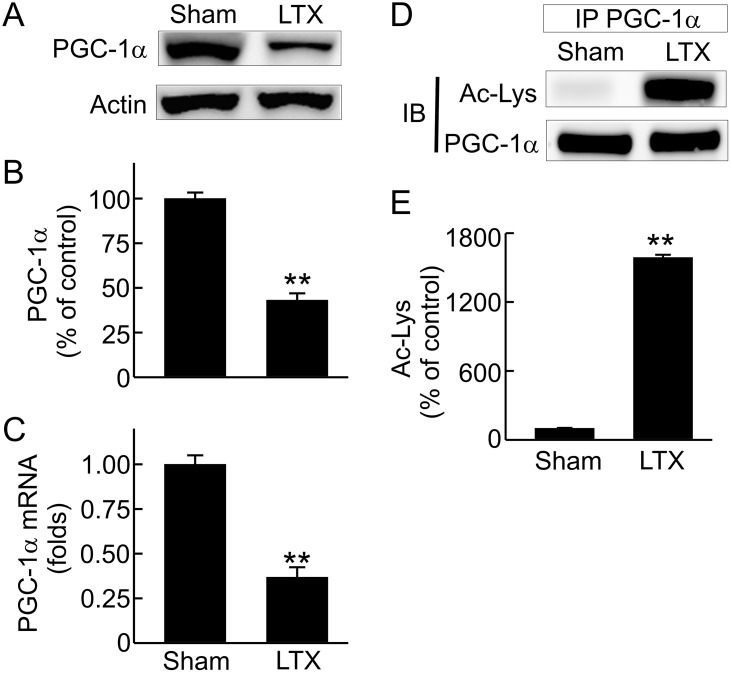
MB is primarily regulated by PGC-1α [40]. To explore why MB is altered after LT, we examined PGC-1α in the kidney. PGC-1α decreased 57% in the kidneys of liver recipients compared to sham-operated rats (Fig 5A and 5B). To explore whether decreased PGC-1α is due to suppressed expression, we examined its mRNA. Renal PGC-1α mRNA decreased 63% after LT (Fig 5C). We also investigated whether PGC-1α activation is altered after LT. PGC-1α activity is higher when de-acetylated [39]. PGC-1α acetylation status was detected by immunoblotting of acetylated lysine residuals in immunoprecipitated PGC-1α. After immunoprecipitation, PGC-1α was equally loaded to each lane to avoid the potential influence of decreased PGC-1α protein on detection of acetylation. Acetylated PGC-1α increased markedly after LT (Fig 5D and 5E), indicating suppressed PGC-1α activation.
Fig 5. Suppressed renal peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) expression and activation after liver transplantation.
Transplantation was performed as described in “Methods” and kidneys were collected 18 h after sham-operation (Sham) or liver transplantation (LTX). A, representative immunoblot images of PGC-1α; B, quantification of immunoblot images of PGC-1α by densitometry; C, PGC-1α mRNA detected by qPCR; D, representative immunoblot images of acetylated lysine residues (Ac-Lys) of immunoprecipitated (IP) PGC-1α; E, quantification of immunoblot images of Ac-Lys. Values are mean ± SEM. **p<0.01 vs sham (n = 3-4/group).
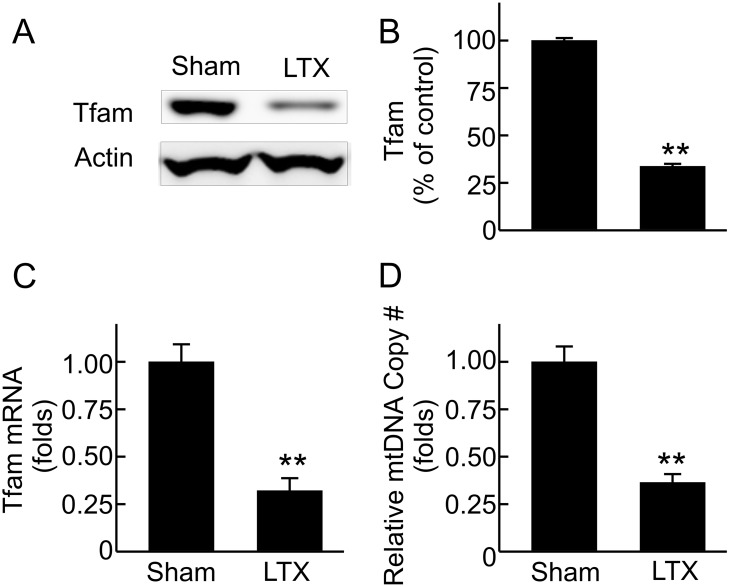
Decreased renal Tfam expression and mtDNA copy number after LT
In addition to controlling transcription of nDNA-encoded OXPHOS proteins, PGC-1α modulates expression of Tfam which regulates the replication and transcription of mtDNA [43]. Tfam decreased 66% and Tfam mRNA decreased 68% after LT (Fig 6A, 6B and 6C), consistent with suppressed Tfam expression. Moreover, renal mtDNA copy number decreased 64% after LT (Fig 6D).
Fig 6. Inhibited renal mitochondrial transcription factor A (Tfam) expression and decreased renal mitochondrial DNA (mtDNA) after liver transplantation.
Transplantation was performed as described in “Methods” and kidneys were collected 18 h after sham-operation (Sham) or liver transplantation (LTX). A, representative immunoblot images of Tfam; B, quantification of immunoblot images of Tfam by densitometry; C, Tfam mRNA detected by qPCR; D, relative mitochondrial DNA copy number detected by qPCR. Values are mean ± SEM. **p<0.01 vs sham (n = 3-4/group).
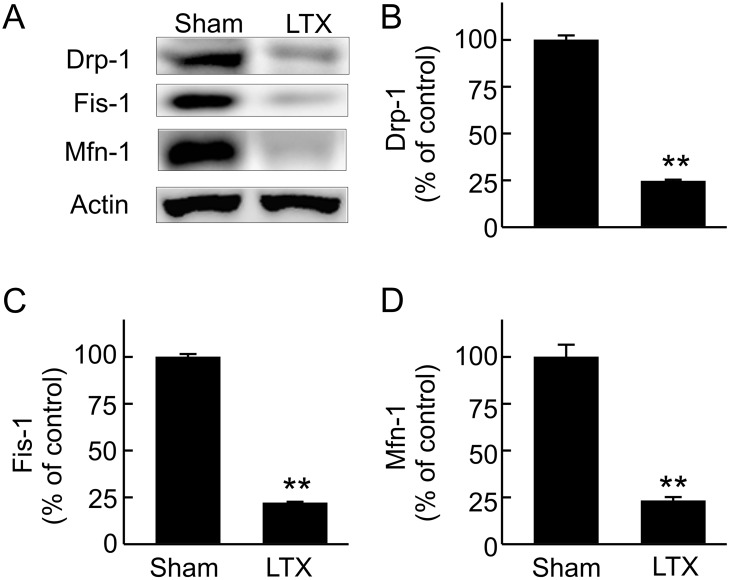
Suppressed renal mitochondrial dynamics after LT
Mitochondria undergo fission and fusion, and mitochondrial fission is required for MB [44]. We therefore investigated whether mitochondrial dynamic processes are altered after LT. Drp-1, the protein that controls mitochondrial fission, and Fis-1, the protein that recruits Drp-1 to the fission site [45], decreased 75% and 78%, respectively, after LT (Fig 7A, 7B and 7C). Mitofusins (Mfn) mediate mitochondrial fusion [46]. Mfn-1 decreased 77% after LT (Fig 7A and 7D). Together, these data reveal that mitochondrial dynamics are suppressed in the kidney after LT.
Fig 7. Suppressed renal mitochondrial dynamics after liver transplantation.
Transplantation was performed as described in “Methods” and kidneys were collected 18 h after sham-operation (Sham) or liver transplantation (LTX). A, representative immunoblot images of Drp-1, fissin-1 (Fis-1) and mitofusin-1 (Mfn-1); B, quantification of immunoblot images of Drp-1 by densitometry; C, quantification of immunoblot images of Fis-1 by densitometry; D, quantification of immunoblot images of Mfn-1 by densitometry. Values are mean ± SEM. **p<0.01 vs sham (n = 3-4/group).
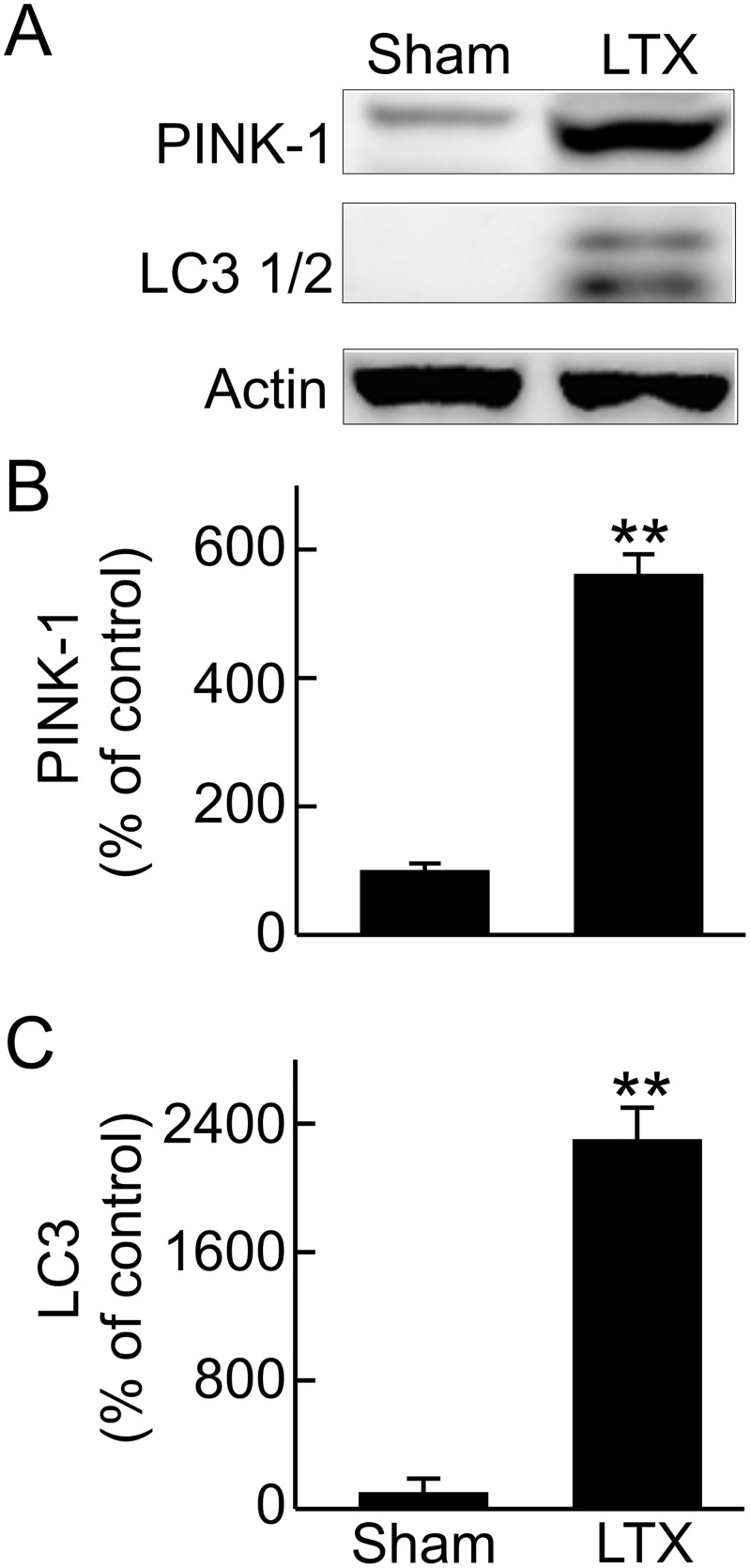
Enhanced renal mitophagy after LT
Mitophagy removes damaged or aged mitochondria [29,30]. PINK-1 which regulates mitophagy [47,48] was expressed at low levels in the kidneys of sham-operated rats but increased 5.6-fold after LT (Fig 8A and 8B). LC3, a mediator of autophagy as well as mitophagy, increased 23-fold in the kidney after LT (Fig 8A and 8C). These data reveal that renal mitophagy is stimulated after LT.
Fig 8. Increased renal mitophagy after liver transplantation.
Transplantation was performed as described in “Methods” and kidneys were collected 18 h after sham-operation (Sham) or liver transplantation (LTX). A, representative immunoblot images of PTEN-induced putative kinase 1 (PINK-1) and microtubule-associated protein 1A/1B-light chain 3 (LC3); B, quantification of immunoblot images of PINK-1 by densitometry; C, quantification of immunoblot images of LC3 by densitometry. Values are mean ± SEM. **p<0.01 vs sham (n = 3-4/group).
Discussion
AKI occurs frequently after LT, which decreases post-transplantation survival [7,10–13]. Therefore, understanding the mechanism and developing effective therapy for prevention and treatment of AKI is important for improvement of the clinical outcomes of LT. Renal dysfunction can occur in the presence (e.g. acute tubular necrosis, ATN) or absence of overt pathological changes (e.g. hepatorenal syndrome) [49–51]. The diagnosis of AKI currently depends on surrogate markers of kidney functions, such as increased serum creatinine and decreased urinary output [50–52]. Liver graft dysfunction is a strong predictor of AKI after LT, and combined liver failure and kidney failure substantially increases mortality [11,19,20]. In this study we show that transplantation of liver grafts after long cold-storage results in renal dysfunction and injury as revealed by increases in serum creatinine, BUN, renal NGAL expression and caspase-3 activation, and pathological changes.
The underlying mechanisms of AKI are poorly understood. In recent years emerging evidences indicate that mitochondrial dysfunction is an important contributor of AKI pathophysiology [21]. Mitochondrial ultrastructural changes such as decreased mitochondrial mass, disrupted cristae, and mitochondrial swelling occur in renal tubular cells during nephrotoxic, ischemic, and septic AKI [53–57]. Mitochondrial ultrastructural changes were observed in kidneys of hepatorenal syndrome patients [58]. Proper mitochondrial function requires maintaining mitochondrial homeostasis through removing damaged/depolarized mitochondria by mitophagy, synthesizing new mitochondrial components (e.g. OXPHOS proteins) and generating new mitochondria by MB, and maintaining mitochondrial dynamics [21,59,60]. Disruption of mitochondrial homeostasis is linked to AKI caused by many renal stressors/toxicants [21]. It is well known that chronic or acute severe liver injury/liver failure (e.g., cirrhosis, cholestasis, alcoholic or viral hepatitis, I/R injury and LT) causes AKI but whether mitochondrial homeostasis is disrupted in these cases remains unclear [61–66].
MB is an important adaptive process counteracting mitochondrial stress and damage (e.g. caused by toxicants and diseases) [23,67,68]. The majority of OXPHOS proteins are encoded by nDNA and transported into mitochondria after synthesis but 13 OXPHOS proteins are encoded by mtDNA and synthesized in mitochondria [68,69]. Recent studies showed that MB suppression occurs in many types of AKI [21]. In this study we demonstrate widespread decreases in renal OXPHOS proteins encoded by both nDNA and mtDNA in association with decreased corresponding mRNAs, indicating suppressed expression of OXPHOS proteins after LT. While sufficient mtDNA copy number is essential for MB and proper mitochondrial function [68], renal mtDNA decreased markedly (Fig 6), also indicating suppressed MB after LT. Together, these data demonstrate clearly that MB suppression also occurs in the kidney and contributes to post-LT AKI.
MB is coordinated by a complicated signaling system, and the transcriptional coactivator PGC-1α is recognized as the governing regulator of MB [70–73]. PGC-1α increases expression of nDNA-encoded OXPHOS enzymes and increases the expression of Tfam, which in turn controls the replication and transcription of mtDNA [70–73]. Therefore, PGC-1α also affects expression of mtDNA encoded OXPHOS proteins. Both expression of PGC-1α and its activation process (deacetylation) were inhibited in the kidney after LT. Consistently, Tfam and mtDNA were also diminished. Thus, suppressed MB after LT is due to inhibited PGC-1α signaling.
Mitophagy and mitochondrial dynamics are important processes that control mitochondrial quality [29,30,74]. Mitophagy occurs in response to starvation, loss of mitochondrial membrane potential or disruption of mitochondrial integrity [29,30]. Removal of damaged mitochondria via mitophagy in a timely manner is critical for cellular homeostasis and function [48,60]. Mitophagy occurs in a parkin-dependent and independent manner [48]. Mitochondria depolarization caused by various insults prevents degradation of PINK1, which in turn promotes parkin translocation to mitochondria. Parkin then enhances recruitment of autophagy receptor proteins (e.g., p62), which further recruit LC3 containing autophagosomes to execute mitophagy. For parkin-independent mitophagy, BNIP3, NIX, or cardiolipin directly interact with LC3 to recruit autophagosomes to remove damaged mitochondria [48,59]. Insufficient mitophagy has been associated with mitochondrial dysfunction and pathogenesis of many diseases such as Parkinson’s disease, cancer, cardiac dysfunction and hypertrophy, steatohepatitis, liver injury caused by hepatotoxicants, and inflammation [60]. However, overactive mitophagy may lead to mitochondrial loss and bioenergetic deficit, as in the case of hepatotoxicity of cadmium [75]. In the kidney, increased mitophagy has been observed in I/R- and sepsis-induced AKI and is thought to be renoprotective, possibly by removal of damaged mitochondria and mitochondria-generated ROS, and prevention of release of mtDNA and cytochrome c that cause inflammation and apoptosis, respectively [21,22,75,76]. In contrast, inhibited mitophagy was observed in high calorie diet-induced or hyperglycemic renal injury [31,77]. Our study showed that mitophagy is stimulated in the kidney after LT, which most likely reflects an attempt after LT to eliminate damaged mitochondria in the kidney.
Mitochondria undergo fission and fusion under physiological conditions [21,59]. Fission is thought to segregate damaged mitochondria which will be removed later, a process critical for mitochondrial quality control. Fission also participates in the process of MB to generate daughter mitochondria. Fusion allows the exchange of material between healthy and damaged mitochondria. Drp1 translocates from the cytosol to the outer mitochondrial membrane and interacts with receptor proteins in mitochondria (e.g., Fis1) to initiate fission [21,59]. Mitochondrial fusion is mediated by mitofusin 1 and 2 (Mfn1; Mfn2) and optic atrophy protein 1 [33,78]. Alterations of mitochondrial fission/fusion occur after I/R- and glycerol-induced AKI [22]. In this study we showed that after LT, Drp-1, Fis1 and Mfn1 all decreased in the kidney, indicating that fission and fusion processes in the kidney are suppressed after LT.
Taken together, MB and mitochondrial dynamics are inhibited in the kidney after LT and mitophagy increased. Such disruption of mitochondrial homeostasis decreases the capability of the kidney to counteract mitochondrial stress, maintain/recover mitochondrial function, and repair mitochondrial and cellular damage. Since renal tubular cells are highly mitochondrial energy-dependent, mitochondrial dysfunction will eventually lead to inhibition of renal function and renal cell damage. Based on these findings, stimulating MB to maintain mitochondrial homeostasis is a promising therapeutic target to prevent/treat AKI after LT. Stimulation of MB has been shown to be effective in several animal models of AKI and would most likely also be protective in AKI after LT.
Data Availability
All relevant data are within the paper.
Funding Statement
This study was supported, in part, by grants DK70844, DK073336 and DK037034 from the National Institutes of Health and grant #81470878 from Chinese National Natural Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Alqahtani SA. Update in liver transplantation. Curr Opin Gastroenterol. 2012; 10.1097/MOG.0b013e3283527f16 [DOI] [PubMed] [Google Scholar]
- 2. Wertheim JA, Baptista PM, Soto-Gutierrez A. Cellular therapy and bioartificial approaches to liver replacement. Curr Opin Organ Transplant. 2012; 10.1097/MOT.0b013e3283534ec9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Harland RC, Platt JL. Prospects for xenotransplantation of the liver. J Hepatol. 1996; 25: 248–258. [DOI] [PubMed] [Google Scholar]
- 4. Fox IJ, Langnas AN, Fristoe LW, Shaefer MS, Vogel JE, Antonson DL, et al. Successful application of extracorporeal liver perfusion: a technology whose time has come. Am J Gastroenterol. 1993; 88: 1876–1881. [PubMed] [Google Scholar]
- 5. Kobayashi N, Fujiwara T, Westerman KA, Inoue Y, Sakaguchi M, Noguchi H, et al. Prevention of acute liver failure in rats with reversibly immortalized human hepatocytes. Science 2000; 287: 1258–1262. [DOI] [PubMed] [Google Scholar]
- 6. Sampaio MS, Martin P, Bunnapradist S. Renal dysfunction in end-stage liver disease and post-liver transplant. Clin Liver Dis. 2014; 18: 543–560. S1089-3261(14)00031-2 [pii]; 10.1016/j.cld.2014.05.003 [DOI] [PubMed] [Google Scholar]
- 7. Pham PT, Slavov C, Pham PC. Acute kidney injury after liver, heart, and lung transplants: dialysis modality, predictors of renal function recovery, and impact on survival. Adv Chronic Kidney Dis. 2009; 16: 256–267. S1548-5595(09)00080-9 [pii]; 10.1053/j.ackd.2009.04.002 [DOI] [PubMed] [Google Scholar]
- 8. Biancofiore G, Davis CL. Renal dysfunction in the perioperative liver transplant period. Curr Opin Organ Transplant. 2008; 13: 291–297. 10.1097/MOT.0b013e328300a058 00075200-200806000-00014 [pii]. [DOI] [PubMed] [Google Scholar]
- 9. Nadim MK, Genyk YS, Tokin C, Fieber J, Ananthapanyasut W, Ye W, et al. Impact of the etiology of acute kidney injury on outcomes following liver transplantation: acute tubular necrosis versus hepatorenal syndrome. Liver Transpl. 2012; 18: 539–548. 10.1002/lt.23384 [DOI] [PubMed] [Google Scholar]
- 10. McCauley J, Van Thiel DH, Starzl TE, Puschett JB. Acute and chronic renal failure in liver transplantation. Nephron 1990; 55: 121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weber ML, Ibrahim HN, Lake JR. Renal dysfunction in liver transplant recipients: evaluation of the critical issues. Liver Transpl. 2012; 18: 1290–1301. 10.1002/lt.23522 [DOI] [PubMed] [Google Scholar]
- 12. O'Riordan A, Dutt N, Cairns H, Rela M, O'Grady JG, Heaton N, et al. Renal biopsy in liver transplant recipients. Nephrol Dial Transplant. 2009; 24: 2276–2282. gfp112 [pii]; 10.1093/ndt/gfp112 [DOI] [PubMed] [Google Scholar]
- 13. Barri YM, Sanchez EQ, Jennings LW, Melton LB, Hays S, Levy MF, et al. Acute kidney injury following liver transplantation: definition and outcome. Liver Transpl. 2009; 15: 475–483. 10.1002/lt.21682 [DOI] [PubMed] [Google Scholar]
- 14. Fraley DS, Burr R, Bernardini J, Angus D, Kramer DJ, Johnson JP. Impact of acute renal failure on mortality in end-stage liver disease with or without transplantation. Kidney Int. 1998; 54: 518–524. 10.1046/j.1523-1755.1998.00004.x [DOI] [PubMed] [Google Scholar]
- 15. Gonwa TA, McBride MA, Anderson K, Mai ML, Wadei H, Ahsan N Continued influence of preoperative renal function on outcome of orthotopic liver transplant (OLTX) in the US: where will MELD lead us? Am J Transplant. 2006; 6: 2651–2659. AJT1526 [pii]; 10.1111/j.1600-6143.2006.01526.x [DOI] [PubMed] [Google Scholar]
- 16. Zhu M, Li Y, Xia Q, Wang S, Qiu Y, Che M, et al. Strong impact of acute kidney injury on survival after liver transplantation. Transplant Proc. 2010; 42: 3634–3638. S0041-1345(10)01302-3 [pii]; 10.1016/j.transproceed.2010.08.059 [DOI] [PubMed] [Google Scholar]
- 17. Mehta RL. Outcomes research in acute renal failure. Semin Nephrol. 2003; 23: 283–294. S0270929503000640 [pii]. [DOI] [PubMed] [Google Scholar]
- 18. Mehta RL, Chertow GM. Acute renal failure definitions and classification: time for change? J Am Soc Nephrol. 2003; 14: 2178–2187. [DOI] [PubMed] [Google Scholar]
- 19. Cabezuelo JB, Ramirez P, Rios A, Acosta F, Torres D, Sansano T, et al. Risk factors of acute renal failure after liver transplantation. Kidney Int. 2006; 69: 1073–1080. 5000216 [pii]; 10.1038/sj.ki.5000216 [DOI] [PubMed] [Google Scholar]
- 20. Iglesias JI, DePalma JA, Levine JS. Risk factors for acute kidney injury following orthotopic liver transplantation: the impact of changes in renal function while patients await transplantation. BMC Nephrol. 2010; 11: 30 1471-2369-11-30 [pii]; 10.1186/1471-2369-11-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stallons LJ, Funk JA, Schnellmann RG. Mitochondrial Homeostasis in Acute Organ Failure. Curr Pathobiol Rep. 2013; 1 10.1007/s40139-013-0023-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Funk JA, Schnellmann RG. Persistent disruption of mitochondrial homeostasis after acute kidney injury. Am J Physiol Renal Physiol. 2012; 302: F853–F864. ajprenal.00035.2011 [pii]; 10.1152/ajprenal.00035.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Attardi G, Schatz G. Biogenesis of mitochondria. Annu Rev Cell Biol. 1988; 4: 289–333. [DOI] [PubMed] [Google Scholar]
- 24. Smith JA, Stallons LJ, Collier JB, Chavin KD, Schnellmann RG. Suppression of mitochondrial biogenesis through toll-like receptor 4-dependent mitogen-activated protein kinase kinase/extracellular signal-regulated kinase signaling in endotoxin-induced acute kidney injury. J Pharmacol Exp Ther. 2015; 352: 346–357. jpet.114.221085 [pii]; 10.1124/jpet.114.221085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stallons LJ, Whitaker RM, Schnellmann RG. Suppressed mitochondrial biogenesis in folic acid-induced acute kidney injury and early fibrosis. Toxicol Lett. 2014; 224: 326–332. S0378-4274(13)01420-3 [pii]; 10.1016/j.toxlet.2013.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jesinkey SR, Funk JA, Stallons LJ, Wills LP, Megyesi JK, Beeson CC, et al. Formoterol restores mitochondrial and renal function after ischemia-reperfusion injury. J Am Soc Nephrol. 2014; 25: 1157–1162. ASN.2013090952 [pii]; 10.1681/ASN.2013090952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Garrett SM, Whitaker RM, Beeson CC, Schnellmann RG. Agonism of the 5-hydroxytryptamine 1F receptor promotes mitochondrial biogenesis and recovery from acute kidney injury. J Pharmacol Exp Ther. 2014; 350: 257–264. jpet.114.214700 [pii]; 10.1124/jpet.114.214700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Korrapati MC, Shaner BE, Schnellmann RG. Recovery from glycerol-induced acute kidney injury is accelerated by suramin. J Pharmacol Exp Ther. 2012; 341: 126–136. jpet.111.190249 [pii]; 10.1124/jpet.111.190249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim I, Rodriguez-Enriquez S, Lemasters JJ. Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys. 2007; 462: 245–253. S0003-9861(07)00162-2 [pii]; 10.1016/j.abb.2007.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lemasters JJ. Variants of mitochondrial autophagy: Types 1 and 2 mitophagy and micromitophagy (Type 3). Redox Biol. 2014; 2: 749–754. 10.1016/j.redox.2014.06.004 S2213-2317(14)00076-7 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gunst J, Derese I, Aertgeerts A, Ververs EJ, Wauters A, Van Den Berghe G, et al. Insufficient autophagy contributes to mitochondrial dysfunction, organ failure, and adverse outcome in an animal model of critical illness. Crit Care Med. 2013; 41: 182–194. 10.1097/CCM.0b013e3182676657 [DOI] [PubMed] [Google Scholar]
- 32. Liesa M, Palacin M, Zorzano A. Mitochondrial dynamics in mammalian health and disease. Physiol Rev. 2009; 89: 799–845. 89/3/799 [pii]; 10.1152/physrev.00030.2008 [DOI] [PubMed] [Google Scholar]
- 33. Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006; 125: 1241–1252. S0092-8674(06)00768-9 [pii]; 10.1016/j.cell.2006.06.010 [DOI] [PubMed] [Google Scholar]
- 34. Suen DF, Norris KL, Youle RJ. Mitochondrial dynamics and apoptosis. Genes Dev. 2008; 22: 1577–1590. 22/12/1577 [pii]; 10.1101/gad.1658508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Olichon A, Baricault L, Gas N, Guillou E, Valette A, Belenguer P, et al. Loss of OPA1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome c release and apoptosis. J Biol Chem. 2003; 278: 7743–7746. 10.1074/jbc.C200677200 C200677200 [pii]. [DOI] [PubMed] [Google Scholar]
- 36. Liu Q, Rehman H, Shi Y, Krishnasamy Y, Lemasters JJ, Smith CD, et al. Inhibition of sphingosine kinase-2 suppresses inflammation and attenuates graft injury after liver transplantation in rats. PLoS One 2012; 7: e41834 10.1371/journal.pone.0041834 PONE-D-12-14479 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhong Z, Ramshesh VK, Rehman H, Currin RT, Sridharan V, Theruvath TP, et al. Activation of the oxygen-sensing signal cascade prevents mitochondrial injury after mouse liver ischemia-reperfusion. Am J Physiol Gastrointest Liver Physiol. 2008; 295: G823–G832. 10.1152/ajpgi.90287.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rehman H, Krishnasamy Y, Haque K, Thurman RG, Lemasters JJ, Schnellmann RG, et al. Green tea polyphenols stimulate mitochondrial biogenesis and improve renal function after chronic cyclosporin a treatment in rats. PLoS One 2013; 8: e65029 10.1371/journal.pone.0065029 PONE-D-13-05193 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Funk JA, Odejinmi S, Schnellmann RG. SRT1720 induces mitochondrial biogenesis and rescues mitochondrial function after oxidant injury in renal proximal tubule cells. J Pharmacol Exp Ther. 2010; 333: 593–601. jpet.109.161992 [pii]; 10.1124/jpet.109.161992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr Rev. 2003; 24: 78–90. [DOI] [PubMed] [Google Scholar]
- 41. Pirola L, Zerzaihi O, Vidal H, Solari F. Protein acetylation mechanisms in the regulation of insulin and insulin-like growth factor 1 signalling. Mol Cell Endocrinol. 2012; 362: 1–10. S0303-7207(12)00305-X [pii]; 10.1016/j.mce.2012.05.011 [DOI] [PubMed] [Google Scholar]
- 42. Goldstein SL. Acute kidney injury biomarkers: renal angina and the need for a renal troponin I. BMC Med. 2011; 9: 135 1741-7015-9-135 [pii]; 10.1186/1741-7015-9-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Virbasius JV, Scarpulla RC. Activation of the human mitochondrial transcription factor A gene by nuclear respiratory factors: a potential regulatory link between nuclear and mitochondrial gene expression in organelle biogenesis. Proc Natl Acad Sci U S A. 1994; 91: 1309–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dikov D, Reichert AS. How to split up: lessons from mitochondria. EMBO J. 2011; 30: 2751–2753. emboj2011219 [pii]; 10.1038/emboj.2011.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Huang P, Galloway CA, Yoon Y. Control of mitochondrial morphology through differential interactions of mitochondrial fusion and fission proteins. PLoS One 2011; 6: e20655 10.1371/journal.pone.0020655 PONE-D-10-02872 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003; 160: 189–200. 10.1083/jcb.200211046 jcb.200211046 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lazarou M, Jin SM, Kane LA, Youle RJ. Role of PINK1 binding to the TOM complex and alternate intracellular membranes in recruitment and activation of the E3 ligase Parkin. Dev Cell. 2012; 22: 320–333. S1534-5807(11)00580-6 [pii]; 10.1016/j.devcel.2011.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jin SM, Youle RJ. PINK1- and Parkin-mediated mitophagy at a glance. J Cell Sci. 2012; 125: 795–799. 125/4/795 [pii]; 10.1242/jcs.093849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wadei HM, Gonwa TA. Hepatorenal Syndrome in the Intensive Care Unit. J Intensive Care Med. 2011; 0885066611408692 [pii]; 10.1177/0885066611408692 [DOI] [PubMed] [Google Scholar]
- 50. Bagshaw SM, Bellomo R, Devarajan P, Johnson C, Karvellas CJ, Kutsiogiannis DJ, et al. Acute kidney injury in critical illness. Can J Anaesth. 2010; 57: 985–998. 10.1007/s12630-010-9375-4 [DOI] [PubMed] [Google Scholar]
- 51. Francoz C, Durand F. A new look at renal dysfunction in the cirrhotic patient. Crit Care 2012; 16: 118 cc11207 [pii]; 10.1186/cc11207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bagshaw SM, Bellomo R, Devarajan P, Johnson C, Karvellas CJ, Kutsiogiannis DJ, et al. Renal support in critical illness. Can J Anaesth. 2010; 57: 999–1013. 10.1007/s12630-010-9376-3 [DOI] [PubMed] [Google Scholar]
- 53. Tran M, Tam D, Bardia A, Bhasin M, Rowe GC, Kher A, et al. PGC-1alpha promotes recovery after acute kidney injury during systemic inflammation in mice. J Clin Invest. 2011; 121: 4003–4014. 58662 [pii]; 10.1172/JCI58662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Parikh SM, Yang Y, He L, Tang C, Zhan M, Dong Z. Mitochondrial function and disturbances in the septic kidney. Semin Nephrol. 2015; 35: 108–119. S0270-9295(15)00012-1 [pii]; 10.1016/j.semnephrol.2015.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Brooks C, Wei Q, Cho SG, Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest. 2009; 119: 1275–1285. 37829 [pii]; 10.1172/JCI37829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zsengeller ZK, Ellezian L, Brown D, Horvath B, Mukhopadhyay P, Kalyanaraman B, et al. Cisplatin nephrotoxicity involves mitochondrial injury with impaired tubular mitochondrial enzyme activity. J Histochem Cytochem. 2012; 60: 521–529. 0022155412446227 [pii]; 10.1369/0022155412446227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Manny J, Livni N, Schiller M, Guttman A, Boss J, Rabinovici N. Structural changes in the perfused canine kidney exposed to the direct action of endotoxin. Isr J Med Sci. 1980; 16: 153–161. [PubMed] [Google Scholar]
- 58. Mandal AK, Lansing M, Fahmy A. Acute tubular necrosis in hepatorenal syndrome: an electron microscopy study. Am J Kidney Dis. 1982; 2: 363–374. S0272638682000432 [pii]. [DOI] [PubMed] [Google Scholar]
- 59. Ni HM, Williams JA, Ding WX. Mitochondrial dynamics and mitochondrial quality control. Redox Biol. 2015; 4: 6–13. S2213-2317(14)00118-9 [pii]; 10.1016/j.redox.2014.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Redmann M, Dodson M, Boyer-Guittaut M, Darley-Usmar V, Zhang J. Mitophagy mechanisms and role in human diseases. Int J Biochem Cell Biol. 2014; S1357-2725(14)00168-X [pii]; 10.1016/j.biocel.2014.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Choi HK, Song YG, Han SH, Ku NS, Jeong SJ, Baek JH, et al. Clinical features and outcomes of acute kidney injury among patients with acute hepatitis A. J Clin Virol. 2011; 52: 192–197. S1386-6532(11)00288-5 [pii]; 10.1016/j.jcv.2011.07.013 [DOI] [PubMed] [Google Scholar]
- 62. Bahirwani R, Shaked O, Bewtra M, Forde K, Reddy KR. Acute-on-chronic liver failure before liver transplantation: impact on posttransplant outcomes. Transplantation 2011; 92: 952–957. 10.1097/TP.0b013e31822e6eda [DOI] [PubMed] [Google Scholar]
- 63. Warner NS, Cuthbert JA, Bhore R, Rockey DC. Acute kidney injury and chronic kidney disease in hospitalized patients with cirrhosis. J Investig Med. 2011; 59: 1244–1251. 10.231/JIM.0b013e3182321471 [DOI] [PubMed] [Google Scholar]
- 64. Lee HT, Park SW, Kim M, D'Agati VD. Acute kidney injury after hepatic ischemia and reperfusion injury in mice. Lab Invest. 2009; 89: 196–208. labinvest2008124 [pii]; 10.1038/labinvest.2008.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Holt S, Marley R, Fernando B, Harry D, Anand R, Goodier D, et al. Acute cholestasis-induced renal failure: effects of antioxidants and ligands for the thromboxane A2 receptor. Kidney Int. 1999; 55: 271–277. 10.1046/j.1523-1755.1999.00252.x [DOI] [PubMed] [Google Scholar]
- 66. Altamirano J, Fagundes C, Dominguez M, Garcia E, Michelena J, Cardenas A, et al. Acute kidney injury is an early predictor of mortality for patients with alcoholic hepatitis. Clin Gastroenterol Hepatol. 2012; 10: 65–71. S1542-3565(11)01000-7 [pii]; 10.1016/j.cgh.2011.09.011 [DOI] [PubMed] [Google Scholar]
- 67. Herrmann JM, Longen S, Weckbecker D, Depuydt M. Biogenesis of mitochondrial proteins. Adv Exp Med Biol. 2012; 748: 41–64. 10.1007/978-1-4614-3573-0_3 [DOI] [PubMed] [Google Scholar]
- 68. Robin ED, Wong R. Mitochondrial DNA molecules and virtual number of mitochondria per cell in mammalian cells. J Cell Physiol. 1988; 136: 507–513. [DOI] [PubMed] [Google Scholar]
- 69. Koehler CM. New developments in mitochondrial assembly. Annu Rev Cell Dev Biol. 2004; 20: 309–335. [DOI] [PubMed] [Google Scholar]
- 70. Huss JM, Torra IP, Staels B, Giguere V, Kelly DP. Estrogen-related receptor alpha directs peroxisome proliferator-activated receptor alpha signaling in the transcriptional control of energy metabolism in cardiac and skeletal muscle. Mol Cell Biol. 2004; 24: 9079–9091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Winder WW, Taylor EB, Thomson DM. Role of AMP-activated protein kinase in the molecular adaptation to endurance exercise. Med Sci Sports Exerc. 2006; 38: 1945–1949. [DOI] [PubMed] [Google Scholar]
- 72. Lee HC, Wei YH. Mitochondrial biogenesis and mitochondrial DNA maintenance of mammalian cells under oxidative stress. Int J Biochem Cell Biol. 2005; 37: 822–834. [DOI] [PubMed] [Google Scholar]
- 73. Scarpulla RC. Nuclear activators and coactivators in mammalian mitochondrial biogenesis. Biochim Biophys Acta. 2002; 1576: 1–14. [DOI] [PubMed] [Google Scholar]
- 74. Hoppins S. The regulation of mitochondrial dynamics. Curr Opin Cell Biol. 2014; 29C: 46–52. S0955-0674(14)00039-8 [pii]; 10.1016/j.ceb.2014.03.005 [DOI] [PubMed] [Google Scholar]
- 75. Pi H, Xu S, Zhang L, Guo P, Li Y, Xie J, et al. Dynamin 1-like-dependent mitochondrial fission initiates overactive mitophagy in the hepatotoxicity of cadmium. Autophagy 2013; 9: 1780–1800. 25665 [pii]; 10.4161/auto.25665 [DOI] [PubMed] [Google Scholar]
- 76. Ishihara M, Urushido M, Hamada K, Matsumoto T, Shimamura Y, Ogata K, et al. Sestrin-2 and BNIP3 regulate autophagy and mitophagy in renal tubular cells in acute kidney injury. Am J Physiol Renal Physiol. 2013; 305: F495–F509. ajprenal.00642.2012 [pii]; 10.1152/ajprenal.00642.2012 [DOI] [PubMed] [Google Scholar]
- 77. Cui J, Shi S, Sun X, Cai G, Cui S, Hong Q, et al. Mitochondrial autophagy involving renal injury and aging is modulated by caloric intake in aged rat kidneys. PLoS One 2013; 8: e69720 10.1371/journal.pone.0069720 PONE-D-13-01396 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hoppins S, Nunnari J. The molecular mechanism of mitochondrial fusion. Biochim Biophys Acta 2009; 1793: 20–26. S0167-4889(08)00255-3 [pii]; 10.1016/j.bbamcr.2008.07.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.