Summary
The aim of this preliminary study was to present a new approach for connectivity analysis in patients with severe acquired brain injury (ABI) that overcomes some of the difficulties created by anatomical abnormalities due to the brain injury.
Using a data-driven approach, resting-state structural MRI (sMRI) and functional MRI (fMRI) data from three severe ABI patients – two with disorders of consciousness (DOC) and one who had recovered consciousness (non-DOC) – were integrated and analyzed. Parameters extracted from the distribution of the connectivity values, such as mean, standard deviation and skeweness, were considered. The distribution parameters estimated seem to provide an accurate multivariate classification of the considered cases that can be summarized as follows: connectivity in the severe ABI patients with DOC was on average lower than in the severe ABI non-DOC patient and healthy subjects. The dispersion of connectivity values of the severe ABI patients, non-DOC and DOC, was comparable, however the shape of the distribution was different in the non-DOC patient. Eventually, seed-based connectivity maps of the default mode network show a pattern of increasing disruption of this network from the healthy subjects to non-DOC and DOC patients. Consistent results are obtained using an ICA-based approach.
Keywords: brain connectivity, disorders of consciousness, magnetic resonance imaging, minimally conscious state, vegetative state
Introduction
High-field (3T) magnetic resonance imaging (MRI) is the technique of choice for the study of acquired brain injury (ABI), especially brainstem and diffuse axonal injury, in clinically stable patients. Compared to low-field (1.5T) MRI, the potential advantages of this technique are its increased spatial contrast and increased spectral and temporal resolution.
Since diagnosis of the vegetative or minimally conscious state is typically based exclusively on a patient’s clinical history and on behavioral observations, the investigation, through high-field MRI studies, of severe ABI in patients also presenting disorders of consciousness (DOC) is a major challenge (Fernandez-Espejo et al., 2010; Rosanova et al., 2012; Laureys and Schiff, 2012; Bruno et al., 2013; Giacino et al., 2014; Crone et al., 2013).
In recent years, functional neuroimaging has been used to demonstrate residual cortical processing in the absence of behavioral signs of consciousness in some non-communicative persons with severe brain injury (Laureys, 2005; Owen et al., 2006; Monti et al., 2010). In particular, to explore structural brain damage and functional residual cognitive functioning, two classes of MRI techniques can be applied: structural MRI (sMRI) and functional MRI (fMRI), which are strictly related, since the latter provides information on the functional association between regions of the anatomical hardware identified by the former.
Structural MRI can be used to quantify lesions and to evaluate the integrity of residual brain tissues, and thus to study patients with severe ABI, who exhibit several kinds of lesions in different brain locations. Macrostructural and microstructural changes induced by severe ABI can be measured using different types of sMRI techniques. Indeed, T1-weighted images can be used to quantify the gray matter, e.g. by means of voxel-based morphometry (Ashburner and Friston, 2000; Tomaiuolo et al., 2004). This method applied in patients in a vegetative state often revealed multilocal structural loss (Juengling et al., 2005). On the other hand, diffusion tensor imaging (DTI) can provide essential information for estimating white matter integrity, as in the case of microscopic disruption of white matter fibers in mild traumatic brain injury (TBI) (Rutgers et al., 2008). In addition, a recent study showed that 3T MRI is almost twice as sensitive as 1.5T MRI in assessing diffuse axonal injury (DAI) in severe TBI (Luccichenti et al., 2010). DAI, associated with de-afferentation and functional rearrangement, probably determines the extent of visualization of lesions (in terms their number and volume) in the chronic phase of TBI. Moreover, DTI tractography, able to investigate macroscopic axon fiber orientation, is currently the only method capable of providing noninvasive in vivo imaging of these structures and of their re-organization during central nervous system functional recovery and plasticity phenomena (Cherubini et al., 2007).
In past decades, fMRI has been used to identify regions specialized in cognitive and/or motor tasks, but more recently interest has shifted towards a novel fMRI approach, i.e. functional connectivity MRI (fcMRI), which focuses on characterization of the brain’s intrinsic functional architecture. It has been shown that the brain architecture is organized in a set of interacting networks that are highly internally coupled (Greicius et al., 2009; Buckner et al., 2008), and network interactions are observed both during the performance of active tasks and at rest. In the first case, typically, data are collected with blocked design experiments in which the contrast between two conditions is evaluated in the presence of auditory, visual, somatosensory or mental imagery stimuli (Laureys, 2005; Owen et al., 2006; Monti et al., 2010, Shiff et al., 2002; Owen et al., 2005; Carter et al., 2012a). At rest, on the other hand, participants do not perform any active task and are simply instructed to remain still with their eyes closed or open, performing fixation in the scanner.
It has been shown that fcMRI at rest has a number of advantages over task-evoked studies for investigating brain networks (Carter et al., 2012b), especially in patients with DOC. First, the resting state is much more easily achieved and tolerated by neurological and neuropsychiatric patient populations, and it is an ideal condition for studying patients with DOC, given their difficulty or inability to participate in active tasks in the scanner. In addition, this approach has been demonstrated to be robust also when investigating functional connectivity at subject level, thus potentially providing the opportunity to use data in single subjects for individual diagnosis, prognosis and recovery monitoring (Carter et al., 2012b). A third major advantage of fcMRI at rest is that it can be used to study multiple networks at once, unlike fMRI in which only the regions involved in the task are examined. These networks observed at rest (resting state networks, RSNs), closely resembling those evoked by tasks, could be an important focus of interest in the study of severe ABI, especially in patients who also present DOC.
Indeed, an innovative approach could be to assess the possible changes in the overall functional cerebral connectivity during the DOC and after recovery of consciousness (Rosanova et al., 2012).
Recently, fcMRI studies have been reported in which RSNs were investigated by means of a seed-based functional connectivity approach. Indeed, the default mode network (DMN) was investigated selecting the posterior cingulate cortex (pCC) as the seed region, whose coordinates were taken from the literature on RSNs in healthy subjects. This step is not always feasible in patients with DOC since the damage in the brain can make it impossible to select the same seeds reported in the literature. Such cases demand a data-driven approach in which:
global properties of the functional connectivity can be investigated by considering all the possible pairs of voxels in the brain;
a subject-specific set of seed locations is estimated from the acquired data, in relation to the individual structural brain lesions.
In this paper, we present a preliminary study in three patients, one with severe ABI and recovered consciousness, and two with severe ABI and persistent DOC (vegetative state), in which sMRI and fMRI data were integrated. In particular, the structural information from T1 and DTI images was integrated with the functional connectivity estimated from blood oxygen level dependent (BOLD) data. The sMRI data were analyzed by estimating the T1 parameters and performing probabilistic fiber tracking (Smith et al., 2004) (www.fmrib.ox.ac.uk/fsl). The fMRI data were analyzed by employing a measure of functional connectivity based on the temporal correlation of the BOLD time series (Smith et al., 2004; Boly et al., 2009; Fox et al., 2005). We propose an example of fMRI data, analyzed using a data-driven approach based on a cross-correlation matrix, computed on all possible pairs of brain voxels without a priori selection of regions of interest (de Pasquale et al., 2013). In particular, we present a multivariate approach in which different parameters, such as the means, standard deviations and skewness of the distribution of the connectivity values, are used.
The aim of this preliminary study was to present a new approach for connectivity analysis in patients with severe ABI that overcomes some of the difficulties created by anatomical abnormalities due to the brain injury.
Materials and methods
Clinical data
The sample included three subjects, one man and two women, with a mean age ± standard deviation of 39±12 years (range: 26–47 years), affected by severe ABI (Glasgow Coma Scale ≤ 8 in the acute phase) (Teasdale and Jennett, 1974), one after recovery of consciousness (non-DOC) and two in a vegetative state (DOC).
The diagnosis of vegetative state followed a period of coma, in one case after hemorrhagic stroke and in the other after subarachnoid hemorrhage. Patients were diagnosed with vegetative state according to the Italian Version of the Coma Recovery Scale-Revised (CRS-R) (Giacino 2004; Lombardi et al., 2007). The patients were in the post-acute or chronic stage, with an interval from coma onset ranging from two to 24 months, and they were recruited in the Post-Coma Unit of the “Fondazione Santa Lucia” Neuro-Rehabilitation Hospital in Rome. This study was approved by the Santa Lucia Foundation Ethics Committee on the 12th February 2009 (Protocol CE/AG-prog.217-218).
Healthy subjects
The control sample, already reported elsewhere (de Pasquale et al., 2013), comprised 20 healthy subjects (9 women and 11 men; mean age ± standard deviation: 30±10 years without any neurological or psychiatric disease and free of drug or toxic substance abuse.
Case 1 (severe ABI, non-DOC)
C.S., female aged 45 years: hemorrhagic stroke with a coma duration of 15 days and complete recovery of consciousness; interval of 2 months from coma onset at the time of the MRI study.
Structural MRI showed an area of hyperintensity in the cortical-subcortical structures, specifically in the temporoparietal region.
Case 2 (DOC1)
D.A., male aged 26 years: hemorrhagic stroke with vegetative state (CRS-R score=7); duration of vegetative state: 7 months at the time of the MRI study.
The patient showed quadriplegia, mutism, dystonic deviation of the head towards the left side and severe spasticity prevalently affecting the upper limbs.
Structural MRI showed ventricle enlargement and lesions in the bilateral frontobasal areas and left insula.
Case 3 (DOC2)
D.R., female aged 47 years: subarachnoid hemorrhage and hydrocephalus with vegetative state (CRS-R score=6); duration of vegetative state: 24 months at the time of the MRI study.
The patient showed quadriplegia, mutism, and severe spasticity of the upper and lower limbs.
Structural MRI showed ventricle enlargement and hypodense areas in bilateral subcortical frontal regions, right frontoparietal areas and cerebellum.
MRI data acquisition
In accordance with a procedure already described elsewhere (de Pasquale et al., 2013; Falletta Caravasso et al., 2015), participants were examined using a 3T Allegra MR Imager (Siemens Medical Solutions, Erlangen, Germany) with a maximum gradient strength of 40 mT/m, using a standard quadrature birdcage head coil for both radiofrequency (RF) transmission and RF reception. All the participants underwent the same MRI protocol, T1-weighted imaging, DTI and fMRI. All planar sequence acquisitions were acquired along the anterior/posterior commissure line. Particular care was taken to center the subject in the head coil and to restrain subject movements with cushions and adhesive medical tape. Diffusion-weighted volumes were acquired using spin-echo echo-planar imaging (echo time/repetition time = 89/8500 ms, bandwidth = 2126 Hz/voxel; matrix size 128 × 128; 80 axial slices, voxel size 1.8 × 1.8 × 1.8 mm3) with 30 isotropically distributed orientations for the diffusion-sensitizing gradients at a b-value of 1000 s mm2 and six b = 0 images). Scanning was repeated three times to increase the signal-to-noise ratio. Whole-brain T1-weighted images were obtained in the sagittal plane using a modified driven equilibrium Fourier transform sequence (Deichaman et al., 2004) (echo time/repetition time = 2.4/7.92 ms, flip angle 15°, voxel size 1 × 1 × 1 mm3). For the fMRI data, we adopted a gradient echoplanar imaging (EPI) sequence, with 38 axial slices with a voxel size of 3 × 3 × 2.5 mm3 (matrix size 64 × 64; FOV 192 × 192 mm2; TR=2470 ms) in ascending order.
The functional tasks consisted of two acquisitions of 200 volumes: i) during rest and ii) during auditory emotional stimulation: the participants listened to recorded accounts of events or people important to them, related by someone close to them (caregiver or significant other).
Data analysis
sMRI
The DTI data were analyzed using tools from the FMRIB Software Library (FSL, www.fmrib.ox.ac.uk/fsl). DTI images were corrected for eddy currents and head motion using affine registration to the average EPI volume with no diffusion weighting. The diffusion tensor was reconstructed and diagonalized in order to obtain basic diffusion parameters such as the fractional anisotropy (FA). The FA was then used to classify each voxel as gray matter (FA < 0.2) or white matter (FA ≥ 0.2) (Basser et al., 2000). Probabilistic diffusion tractography was carried out estimating a probability distribution function of the principal fiber direction at each voxel in the diffusion space (Behrens et al., 2003). Connectivity maps between seed voxels and all other points in the brain were generated by repeatedly sampling connected pathways through the probability density function field. The seed voxels were located in the right and in the left cerebral peduncle to verify the integrity of the corticospinal tract in the left and in the right hemisphere.
fMRI
Our pre-processing pipeline consists of several steps performed using a combination of tools from the FSL (www.fmrib.ox.ac.uk/fsl). The data are initially motion corrected using the FSL–MCFLIRT tool (Jenkinson et al., 2002). We note that while this step was able to realign all the volumes acquired for the healthy subjects, in the two DOC patients 6% and 7% of the volumes were excluded from the analyses because of excessively large motion artifacts, which greatly influence connectivity findings (Van Dijk et al., 2012). This step was performed by comparing the motion parameters with the distribution obtained from the healthy sample. Volumes showing motion parameters significantly different from the healthy sample were excluded. Then, the FSL–BET and FSL–FAST tools were used to extract brain volumes and to estimate a gray matter mask. Finally, we performed mean-based intensity normalization of all volumes, high-pass temporal filtering, Gaussian low-pass temporal filtering (full-width half maximum, FWHM, sigma = 2.8), and coregistration to the MNI152 standard space (FSL–FNIRT tool). The connectivity analyses focused only on voxels classified as belonging to gray matter. The parameter used to assess functional connectivity is the temporal cross-correlation at lag zero (Fox et al., 2005). It is an interesting measure since it is not affected by scale effects such as the BOLD signal amplitude. The estimate of the correlation is repeated for every pair of voxels in order to obtain a matrix C representing the whole-brain connectivity of a given subject for a given session. This measure of connectivity will not depend on the amplitude and baseline of BOLD signals. This allows a group analysis in which the connectivity of different subjects is compared. In particular, in this paper, different parameters, obtained from C, will be used to characterize the overall connectivity:
- the mean value of C;
- the standard deviation and skewness of the distribution of the C values, to characterize the shape of the distribution (Kenney and Keeping, 1962). Since the correlation ranges from −1 to 1, a significant deviation from zero of this parameter indicates that the correlation values are not evenly distributed around zero and the bulk of the estimated values are either positive (negative skewness) or negative (positive skewness);
- the spatial topography of C corresponding to different conditions, e.g. presence or absence of an auditory stimulus (see above for the description of the experimental paradigm). This makes it possible to observe a variation in connectivity (increase or decrease), corresponding to the different conditions, and this parameter could represent, as a future development, a basis for identifying the areas involved in this variation.
Since the estimate of C when all voxels in the brain are considered is computationally expensive, the size of the fMRI data was reduced by means of smoothing and down-sampling. First, fMRI volumes were convolved with a 2D mean filter so that the value at each voxel was replaced with the average computed on its second order neighbors. Then the images were down-sampled by a factor of 2, to obtain an actual axial voxel size of 6×6 mm2.
We note that in this work, we did not adopt any signal regression step, e.g. global signal regression. There are several reasons for this. The utility of global signal regression is still under debate in the fMRI community (Birn, 2012; Braun et al., 2011; Chang and Glover, 2010; Fox et al., 2009; Murphy et al., 2009; Scholvinck et al., 2010; Schwarz and McGonigle, 2011). Indeed, although it aims to remove non-neural noise, such as respiration-induced or other types of slow artifactual fluctuations, it might have a great impact on the amplitude of functional coupling. Moreover, another disadvantage of global signal regression is that it may greatly reduce the reliability of region of interest-based correlation analyses (Braun et al., 2011; Guo et al., 2012; Schwarz and McGonigle, 2011) and it markedly shifts the distribution of correlation values, reducing the number of positive correlations and inducing negative ones (Murphy et al. 2009). These effects, evident on connectivity estimated in healthy subjects, might have a dramatic effect on our data in which we already expect a strong reduction of connectivity due to the patients’ conditions. We acknowledge that our results might be affected by these artifacts to some extent.
Results
sMRI
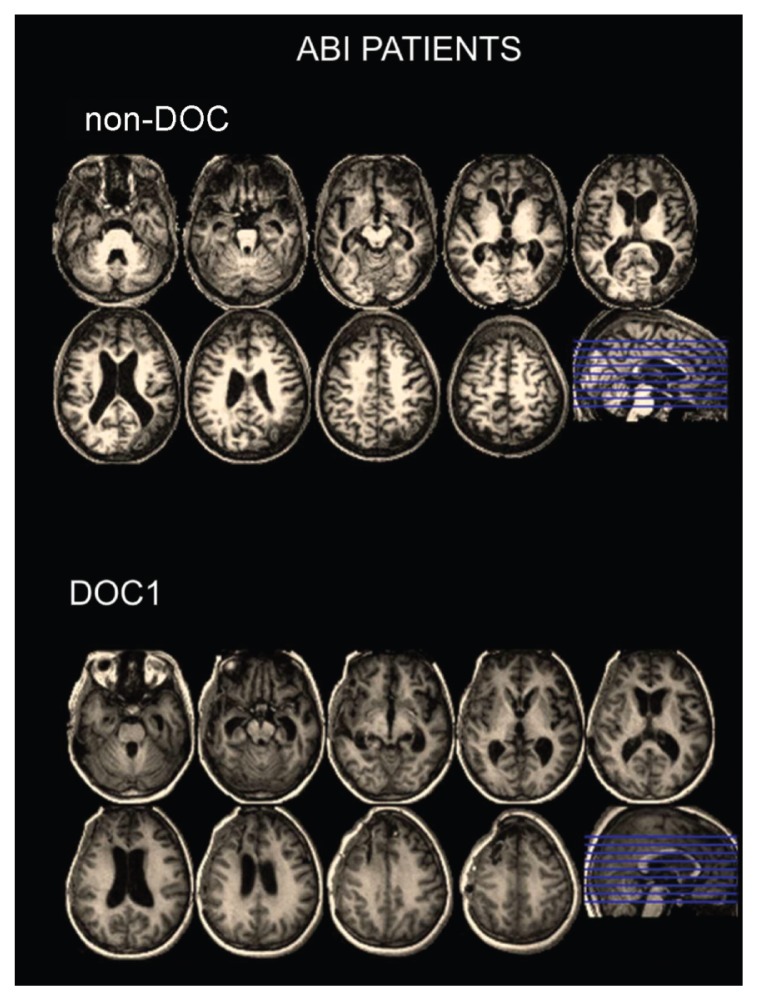
In figure 1, structural T1-weighted MRI from two patients with severe ABI are shown. In case 1, a patient who had recovered consciousness (top panel, non-DOC), the MRI study shows large stable encephalomalacic areas, hypointense on T1-weighted images, involving the gray and white matter of both the temporal and the frontal lobes bilaterally and the left parietal lobe. The ventricles and the sulci adjacent to the lesions are dilated, reflecting a loss of cerebral tissue volume. In case 2, a patient diagnosed with vegetative state (bottom panel, DOC1), the MRI study shows encephalomalacic areas, hypointense on T1-weighted images, involving both the gray and the white matter of the right temporal lobe and of the frontal lobes. The right ventricles and the sulci adjacent to the lesions are dilated, reflecting cortical and subcortical atrophy.
Figure 1.
Structural T1-weighted MRI from two patients with severe ABI.
Top panel: severe ABI patient with normal consciousness (non-DOC).
The MRI study shows large, stable encephalomalacic areas, hypointense on T1-weighted images, involving the gray and white matter of both temporal and both frontal lobes and the left parietal lobe.
Bottom panel: severe ABI patient with DOC, i.e. vegetative state (DOC1)
The MRI study shows encephalomalacic areas, hypointense on T1-weighted images, involving both the gray and the white matter of the right temporal lobe and of the frontal lobes.
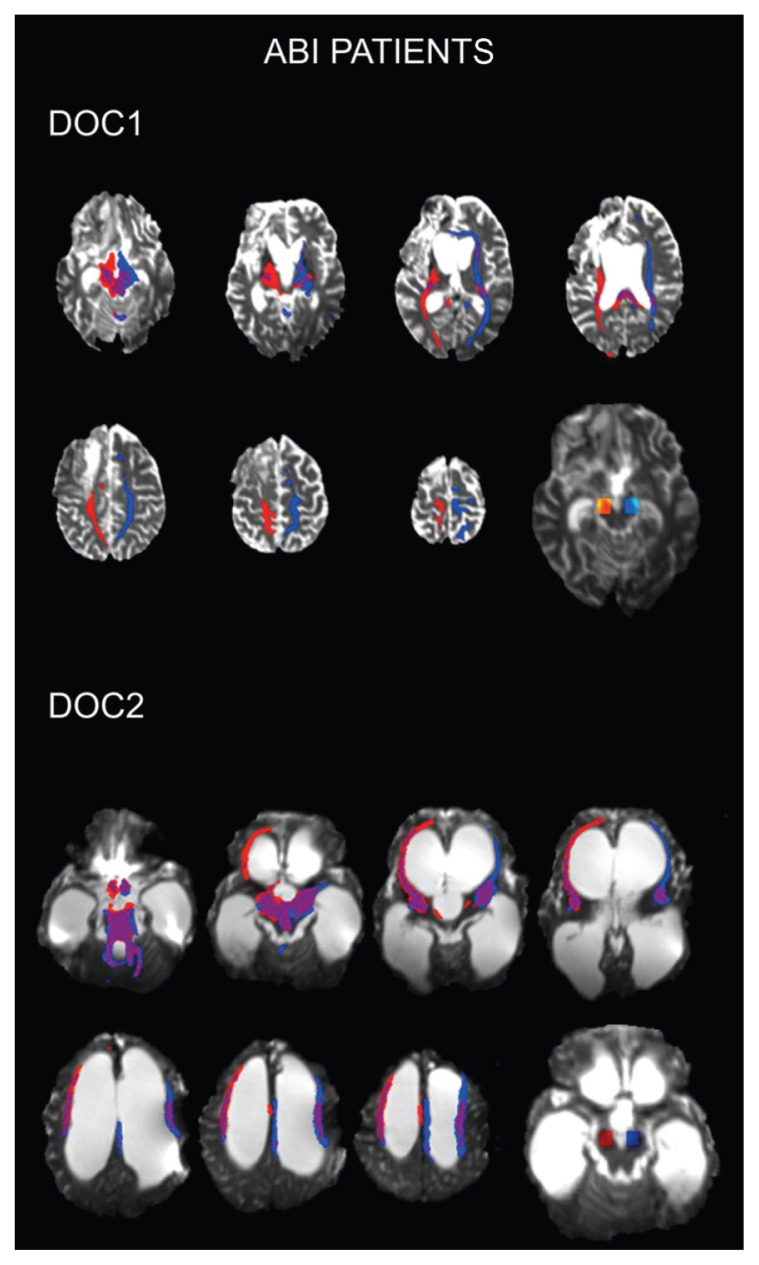
Figure 2 shows the structural DTI MRI from the two severe ABI patients also affected by a DOC (a diagnosis of vegetative state). In case 2 (DOC1, top panel) MRI tractography shows a lack of frontal pathways mainly in the left hemisphere. In case 3 (DOC2, bottom panel), MRI tractography shows normal reconstruction of frontal fibers, also taking into account the significant ventricular dilatation.
Figure 2.
Structural DTI MRI from two severe ABI patients with DOC (vegetative state).
Top panel: (DOC1) MRI tractography shows a lack of frontal pathways mainly in the left hemisphere.
Bottom panel: (DOC2) MRI tractography shows normal reconstruction of frontal fibers, also taking into account the significant ventricular dilatation.
fMRI
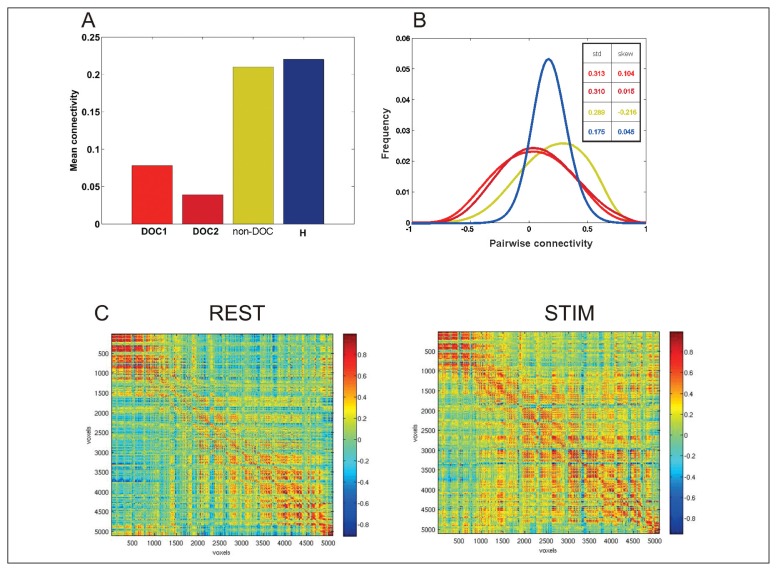
Figure 3A reports the mean values of connectivity estimated at rest in the two severe ABI patients with DOC (DOC1 and DOC2, red bars), in the severe ABI patient without DOC (non-DOC, yellow bar), and in the sample of healthy controls (H, blue bar). The average values for the two DOC patients, namely 0.04 and 0.08, are smaller than the corresponding values for both the non-DOC patient and the healthy subjects (0.20 and 0.22 respectively). This shows, as expected, that average overall connectivity in the vegetative state was lower than in the non-DOC and healthy state. As can be noted in figure 3B, the shape of the distribution in these cases was also very different. Both the subject with DOC and the non-DOC patient were characterized by a large spread of connectivity values around a small average value. In fact, the sample standard deviations for these cases were very similar (approximately 0.31), and found to be twice the sample standard deviation obtained for the healthy subjects (approximately 0.17). This shows that in the healthy subjects the connectivity was stronger on average and also tightly centered around its mean value, i.e. the connectivity dispersion was smaller. Another interesting point is that the dispersion was comparable between the non-DOC and the DOC patients (the sample standard deviation is approximately the same), but the shape of the distribution was found to be very different in the non-DOC patient. Indeed, the skewness of this distribution as an absolute value was higher than in all the other cases, namely s=-0.21. This indicates that in the severe ABI patient with recovered consciousness (non-DOC), although the distribution showed a similar dispersion to that seen in the vegetative state cases, the values were not evenly distributed. The frequency of the positive values was higher than the frequency of the negative values and this explains why the average value is comparable to the value for the healthy subjects. This is interesting since it shows that to characterize connectivity accurately, the average and the standard deviations are not sufficient. The non-DOC patient could not be distinguished from the vegetative state (DOC) patients on the basis of connectivity dispersion, and could not be distinguished from the healthy subjects on the basis of the average connectivity value. Only a multivariate approach in which these three parameters are taken into account simultaneously succeeded in classifying the three cases correctly.
Figure 3.
Functional connectivity in resting condition and after auditory emotional stimulation
A) The mean connectivity values estimated at rest in three patients with severe ABI – two DOC patients (DOC1, DOC2, red bars) and a non-DOC patient (non-DOC, yellow bar) – and in a representative healthy subject (H, blue bar). The DOC patients both show smaller overall connectivity, while the non-DOC patient and the healthy subject have similar connectivity values. B) Distribution of overall connectivity at rest. The standard deviation shows that the distribution of connectivity in the two DOC cases and in the non-DOC patient shows greater spread than in a healthy subject. The skewness shows that although the DOC and non-DOC patients have a similar spread of connectivity, in the non-DOC patient there is a prevalence of positive values of connectivity. C) The cross-correlation matrix estimated at rest before (REST) or during an auditory stimulation (STIM) in both the vegetative state patients. A 60% increase in the connectivity can be noted between the two conditions.
Figure 3C, as an example, reports the cross-correlation matrix for a DOC patient at rest obtained before (REST) and during (STIM) an auditory emotional stimulation (see above). It can be noted that in the STIM condition the connectivity increased. This increase represented a 60% increase in the average connectivity value in the STIM versus the REST condition.
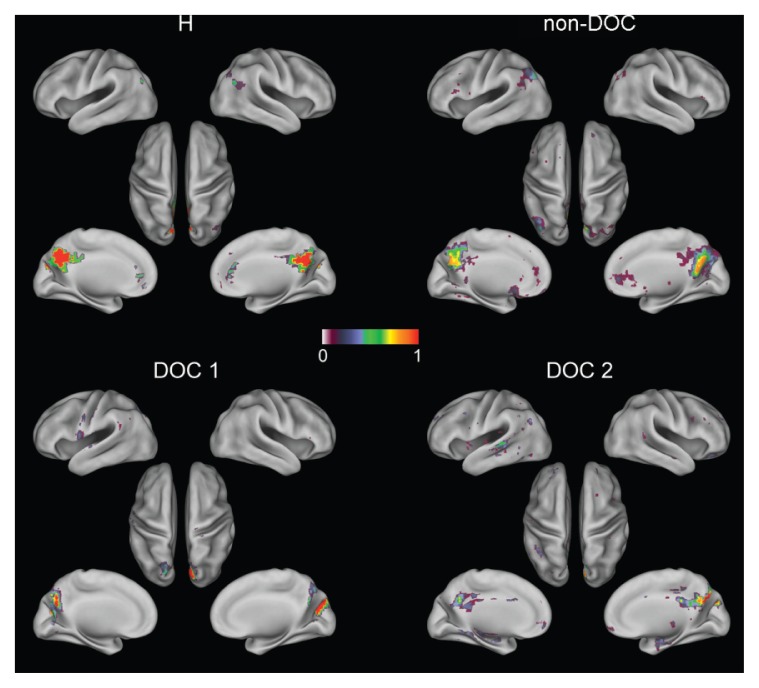
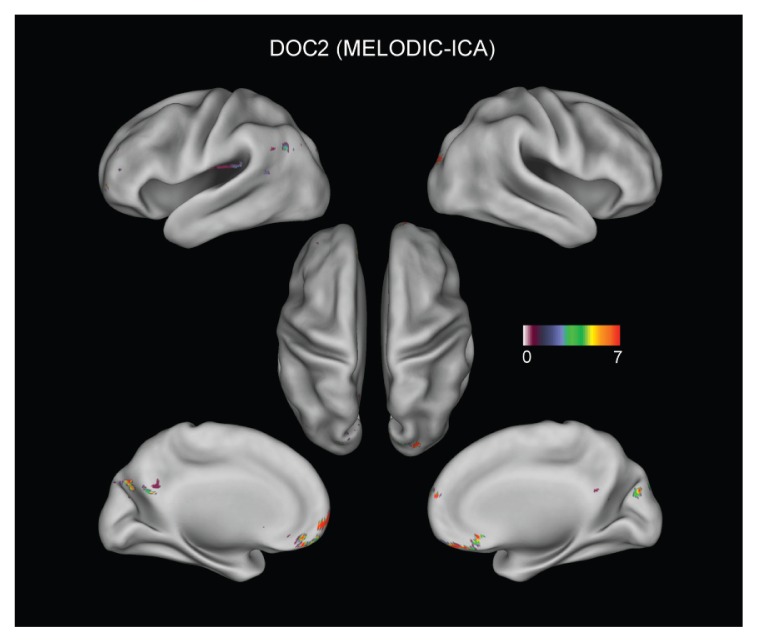
In order to investigate the spatial structure of connections, the topography of the DMN was investigated in the above patients. Figure 4 shows the significant connections (p<0.05, false discovery rate corrected) when the pCC was adopted as the seed region (de Pasquale et al., 2012). Although the Fisher transform was used to assess significance, for reasons of clarity the figure shows only the original correlation values. In the upper-left panel (H) the DMN topography obtained from the sample of healthy subjects is reported. The typical spatial structure reported in the literature, comprising the left and right angular gyri, dorsal and ventral medial prefrontal cortex, can be noted. The upper-right panel reports the results in the patient without the vegetative state (non-DOC). Interestingly, although some disruption is emerging, the DMN topography is still preserved. Although weaker connections between the pCC and the angular gyri can be noted, there are still significant connections with the frontal regions of this network. The disruption of the DMN is more evident in the two DOC patients (lower panels): in these cases the pCC seems disconnected from the rest of the DMN and only in one patient can weak connections with the frontal regions be detected. In order to verify that these results were not biased by the particular seed-based approach adopted, in figure 5 we report, as an example, the results obtained in DOC2 when using the MELODIC ICA (Multivariate Exploratory Linear Optimized Decomposition into Independent Components) FSB tool to extract RSNs. Of note, the first 50 components obtained did not show any interesting spatial structure. The only independent component resembling the DMN is reported in figure 5. We noted a good agreement between the seed-based and ICA-based results since both showed only partial frontal connections with the pCC.
Figure 4.
Seed-based functional connectivity (seed=pCC) for the default mode network in a healthy sample (H), non-DOC patient (non-DOC) and two DOC patients (DOC1 and DOC2).
A pattern of increasing DMN disruption is evident from healthy to DOC patients.
Figure 5.
MELODIC ICA-based results for patient DOC2.
The independent component resembling the DMN topography is reported. In agreement with the previous figure, only partial frontal connections are obtained.
Discussion
This paper describes a combined sMRI-fMRI study performed to investigate structural and functional brain connectivity in two severe ABI patients with DOC and one severe ABI patient who had recovered consciousness (non-DOC). We underline that the main results presented in this paper are not interesting per se, but rather because they may provide validation of the proposed methodology (de Pasquale et al., 2013) in cases with severe diffuse brain damage and previous or persistent DOC.
In medical practice, sMRI data provide clinicians with important information on diffuse brain damage and the stability of the clinical picture. Nonetheless, volumetric T1 studies often do not correlate with the outcome. In this work, for example, the three cases with severe ABI differed in the extent and distribution of the lesions. In the first patient, with recovered consciousness, the T1-weighted MRI study indicated the presence of more extensive brain lesions, bilaterally distributed, compared to the unilateral brain lesions observed in the other patient with severe ABI and DOC. On the other hand, DTI tractography in the two cases with severe ABI and DOC (vegetative state) showed a lack of frontal pathways mainly in the left hemisphere in case 2 (DOC1), whereas in case 3 (DOC2), it revealed reconstruction of the frontal fibers already found in normal subjects (de Pasquale et al., 2013), also taking into account the significant ventricular dilatation. Again, this important difference in the anatomical connectivity did not seem to discriminate between the clinical conditions of the two patients, both of whom were diagnosed with vegetative state. Therefore, structural imaging remains an important evaluation but it needs to be integrated, in the same protocol, with a functional study.
The multivariate fMRI approach showed some interesting aspects of the functional connectivity in these patients. Both the average connectivity values and the spread of the overall functional connectivity in the two patients with DOC were very similar. This result is interesting since the residual brain structures were dramatically different between DOC1 and DOC2, as can be observed in figure 2. As expected, the average connectivity for the two patients with DOC was weaker than in the patient with severe ABI and recovered consciousness (non-DOC) and the healthy subjects. Interestingly, the non-DOC patient showed an average connectivity value similar to that of the healthy subjects but a spread of connectivity similar to the DOC patients. The shape of the distribution of the connectivity, in the patient with severe ABI and recovered consciousness (non-DOC), showed that the distribution was more skewed towards positive values of connectivity. Thus, only the integration of these three parameters (mean, standard deviation and skewness of the connectivity distribution) may allow more accurate classification of these three severe brain injury patients on the basis of functional data. These results of our data-driven approach from two patients with DOC with large structural brain differences highlighted by previous sMRI investigations bear out interesting data about the possible residual, hidden cognitive abilities of our patients, in view of the activation changes found during the auditory emotional stimulation. During the experiment, patients listened to a recorded voice of a close relative evoking personal and emotionally significant events from their lives. Interestingly, we observed a 60% increase in the average connectivity value between the resting condition and after the significant emotional stimulation. This result nicely relates to the hypothesis reported in the literature in which some patients with DOC, such as vegetative or minimally conscious state, may have brain activation reflecting some residual cognition, even in the absence of any behavioral interaction with the environment (Owen et al., 2006; Monti et al., 2010).
The preliminary results of an innovative analysis of brain connectivity presented in this paper may represent the basis for future studies on functional connectivity in individuals with DOC. In the literature, functional connectivity has been investigated in different pathologies such as vegetative and minimally conscious state (Rosanova et al., 2012), schizophrenia (Bassett et al., 2008), Alzheimer’s disease (Sanz-Arigita et al., 2010) and stroke (Carter et al., 2012a). Nevertheless, in these studies no multimodal integration of different imaging techniques was presented and the connectivity was computed from prior seeds. In fact, popular seed connectivity methods rely largely on a set of node coordinates from the fMRI literature on healthy subjects (Fox et al., 2005; de Pasquale et al., 2010). These sets cannot always be used in individuals with DOC and other brain pathologies because of their structural brain damage. In these cases, a data-driven approach, such as the one proposed here, can be used to identify ad-hoc seed locations specific for the patients under investigation, taking into account the anatomical abnormalities due to their multifocal or diffuse brain injury (de Pasquale et al., 2013). This could be regarded as a preliminary step for subsequent network analyses, e.g. a graph theory based approach, in which the node grid is identified based on the patient data (Sporns, 2006; Stam et al., 2009). This approach is particularly promising since it can provide different parameters able to characterize important global and local network features (degree of clustering, mean path length and modularity). In addition, these parameters can also be obtained in a follow-up study to describe the cerebral network plasticity after the acquired brain injury (Wang et al., 2010). This might be important from a clinical point of view since these analyses could critically improve the clinical evaluation of these patients and may represent a promising avenue for further investigation.
The present study has some limitations. First, the very small and heterogeneous sample studied does not allow any definitive interpretation of the study findings. Additionally, we propose a longitudinal follow-up of functional connectivity over time to confirm our preliminary findings. This could be affected more by noise than other parameters such as, for example, the spectral coherence. Thus, the adoption of different parameters reported in the functional connectivity literature could be the objective of future work (Li et al., 2009). Finally, computation of the cross-correlation matrix for every pair of voxels within the brain is computationally onerous and time consuming. For this reason, the data were down-sampled. This certainly limits the spatial resolution of this approach. Finally, in this work physiological noise correction was not performed in order not to lower the already small effects of functional coupling. However, we acknowledge that this is a limitation of our study and such corrections might be the topic of a future work.
Acknowledgments
The study was supported by the Italian Ministry of Health [grant RF2008 n.31].
References
- Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pajevic S, Pierpaoli C, et al. In vivo fiber tractography using DT-MRI data. Magn Reson Med. 2000;44:625–632. doi: 10.1002/1522-2594(200010)44:4<625::aid-mrm17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Bassett DS, Bullmore E, Verchinski BA, et al. Hierarchical organization of human cortical networks in health and schizophrenia. J Neurosci. 2008;28:9239–9248. doi: 10.1523/JNEUROSCI.1929-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Jenkinson M, et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003;50:1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Birn RM. The role of physiological noise in resting-state functional connectivity. Neuroimage. 2012;62:864–870. doi: 10.1016/j.neuroimage.2012.01.016. [DOI] [PubMed] [Google Scholar]
- Boly M, Tshibanda L, Vanhaudenhuyse A, et al. Functional connectivity in the default network during resting state is preserved in a vegetative but not in a brain dead patient. Hum Brain Mapp. 2009;30:2393–2400. doi: 10.1002/hbm.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun U, Plichta MM, Esslinger C, et al. Test–retest reliability of resting-state connectivity network characteristics using fMRI and graph theoretical measures. Neuroimage. 2012;59:1404–1412. doi: 10.1016/j.neuroimage.2011.08.044. [DOI] [PubMed] [Google Scholar]
- Bruno MA, Laureys S, Demertzi A. Coma and disorders of consciousness. Handb Clin Neurol. 2013;118:205–213. doi: 10.1016/B978-0-444-53501-6.00017-2. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Carter AR, Patel KR, Astafiev SV, et al. Upstream dysfunction of somatomotor functional connectivity after corticospinal damage in stroke. Neurorehabil Neural Repair. 2012a;26:7–19. doi: 10.1177/1545968311411054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AR, Shulman GL, Corbetta M. Why use a connectivity-based approach to study stroke and recovery of function? Neuroimage. 2012b;62:2271–2280. doi: 10.1016/j.neuroimage.2012.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Glover GH. Time–frequency dynamics of resting-state brain connectivity measured with fMRI. Neuroimage. 2010;50:81–98. doi: 10.1016/j.neuroimage.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherubini A, Luccichenti G, Péran P, et al. Multimodal fMRI tractography in normal subjects and in clinically recovered traumatic brain injury patients. Neuroimage. 2007;34:1331–1341. doi: 10.1016/j.neuroimage.2006.11.024. [DOI] [PubMed] [Google Scholar]
- Crone JS, Soddu A, Höller Y, et al. Altered network properties of the fronto-parietal network and the thalamus in impaired consciousness. Neuroimage Clin. 2013;26:240–248. doi: 10.1016/j.nicl.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pasquale F, Della Penna S, Snyder AZ, et al. Temporal dynamics of spontaneous MEG activity in brain networks. Proc Natl Acad Sci USA. 2010;107:6040–6045. doi: 10.1073/pnas.0913863107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pasquale F, Della Penna S, Snyder AZ, et al. A cortical core for dynamic integration of functional networks in the resting human brain. Neuron. 2012;74:753–764. doi: 10.1016/j.neuron.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pasquale F, Sabatini U, Della Penna S, et al. The connectivity of functional cores reveals different degrees of segregation and integration in the brain at rest. Neuroimage. 2013;69:51–61. doi: 10.1016/j.neuroimage.2012.11.051. [DOI] [PubMed] [Google Scholar]
- Deichmann R, Schwarzbauer C, Turner R. Optimisation of the 3D MDEFT sequence for anatomical brain imaging: technical implications at 1.5 and 3 T. Neuroimage. 2004;21:757–767. doi: 10.1016/j.neuroimage.2003.09.062. [DOI] [PubMed] [Google Scholar]
- Falletta Caravasso C, de Pasquale F, Ciurli P, et al. The Default Mode Network connectivity predicts cognitive recovery in severe acquired brain injury patients: a longitudinal study. J Neurotrauma. 2015 doi: 10.1089/neu.2015.4003. (in press) [DOI] [PubMed] [Google Scholar]
- Fernández-Espejo D, Junque C, Cruse D, et al. Combination of diffusion tensor and functional magnetic resonance imaging during recovery from the vegetative state. BMC Neurol. 2010;3:10–77. doi: 10.1186/1471-2377-10-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, et al. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 2009;101:3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacino JT. The vegetative and minimally conscious states: consensus-based criteria for establishing diagnosis and prognosis. NeuroRehabilitation. 2004;19:293–298. [PubMed] [Google Scholar]
- Giacino JT, Fins JJ, Laureys S, et al. Disorders of consciousness after acquired brain injury: the state of the science. Nat Rev Neurol. 2014;10:99–114. doi: 10.1038/nrneurol.2013.279. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, et al. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo CC, Kurth F, Zhou J, et al. One-year test–retest reliability of intrinsic connectivity network fMRI in older adults. Neuroimage. 2012;61:1471–1483. doi: 10.1016/j.neuroimage.2012.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, et al. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Juengling FD, Kassubek J, Huppertz HJ, et al. Separating functional and structural damage in persistent vegetative state using combined voxel-based analysis of 3-D MRI and FDG-PET. J Neurol Sci. 2005;228:179–184. doi: 10.1016/j.jns.2004.11.052. [DOI] [PubMed] [Google Scholar]
- Kenney JF, Keeping ES. Mathematics of Statistics. Pt 1. 3rd ed. Vol. 101. Princeton, NJ: Van Nostrand; 1962. p. 1962. [Google Scholar]
- Laureys S, Schiff ND. Coma and consciousness: paradigms (re)framed by neuroimaging. Neuroimage. 2012;61:478–491. doi: 10.1016/j.neuroimage.2011.12.041. [DOI] [PubMed] [Google Scholar]
- Laureys S. The neural correlate of (un)awareness: lessons from the vegetative state. Trends Cogn Sci. 2005;9:556–559. doi: 10.1016/j.tics.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Li K, Guo L, Nie J, et al. Review of methods for functional brain connectivity detection using fMRI. Comput Med Imaging Graph. 2009;33:131–139. doi: 10.1016/j.compmedimag.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi F, Gatta G, Sacco S, et al. The Italian version of the Coma Recovery Scale-Revised (CRS-R) Funct Neurol. 2007;22:47–61. [PubMed] [Google Scholar]
- Luccichenti G, Giugni E, Péran P, et al. 3 Tesla is twice as sensitive as 1.5 Tesla magnetic resonance imaging in the assessment of diffuse axonal injury in traumatic brain injury patients. Funct Neurol. 2010;25:109–114. [PubMed] [Google Scholar]
- Monti MM, Vanhaudenhuyse A, Coleman MR, et al. Willful modulation of brain activity in disorders of consciousness. N Engl J Med. 2010;362:579–589. doi: 10.1056/NEJMoa0905370. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker D, et al. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, Coleman MR, Menon DK, et al. Residual auditory function in persistent vegetative state: a combined PET and fMRI study. Neuropsychol Rehabil. 2005;15:290–306. doi: 10.1080/09602010443000579. [DOI] [PubMed] [Google Scholar]
- Owen AM, Coleman MR, Boly M, et al. Detecting awareness in the vegetative state. Science. 2006;313:1402. doi: 10.1126/science.1130197. [DOI] [PubMed] [Google Scholar]
- Rosanova M, Gosseries O, Casarotto S, et al. Recovery of cortical effective connectivity and recovery of consciousness in vegetative patients. Brain. 2012;135:1308–1320. doi: 10.1093/brain/awr340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutgers DR, Toulgoat F, Cazejust J, et al. White matter abnormalities in mild traumatic brain injury: a diffusion tensor imaging study. AJNR Am J Neuroradiol. 2008;29:514–519. doi: 10.3174/ajnr.A0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz-Arigita EJ, Schoonheim MM, Damoiseaux JS, et al. Loss of ‘small-world’ networks in Alzheimer’s disease: graph analysis of FMRI resting-state functional connectivity. PLoS One. 2010;5:e13788. doi: 10.1371/journal.pone.0013788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff ND, Ribary U, Moreno DR, et al. Residual cerebral activity and behavioral fragments can remain in the persistently vegetative brain. Brain. 2002;125:1210–1234. doi: 10.1093/brain/awf131. [DOI] [PubMed] [Google Scholar]
- Schölvinck ML, Maier A, Ye FQ, et al. Neural basis of global resting-state fMRI activity. Proc Natl Acad Sci USA. 2010;107:10238–10243. doi: 10.1073/pnas.0913110107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz AJ, McGonigle J. Negative edges and soft thresholding in complex network analysis of resting state functional connectivity data. Neuroimage. 2011;55:1132–1146. doi: 10.1016/j.neuroimage.2010.12.047. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Sporns O. Small-world connectivity, motif composition, and complexity of fractal neuronal connections. Biosystems. 2006;85:55–64. doi: 10.1016/j.biosystems.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Stam CJ, de Haan W, Daffertshofer A, et al. Graph theoretical analysis of magnetoencephalographic functional connectivity in Alzheimer’s disease. Brain. 2009;132:213–224. doi: 10.1093/brain/awn262. [DOI] [PubMed] [Google Scholar]
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- Tomaiuolo F, Carlesimo GA, Di Paola M, et al. Gross morphology and morphometric sequelae in the hippocampus, fornix, and corpus callosum of patients with severe non-missile traumatic brain injury without macroscopically detectable lesions: a T1 weighted MRI study. J Neurol Neurosurg Psychiatry. 2004;75:1314–1322. doi: 10.1136/jnnp.2003.017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Yu C, Chen H, et al. Dynamic functional reorganization of the motor execution network after stroke. Brain. 2010;133:1224–1238. doi: 10.1093/brain/awq043. [DOI] [PubMed] [Google Scholar]