Summary
This study was conducted to assess the efficacy of oral zinc supplementation in children with intractable epilepsy. Forty-five children aged between three and 12 years and diagnosed with idiopathic intractable epilepsy at Assiut University Hospital, Assiut, Egypt were recruited. The patients were randomly allocated to two groups: the intervention group received oral zinc supplementation (1 mg/kg/day) while the placebo group received placebo, each for six months. The parents of each child filled in a detailed questionnaire that covered demographic characteristics, type of seizures, frequency, duration of seizures, previous hospital admissions, postictal phenomena and the occurrence of status epilepticus. The primary outcome (frequency of seizures) was compared between the two groups. Zinc supplementation resulted in a significant reduction of seizure frequency in 31% of the treated children.
Zinc is an important trace element. Our results suggest that it has mildly beneficial effects in children with intractable epilepsy. We recommend further investigation of oral zinc supplementation as an adjunctive therapy for managing intractable epilepsy in children. Zinc therapy may be an option in treatment protocols for intractable epilepsy in the near future.
Keywords: children, intractable epilepsy, zinc
Introduction
Zinc is an important trace element whose intake is related to protein intake. As a result, zinc deficiency is an important component of nutrition-related morbidity worldwide. Zinc supplementation in populations at possible risk of zinc deficiency appears to have beneficial effects on the incidence and outcome of serious childhood diseases (Abrams, 2014; Crepin et al., 2009). Zinc is an activating cofactor for more than 70 important enzyme systems, including carbonic anhydrases, alkaline phosphatases, dehydrogenases and carboxypeptidases. It is also involved in the regulation of nucleoproteins and the activity of various inflammatory cells and plays an important biological role in the central nervous system (CNS) (Abrams, 2014; Crepin et al., 2009; Saad et al., 2014). The hypothalamus contains high levels of zinc and is sensitive to its deficiency. At neuronal level, zinc, by decreasing the extracellular glutamate concentration and increasing the extracellular concentration of gamma amino butyric acid (GABA), acts as a neurotransmitter in glutaminergic and GABAergic transmission. Low levels of zinc have been observed in seizures as hypozincemia activates the N-methyl-D-aspartate receptor which may play an important role in the induction of epileptic discharges (Crepin et al., 2009; Lee and Chung, 2010; Saad et al., 2014). In a previous work (Saad et al., 2014), we found significantly lower zinc levels in the serum of children with refractory epilepsy in comparison with healthy control children. Similarly, in a recent study, Kheradmand et al. (2014) reported significantly lower serum zinc levels in patients with intractable epilepsy in comparison with a controlled epilepsy group. These data suggest that children with refractory epilepsy may potentially benefit from zinc supplementation treatment. This study was conducted to evaluate the efficacy of oral zinc supplementation as an adjunctive therapy in an Egyptian cohort of children with intractable epilepsy.
Materials and methods
The Ethics Committee of Assiut University, Assiut, Egypt, approved the study. All methods and procedures used in this study were approved by the Institutional Review Board (IRB) of the same university. Informed consent was obtained in accordance with the guidelines set forth by the Assiut University IRB: subjects were provided with a brief description of the study procedures, and informed about: the potential benefits and risks, the voluntary nature of the study, their right to withdraw without consequences, and the confidentiality of the information collected. Informed consent was obtained using language (Arabic dialect) appropriate for all the participants’ parents/caregivers. Prior to taking part in the study, children were required to give their assent and their parents/caregivers were required to give their consent to the child’s participation. Finally, the participants were given a Participant’s Bill of Rights. The study was a six-month, randomized, double-blind, placebo-controlled trial performed at the outpatient neurology clinic at Assiut University Hospital and at three private centers in Assiut, Egypt.
Patients
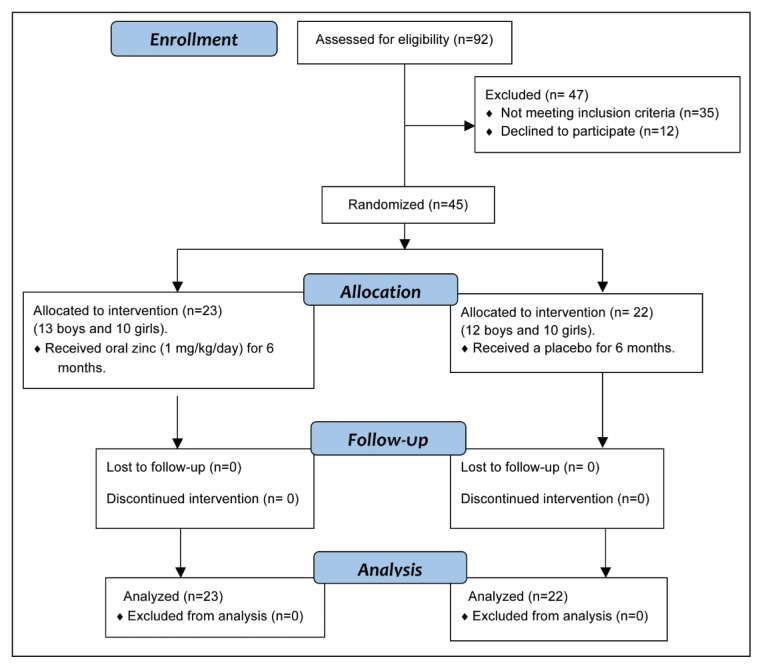
The patients included 45 outpatients (24 boys and 21 girls) aged between three and 12 years. All the patients were recruited from the neuropediatric clinics at Assiut University Hospital and from three private centers in Assiut City, Upper Egypt. In total, 92 patients were screened for eligibility, and 45 were enrolled (consent was not given in 12 cases and 35 patients did not meet eligibility criteria and/or met one or more of the exclusion criteria).
Inclusion criteria
Children were eligible for inclusion if they presented with idiopathic generalized intractable epilepsy (one or more seizures per month) and failure of adequate trials of at least two tolerated, appropriately chosen and administered antiepileptic drugs (whether as monotherapy or in combination in proper doses, and within therapeutic range) to achieve seizure freedom for a period of six months or more (Saad et al., 2014; Sirven et al., 2014).
Exclusion criteria
Epilepsy patients with structural CNS abnormalities, head trauma, neonatal insults, hypoxic ischemic encephalopathy, cerebral palsy, metabolic diseases, chronic medical conditions including anemia and gastrointestinal disease associated with malabsorption, neuropsychological diseases and neurodegenerative disorders were excluded from the study. Patients whose epilepsy was controlled with antiepileptic drug therapy were also excluded. Finally, no patient was started on any other therapies, such as vitamins or antioxidants, during the four weeks prior to the study or during the study period.
Methodology
The evaluation of the patients consisted of a thorough disease history including the age at onset of epileptic seizures, the type(s), frequency and duration of seizures, the occurrence of status epilepticus, the occurrence of pre- and postictal phenomena, the history of drugs used and compliance, as well as family history of similar conditions.
In our randomized, double-blind clinical trial, a random number generator was used to randomize patients to receive zinc supplementation, 1 mg/kg/day (Schwartz, 2012) (the intervention group) or a placebo solution (placebo group). Both zinc and placebo were administered in syrup form and had identical packaging and nearly the same taste and color (no differences were detected). The assignments were kept in sealed envelopes until data analysis. Randomization and allocations were blind. Figure 1 shows the number of patients enrolled in the study and randomized to each group. The intervention group comprised 23 patients (13 males and 10 females) randomly allocated to receive zinc syrup (10 mg/5ml zinc sulfate). Each 100 ml of syrup contained zinc sulfate heptahydrate, 0.88 mg, which is equivalent to 0.2 g zinc. The placebo group comprised 22 patients (12 males and 10 females) who received the placebo solution for six months. Throughout the study, during which medications were administered by the patients themselves or by their parents/caregivers, the patients and their parents/caregivers were blind to assignments. The patients were evaluated for seizure response and adverse events at weekly clinical visits during the first four weeks after the start of the trial and on a monthly basis thereafter. All the patients were monitored for six months. Seizure frequency was recorded using a detailed questionnaire designed by the authors and filled in by the parents/caregivers of each patient. This questionnaire collected patient demographic data and information on seizure type and frequency, specific epilepsy syndrome, age at seizure onset, age at the start of antiepileptic therapy, duration of epilepsy before the study, concomitant antiepileptic drugs used, and information regarding these drugs: starting dose and maintenance dose, efficacy, adverse events, and reasons for drug discontinuation, as well as any clinical problems during the study. Baseline seizure frequency was evaluated for two months before therapy was begun. Efficacy was based on a change of seizure frequency, with data obtained from the questionnaires kept by parents or caregivers. Seizure reduction after the therapy was classified relative to baseline seizure frequency, as follows: seizure-free (100% control of seizures), good response (from 50% to <100% reduction in seizure frequency), no response (<50% reduction of seizure frequency or no effect), or worsening (increase in seizure frequency). Patients in the first two groups, i.e. who achieved 50%-100% decrease in seizure frequency compared with the baseline seizure frequency, were defined as good responders. Side effects were recorded throughout the study and were assessed using a checklist every month throughout the study period.
Figure 1.
Consort flow diagram.
Statistical analysis
The Statistical Package for Social Sciences (SPSS) version 22 was used for data analysis. To assess the comparability of the groups (intervention and placebo) and study centers, age, weight, age at first seizure, frequency of convulsions and outcome were compared using two-way analysis of variance, with center and treatment as the fixed factors, and Mantel-Haenszel chi-square tests, adjusting for the center. A p value of <0.05 denoted the presence of a statistically significant difference.
Results
Table I shows the clinical characteristics of the studied groups. The intervention group and the placebo group had a mean age±SD of 9.4±2.1 years and 8.28±4.0 years, respectively, and in both groups the age of the participants ranged from 3 to 12 years. Males accounted for 56.5% of the intervention group and 54.5% of the placebo group. All the patients were of normal weight for age. No significant differences were found between the two groups in demographic data including age, gender, weight and clinical features, such as age at seizure onset, duration of epilepsy before the study and number of concomitant antiepileptic drugs. Table I details the types of antiepileptic drugs used in both groups and the duration of treatment.
Table I.
Patient characteristics (n=45).
| Item | Intervention group (zinc therapy) | Placebo group | p-value |
|---|---|---|---|
| Age (years) | 0.62† | ||
| - Mean ± SD | 9.4 ± 2.1 | 8.28 ± 4.0 | |
| - Range | 3 – 12 | 3 – 12 | |
| 0.74* | |||
| Males, n (%) | 13 (56.5%) | 12 (54.5%) | |
| Females n (%) | 10 (43.5%) | 10 (45.5%) | |
| Body weight (kg) | 0.31† | ||
| - Mean ± SD | 24.8 ± 8.1 | 25 ± 10.3 | |
| - Range | 12 – 34.5 | 11.5 – 37 | |
| Age at first seizure (years) | 0.62† | ||
| - Mean ± SD | 3.58 ± 3.05 | 3.8 ± 1.6 | |
| - Range | 1.75 – 6.3 | 1.5 – 5.75 | |
| Antiepileptic drugs used | valproate and levetiracetam | valproate and levetiracetam | - |
| Months of antiepileptic drug use | 0.31† | ||
| - Mean ± SD | 6.7 ± 2.4 | 7.1 ± 1.9 | |
| - Range | 10 – 44 | 11 – 37 |
Two-way analysis of variance with factors: treatment group, center, and treatment by center.
Mantel-Haenszel summary chi-square, stratified by center.
As regards the frequency of seizures, 13% of the patients in the intervention group had more than five epileptic seizures per day, versus 14% in the placebo group. A seizure frequency of 1–5/day was recorded in 9% of the intervention group and in 14% of the placebo group. Seizures occurred from one to five times per week in 43% of the treated patients and 36% of the controls, and from one to five times per month in 35% and 36% of the intervention and placebo group respectively (Table II). There was no significant difference in seizure frequency between the groups before treatment (p=0. 31).
Table II.
Baseline (pre-treatment) frequency of seizures in patients receiving zinc supplementation versus placebo.
| Frequency | Zinc n (%) |
Placebo n (%) |
p-value |
|---|---|---|---|
| > 5/day | 3 (13%) | 3 (14%) | 0.31* |
| 1–5/day | 2 (9%) | 3 (14%) | |
| 1–5/week | 10 (43%) | 8 (36%) | |
| 1–5/month | 8 (35%) | 8 (36%) | |
| Total | 23 (100%) | 22 (100%) |
Mantel-Haenszel summary chi-square, stratified by center.
The side effects noted by the research team during the six-month study period included itching, abdominal pain, nausea and vomiting. All were mild and transient and all the patients continued the study.
Table III shows the outcome of oral zinc supplementation versus placebo in the two groups. A seizure-free status was attained by two patients (9%) in the intervention group receiving adjunctive oral zinc therapy, but in no patient in the placebo group. Five of the patients (31%) in the intervention group were considered good responders (experiencing a >50% reduction in seizure frequency) versus 4.5% of the placebo group. The no response (0 – <50% reduction in seizure frequency) rate was significantly lower in the intervention group (69%) than in the placebo group (95.5%). Overall, an improvement occurred in 31% of the intervention group versus 4.5% of the placebo group. There was a significant difference in seizure frequency between the two groups after zinc treatment (p=0.02) (Table III).
Table III.
Outcome after therapy in patients receiving zinc supplementation versus placebo.
| % reduction in seizures | Zinc n (%) |
Placebo n (%) |
p-value |
|---|---|---|---|
| 100% | 2 (9%) | 0 (0%) | 0.02* |
| ≥50% – <100% | 5 (22%) | 1 (4.5%) | |
| <50% | 4 (17%) | 3 (13.5%) | |
| 0% | 12 (52%) | 18 (82%) | |
| Total | 23 (100%) | 22 (100%) |
Mantel-Haenszel summary chi-square, stratified by center.
Discussion
Epilepsy is the most common neurological disorder among children and it is a significant public health concern in this population. It has a significant physical, psychological, economic and social toll on children and their caregivers. There are multiple treatment options for children with epilepsy. In many cases, treatment begins with a monotherapy and antiepileptic drugs are added (polytherapy) if seizures are not controlled with monotherapy. Only about 3% of patients who fail to respond to the first two antiepileptic drugs become seizure free with the third antiepileptic drug used, and in about one-third of patients with epilepsy the seizures are intractable (Kwan and Brodie, 2000; Sirven et al., 2014; Saad, 2014). Because of unstandardized definitions as well as misdiagnoses, the incidence and prevalence of intractable epilepsy are somewhat uncertain (Sirven et al., 2014). Furthermore, the mechanisms of epileptogenesis are not well established. Experimental animal models of epilepsy suggested that alteration of homeostasis of some trace elements in the brain may be involved in the pathogenesis of seizures. Theoretically, trace elements may play a role in the production and control of seizures in humans. This concept has led to several studies. However, the relationship between epilepsy and trace elements is still poorly understood (Hirate et al., 2002; Saad et al., 2014; Sirven et al., 2014).
Zinc is the second most abundant trace element in the body. It regulates the CNS through the modulation of glutamate and GABA receptor activity (Kheradmand et al., 2014; Seven et al., 2013). Several studies, including a recent work by our group, reported a significant decrease in serum zinc levels in patients with intractable epilepsy (Kheradmand et al., 2014; Saad et al., 2014; Seven et al., 2013). Other studies found lower levels of zinc in the serum as well as in other tissues (nails and hair) of epileptic patients (Ashraf et al., 1995; Barbeau and Donaldson, 1974; Ilhan et al., 2004; Wojciak et al., 2013). On the basis of previous studies, we hypothesized that children with refractory epilepsy may potentially benefit from oral zinc as an adjunctive to antiepileptic drugs. Therefore, this study set out to evaluate the efficacy of oral zinc supplementation as an adjunctive therapy in children with intractable epilepsy.
Our double-blind, placebo-controlled trial is the first trial of oral zinc supplementation in this population. We found that 31% of children with intractable epilepsy receiving zinc therapy for six months showed a significant improvement (>50–100% reduction in seizure frequency), compared with only 4.5% of patients receiving placebo (p<0.05). In our view, these are findings of clinical importance and are encouraging as regards the possible use of zinc in children with intractable epilepsy. The primary limitation of our study was the small sample size. In addition, we are from a developing country and were not able to obtain any pre and post-intervention serum zinc levels. There is an urgent need for larger randomized controlled trials with the measurement of pre- and post-intervention serum zinc levels and with long-term follow-up.
In conclusion, zinc is an important trace element. Our results supported mildly beneficial effects in children with intractable epilepsy. We recommend further investigation of oral zinc supplementation as an adjunctive therapy for managing intractable epilepsy in children. Zinc therapy may be an option in treatment protocols for intractable epilepsy in the near future.
References
- Abrams SA. Zinc deficiency and supplementation in children and adolescents. 2014. [Accessed Jan 2015]. Available at: http://www.uptodate.com/contents/zinc-deficiency-and-supplementation-inchildren-and-adolescents.
- Ashraf W, Jaffar M, Mohammed D, et al. Utilization of scalp hair for evaluating epilepsy in male and female groups of the Pakistan population. Sci Total Environ. 1995;164:69–73. doi: 10.1016/0048-9697(95)04457-c. [DOI] [PubMed] [Google Scholar]
- Barbeau A, Donaldson J. Zinc, taurine and epilepsy. Arch Neurol. 1974;30:52–58. doi: 10.1001/archneur.1974.00490310054009. [DOI] [PubMed] [Google Scholar]
- Crepin S, Godet B, Chassain B, et al. Malnutrition and epilepsy: a two-way relationship. Clin Nutr. 2009;28:219–225. doi: 10.1016/j.clnu.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Hirate M, Takeda A, Tamano H, et al. Distribution of trace elements in the brain of EL (epilepsy) mice. Epilepsy Res. 2002;51:109–116. doi: 10.1016/s0920-1211(02)00098-0. [DOI] [PubMed] [Google Scholar]
- Ilhan A, Ozerol E, Güleç M, et al. The comparison of nail and serum trace elements in patients with epilepsy and healthy subjects. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:99–104. doi: 10.1016/j.pnpbp.2003.09.025. [DOI] [PubMed] [Google Scholar]
- Kheradmand Z, Yarali B, Zare A, et al. Comparison of serum zinc and copper levels in children and adolescents with intractable and controlled epilepsy. Iran J Child Neurol. 2014;8:49–54. [PMC free article] [PubMed] [Google Scholar]
- Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- Lee SW, Chung SS. A review of the effects of vitamins and other dietary supplements on seizure activity. Epilepsy Behav. 2010;18:139–150. doi: 10.1016/j.yebeh.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Saad K. Childhood epilepsy: an update on diagnosis and management. American Journal of Neuroscience. 2014;5:36–51. [Google Scholar]
- Saad K, Hammad E, Hassan AF, et al. Trace element, oxidant, and antioxidant enzyme values in blood of children with refractory epilepsy. Int J Neurosci. 2014;124:181–186. doi: 10.3109/00207454.2013.831851. [DOI] [PubMed] [Google Scholar]
- Schwartz MW. The 5 Minute Pediatric Consult. Philadelphia, PA 19106: Lippincott Williams & Wilkins; 2012. [Google Scholar]
- Seven M, Basaran SY, Cengiz M, et al. Deficiency of selenium and zinc as a causative factor for idiopathic intractable epilepsy. Epilepsy Res. 2013;104:35–39. doi: 10.1016/j.eplepsyres.2012.09.013. [DOI] [PubMed] [Google Scholar]
- Sirven JI, Pedley TA, Wilterdink JL. Evaluation and management of drug-resistant epilepsy. 2014. [Accessed Jan 2015]. Available at: http://www.uptodate.com/contents/evaluation-and-management-of-drug-resistant-epilepsy.
- Wojciak RW, Mojs E, Stanislawska-Kubiak M, et al. The serum zinc, copper, iron, and chromium concentrations in epileptic children. Epilepsy Res. 2013;104:40–44. doi: 10.1016/j.eplepsyres.2012.09.009. [DOI] [PubMed] [Google Scholar]