Summary
This study was performed with the aim of assessing dispositional optimism (DO) in a sample of Parkinson’s disease (PD) patients, in order to evaluate its association with clinical outcomes and its impact on rehabilitation.
Before entering an outpatient rehabilitation program, 58 participants suffering from idiopathic PD completed the Life Orientation Test-Revised (LOT-R) to evaluate their level of DO, the WHO-5 scale to evaluate their health-related quality of life (HR-QoL), the Hospital Anxiety and Depression Scale (HADS) to identify emotional distress, and the Barthel Index to evaluate their level of disability.
All the measures were repeated four months later, at their discharge from the program. Disease stage and severity measures (Unified Parkinson’s Disease Rating Scale) were also taken into consideration. Correlations and multivariate regression analyses compared DO with the health-related variables.
On admission a high level of DO was found to be associated with less severe disease, a better quality of life (QoL) and lower emotional distress, but not with level of disability (Barthel Index). Consistent results were found at discharge. The level of DO did not change after rehabilitation, while anxiety was significantly reduced, especially in subjects with low LOT-R and high HADS scores. The Barthel Index values significantly improved. At discharge, participants with high DO showed the best improvements in disability and in QoL.
In conclusion, a high level of DO was associated with QoL, HADS and UPDRS both on admission and at discharge. The level of DO remained stable after rehabilitation, while disability and anxiety were reduced. Participants with high DO generally had better QoL, and better clinical and psychological performances.
Keywords: disability, dispositional optimism, mood, Parkinson’s disease, quality of life, rehabilitation
Introduction
Parkinson’s disease (PD) is a disabling and progressive disease. Nevertheless, dispositional optimism (DO) has recently become a topic of growing interest and research within the field of PD. The mental attitude, or outlook on life, of individuals with DO is characterized by positive expectations and confidence in a secure future. They also view events and situations, including difficulties, in a positive light. In recent years, there has been an increase in studies exploring emotional responses, adaptive behavior and coping strategies under stressful conditions (see Chiesi et al., 2013).
Three early studies investigated the role of DO on disability and health-related quality of life (HR-QoL) in PD. While a longitudinal study in 12 PD patients found no significant relationship between disease severity and DO (Shifren, 1996), the Global Parkinson’s Disease International Survey (2002) found a statistically significant effect of DO on HR-QoL in 1020 PD patients. Several years later, the presence of low DO or high pessimism was found to be associated with reduced QoL in 99 PD patients (Gruber-Baldini et al., 2009). More recently, we found high DO to be associated with a satisfactory quality of life (QoL), low emotional distress, and reduced disease severity in PD (Gison et al., 2014). None of the above studies considered the effect of rehabilitation.
The present study was conducted with two aims: i) to evaluate the presence of correlations between PD patients’ levels of DO and major measures of well-being, both on admission to and at discharge from an outpatient rehabilitation program; ii) to examine the effect of baseline DO (on admission) on the rehabilitation outcome.
Materials and methods
Participants and recruitment
Between 2006 and 2012, 112 patients with idiopathic PD diagnosed by movement disorder specialists were referred to us by general practitioners in our local health district. To be included in this study, patients had to have a confirmed diagnosis of idiopathic PD, and have received more than five years of education. The exclusion criteria were: an advanced stage of disease; mental impairment, corresponding to a score <24 on the Mini-Mental State Examination (MMSE) (Folstein et al., 1975); a high level of comorbidity, liable to hinder physical treatment; and lack of informed consent. The diagnosis of idiopathic PD was not confirmed in 24 patients and a further 18 patients were not included due to the presence of mental impairment. Twelve participants did not complete the rehabilitation program (mainly for logistic reasons) and were considered lost at retest. Thus, the study sample comprised 58 subjects. The study was approved by the San Raffaele Pisana Ethics Committee and written consent was a strict requirement for the patients’ participation in the study.
Measures
Independent variable
Dispositional optimism was assessed by means of the Life Orientation Test-Revised (LOT-R), which consists of three items in a positive direction and three items in a negative direction, plus four ’filler’ items (Scheier et al., 1994). The Italian version of the LOT-R does not distinguish between DO and pessimism (Anolli, 2005); each item is scored 1–5, and high values indicate the presence of DO. The total score, ranging from 6 to 30, is the sum of all six (non-neutral) items. The raw values are converted into percentiles. There are no cutoffs. The brevity of this test makes it suitable for use in projects involving elderly people.
Outcome variables
Health-related QoL was assessed using the WHO-Five Well-being Index (WHO-5), which is a self-administered five-item scale (WHO, 1993; Bech, 2004; Schneider et al., 2010). Each item assesses the degree of positive well-being during the past two weeks on a six-point Likert scale graded from 0 (“at no time”) to 5 (“all of the time”). The raw score ranges from 0 to 25. In order to obtain a score on a scale from 0 to 100 (best conceivable level of well-being) the raw scores are multiplied by four. A validated Italian translation was used (De Girolamo et al., 2000).
Emotional distress was assessed using the Hospital Anxiety and Depression Scale (HADS), which is a self-assessment scale (Zigmond and Snaith, 1983). Higher scores indicate a higher level of psychological distress. The cut-off scores are fixed at 5 for the sub-scales and at 10 for the total score. The subscales are also valid measures of the severity of the emotional disorder. A validated Italian translation was used (Costantini et al., 1999).
Disability was assessed using the Barthel Index (Shah et al., 1989). This instrument is widely used to evaluate activities of daily living (ADLs). The scale consists of 10 items that measure: feeding, moving from wheelchair to bed and back, grooming, toilet use (transferring to and from a toilet), bathing, walking on a level surface, going up and down stairs, dressing, bowel continence, and bladder continence. The highest score is 100 and corresponds to total independence. Since motor fluctuations are common in PD, the best score for each item in the last week was taken.
Covariates
A demographics questionnaire was completed gathering information that included age, gender, living situation (living alone or with spouse/family), education, working status, and marital status. Disease severity measures included the Hoehn and Yahr (HY) stage (Hoehn and Yahr, 1967), the Unified Parkinson’s Disease Rating Scale (UPDRS) (Fahn et al., 1987), the MMSE (Folstein et al., 1975), and the disease duration (years from the apparent onset of PD). The HY stages range from 1 to 5 according to the degree of severity. Patients presenting with high severity, i.e. stages 4 and 5 were not enrolled. The UPDRS contains separate sections: I. Mentation, II. ADLs, III. Motor Examination, and IV. Complications of therapy, as well as a Total score (I + II + III). PD subjects with a UPDRS score >80 were not included. The MMSE is the most commonly used cognitive test and has a maximum score of 30 points. In general, MMSE scores ≥24 are considered normal. The Italian version was used (Magni, 1996) and patients presenting with a value <24 were not included in the present study. Finally, comorbidity was assessed using the Cumulative Illness Rating Scale (Linn et al., 1968).
Drug therapy
The participants had all been receiving optimized levodopa/dopamine decarboxylase inhibitor therapy and dopamine agonists. Their medication intake, ensuring a good benefits/side effects ratio, was noted before they started the rehabilitation program and was kept stable throughout the observation period. Only 9/58 patients (15.5%) had a HADS score >10 on admission and they received anti-depressant drugs. This percentage is much lower than those reported in other studies on PD. This discrepancy is due to the fact that the most severely depressed patients referred to our day hospital unit either failed to give their informed consent or refused to undergo testing.
Sleep aids were used in 14 participants (24%) while antipsychotics were never used in our sample. Incidentally, in our clinical practice we use clozapine in cases of psychosis or brisk resting tremor, but none of the participants presented these disturbances.
Rehabilitation
The objective of physical therapy in PD is to improve the patient’s HR-QoL by maintaining or increasing his levels of independence, safety and well-being in the performance of ADLs. This is achieved through prevention of inactivity and falls, improving functional activity and physical capacity (aerobic capacity, muscle strength and joint mobility), and decreasing limitations, especially of posture and movement.
The rehabilitation program focused on the following problem areas: transfers, body posture, grasping, balance, gait, speech, and cognitive and emotional impairment. A multidisciplinary treatment approach was adopted, particularly in the case of patients with a complex presentation. Accordingly, different specialists were involved: a rehabilitation physician, a neurologist, a physical therapist, an occupational therapist, a speech therapist and a psychologist. The treatment consisted of three 3-hour treatment sessions per week for 16 weeks. In detail, in accordance with the Dutch Guidelines (Keus et al., 2004), the patients underwent the following rehabilitation interventions:
physical therapy, based on cognitive movement strategies, cueing strategies, improvement of the performance of transfers, normalization of body posture, stimulation of static and dynamic balance, improvement of gait speed and safety, maintenance or improvement of physical capacity, and avoidance of dual tasking;
group treatment, geared at improving physical capacity and increasing well-being;
occupational therapy, aimed at solving practical problems arising in daily activities, for example in self-care, work, hobbies and recreation, transport, housekeeping and communication;
speech therapy, aimed at teaching patients how to cope with, or reduce, limitations and social participation problems connected with communication, eating and drinking;
neuropsychological therapy, based on the activation and stimulation of cognitive functions like memory, attention/reaction time and logical-deductive reasoning.
Data analysis
The presence of bivariate correlations between the parameters collected on admission (scores on LOT-R and clinical scales of QoL, measures of emotional distress and disability) was evaluated and Pearson correlation coefficients were measured. The correlation between UPDRS and MMSE on admission was borderline significant (p<0.05). The same analysis was performed at discharge to assess whether LOT-R status had remained the same. None of the variables included in the statistical models showed significant departures from normality (KS test), and the evaluation of the non-parametric correlation coefficients (Kendall’s tau and Spearman’s rho) confirmed that they remained unchanged over the study period. However, the variables showed a certain tendency to asymmetrical distribution, and to take this into account log-transformed data were used in most analyses and parametric tests were applied.
The association, on admission, between the level of DO and the other parameter scores was analyzed with a log-normal regression model, after adjusting for age, gender (male vs female), education (<8 years; 8–13 years; >13 years overall), severity of PD (UPDRS), and cognitive status (MMSE). The level of DO as effect modifier of all the clinical outcomes of the rehabilitation was evaluated with univariate stratified analysis, and a multiple regression analysis was performed for each parameter using a log-linear model adjusted for age, gender, education, UPDRS, MMSE and for the baseline level of the corresponding parameter. All analyses were performed with SPSS and Stata statistical software.
Results
Sample description
The study sample consisted of 37 men (63.8%) and 21 women (36.2%) with a mean age of 68.2 years (SD 10.4). Fifteen subjects (26.8%) had received less than eight years of education, 22 (39.3%) between eight and 13 years, and 19 (33.9%) more than 13 years. Eighty-one percent of the participants were below the HY stage 3. The mean disease duration was 6.7 years (SD 5.5). The UPDRS was administered in the on-medication state and the median total UPDRS score was 36.
Results on admission
A statistical description of the variables and their correlations on admission is provided below. It emerged that high LOT-R values were associated with a lower severity of disease, better QoL, better mood (as measured by both the anxiety and depression HADS subscales), but not with level of disability (Barthel Index). All the parameters considered on admission were reciprocally correlated except for disability, as shown in Table I.
Table I.
Descriptive statistics and bivariate correlation coefficients between parameters investigated on admission.
| Variable | Mean | Range | MMSE | UPDRS | LOT-R | WHO | HADS Total | HADS Anxiety | HADS Depression |
|---|---|---|---|---|---|---|---|---|---|
| MMSE | 28.0 (1.8) | 24–30 | |||||||
| UPDRS | 38.8 (14.4) | 14–78 | −.269* | ||||||
| LOT-R | 44.3 (27.6) | 6–90 | .203 | −.305* | |||||
| WHO-5 | 44.2 (20.0) | 0–84 | −.033 | −.275* | .506** | ||||
| HADS Total | 13.4 (7.7) | 0–32 | −.221 | .585** | −.636** | −.644** | |||
| HADS-Anxiety | 7.1 (4.4) | 0–17 | −.201 | .539** | −.562** | −.506** | .884** | ||
| HADS-Depression | 6.3 (4.3) | 0–21 | −.190 | .495** | −.561** | −.633** | .881** | .559** | |
| Barthel | 73.3 (11.7) | 45–100 | .126 | .01** | .169 | .121 | .048 | .041 | .044 |
Abbreviations: MMSE=Mini-Mental State Examination; UPDRS=Unified Parkinson’s Disease Rating Scale; LOT-R =Life Orientation Test-Revised; WHO-5=WHO-Five Well-being Index; HADS=Hospital Anxiety and Depression Scale. Data are given as mean values and SD;
p<0.05;
p<0.01.
Table II illustrates the association found in the PD patients between DO, categorized in three levels, and measures of disability, HR-QoL and emotional distress. For each outcome, mean ratio estimates were calculated in participants with LOT-R scores in the intermediate (21–60) or high range (>60) vs those with low LOT-R scores (≤20). The QoL of the participants with high LOT-R scores was found to be significantly (1.89 times) better than that of the participants with low LOT-R scores, while the level of emotional distress in the group with high LOT-R scores was significantly lower (HADS Total 0.48; 95% CI 0.32–0.71) than in participants with a low level of DO. No association was found with the level of disability, although patients with intermediate and high levels of DO showed higher Barthel Index values, i.e., 5% and 8% higher, respectively.
Table II.
Influence of level of optimism (Life Orientation Test-Revised score) on selected parameters on admission.
| Barthel Index mean ratio (95% CI) | WHO-5 mean ratio (95% CI) | HADS Total mean ratio (95% CI) | HADS Anxiety mean ratio (95% CI) | HADS Depression mean ratio (95% CI) | |
|---|---|---|---|---|---|
| Low optimism (≤20) | 1.00 (-) | 1.00 (-) | 1.00 (-) | 1.00 (-) | 1.00 (-) |
| Intermediate optimism (21–60) | 1.05 (0.94–1.17) | 1.75 (1.17–2.62) | 0.76 (0.53–1.09) | 0.85 (0.60–1.19) | 0.81 (0.52–1.26) |
| High optimism (>60) | 1.08 (0.95–1.22) | 1.89 (1.22–2.95) | 0.48 (0.32–0.71) | 0.48 (0.33–0.69) | 0.43 (0.28–0.69) |
Abbreviations: WHO-5=WHO-Five Well-being Index; HADS=Hospital Anxiety and Depression Scale.
Mean ratio and 95% confidence intervals are adjusted for age, gender, education, and UPDRS and MMSE scores.
Results at re-test
These correlations were maintained even after the completion of the rehabilitation program, i.e. LOT-R was still significantly correlated with WHO-5, HADS (both subscales) and QoL, but not with the Barthel Index (data non shown).
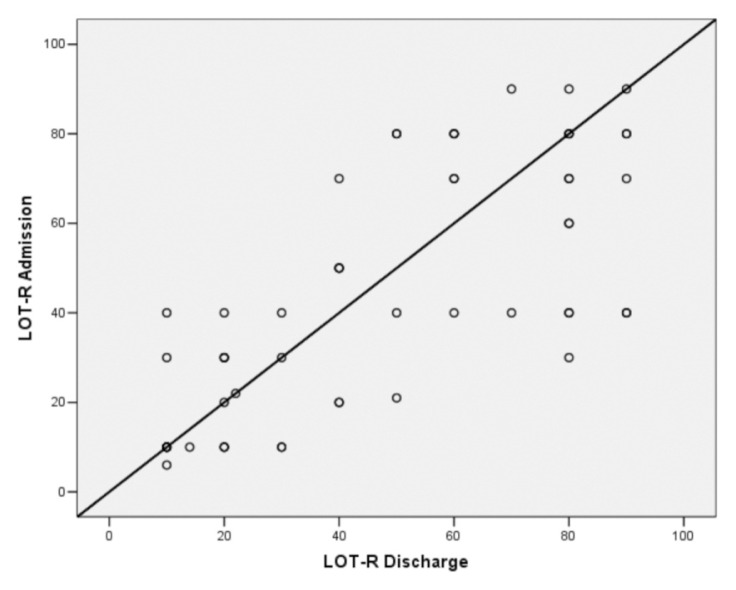
Similarly, as shown in Figure 1, the level of DO in PD participants did not change significantly after the rehabilitation treatment (increasing from a mean value of 44.3 to a mean value of 47.7, p=0.201).
Figure 1.
Comparison of LOT-R scores on admission and at discharge (r = 0.557).
By stratifying the LOT-R score into three levels, we observed that low scores on admission tended to remain low at retest (12 out of 16 patients). Likewise, high values remained high (11 out of 19 patients). By contrast, we observed a clear change in Barthel Index values after rehabilitation, thus indicating a significant reduction of disability. The other variable showing a significant improvement after treatment was the HADS-Anxiety subscale score (Table III).
Table III.
The effect of rehabilitation on QoL, emotional distress and disability evaluated by comparing mean values on admission and at discharge.
| Variable | Mean (SD) Admission | Mean (SD) Discharge | Δ (T1 – T0) | p-value |
|---|---|---|---|---|
| LOT-R | 44.3 (27.6) | 47.7 (28.6) | + 3.4 | n.s. |
| WHO-5 | 44.2 (20.0) | 47.1 (21.7) | + 2.9 | n.s. |
| HADS Total | 13.4 (7.7) | 12.4 (7.1) | − 1.0 | 0.085 |
| HADS-Anxiety | 7.1 (4.4) | 6.2 (3.8) | − 0.9 | 0.011 |
| HADS-Depression | 6.3 (4.3) | 6.2 (4.2) | − 0.1 | n.s. |
| Barthel Index | 73.3 (11.7) | 84.5 (10.9) | + 11.2 | <0.001 |
Abbreviations: LOT-R =Life Orientation Test-Revised; WHO-5=WHO-Five Well-being Index; HADS=Hospital Anxiety and Depression Scale; n.s.=not significant.
As shown in Table IV, after the stratification of DO into three levels, the pattern of post-rehabilitation changes in selected outcome variables was found to differ between the subgroups. The participants with high DO showed the best improvement in HR-QoL and in reduction of disability, while those in the low subgroup showed the largest reduction of emotional distress, especially anxiety. None of these interactions was found to be significant, as confirmed by the multivariate linear regression analyses, which were adjusted for all covariates and for the baseline score of the parameter considered in the model.
Table IV.
Effect of rehabilitation on QoL, emotional distress and disability. Mean values on admission and at discharge (stratified by level of optimism at baseline).
| Variable | Mean (SD) Admission | Mean (SD) Discharge | Δ (T1 – T0) | p-value |
|---|---|---|---|---|
| WHO-5 | ||||
| Low LOT-R score | 27.0 (15.4) | 28.3 (16.3) | + 1.3 | n.s. |
| Interm. LOT-R score | 48.5 (18.0) | 51.4 (19.5) | + 2.9 | n.s. |
| High LOT-R score | 53.5 (17.3) | 57.7 (18.7) | + 4.2 | n.s. |
| HADS Total | ||||
| Low LOT-R score | 19.4 (7.1) | 17.0 (7.1) | − 2.4 | <0.05 |
| Interm. LOT-R score | 14.4 (7.1) | 13.6 (6.6) | − 0.8 | n.s. |
| High LOT-R score | 7.2 (3.2) | 7.2 (3.8) | − 0.0 | n.s. |
| HADS-Anxiety | ||||
| Low LOT-R score | 10.2 (4.3) | 8.3 (3.7) | − 1.9 | <0.05 |
| Interm. LOT-R score | 6.3 (4.3) | 6.2 (4.2) | − 0.1 | n.s. |
| High LOT-R score | 3.4 (1.6) | 3.4 (2.0) | − 0.0 | n.s. |
| HADS-Depression | ||||
| Low LOT-R score | 9.3 (4.4) | 8.8 (4.3) | − 0.5 | n.s. |
| Interm. LOT-R score | 6.6 (4.3) | 6.7 (3.7) | + 0.1 | n.s. |
| High LOT-R score | 3.4 (2.2) | 3.4 (2.8) | − 0.0 | n.s. |
| Barthel Index | ||||
| Low LOT-R score | 69.7 (11.2) | 79.8 (11.5) | + 10.1 | <0.001 |
| Interm. LOT-R score | 73.4 (13.1) | 84.7 (11.3) | + 11.3 | <0.001 |
| High LOT-R score | 76.2 (10.1) | 88.2 (8.7) | + 12.0 | <0.001 |
Abbreviations: LOT-R =Life Orientation Test-Revised; WHO-5=WHO-Five Well-being Index; HADS=Hospital Anxiety and Depression Scale.
Discussion
The present study is the first to investigate the value of DO in PD subjects participating in a rehabilitation program. DO is a dispositional attitude or world view that leads those who have it to have positive expectancies for the future. Higher LOT-R values on admission were associated with a higher HR-QoL. The Global Parkinson’s Disease Survey (2002) also found that DO had a significant impact on HR-QoL assessed on the basis of a single item. Our observations are in line with the findings of previous cross-sectional studies (Gruber-Baldini et al., 2009; Gison et al., 2014), showing that greater DO (and less pessimism) was associated with better mental health and a better QoL, important goals to reach in all chronic illnesses. A new finding, emerging from the present study, was the stability of the correlations throughout the four-month rehabilitation program.
At discharge, the participants had less psychological distress and the data analysis showed a significant reduction in their anxiety levels. A further finding was the clear reduction of disability. On admission, DO was uncorrelated with disability, as previously found by others (Gruber-Baldini et al., 2009; Gison et al., 2014), although at discharge the best improvements in disability and in HR-QoL were shown by the participants with high DO. While previous studies on rehabilitation in PD did not consider DO, in the field of traumatic brain injury higher levels of DO were found to predict better psychological functioning and improved cognitive and functional outcomes (Ramanathan et al., 2011). We speculate that the physical training in our patients was certainly facilitated by anxiety reduction and by relaxation, leading to marked improvements in their Barthel Index scores. The lower levels of anxiety probably favored a reduction of rigidity, and may also have increased the subjects’ available attention resources and made them better able to multitask. Indeed, concentration and proper evaluation of rhythm and step improve walking (Jones et al., 2008). Another possible explanation may be psychophysiological. Regional cerebral blood flow in healthy volunteers is enhanced in tasks followed by monetary reward, which activates several cortical and subcortical areas. This pattern demonstrates the role played by mesolimbic dopamine pathways in reward processing. PD patients show compensatory cortical loops (Künig et al., 2000). However, PD participants enrolled in a rehabilitation program are different from PD patients who remain at home and do not undergo rehabilitation. Multi-dimensional training may constitute a rewarding experience capable, to an extent, of fostering physiological mesolimbic dopamine turnover. It would be advisable to verify, through neuroimaging techniques, the hypothesis that rehabilitation fosters mesolimbic dopamine turnover.
Our results also indicate that PD patients can remain optimistic in spite of the progressive nature of their disease. Early studies argued that people who present with DO have generally positive expectancies for the future and experience less distress when coping with difficult situations (Andersson, 1996). As we have previously speculated (Gison et al., 2014), patients’ DO could be reinforced by external factors, such as the pleasant setting offered by a rehabilitation center staffed by skilled and courteous operators, and their perception of the quality of the work done there. Moreover, improved motor performances and the acquisition of new abilities could also reinforce DO. In spite of these considerations, and in spite of a clear improvement in the patients’ performance of ADLs, their DO was not found to have increased at discharge, a finding that confirms the stability of this personality trait. In other words, optimistic people are unlikely to become more optimistic. Whereas PD is a progressive disease, DO remains stable over time (at least in the time frame of our study); this was confirmed by the high correlation coefficient between LOT-R on admission and at discharge (r= .57).
The pre- and post-intervention comparison in our sample showed a significant improvement in HADS-Anxiety subscale scores, an improvement that was predicted by DO levels. However, depression was not influenced by the treatment, in spite of its leading to reduced disability and reduced anxiety. Thus, DO and depression were two stable variables in our study. Our findings are very similar to the conclusions that emerged in a study on women surgically treated for breast cancer. In that study, DO remained stable over the follow-up period, whether the patients received bad news or not; depression, too, remained fairly stable even among the women who received bad news (Schou et al., 2005). According to Geriatric Depression Scale score changes over a nine-year period, 34% of 184 PD patients remained stable, 35% showed an improvement, and 30.9% were worse in the follow-up study. Gender, age, age at onset, HY stage, UPDRS score, and PD duration were not related to depression outcome (Rojo et al., 2003). In our cases, the level of depression remained stable over time, possibly a consequence of impaired neurotransmission, whereas a reduction in the level of anxiety was found at discharge, which may have been a psychological effect of the subjects receiving reassurance. On admission, 18 of the 58 participants in our sample (31%) were in the fourth quartile of the LOT-R score distribution, while the same quartile of the HADS score distribution included 17 participants (29%). The different evolution of anxiety and depression over the four months of this study illustrates the usefulness of HADS, which allows the identification of these conditions. Mutual influences between PD and depression are known to occur. For example, in a large sample of subjects, the risk of developing PD was significantly increased in those with affective disorder compared with other chronic pathologies (Nilsson et al., 2001); this study supports the hypothesis of a common etiology for major affective disorder and PD.
Some limitations of the present study should be acknowledged. First of all, the conclusions drawn refer only to patients in the earliest HY stages, since those in stages 4 and 5 were excluded. In stage 4, one finds patients with severe disability and no handwriting, although they are still able to walk or stand unassisted, while those in stage 5 are in a wheelchair or bedridden unless aided. To test such patients with the scales used in the present research would be highly impractical. Second, the Italian version of the LOT-R scale is slightly different from the LOT-R scale used elsewhere (Gruber-Baldini et al., 2009), although the conclusions are the same. Indeed, the accuracy of LOT-R was recently confirmed in young Italian volunteers (Chiesi et al., 2013). Finally, the influence of social support in our participants is unknown. Social support is mainly provided by families, caregivers, friends, colleagues, neighbors and health care professionals.
In spite of these limitations, our results nevertheless indicate that it is advisable to integrate the evaluation of personality traits for a better prediction of outcome of PD participants under rehabilitation. A working hypothesis to test is the better adaptation of optimistic people to the different types of social settings in their everyday life (Ravenek and Schneider, 2009). A further working hypothesis is the better adaptation of optimistic people suffering from PD in the domains of the International Classification of Functioning, Disability and Health (ICF) that concern the concept of participation. Today, objectives beyond those usually considered in PD evaluation and rehabilitation, e.g. personal relationships and environmental facilitators and barriers, are an increasing focus of study and attention (Raggi et al., 2010).
The conclusions of this study, with implications for rehabilitation, are the following: i) personality traits should be considered in PD because they may influence outcome; ii) DO is predictive of HR-QoL and anxiety levels both on admission and at discharge after 4 months; iii) DO and depression scores are unchanged by the rehabilitation intervention.
References
- Andersson G. The benefits of optimism: a meta-analytic review of the life orientation test. Pers Individ Dif. 1996;21:719–725. [Google Scholar]
- Anolli L. Versione Italiana “L’ottimismo”. Bologna: Il Mulino; 2005. Life Orientation Test-r. [Google Scholar]
- Bech P. Measuring the dimension of psychological general well-being by the WHO-5 119. Quality of Life Newsletter. 2004;32:15–16. [Google Scholar]
- Chiesi F, Galli S, Primi C, et al. The accuracy of the Life Orientation Test-Revised (LOT-R) in measuring dispositional optimism: evidence from item response theory analyses. J Pers Assess. 2013;95:523–529. doi: 10.1080/00223891.2013.781029. [DOI] [PubMed] [Google Scholar]
- Costantini M, Musso M, Viterbori P, et al. Detecting psychological distress in cancer patients: validity of the Italian version of the Hospital Anxiety and Depression Scale. Support Care Cancer. 1999;7:121–127. doi: 10.1007/s005200050241. [DOI] [PubMed] [Google Scholar]
- De Girolamo G, Rucci P, Scocco P, et al. Quality of life assessment: validation of the Italian version of the WHOQOL-Brief. Epidemiol Psichiatr Soc. 2000;9:45–55. doi: 10.1017/s1121189x00007740. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton RL . Members of the UPDRS Development Committee. Unified Parkinson’s disease rating scale. In: Fahn S, Marsden CD, Calne DB, et al., editors. Recent Developments in Parkinson’s Disease. Hants: Macmillan Healthcare Information; 1987. pp. 153–164. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-Mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gison A, Dall’Armi V, Donati V, et al. Dispositional optimism, depression, disability and quality of life in Parkinson’s disease. Funct Neurol. 2014;29:113–119. [PMC free article] [PubMed] [Google Scholar]
- Global Parkinson’s Disease Survey Steering Committee. Factors impacting on quality of life in Parkinson’s disease: results from an international survey. Mov Disord. 2002;17:60–67. doi: 10.1002/mds.10010. [DOI] [PubMed] [Google Scholar]
- Gruber-Baldini AL, Ye J, Anderson KE, et al. Effects of optimism/pessimism and locus of control on disability and quality of life in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15:665–669. doi: 10.1016/j.parkreldis.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression, and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Jones D, Rochester L, Birleson A, et al. Everyday walking with Parkinson’s disease: understanding personal challenges and strategies. Disabil Rehabil. 2008;30:1213–1221. doi: 10.1080/09638280701828955. [DOI] [PubMed] [Google Scholar]
- Künig G, Leenders KL, Martin-Sölch C, et al. Reduced reward processing in the brains of Parkinsonian patients. Neuroreport. 2000;11:3681–3687. doi: 10.1097/00001756-200011270-00019. [DOI] [PubMed] [Google Scholar]
- Linn BS, Linn MW, Gurel L. Cumulative Illness Rating Scale. J Am Geriatr Soc. 1968;16:622–626. doi: 10.1111/j.1532-5415.1968.tb02103.x. [DOI] [PubMed] [Google Scholar]
- Keus SHJ, Hendriks HJM, Bloem BR, et al. KNGF Guidelines for physical therapy in patients with Parkinson’s disease. Supplement to the Dutch Journal of Physiotherapy. 2004;114(3) [Google Scholar]
- Magni E, Binetti G, Bianchetti A, et al. Mini-Mental State Examination: a normative study in Italian elderly population. Eur J Neurol. 1996;3:198–202. doi: 10.1111/j.1468-1331.1996.tb00423.x. [DOI] [PubMed] [Google Scholar]
- Nilsson FM, Kessing LV, Bolwig TG. Increased risk of developing Parkinson’s disease for patients with major affective disorder: a register study. Acta Psychiatr Scand. 2001;104:380–386. doi: 10.1034/j.1600-0447.2001.00372.x. [DOI] [PubMed] [Google Scholar]
- Raggi A, Leonardi M, Ajovalasit D, et al. Functioning and disability in Parkinson’s disease. Disabil Rehabil. 2010;32(Suppl 1):S33–S41. doi: 10.3109/09638288.2010.511688. [DOI] [PubMed] [Google Scholar]
- Ramanathan DM, Wardecker BM, Slocomb JE, et al. Dispositional optimism and outcome following traumatic brain injury. Brain Inj. 2011;25:328–337. doi: 10.3109/02699052.2011.554336. [DOI] [PubMed] [Google Scholar]
- Ravenek MJ, Schneider MA. Social support for physical activity and perceptions of control in early Parkinson’s disease. Disabil Rehabil. 2009;31:1925–1936. doi: 10.1080/09638280902850261. [DOI] [PubMed] [Google Scholar]
- Rojo A, Aguilar M, Garolera MT, et al. Depression in Parkinson’s disease: clinical correlates and outcome. Parkinsonism Relat Disord. 2003;10:23–28. doi: 10.1016/s1353-8020(03)00067-1. [DOI] [PubMed] [Google Scholar]
- Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a reevaluation of the Life Orientation Test. J Pers Soc Psychol. 1994;67:1063–1078. doi: 10.1037//0022-3514.67.6.1063. [DOI] [PubMed] [Google Scholar]
- Schou I, Ekeberg Ø, Ruland CM. The mediating role of appraisal and coping in the relationship between optimism-pessimism and quality of life. Psychooncology. 2005;14:718–727. doi: 10.1002/pon.896. [DOI] [PubMed] [Google Scholar]
- Schneider CB, Pilhatsch M, Rifati M, et al. Utility of the WHO-Five Well-being Index as a screening tool for depression in Parkinson’s disease. Mov Disord. 2010;25:777–783. doi: 10.1002/mds.22985. [DOI] [PubMed] [Google Scholar]
- Shah S, Vanclay F, Cooper B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J Clin Epidemiol. 1989;42:703–709. doi: 10.1016/0895-4356(89)90065-6. [DOI] [PubMed] [Google Scholar]
- Shifren K. Individual differences in the perception of optimism and disease severity: a study among individuals with Parkinson’s disease. J Behav Med. 1996;19:241–271. doi: 10.1007/BF01857768. [DOI] [PubMed] [Google Scholar]
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- WHO. Study protocol for the World Health Organization project to develop a Quality of Life assessment instrument (WHOQOL) Qual Life Res. 1993;2:153–159. [PubMed] [Google Scholar]