Abstract
The effect of macromolecular crowding on the structure, dynamics, and reactivity of biomolecules is well-established and the relevant research has been extensively reviewed. Herein, we focus our discussion on crowding effects arising from small co-solvent molecules and densely packed surface conditions. In addition, we highlight recent efforts that capitalize on the excluded volume effect for various tailored biochemical and biophysical applications. Specifically, we discuss how a targeted increase in local mass density can be exploited to gain insight into the folding dynamics of the protein of interest and how confinement via reverse micelles can be used to study a range of biophysical questions, from protein hydration dynamics to amyloid formation.
A large number of macromolecules and molecular complexes exist inside a living cell, leading to a very crowded environment.1 Due to volume exclusion, the accessible free space and available water molecules become limited for all cellular components, which, in principle, can have a profound effect on the physical and chemical properties of the biomolecules confined inside the cell. To characterize the excluded volume effect on various biophysical and biochemical processes, in vitro studies have employed a variety of macromolecules, such as polymers (e.g., dextran and Ficoll) and large proteins (e.g., lysozyme and bovine serum albumin), to tune the degree of crowding of the system of interest. As expected, these studies revealed that macromolecular crowding can indeed affect, sometimes significantly, the thermodynamic and/or kinetic properties of the biological molecule(s) in question. Interested readers can find several up-to-date reviews on this very active research area.2–5 What is surprising, however, is that a number of recent studies have indicated that the excluded volume effect can also arise from unexpected sources, such as trimethylamine N-oxide (TMAO) and trifluoroethanol (TFE). Findings from these studies suggest that molecular crowding arising from molecules that are a fraction of the size of macromolecular crowders may be more common than previously assumed and should be considered when interpreting experimental results. Below, we will first discuss this phenomenon in the context of biomolecular folding and then highlight recent advances in using specifically engineered molecular-level crowding and/or confinement to achieve various goals in biophysical chemistry.
Nano-Crowding Arising from Solvents
The phrase “nano-crowding” was first coined by Thirumalai, Straub, and coworkers.6 In an effort to understand the stabilizing mechanism of a commonly encountered osmolyte, trimethylamine N-oxide (TMAO), they conducted all-atom molecular dynamics (MD) simulations on a series of peptides in a 1 M TMAO solution. For short dipeptides, which were used as models of the unfolded state, they found that TMAO molecules preferentially form hydrogen bonds (H-bonds) with peptide backbone units and basic sidechains, thus limiting their conformational degrees of freedom. For longer polypeptides that are capable of forming intramolecular H-bonds, they found that addition of TMAO increased the population of α-helical conformations as the co-solvent molecules are depleted from the backbone. As shown (Figure 1), simulations of the Aβ16–22 peptide in various concentrations of TMAO yielded similar results, as the peptide formed a more compact structure with increasing concentrations of co-solvent. These findings thus prompted the authors to conclude that TMAO can act as a nano-crowding agent by entropically destabilizing a peptide’s unfolded state while stabilizing the folded state via the excluded volume effect. A recent simulation study of Shea and coworkers7 on how osmolytes affect the structural changes and aggregation propensities of the amyloid protein tau also supports this mechanism.
Figure 1.
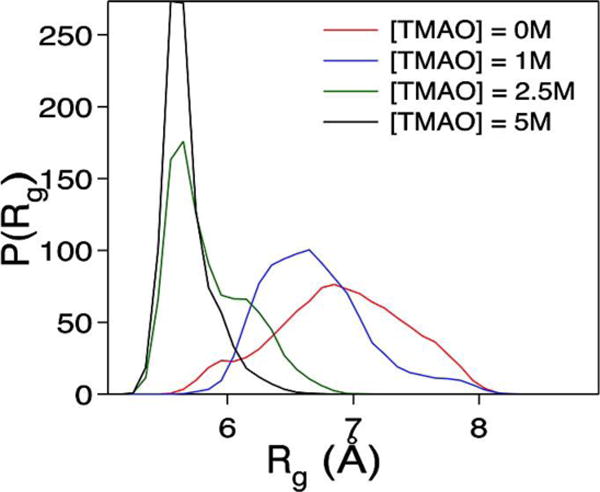
The probability distribution of the radius of gyration, P(Rg), of Aβ16–22 in varying TMAO concentrations, as indicated. As the concentration of TMAO increased, the Rg decreased indicating that the peptide became more compact. Reprinted with permission from ref. 6. Copyright 2011 American Chemical Society.
While the proposed crowding role of TMAO is corroborated by its ability to promote compact protein structures,8,9 a more direct experimental validation of this phenomenon came from a recent study by Ma et al.10 Specifically, they used two-dimensional infrared (2D IR) spectroscopy and the nitrile stretching vibration of p-cyanophenylalanine (PheCN) as a site-specific IR reporter11 to directly assess the excluded volume effect of TMAO. Their experimental design was based on the notion that any entropic effect arising from volume exclusion of the co-solvent molecules will lead to an increase in the degree of conformational rigidity of the peptide and protein systems in question, thereby resulting in a longer spectral diffusion time of the IR reporter.12 The latter is a measure of the dynamics that lead to fluctuations in the vibrational frequency of the IR probe. As indicated (Figure 2), their 2D IR results showed that in the presence of TMAO, the spectral diffusion kinetics of the nitrile probe became slower, as evidenced by an increase in the offset of the respective kinetic traces. Since this kinetic offset results from protein or peptide conformational dynamics that contribute to the nitrile frequency fluctuations but are too slow to be resolved in these experiments, their finding thus corroborates the idea that TMAO can influence protein conformational stability and dynamics via the excluded volume effect.
Figure 2.
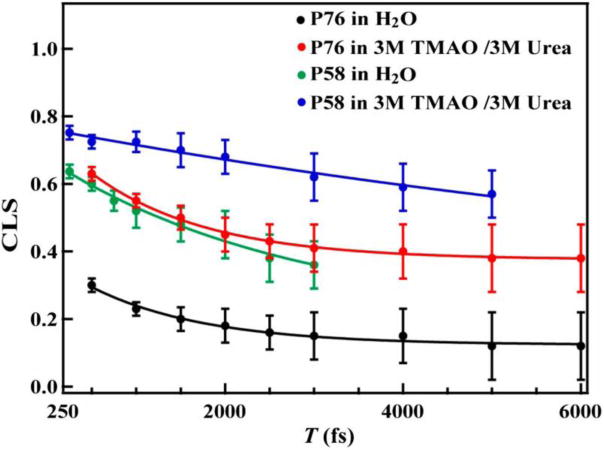
Spectral diffusion dynamics of the nitrile stretching vibration of PheCN in HP35-P76 and HP35-P58 obtained under different solvent conditions, as measured by the center line slope (CLS) of the corresponding 2D IR spectrum as a function of the waiting time (T). Addition of TMAO leads to an increase in the vertical offset of the kinetics, indicating an increase in the percentage of conformational states that interconvert on a timescale that is slower than the time window of the experiment. Reprinted with permission from ref. 10. Copyright 2014 Proceedings of the National Academy of Science.
Another piece of experimental evidence supporting this conclusion came from a recent study of Nesbitt and coworkers.13 Using single-molecule fluorescence resonance energy transfer (smFRET) spectroscopy, they examined the folding/unfolding kinetics of an 8 base pair DNA duplex and found that TMAO stabilized the folded structure by both increasing the folding rate and decreasing the unfolding rate. However, further van’t Hoff and Eyring analyses led them to conclude that the TMAO-induced stabilization of the folded state largely resulted from an increased entropic cost of the unfolding process. The authors suggested that such an entropic mechanism could be due to TMAO acting as a nano-crowder. Their conclusion was in line with an earlier computational study14 on RNA hairpin formation in the presence of TMAO, which showed that if the interactions between TMAO and the RNA are repulsive, then this osmolyte is effectively acting as a small crowding agent that stabilizes the folded state via the excluded volume effect.
Such a crowding effect is not unique to TMAO, as other osmolyte molecules have also been shown or proposed to exhibit similar actions. For example, the statistical study of Graziano15 on a model protein suggested that the ability of trehalose to counteract the denaturation effect of urea is due to the increased cost of cavity creation in trehalose/urea solutions, which outweighs the destabilizing interactions of the protein with urea molecules. This essentially suggests that trehalose protects the folded state from unfolding via the excluded volume effect. Additionally, a recent study by Jain et al.16 hypothesized that the ability of the osmolyte glycine betaine (GB) to counteract the destabilizing effect of chemical denaturants and temperature changes on ferricytochrome c and ferrocytochrome c is partially the result of zwitterionic GB being excluded from the protein surface in much the same way as TMAO. Moreover, using MD simulations, Zhou and coworkers17 observed that in the presence of guanidinium chloride (GdmCl), urea actually induced a crowding effect on two proteins, hen egg-white lysozyme and protein L, causing them to collapse. In this case, GdmCl, which has a stronger electrostatic interaction with the protein, caused urea to be depleted from the protein surface. However, the displaced urea molecules accumulated near the protein, producing a “local crowding” effect. Taken together, these studies suggest that when a co-solvent molecule is preferentially depleted from the surface of a biomolecule, a measurable excluded volume effect may arise.
Motivated by these previous studies and also the fact that trifluoroethanol (TFE), a co-solvent commonly used to enhance protein secondary structure formation, can form clusters at certain volume percentages,18 Culik et al.19 hypothesized that TFE could act as a nano-crowder to increase the rate of protein folding. To test this hypothesis, they studied the effect of various percentages of TFE in water on the folding rate of an intrinsically disordered peptide, the phosphorylated kinase inducible domain (pKID) peptide. As shown (Figure 3), upon increasing the TFE percentage from 5% to 15%, there was an increase in the relaxation rate and hence, the folding rate of the peptide, which remained unchanged up to 30% TFE. Further increase of the TFE percentage led to a decrease in the relaxation rate. Since the cluster formation of TFE was previously shown to reach its maximum around 30% TFE, these results thus support the idea that TFE can affect the structure and dynamics of polypeptides via volume exclusion.
Figure 3.
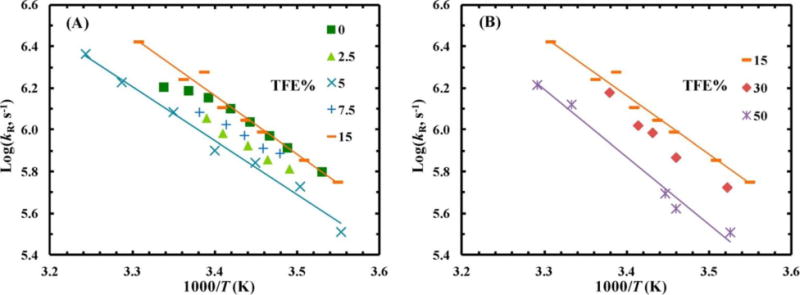
Conformational relaxation rate constant, kR, of pKID versus temperature, T, for various TFE percentages, as indicated. The solid lines are to guide the eye. Reprinted with permission from ref. 19. Copyright 2014 American Chemical Society.
We hope these abovementioned examples provide compelling evidence that small molecules can act like macromolecules to crowd biopolymers and the resultant excluded volume effect can be significant. In addition, they demonstrate the need for examining other commonly used co-solvents in biophysics and biochemistry for this effect.
Nano-Crowding Arising from Molecular Constraints
The degree of crowding of a solution can be tuned by the addition of an appropriate amount of a co-solvent or co-solute. However, this approach would not be useful in applications where only a specific region of the biomolecule of interest needs to be crowded. One promising and practical approach of site-specifically increasing the local mass density and hence, the degree of crowding, is to insert a specific construct to the desired molecular location. Indeed, several studies have taken advantage of recent developments in peptide chemistry and protein design and used molecular cross-linkers,20 such as azobenzene and m-xylene, to manipulate molecular crowding in a site-specific manner. For example, in order to mimic the crowded environment within which α-helix folding takes place in proteins, Hamm and coworkers21 employed a photo-switchable azobenzene moiety to simultaneously serve as a photo-trigger of the folding kinetics of an alanine-rich peptide and a structural constraint to reduce its conformational flexibility. They found, akin to the excluded volume effect, that this constraint decreased the entropic cost of folding by limiting the conformational motions of the unfolded peptide. In addition, they found that the azobenzene cross-linker stabilized kinetic traps along the folding pathway that were not present in the unconstrained system and that escape from these traps was the rate-limiting step to complete α-helix formation. Moreover, by altering the bulkiness of the sidechains in the peptide near the azobenzene cross-linker, Paoli et al.22 found that peptides with bulkier sidechains folded slower than the alanine-rich peptide. This was attributed to an increased steric hindrance of the cross-linker on the rearrangement of these sidechains.23
In a similar study, Markiewicz et al.24 used a molecular cross-linker to help assess the effect of internal friction on protein folding dynamics (Figure 4). While it is well known that internal friction arising from adjacent structural elements, due to interactions and/or sterics, can decrease the folding rate as the polypeptide chain becomes increasingly compact throughout the folding process, a direct measurement of its effect has been difficult. Using the mini-protein Trp-cage as an example, the authors showed that it is possible to employ an “inert” cross-linker to strategically increase the local mass density and hence allow a direct estimate, via folding kinetics measurements, of the magnitude of internal friction arising from such a mimic of protein secondary structural elements. Specifically, they placed an m-xylene cross-linker in a congested region of the α-helix of Trp-cage and examined the effect of this moiety on the folding thermodynamics and kinetics of this mini-protein. While the m-xylene cross-linker did not induce any appreciable change in the thermal stability of Trp-cage, it did slow down both the folding and unfolding rates in comparison to those of the unconstrained protein. These rate decreases are consistent with the notion that, in this case, the m-xylene cross-linker acts as a local crowder to increase the frictional drag or roughness of the underlying folding energy landscape. Moreover, using these kinetic rates, an equation for the roughness of barrier-crossing,25 and transition state theory, the authors estimated the increase in the internal friction due to the cross-linker to be between 0.4–1.0 kBT.
Figure 4.
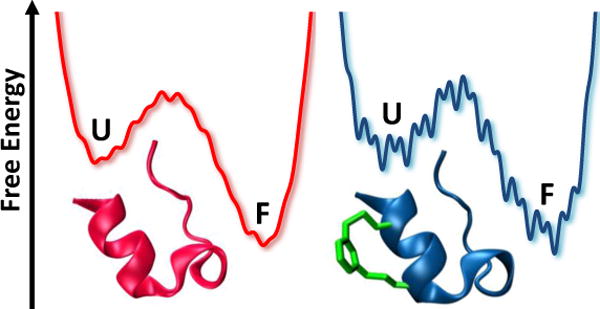
Cartoon illustration of the free energy landscape of the unconstrained (pink) and constrained (blue) Trp-cage peptide, showing that the cross-linker increases the internal friction of the folding process.
While these examples demonstrate the possibility of using a cross-linker to evaluate the effect of localized crowding on protein folding dynamics, care must be taken when utilizing this approach, as incorporation of an external structural element into a protein can lead to changes in other structural and/or physical properties of the protein26 besides the intended change in local mass density. Despite this shortcoming, we believe that this strategy, when implemented appropriately, offers a convenient means to tune the excluded volume in the region of interest, thus allowing assessment of its effect in a site-specific manner.
Nano-Crowding via Molecular Packing
Many biophysical and/or biochemical techniques require coating of a substrate surface with specific biomolecules. For example, DNA microarrays rely on functional DNA molecules tethered to a solid support to monitor gene expression.27 To achieve high throughput and sensitivity, it is common to use densely-packed surfaces in these types of applications. However, crowding effects incurred from densely-attached molecular moieties can significantly alter their structure,28 structural transition,29 functionality,30 and reactivity. For example, the study of Peterson et al.31 showed that the DNA hybridization rate and efficiency to bind DNA probes immobilized on gold surfaces depended strongly on the probe density. In the lowest probe densities studied, 100% hybridization was possible, whereas only ~10% efficiency was obtained when the probe density reached 12.0 × 1012 molecules/cm2. Similarly, the study of Majumdar and coworkers32 on the effect of salt concentration on DNA immobilization and hybridization found that the hybridization density was the largest for 200–400 mM salt solutions since the probe density decreased at lower salt concentrations yet surface crowding caused repulsive interactions at higher salt concentrations. In another study of surface immobilized DNA molecules, Scoles and coworkers33,34 found that the ability of a restriction enzyme to cut the attached DNA in half is highly dependent on the packing density of the DNA with a threshold density above which the reaction was impeded. By analyzing the experimental data using a semiquantitative model, they concluded that the restriction enzyme must have entered the DNA matrix from the side as access from the top was hindered. However, the two-dimensional diffusion of the enzyme became highly restricted in high DNA densities due to crowding, resulting in incomplete or negligible cleavage of the matrix. As shown by Saha et al,35 the density of surface packing can also have a significant effect on the enzymatic activity of proteins. By studying the reactivity of trypsin protease immobilized on copper sulfide nanoparticles, the authors found that at monolayer surface coverage (~2.0 mg/m2), 98% of the enzymatic activity was retained, but under more densely packed conditions (e.g., ~14.0 mg/m2) the enzyme lost 23% of its catalytic activity. They ascribed this loss of activity to a change in the tertiary structure of the attached protein molecules caused by steric crowding effects on the surface.
It is clear that packing density can have a profound influence on the physical and/or chemical properties of biological molecules that are adsorbed on or attached to the surface of an engineered substrate, and the resulting effect can be quantified in many cases. However, for naturally-occurring biological surfaces such as cell membranes, where a large number of different molecules co-exist, the effects of crowding are much less explored and understood. In one computational study, Phillips and coworkers36 found that a crowding-induced entropic tension in the membrane can change the gating energies of bacterial mechanosensitive membrane channels by more than 2 kBT. Other studies37–39 have shown that protein crowding can cause membrane buckling and deformation. Because cell membranes play a critical role in many cellular processes, it is expected that more studies relating crowding to biological membrane activity will be carried out in the future.
Nano-Confinement via Inclusion
Another mechanism by which the excluded volume effect on a biophysical system can be studied is through confinement. By restricting the volume of a molecule of interest through a confining boundary, an excluded volume effect is produced, similar to the way in which solvent molecules and molecular constraints can crowd a molecule of interest. While there are many strategies to experimentally and computationally confine a molecule, including liposomes,40,41 sol-gel matrices,42,43 nanofluidics,44,45 among others,46–52 herein we only focus on reverse micelle (RM) encapsulation, a widely used technique in the field of biophysical chemistry. A RM consists of self-aggregating surfactants that form a spherical shell in an organic solvent, with the nonpolar tails of the surfactants oriented outwards into the solvent and the polar head groups of the surfactants sequestering a nanopool of water inside the RM. While many surfactants, such as anionic sodium dodecyl sulfate (SDS), cationic hexadecyltrimethylammonium bromide (CTAB), and neutral Triton X-100 (TX-100), are capable of forming RMs,53 anionic sodium bis(2-ethylhexyl) sulfosuccinate (AOT) is the most commonly used surfactant in biophysical studies. By increasing the water loading parameter (w0), defined as the ratio of the concentration of water to surfactant, the size of the nanopool of water, and thus the RM, also increases. Therefore, one can control the number of water molecules inside a RM, providing an easy control over the degree of hydration of the encapsulated guest molecules. As the activity of confined water differs from that of bulk water,54 RMs of relatively low w0 values have been used extensively to study the chemical, physical, or photophysical properties of a wide variety of molecular systems. Because of space limitation, below we only discuss studies that utilize RMs to study how hydration and confinement mediate the structure, dynamics, and function of biomolecules. For readers interested in other applications and aspects of RMs, we direct their attention to several recent reviews.55–58
The work of Wand and coworkers59,60 demonstrated that AOT RMs in low-viscosity solvents are ideally suited to study protein hydration dynamics using NMR spectroscopy. This is because the confinement of both the water and the protein inside the RM slows down the residence time of the water on the protein and removes any ambiguity between long-range contributions from bulk water molecules and those from the hydration water molecules, i.e., the water molecules in the first and second layer around the protein, thereby allowing high-resolution NMR techniques to be used. For example, using both 15N- and 13C-resolved NOESY and ROESY experiments, they initially studied the protein ubiquitin and determined the location of hydration waters on the surface of the protein and the effective correlation time between the water and the protein at each location, effectively mapping the hydration dynamics of the protein surface (Figure 5). Interestingly, the areas of restricted hydration dynamics were found to be areas of ubiquitin that are important to its binding interactions with other proteins. After this initial application, Wand and coworkers have extended and optimized this RM encapsulation strategy for many other proteins, including cytochrome c and flavodoxin, and have used this method to investigate ligand binding, among other processes.61
Figure 5.
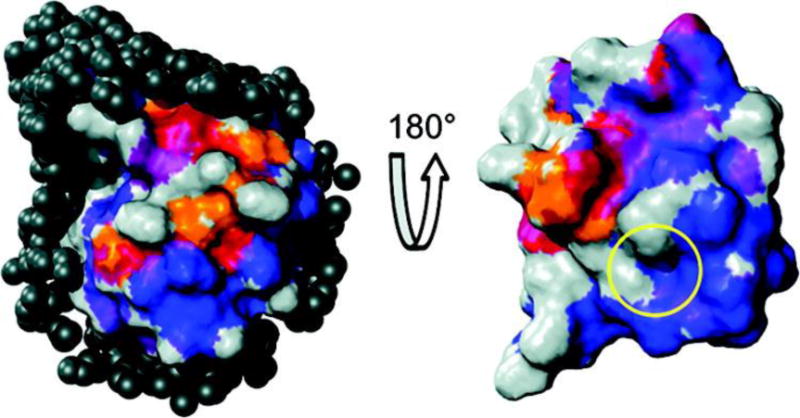
Ubiquitin hydration surface and comparison to its protein-protein interaction surface, shown from two perspectives. Average σNOE/σROE ratios from −0.5 (blue) to 0 (red) are mapped to the surface, showing the regions of slower hydration dynamics (blue) and faster hydration dynamics (red). The yellow circle shows the region of ubiquitin binding interactions. Reprinted with permission from ref. 59. Copyright 2011 American Chemical Society.
In a different application, Mukherjee et al.62,63 utilized AOT RMs to investigate the effect of hydration and confinement on the helix-coil equilibrium of alanine-based peptides. They found that as w0 decreased (i.e., from 20 to 6), the helicity of the peptide increased, indicating that dehydration of the peptide backbone preferentially stabilized helical structure. However, further decreasing w0 to 4 resulted in a decrease in helicity, indicating that the excluded volume effect becomes dominant under this confinement condition, forcing the peptide to adopt an ensemble of conformations that are shorter than the α-helical structure. In addition, their results demonstrated that the degree of dehydration of the peptide backbone can be quantified via IR spectroscopy, as the hydrated and dehydrated backbone units show distinguishable amide I bands.64,65 The latter finding suggests that it is possible to use this approach (i.e., the systematic variation of w0) and isotope-editing66 to determine the mechanism of backbone dehydration in a site-specific manner.
The MD simulations of Straub and coworkers67 provided further atomistic details of the abovementioned peptide system in spherically restricted and unrestricted RMs of w0 = 6. They found that α-helical structure was stable under both conditions, consistent with the experimental observation. In addition, they found that in restricted RMs, the amount of water molecules within 4 Å of each residue varied between 8 and 23; however, most residues had about 12 water molecules nearby while only the N- and C-terminal residues were close to the AOT tail groups. Conversely, in unrestricted RMs, they found that the degree of hydration was much smaller with about 6 water molecules per residue and that the peptide interacted more with the AOT tails (Figure 6). The latter indicates that there is more “free water” in the unrestricted RM, which may increase the entropy of the system. Similar results of peptide interactions with the water/AOT interface were observed by Garcia and coworkers.68 Interestingly, using PheCN as a site-specific probe, Mukherjee et al.69 further showed that when confined in AOT RMs of w0 = 6, a membrane-binding and amphipathic peptide mastoparan × (MPx) was found to be located in the interfacial region of RMs. Additionally, their results suggested that the hydrophobic face of the amphipathic MPx α-helix was actually directed towards the polar core of the RM such that the positively-charged, hydrophilic side of the helix could face the negatively-charged AOT head groups.
Figure 6.
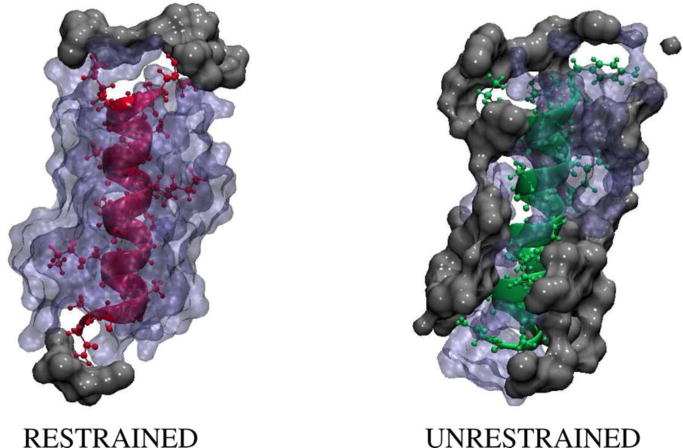
MD Snapshots of an alanine-based peptide in a spherically restrained (left, pink) and a spherically unrestrained (right, green) RM, showing the interaction between the peptide, the surrounding water molecules (blue), and the AOT tail groups (gray). Reprinted with permission from ref. 66. Copyright 2014 American Chemical Society.
For proteins, the stabilizing or destabilizing effect arising from RM confinement may be more complicated. For example, Chowdhury and coworkers70 found that confining myoglobin (MB) in relatively small AOT RMs (w0 = 4 to 12) led to a decrease in its secondary structure with a trend that increasing w0 induced a greater destabilization. Their results suggest that effects beyond simple confinement are at play. In addition, they found that while MB unfolded cooperatively in buffer, it exhibited noncooperative unfolding transitions in RMs. They attributed those behaviors to two factors: (1) the size of the protein was comparable to the size of the confining cage, i.e., the RM, and (2) the polarity of the RM head groups had a destabilizing effect on the protein.
Further experimental evidence that the RM polarity can affect the conformation of a confined protein came from the work of Pal and coworkers,71 who studied the electron transfer yield from riboflavin binding protein (RBP) to the bound cofactor riboflavin (Rf) in both anionic AOT and cationic CTAB RMs. They found that, in similar size water pools, the electron transfer was observed to occur in CTAB RMs but not in AOT RMs. They attributed this difference to the polarity of the confining surface; the anionic AOT interacted strongly with the negatively-charged protein, which in turn affected the Rf binding site such that Rf bound to RBP differently, destroying the electron transfer pathway. Meanwhile, the cationic CTAB did not disrupt the protein conformation and thus, the binding, allowing the electron transfer to occur similarly to the process observed in buffer. Using molecular dynamics simulations, Garcia and coworkers72 also showed that the stability of the miniprotein Trp-cage confined in fullerene balls depended on the polarity of the confining wall. Their results indicated that under nonpolar confinement, the folded state of the protein was stabilized while polar confinement destabilized the folded state because of interactions with the walls of the confining cage. Additionally, Li et al.,73 through Gō-model simulations, observed more conformational switching of adenylate kinase from the active state to the inactive state when the attractive interactions between the confining cage and the protein were increased. Taken together, the effect of the confinement polarity appears to be both sequence- and structure-specific to the protein encapsulated.
It has been predicted that dehydration plays an important role in the formation and growth of amyloid fibrils.74 To test this hypothesis, Mukherjee et al.75 employed AOT RMs of various sizes to systematically tune the degree of hydration of two model amyloid peptides, Aβ16–22 and Sup357–13, and used infrared spectroscopy to follow their aggregation kinetics under those confined conditions. Their results indeed showed that in a more dehydrated environment (e.g., w0 = 6) these peptides aggregated much faster than in a less dehydrated condition (e.g., w0 = 20). Similar results were also obtained in a molecular simulation study.76 Additionally, Axelsen and coworkers77 found that monomeric Aβ1–40 formed extended β-strands in AOT RM using FTIR spectroscopy, suggesting that this conformation could “seed” the formation of amyloid fibrils.
The examples discussed above provide compelling evidence that small RMs are versatile nano-confining cages of biological molecules and afford a wide range of applications. However, such confinement does not only limit the free space accessible by the encapsulated molecules, but can also affect their properties via other factors, including the water density and activity, specific and non-specific interactions with the confining cage, and the ability to exchange contents between RM cavities. Thus, in practice, potential complications arising from these non-excluded volume factors need to be considered when designing confinement experiments using RMs.
Summary and Outlook
Since the cellular environment is highly crowded due to the co-existence of various macromolecules and their complexes, the study of how macromolecular crowding affects the structure, dynamics, and function of biological molecules is currently a very active research area. As discussed above, however, several recent studies indicate that even small molecules, such as TMAO and TFE, can also act as effective crowding agents to influence, for example, the conformational dynamics and stability of proteins. Thus, these studies highlight the need to examine the potential crowding ability of other commonly encountered small co-solvent molecules in biochemical and biophysical studies. For instance, considering that TMAO is one of the many small organic molecules that nature uses to cope with various stresses, it is possible that other osmolytes, such as glycine, taurine, and dimethylsulfoniopropionate, could exert a similar entropic effect as TMAO to stabilize the folded state of proteins. Hence, it would be particularly worthwhile to verify this point in future studies. In addition, as highlighted above, a specifically engineered crowded environment can be created, for example, by increasing the local mass density or through confinement and the resultant crowding effect can be investigated to provide insight into various biochemical and biophysical questions. In particular, we believe that the strategy of using a structural motif to crowd or constrain a specific volume element in the biomolecule of interest has the potential to be useful in the following applications: (1) to help assess the magnitude of internal friction encountered in protein folding dynamics, (2) to trap a high free energy state,78,79 and (3) to modulate the on- and off-rates of ligand binding by exposing binding sites in proteins using a photolyzable constraint. Similarly, the confining strategy via RM inclusion could be extended to, for example, (1) study the excluded volume effect on the structure and dynamics of intrinsically disordered proteins or other metastable proteins,80 (2) study the structure and folding dynamics of peptides and proteins that develop secondary and/or tertiary interactions at a low degree of hydration such as the late embryogenesis abundant (LEA) proteins, which fold into α-helices upon desiccation,81 (3) expand the time window of observation in laser-induced temperature-jump experiments82 using an organic solvent (e.g, isooctane) that has a smaller thermal conductivity than water to slow down the rate of heat transfer from the water pool in the RMs to the surroundings, and (4) investigate the water molecules inside of the cytosol of E. coli cells using infrared spectroscopy by removing bulk water contributions through RM encapsulation.
QUOTES.
What is surprising, however, is that several recent studies have indicated that the excluded volume effect can also arise from unexpected sources, such as TMAO and TFE.
One promising and practical approach of site-specifically increasing the local mass density and hence, the degree of crowding, is to insert a specific construct to the desired molecular location.
Acknowledgments
We thank the National Institutes of Health (GM-065978 and P41-GM104605) for funding. M.R.H. and R.M.A. are NSF Graduate Research Fellows (DGE-1321851).
Biographies
Mary Rose Hilaire is a Ph.D. candidate in the graduate group of Chemistry at the University of Pennsylvania and a NSF Graduate Research Fellow. She graduated with a B.S. in Chemistry from Temple University in 2012. Her research interests include using non-natural amino acids to probe protein folding and protein-ligand binding events using fluorescence and infrared spectroscopies.
Rachel M. Abaskharon is a Ph.D. candidate in the Chemistry Department at the University of Pennsylvania and a NSF Graduate Research Fellow. She graduated from The College of New Jersey in 2012 with a B.S. in Chemistry and a B.A. in Mathematics. Her current research interests lie in using photochemical triggers to initiate protein folding reactions and gain new insights into the complexities of the protein folding free energy landscape.
Feng Gai is the Edmund J. and Louise W. Kahn Endowed Term Professor of Chemistry at the University of Pennsylvania. His current research interests focus on protein folding, the protein structure-dynamic-function relationship, as well as the development of new spectroscopic probes and methods. http://gaigroup.chem.upenn.edu/
References
- 1.Ellis RJ, Minton AP. Cell Biology: Join the Crowd. Nature. 2003;425:27–28. doi: 10.1038/425027a. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharya A, Kim YC, Mittal J. Protein-protein Interactions in a Crowded Environment. Biophys Rev. 2013;5:99–108. doi: 10.1007/s12551-013-0111-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christiansen A, Wang Q, Cheung MS, Wittung-Stafshede P. Effects of Macromolecular Crowding Agents on Protein Folding In Vitro and In Silico. Biophys Rev. 2013;5:137–145. doi: 10.1007/s12551-013-0108-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phillip Y, Schreiber G. Formation of Protein Conplexes in Crowded Environments – From In Vitro to In Vivo. FEBS Lett. 2013;587:1046–1052. doi: 10.1016/j.febslet.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuznetsova IM, Turoverov KK, Uversky VN. What Macromolecular Crowding Can Do to a Protein. Int J Mol Sci. 2014;15:23090–23140. doi: 10.3390/ijms151223090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho SS, Reddy G, Straub JE, Thirumalai D. Entropic Stabilization of Proteins by TMAO. J Phys Chem B. 2011;115:13401–13407. doi: 10.1021/jp207289b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levine ZA, Larini L, LaPointe NE, Feinstein SC, Shea J-E. Regulation and Aggregation of Intrinsically Disordered Peptides. Proc Natl Acad Sci U S A. 2014;112:2758–2763. doi: 10.1073/pnas.1418155112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baskakov I, Bolen DW. Forcing Thermodynamically Unfolded Proteins to Fold. J Biol Chem. 1998;273:4831–4834. doi: 10.1074/jbc.273.9.4831. [DOI] [PubMed] [Google Scholar]
- 9.Uversky VN, Li J, Fink AL. Trimethylamine-N-Oxide-Induced Folding of α-Synuclein. FEBS Lett. 2001;509:31–35. doi: 10.1016/s0014-5793(01)03121-0. [DOI] [PubMed] [Google Scholar]
- 10.Ma J, Pazos IM, Gai F. Microscopic Insights Into the Protein-Stabilizing Effect of Trimethylamine N-Oxide (TMAO) Proc Natl Acad Sci U S A. 2014;111:8476–8481. doi: 10.1073/pnas.1403224111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma J, Pazos IM, Zhang W, Culik RM, Gai F. Site-Specific Inrared Probes of Proteins. Annu Rev Phys Chem. 2015;66:357–377. doi: 10.1146/annurev-physchem-040214-121802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung JK, Thielges MC, Fayer MD. Conformational Dynamics and Stability of HP35 Studied with 2D IR Vibrational Echoes. J Am Chem Soc. 2012;134:12118–12124. doi: 10.1021/ja303017d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holmstrom ED, Dupuis NF, Nesbitt DJ. Kinetic and Thermodynamic Origins of Osmolyte-Influenced Nucleic Acid Folding. J Phys Chem B. 2015;119:3687–3696. doi: 10.1021/jp512491n. [DOI] [PubMed] [Google Scholar]
- 14.Pincus DL, Hyeon C, Thirumalai D. Effects of Trimethylamine N-Oxide (TMAO) and Crowding Agents on the Stability of RNA Hairpins. J Am Chem Soc. 2008;130:7364–7372. doi: 10.1021/ja078326w. [DOI] [PubMed] [Google Scholar]
- 15.Graziano G. On the Ability of Trehalose to Offset the Denaturing Activity of Urea. Chem Phys Lett. 2013;556:292–296. [Google Scholar]
- 16.Jain R, Sharma D, Kumar S, Kumar R. Factor Defining the Effects of Glycine Betaine on the Thermodynamic Stability and Internal Dynamics of Horse Cytochrome C. Biochemistry. 2014;53:5221–5235. doi: 10.1021/bi500356c. [DOI] [PubMed] [Google Scholar]
- 17.Xia Z, Das P, Shakhnovich EI, Zhou R. Collapse of Unfolded Proteins in a Mixture of Denaturants. J Am Chem Soc. 2012;134:18266–18274. doi: 10.1021/ja3031505. [DOI] [PubMed] [Google Scholar]
- 18.Hong D-P, Hoshino M, Kuboi R, Goto Y. Clustering of Fluorine-Substituted Alcohols as a Factor Responsible for Their Marked Effects on Proteins and Peptides. J Am Chem Soc. 1999;121:8427–8433. [Google Scholar]
- 19.Culik RM, Abaskharon RM, Pazos IM, Gai F. Experimental Validation of the Role of Trifluoroethanol as a Nanocrowder. J Phys Chem B. 2014;118:11455–11461. doi: 10.1021/jp508056w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markiewicz BN, Culik RM, Gai F. Tightening up the Structure, Lighting up the Pathway: Application of Molecular Constraints and Light to Manipulate Protein Folding, Self-assembly and Function. Sci China: Chem. 2014;57:1615–1624. doi: 10.1007/s11426-014-5225-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ihalainen JA, Paoli B, Muff S, Backus EHG, Bredenbeck J, Woolley GA, Caflisch A, Hamm P. Alpha-Helix Folding in the Presence of Structural Constraints. Proc Natl Acad Sci U S A. 2008;105:9588–9593. doi: 10.1073/pnas.0712099105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paoli B, Seeber M, Backus EHG, Ihalainen JA, Hamm P, Caflisch A. Bulky Side Chains and Non-Native Salt Bridges Slow Down the Folding of a Cross-Linked Helical Peptide: A Combined Molecular Dynamics and Time-Resolved Infrared Spectroscopy Study. J Phys Chem B. 2009;113:4435–4442. doi: 10.1021/jp810431s. [DOI] [PubMed] [Google Scholar]
- 23.Paoli B, Pellarin R, Caflisch A. Slow Folding of Cross-Linked α-Helical Peptides Due To Steric Hindrance. J Phys Chem B. 2010;114:2023–2027. doi: 10.1021/jp910216j. [DOI] [PubMed] [Google Scholar]
- 24.Markiewicz BN, Jo H, Culik RM, DeGrado WF, Gai F. Assessment of Local Friction in Protein Folding Dynamics Using a Helix Cross-Linker. J Phys Chem B. 2013;117:14688–14696. doi: 10.1021/jp409334h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sagnella DE, Straub JE, Thirumalai D. Time Scales and Pathways for Kinetic Energy Relaxation in Solvated Proteins: Application to Carbonmonoxy Myoglobin. J Chem Phys. 2000;113:7702–7711. [Google Scholar]
- 26.Abaskharon RM, Culik RM, Woolley GA, Gai F. Tuning the Attempt Frequency of Protein Folding Dynamics via Transition-State Rigidification: Application to Trp-Cage. J Phys Chem Lett. 2015;6:521–526. doi: 10.1021/jz502654q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sassolas A, Leca-Bouvier BD, Blum LJ. DNA Biosensors and Microarrays. Chem Rev. 2008;108:109–139. doi: 10.1021/cr0684467. [DOI] [PubMed] [Google Scholar]
- 28.Giacomelli CE, Norde W. Conformational Changes of the Amyloid β-Peptide (1–40) Adsorbed on Solid Surfaces. Macromol Biosci. 2005;5:401–407. doi: 10.1002/mabi.200400189. [DOI] [PubMed] [Google Scholar]
- 29.Watkins HM, Simon AJ, Ricci F, Plaxco KW. Effects of Crowding on the Stability of a Surface-Tethered Biopolymer: An Experimental Study of Folding in a Highly Crowded Regime. J Am Chem Soc. 2014;136:8923–8927. doi: 10.1021/ja411486g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zanetti-Polzi L, Daidone I, Bortolotti CA, Corni S. Surface Packing Determines the Redox Potential Shift of Cytochrome C Adsorbed on Gold. J A Chem Soc. 2014;136:12929–12937. doi: 10.1021/ja505251a. [DOI] [PubMed] [Google Scholar]
- 31.Peterson AW, Heaton RJ, Georgiadis RM. The Effect of Surface Probe Density on DNA Hybridization. Nucleic Acids Res. 2001;29:5163–5168. doi: 10.1093/nar/29.24.5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castelino K, Kannan B, Majumdar A. Characterization of Grafting Density and Binding Efficiency of DNA and Proteins on Gold Surfaces. Langmuir. 2005;21:1956–1961. doi: 10.1021/la047943k. [DOI] [PubMed] [Google Scholar]
- 33.Castronovo M, Radovic S, Grunwald C, Casalis L, Morgante M, Scoles G. Control of Steric Hindrance on Restriction Enzyme Reactions With Surface-Bound DNA Nanostructures. Nano Lett. 2008;8:4140–4145. doi: 10.1021/nl802370g. [DOI] [PubMed] [Google Scholar]
- 34.Castronovo M, Lucesoli A, Parisse P, Kurnikova A, Malhotra A, Grassi M, Grassi G, Scaggiante B, Casalis L, Scoles G. Two-Dimensional Enzyme Diffusion in Laterally Confined DNA Monolayers. Nat Commun. 2011;2:297. doi: 10.1038/ncomms1296. [DOI] [PubMed] [Google Scholar]
- 35.Saha B, Saikia J, Das G. Correlating Enzyme Density, Conformation and Activity on Nanoparticle Surfaces in Highly Functional Bio-Nanocomposites. Analyst. 2015;140:532–542. doi: 10.1039/c4an01639d. [DOI] [PubMed] [Google Scholar]
- 36.Lindén M, Sens P, Phillips R. Entropic Tension in Crowded Membranes. PLoS Comput Biol. 2012;8:e1002431. doi: 10.1371/journal.pcbi.1002431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stachowiak JC, Hayden CC, Sasaki DY. Steric Confinement of Proteins on Lipid Membranes Can Drive Curvature and Tubulation. Proc Natl Acad Sci U S A. 2010;107:7781–7786. doi: 10.1073/pnas.0913306107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stachowiak JC, Schmid EM, Ryan CJ, Ann HS, Sasaki DY, Sherman MB, Geissler PL, Fletcher DA, Hayden CC. Membrane Bending by Protein-Protein Crowding. Nat Cell Biol. 2012;14:944–949. doi: 10.1038/ncb2561. [DOI] [PubMed] [Google Scholar]
- 39.Zhu C, Das SL, Baumgart T. Nonlinear Sorting, Curvature Generation, and Crowding of Endophilin N-BAR on Tubular Membranes. Biophys J. 2012;102:1837–1845. doi: 10.1016/j.bpj.2012.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Nies P, Nourian Z, Kok M, van Wijk R, Moeskops J, Westerlaken I, Poolman JM, Eelkema R, van Esch JH, Kuruma Y, et al. Unbiased Tracking of the Progression of mRNA and Protein Synthesis in Bulk and in Liposome-Confined Reactions. ChemBioChem. 2013;14:1963–1966. doi: 10.1002/cbic.201300449. [DOI] [PubMed] [Google Scholar]
- 41.Zhang D, Zhao Y-X, Qiao Z-Y, Mayerhöffer U, Spenst P, Li X-J, Würthner F, Wang H. Nano-Confined Squaraine Dye Assemblies: New Photoacoustic and Near-Infrared Fluorescence Dual-Modular Imaging Probes in Vivo. Bioconjug Chem. 2014;25:2021–2029. doi: 10.1021/bc5003983. [DOI] [PubMed] [Google Scholar]
- 42.Eggers DK, Valentine JS. Molecular Confinement Influences Protein Structure and Enhances Thermal Protein Stability. Protein Sci. 2001;10:250–261. doi: 10.1110/ps.36201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buthe A. Entrapment of Enzymes in Nanoporous Sol-Gels. Methods Mol Biol. 2011;743:223–237. doi: 10.1007/978-1-61779-132-1_18. [DOI] [PubMed] [Google Scholar]
- 44.Wang C, Sheng Z-H, Ouyang J, Xu J-J, Chen H-Y, Xia X-H. Nanoconfinement Effects: Glucose Oxidase Reaction Kinetics in Nanofluidics. ChemPhysChem. 2012;13:762–768. doi: 10.1002/cphc.201100842. [DOI] [PubMed] [Google Scholar]
- 45.Wang C, Ye D-K, Wang Y-Y, Lu T, Xia X-H. Insights into the “Free State” Enzyme Reaction Kinetics in Nanoconfinement. Lab Chip. 2013;13:1546–1553. doi: 10.1039/c3lc41319e. [DOI] [PubMed] [Google Scholar]
- 46.Klimov DK, Newfield D, Thirumalai D. Simulations of β-hairpin Folding Confined to Spherical Pores using Distributed Computing. Proc Natl Acad Sci U S A. 2002;99:8019–8024. doi: 10.1073/pnas.072220699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baumketner A, Jewett A, Shea JE. Effects of Confinement in Chaperonin Assisted Protein Folding: Rate Enhancement by Decreasing the Roughness of the Folding Energ Landscape. J Mol Biol. 2003;332:701–713. doi: 10.1016/s0022-2836(03)00929-x. [DOI] [PubMed] [Google Scholar]
- 48.Ziv G, Haran G, Thirumalai D. Ribosome Exit Tunnel Can Entropically Stabilize α-helices. Proc Natl Acad Sci U S A. 2005;102:18956–18961. doi: 10.1073/pnas.0508234102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guo L, Chowdhury P, Fang J, Gai F. Heterogeneous and Anomalous Diffusion inside Lipid Tubules. J Phys Chem B. 2007;111:14244–14249. doi: 10.1021/jp076562n. [DOI] [PubMed] [Google Scholar]
- 50.Lucent D, Vishal V, Pande V. S. Protein Folding under Confinement: A Role for Solvent. Proc Natl Acad Sci U S A. 2007;104:10430–10434. doi: 10.1073/pnas.0608256104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mittal J, Best RB. Thermodynamics and Kinetics of Protein Folding under Confinement. Proc Natl Acad Sci U S A. 2008;105:20233–20238. doi: 10.1073/pnas.0807742105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bhattacharya A, Best RB, Mittal J. Smoothing of the GB1 Hairpin Folding Landscape by Interfacial Confinement. Biophys J. 2012;103:596–600. doi: 10.1016/j.bpj.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yeung PS-W, Eskici G, Axelsen PH. Infrared Spectroscopy of Proteins in Reverse Micelles. Biochim Biophys Acta - Biomembr. 2013;1828:2314–2318. doi: 10.1016/j.bbamem.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou HX. Helix Formation Inside a Nanotube: Possible Influence of Backbone-Water Hydrogen Bonding by the Confining Surface through Modulation of Water Activity. J Chem Phys. 2007;127:245101. doi: 10.1063/1.2812282. [DOI] [PubMed] [Google Scholar]
- 55.Fayer MD, Levinger NE. Analysis of Water in Confined Geometries and at Interfaces. Annu Rev Anal Chem. 2010;3:89–107. doi: 10.1146/annurev-anchem-070109-103410. [DOI] [PubMed] [Google Scholar]
- 56.Fayer MD. Water in a Crowd. Physiology. 2011;26:381–392. doi: 10.1152/physiol.00021.2011. [DOI] [PubMed] [Google Scholar]
- 57.Fayer MD. Dynamics of Water Interacting with Interfaces, Molecules, and Ions. Acc Chem Res. 2012;45:3–14. doi: 10.1021/ar2000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Correa NM, Silber JJ, Riter RE, Levinger NE. Nonaqueous Polar Solvents in Reverse Micelle Systems. Chem Rev. 2012;112:4569–4602. doi: 10.1021/cr200254q. [DOI] [PubMed] [Google Scholar]
- 59.Nucci NV, Pometun MS, Wand AJ. Site-Resolved Measurement of Water-Protein Interactions by Solution NMR. Nat Struct Mol Biol. 2011;18:245–249. doi: 10.1038/nsmb.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nucci NV, Pometun MS, Wand AJ. Mapping the Hydration Dynamics of Ubiquitin. J Am Chem Soc. 2011;133:12326–12329. doi: 10.1021/ja202033k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nucci NV, Valentine KG, Wand AJ. High-Resolution NMR Spectroscopy of Encapsulated Proteins Dissolved in Low-Viscosity Fluids. J Magn Reson. 2014;241:137–147. doi: 10.1016/j.jmr.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mukherjee S, Chowdhury P, Gai F. Tuning the Cooperativity of the Helix-Coil Transition by Aqueous Reverse Micelles. J Phys Chem B. 2006;110:11615–11619. doi: 10.1021/jp062362k. [DOI] [PubMed] [Google Scholar]
- 63.Mukherjee S, Chowdhury P, Gai F. Infrared Study of the Effect of Hydration on the Amide I Band and Aggregation Properties of Helical Peptides. J Phys Chem B. 2007;111:4596–4602. doi: 10.1021/jp0689060. [DOI] [PubMed] [Google Scholar]
- 64.Manas ES, Getahun Z, Wright WW, DeGrado WF, Vanderkooi JM. Infrared Spectra of Amide Groups in a-Helical Proteins: Evidence for Hysdrogen Bonoding to Water. J Am Chem Soc. 2000;122:9883–9890. [Google Scholar]
- 65.Huang CY, Klemke JW, Getahun Z, DeGrado WF, Gai F. Temperature-Dependent Helix-Coil Transition of an Alanine Based Peptide. J Am Chem Soc. 2001;123:9235–9238. doi: 10.1021/ja0158814. [DOI] [PubMed] [Google Scholar]
- 66.Huang C-Y, Getahun Z, Wang T, DeGrado WF, Gai F. Time-Resolved Infrared Study of the Helix-Coil Transition Using 13C-Labeled Helical Peptides. J Am Chem Soc. 2001;123:12111–12112. doi: 10.1021/ja016631q. [DOI] [PubMed] [Google Scholar]
- 67.Martinez AV, Małolepsza E, Domínguez L, Lu Q, Straub JE. Role of Charge and Solvation in the Structure and Dynamics of Alanine-Rich Peptide AKA2 in AOT Reverse Micelles. J Phys Chem B. 2014 doi: 10.1021/jp508813n. Article ASAP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tian J, Garcia AE. An Alpha-Helical Peptide in AOT Micelles Prefers to Be Localized at the Water/headgroup Interface. Biophys J. 2009;96:57–59. doi: 10.1016/j.bpj.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mukherjee S, Chowdhury P, DeGrado WF, Gai F. Site-Specific Hydration Status of an Amphipathic Peptide in AOT Reverse Micelles. Langmuir. 2007;23:11174–11179. doi: 10.1021/la701686g. [DOI] [PubMed] [Google Scholar]
- 70.Malik A, Kundu J, Mukherjee SK, Chowdhury PK. Myoglobin Unfolding in Crowding and Confinement. J Phys Chem B. 2012;116:12895–12904. doi: 10.1021/jp306873v. [DOI] [PubMed] [Google Scholar]
- 71.Saha R, Rakshit S, Verma PK, Mitra RK, Pal SK. Protein-Cofactor Binding and Ultrafast Electron Transfer in Riboflavin Binding Protein under the Spatial Confinement of Nanoscopic Reverse Micelles. J Mol Recognit. 2013;26:59–66. doi: 10.1002/jmr.2246. [DOI] [PubMed] [Google Scholar]
- 72.Tian J, Garcia AE. Simulation Studies of Protein Folding/Unfolding Equilibrium Under Polar and Nonpolar Confinement. J Am Chem Soc. 2011;133:15157–15164. doi: 10.1021/ja2054572. [DOI] [PubMed] [Google Scholar]
- 73.Li M, Xu W, Zhang JZH, Xia F. Combined Effect of Confinement and Affinity of Crowded Environment on Conformation Switching of Adenylate Kinase. J Mol Model. 2014;20:2530–2540. doi: 10.1007/s00894-014-2530-z. [DOI] [PubMed] [Google Scholar]
- 74.Thirumalai D, Reddy G, Straub JE. Role of Water in Protein Aggregation and Amyloid Polymorphism. Acc Chem Res. 2012;45:83–92. doi: 10.1021/ar2000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mukherjee S, Chowdhury P, Gai F. Effect of Dehydration on the Aggregation Kinetics of Two Amyloid Peptides. J Phys Chem B. 2009;113:531–535. doi: 10.1021/jp809817s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Martinez AV, Małolepsza E, Rivera E, Lu Q, Straub JE. Exploring the Role of Hydration and Confinement in the Aggregation of Amyloidogenic Peptides Aβ16–22 and Sup357–13 in AOT Reverse Micelles. J Chem Phys. 2014;141:22D530. doi: 10.1063/1.4902550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yeung PS-W, Axelsen PH. The Crowded Environment of a Reverse Micelle Induces the Formation of β-Strand Seed Structures for Nucleating Amyloid Fibril Formation. J Am Chem Soc. 2012;134:6061–6063. doi: 10.1021/ja3004478. [DOI] [PubMed] [Google Scholar]
- 78.Markiewicz BN, Yang L, Culik RM, Gao YQ, Gai F. How Quickly Can a β-Hairpin Fold from Its Transition State? J Phys Chem B. 2014;118:3317–3325. doi: 10.1021/jp500774q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pearson AD, Mills JH, Song Y, Nasertorabi F, Han GW, Baker D, Stevens RC, Schultz PG. Trapping a Transition State in a Computationally Designed Protein Bottle. Science. 2015;347:863–867. doi: 10.1126/science.aaa2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Peterson RW, Anbalagan K, Tommos C, Wand AJ. Forced Folding and Structural Analysis of Metastable Proteins. J Am Chem Soc. 2004;126:9498–9499. doi: 10.1021/ja047900q. [DOI] [PubMed] [Google Scholar]
- 81.Shih M-D, Hoekstra FA, Hsing Y-IC. Late Embroyogenesis Abundant Proteins. Adv Bot Res. 2008;48:211–255. [Google Scholar]
- 82.Serrano AL, Waegele MM, Gai F. Spectroscopic Studies of Protein Folding: Linear and Nonlinear Methods. Protein Sci. 2012;21:157–170. doi: 10.1002/pro.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


