Summary
The intentional activation of autonomic dysreflexia (AD, also called “boosting”), a practice sometimes used by athletes affected by spinal cord injury (SCI), is banned by the International Paralympic Committee (IPC). Although various studies have addressed doping and AD as separate issues, studies evaluating AD as a doping method are lacking. The aim of this brief review is to contribute to better understanding of the relationship between doping and AD.
We conducted a literature search of the PubMed database (from 1994 onwards). The key search terms “autonomic dysreflexia” and “boosting” were cross-referenced with “sport performance”. The official Paralympic website was also viewed. AD is a potent sympathetic reflex, due to a massive release of noradrenaline, that results in marked vasoconstriction distal to the level of the lesion. Athletes with SCI often self-inflict physical suffering in order to induce this phenomenon, which carries high health risks (i.e., hypertension, cerebral hemorrhage, stroke and sudden death). Boosting is a practice that can be compared to doping methods and the IPC expressly prohibits it. Any deliberate attempt to induce AD, if detected, will lead to disqualification from the sporting event and subsequent investigation by the IPC Legal and Ethics Committee.
Keywords: autonomic dysreflexia, boosting, doping, International Paralympic Committee
Introduction
Doping in sport is a well-known phenomenon that has been studied mainly from a biomedical standpoint, even though it also has important psychosocial implications, recently highlighted by Morente-Sánchez and Zabala (2013). Without going into the various aspects of this multi-faceted problem, an irrefutable fact is that doping is against the law. The purposes of the World Anti-Doping Program and the World Anti-Doping Code are to protect the athlete’s fundamental right to participate in doping-free sport, and thus to promote health, fairness and equality for athletes worldwide.
Athletes are always looking to gain the edge over their opponents, by fair means or otherwise in some cases. Athletes who resort to doping expose themselves to health risks in the pursuit of sporting excellence. The increasingly high profile of Paralympic sport, and its potential rewards, has led some athletes with disabilities to seek improved performance through the use of prohibited substances (Webborn and Van de Vliet, 2012; Van de Vliet, 2012). In the context of the Paralympic Games, efforts to combat doping date back to 1984, the year when the first doping controls were conducted in this setting. In disability sport, however, there exists a particular doping method known as “boosting”. Boosting is the intentional induction of autonomic dysreflexia (AD) with the aim of enhancing performance. It results in a dramatic increase in blood pressure (BP) just prior to competition (Blauwet et al., 2013). AD is an acute syndrome that occurs in people affected by spinal cord injury (SCI) due to excessive sympathetic output (Bhambhani, 2002; Bhambhani et al., 2010).
As a rule, subjects with SCI experience paralysis and loss of sensation below the level of the lesion; furthermore, they often exhibit dysautonomic disorders affecting BP, heart rate and bladder and bowel control (Bhambhani, 2002). Consequently, during competition, a wheelchair athlete’s heart rate fails to increase according to the demands of his/her body, and this can lead to low BP, fatigue, often a poor performance, and a loss of endurance (Moreno et al., 2012). An athlete with a high-level SCI has reduced physiological resources for improving cardiac output and obtaining maximal oxygen uptake, and thus for maintaining endurance when competing (Bhambhani et al., 2012). Indeed, in such athletes, impaired sympathetic cardiac innervation results in a maximum heart rate of 110–130 beats per minute, determined by sinoatrial activity alone; furthermore, their restricted heart rate reserve and reduced stroke volume are compounded by a deficit of catecholamine response to exercise and by absence of the muscular venous pump in the lower limbs (Bhambhani, 2002). Because of these physiological limitations, some athletes with high-level SCI try to induce the dysreflexic state. This state is elicited by a triggering stimulus below the level of the lesion that generates a generalized efferent sympathetic response; this, in turn, produces vasoconstriction below the neurological lesion (Blackmer, 2003). Consequently, the subject exhibits increased BP and blood flow to working muscles, and thus improved performance. The amplified sympathetic discharge is most likely attributable to denervation hypersensitivity of sympathetic spinal, ganglionic or peripheral receptor sites, loss of supraspinal inhibitory control, and formation of abnormal synaptic connections resulting from axonal resprouting (Bhambhani, 2002).
The aim of this paper is to increase awareness and understanding of this serious medical condition that is self-induced by some athletes in order to gain an unfair advantage in sports competitions. We also wish to underline that, although this practice serves the same purpose as doping, it is not included in the World Anti-Doping Agency (WADA) list of doping methods, and therefore not prohibited by this agency.
Search strategy
We performed a literature search of the PubMed database (from 1994 onwards). The key terms “autonomic dysreflexia” and “boosting” were searched alone and then both were cross-referenced with “sport performance”. The search was last updated on May 20, 2015.
Results
The terms “autonomic dysreflexia” and “boosting” alone retrieved 748 and 5709 citations, respectively. Conversely, only seven contributions were retrieved when the search was refined; two reported data from studies conducted in athletes with SCI by means of physiological tests or self-administered questionnaire, whereas the remaining five were systematic or critical reviews. Table I lists the contributions and summarizes their main results and conclusions.
Table I.
Contributions retrieved from the PubMed database (from 1994 onwards) using the key serach terms autonomic dysreflexia, boosting, sport performance.
| Authors | Contribution | Results/Conclusions |
|---|---|---|
| Harris, 1994 | Review/Editorial | It is recommended that all tetraplegic sports people, instructors, trainers and organizers and also the authorities be made fully aware of the technique known as “boosting to produce autonomic dysreflexia”. Investigations and discussions are considered to be necessary and decisions should be taken in order to control the procedure; indeed it would be best if appropriate actions were taken to forbid self-induced autonomic dysreflexia in tetraplegic sports people. |
| Bhambhani, 2002 | Review | Athletes with spinal cord injury have an impaired thermoregulatory capacity and may be more susceptible to thermal stress when compared with able-bodied athletes. Therefore, they should take precautions to minimize the effects of dehydration, heat exhaustion and heat stroke during distance racing events. Wheelchair athletes with quadriplegia who voluntarily induce AD, commonly known as boosting, may enhance distance racing performance by increasing their aerobic power. However, this practice is banned by the IPC not because of its performance enhancing capabilities, but because it could be dangerous to the athletes’ health. |
| Bhambhani et al., 2010 | Study by self-report questionnaire on 99 participants in the Paralympic games | “Of 99 participants, 54.5% had previously heard of AD while 39.4% were unaware; 16.7%, all males, had used AD to enhance performance. Participants reported that AD was (1) useful for middle (78.6%) and long distance (71.4%), marathon (64.3%) and wheelchair rugby (64.3%); (2) somewhat dangerous (48.9%), dangerous (21.3%) or very dangerous (25.5%) to health. Results were not influenced by age, injury level or injury duration. Findings indicate the need for educational programs aimed at enhancing the AD knowledge of rehabilitation professionals, coaches and trainers working with SCI individuals.” |
| Mills and Krassioukov, 2011 | Systematic review | “Boosting has been shown to improve sporting performance but can also cause serious complications due to extreme rises in BP. Therefore, boosting has been banned by the IPC. Despite this ban some elite high-level SCI athletes continue to boost. The IPC recognizes that the current classification systems are not the gold standard and further work is needed to create a more evidence-based classification. Further research is needed to determine if the inclusion of ANS parameters contributes to strengthen classifications systems in Paralympic sports. This includes the development of a simple, valid and reliable bedside assessment of autonomic function that can be used to reliably compare athletes with or without ANS dysfunction, thereby enabling further research into the isolated effect of ANS dysfunction on sporting performance.” |
| Krassioukov, 2012 | Systematic review | “Autonomic dysreflexia occurs in up to 90% of individuals with a cervical or high-thoracic SCI and requires prompt intervention. It also is known that, during athletic activities, self-induced AD is used by some individuals to improve their performance, a technique known as “boosting.” For health safety reasons, boosting is officially banned by the IPC. Devastating paralysis, a variety of autonomic dysfunctions, and abnormal cardiovascular control after SCI present significant challenges in terms of individuals remaining active in competitive sports. Medical practitioners who are involved in the care of wheelchair athletes should be aware of the unique cardiovascular dysfunction that results from SCI and may occur at any time, even with seemingly innocuous triggers. Prompt recognition and appropriate management of these conditions, including episodes of AD, could be life saving.” |
| Blauwet et al., 2013 | Study testing the presence of AD in Paralympic athletes prior to competition | “Testing was performed at three major international Paralympic events. […] A total of 78 tests for the presence of AD were performed during the three games combined. No athlete tested positive. […] No athletes were withdrawn from competition due to the presence of AD. […] Knowledge gained during these early testing experiences will be used to guide ongoing refinement of the testing protocol and the development of further educational initiatives.” |
| Krassioukov and West, 2014 | Review/Expert Opinion | “Athletes with SCI have been documented to self-induce autonomic dysreflexia before competition with a view of increasing BP and improving their performance, a technique known as “boosting”. For health safety reasons, boosting is officially banned by the IPC.” |
Abbreviations: AD=autonomic dysreflexia; ANS=autonomic nervous system; SCI=spinal cord injury; IPC=International Paralympic Committee; BP=blood pressure
Autonomic dysreflexia and physiological effects
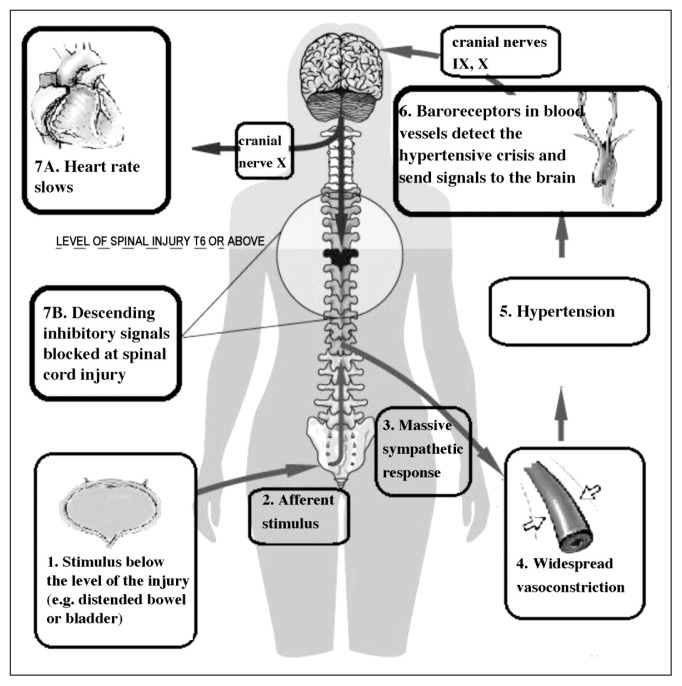
Autonomic dysreflexia is a phenomenon mediated by spinal reflex mechanisms that remain intact in spite of the patient’s injury (Blackmer, 2003) and it is attributable to an overactive autonomic nervous system (ANS): a strong sympathetic discharge causes the abrupt onset of high BP. People with SCI at level T6 or higher may suffer from episodes of AD that can greatly reduce their quality of life (Thumbikat and Tophill, 2003). AD occurs in the presence of a stimulus to the body below the level of the SCI (e.g., an overfull bladder) and it occurs both in complete and incomplete spinal injury cases, although it seems to be more severe in the latter (Milligan et al., 2012). In response to the stimulus, nerve impulses are sent to the spinal cord, where they travel upwards until they are blocked at the level of the lesion. Since the impulses cannot reach the brain, a reflex is activated that increases the activity of the sympathetic ANS. This results in spasms and contraction of the blood vessels, and an increase in BP (Bhambhani, 2002; Blackmer, 2003; Krassioukov and West, 2014) (Fig. 1).
Figure 1.
The body’s reaction following a stimulus triggering autonomic dysreflexia.
The afferent stimulus, in this case a distended bladder, triggers a peripheral sympathetic response, which results in vasoconstriction and hypertension. Descending inhibitory signals, which would normally counteract the rise in blood pressure, are blocked at the level of the spinal cord injury.
The bladder, bowel and skin are the main sources of AD triggers (Karlsson, 1999); irritation of these organs is a common cause of the phenomenon. AD is associated with a large set of symptoms; these include: a 20–40 mmHg increase above resting systolic BP; bradycardia; sweating above the level of the injury; pounding headache; shortness of breath; nasal congestion; restlessness or anxiety; flushing or blotching of skin (Milligan et al., 2012).
It must be recalled that BP in subjects with SCI is lower than in healthy people (i.e., about 15–20 mmHg lower); as a consequence, a 20 to 40 mmHg increase should be considered a red flag indicating an emergency (Legg and Mason, 1998).
Autonomic dysreflexia is thus the result of a strong sympathetic discharge activated by a stimulus below the level of the spinal cord injury (Karlsson, 1999). The symptoms are usually short lasting. Nevertheless, when trigged by certain, specific stimuli, the syndrome can last for periods ranging from days to weeks (Legg and Mason, 1998).
To better understand how AD occurs, it must be remembered that, in normal conditions, the nervous system has several mechanisms allowing it to quickly detect and react to painful stimuli (Marsh and Weaver, 2004). This is made possible by the direct communication between the two branches, sympathetic and parasympathetic, of the ANS. The first of these allows us to react in the presence of danger; indeed, the sympathetic branch is commonly recognized to be responsible for “fight or flight” behavior. The parasympathetic branch, on the other hand, mitigates the sympathetic response, thus protecting the organism from the consequences of hyper-arousal. These two branches play complementary roles: when one is active, the other is suppressed, and this is possible because they have separate paths (Legg and Mason, 1998; Schmid et al., 2001).
Boosting and sports
Man has long looked for artificial means of boosting his physical performances (Santamaria et al., 2013). The reasons for this are various and have changed over time. Primitive man, for example, needed to find ways to be a better hunter. Today, some athletes artificially modify their own performances in pursuit of profit. After all, the best performances bring the greatest earnings (Lippi et al., 2010); this desire for enhanced physical abilities is also seen among disabled athletes. A study by Bhambhani et al. (2010) indicates that more than 15% of athletes with SCI above T6 have voluntarily induced AD in order to boost their athletic performance.
Athletes with SCI exhibit a peculiar response to pain. In fact, the stimulus potentially inducing pain cannot exceed the level of the lesion; at the same time, the stimuli from the brain cannot be sent down the spinal cord to eliminate the effects of the activity of sympathetic cells (Karlsson, 1999). The fact that the athlete does not feel the pain results in an uncontrolled and exaggerated sympathetic response. When this response occurs, the body tries to compensate for it by means of different mechanisms. Although the parasympathetic system is able to recognize what is happening, and can slow the heart rate (Karlsson, 1999), since it is not able to communicate with the part of the body below the lesion, it is as though one-half of the body is trying to relax, while the other half remains in “fight or flight” mode. Thus, these different signals cause the heart rate to lower and, at the same time, the blood vessels to constrict; the co-occurrence of these two reactions immediately results in a huge boost of energy (Legg and Mason, 1998), and while this is what the athlete is seeking to obtain, it may also result in a medical emergency (Blackmer, 2003). It is known that higher the level of the lesion, greater the degree of cardiovascular dysfunction. Another important factor related to the severity of AD is the completeness of the spinal cord injury: only 27% of incomplete quadriplegic patients show signs of this condition, as compared with 91% of quadriplegics with complete spinal damage (Legg and Mason, 1998). The main reason why Paralympic athletes self-induce the AD syndrome is that, during training and competition, the dysreflexic state reduces the rating of perceived exertion, and increases their top speed (Webborn, 1999). Athletes with a high-level SCI (T6 and above) who voluntarily induce an episode of AD prior to or during an event in order to enhance their performance may even inflict suffering on parts of their body below the lesion where pain perception is lacking (Marsh and Weaver, 2004; Harris, 1994). This kind of self-harm is induced in various ways, e.g., i) by winding leg straps too tightly; ii) by delivering electric shocks to the muscles; iii) by constriction of the feet, legs, scrotum or testicles; iv) by bone fracture (usually the toes); v) by catheter locking to allow overfilling of the bladder (Legg and Mason, 1998).
Self-injury is not common in able-bodied competitors given that, in these subjects, in contrast to those suffering from SCI, exercise increases the natural heart rate and BP (Bhambhani, 2002).
The most important issues concerning boosting and its implications are probably those highlighted by the cooperative study conducted by the WADA, the International Paralympic Committee (IPC) and the University of Alberta (Canada). The study lasted from March 2007 to February 2009, and its aim was to provide a snapshot of the spread of this practice and of the level of knowledge of it among athletes. The findings highlighted a need for educational programs, run by rehabilitation professionals, coaches and trainers, aimed at improving knowledge of AD (Webborn and Van de Vliet, 2012).
The medical complications of AD include both short-and long-term ones. The short-term symptoms may be: i) cardiovascular (rise in systemic BP, cardiac arrhythmias, pulmonary edema, cardiac arrest and death); neurological (moderate or severe headache, cerebral hemorrhage, seizures, aphasia); ocular (blindness, retinal hemorrhage); pulmonary (apnea); and vegetative and metabolic (excessive sweating, hyperthermia, hyponatremia). Furthermore, many of the stimuli used to induce AD (such as bladder or bowel distention and skin trauma) can cause surgical diseases (e.g., hydronephrosis, pyelonephritis, and skin infections). Urinary complications and renal failure were the major causes of death in patients with SCI until intermittent catheterization was added to the treatment of high-level spinal injuries. Little is known about the long-term effects of boosting. However, it is possible that increased BP could accelerate the atherosclerotic process (Shepard, 2003).
The position of the International Paralympic Committee
Boosting is potentially dangerous and is considered a doping offence by the IPC (Bhambhani et al., 2010). The IPC is a non-profit international organization that governs the Paralympic Movement. It organizes the Summer and Winter Paralympic Games, and serves as the international federation for 12 sports, supervising and coordinating the organization of their World Championships and other competitions (Blauwet et al., 2013). The mission of the IPC is to allow disabled athletes to achieve sporting excellence and to create opportunities for everyone at any level. A further aim of the IPC is to promote the Paralympic values of courage, determination, inspiration and equality (Van de Vliet, 2012).
The problem of boosting came clearly to the fore at the Paralympic Games in Atlanta in 1996 and prompted the IPC to address and highlight the ethical and, in particular, the health protection aspects of the problem. It is interesting, in this regard, to compare the position of the IPC with that of the WADA. Boosting, although it is a method used to improve performance, and is therefore comparable to doping methods banned by the WADA, is not actually prohibited by the WADA (Blackmer, 2003).
The WADA Prohibited List of substances and methods was first published in 1963 under the leadership of the International Olympic Committee. Reviewing this list with reference to the past 10 years, it emerges that voluntary induction of AD is not considered a doping method by this agency (Table II) (Santamaria et al., 2013). Athletes with clear symptoms of AD will not be liable to any penalty, since they have not committed any type of offense (Fraser, 2004).
Table II.
The WADA Prohibited List for 2014.
|
Substances and methods prohibited at all times (in- and out-of-competition) S1. Anabolic agents S2. Peptide hormones, growth factors and related substances S3. Beta-2 agonists S4. Hormone antagonists and modulators S5. Diuretics and other masking agents M1. Enhancement of oxygen transfer M2. Chemical and physical manipulation M3. Gene doping |
|
Substances and methods prohibited in competition In addition to the categories S1 to S5 and M1 to M3 defined above, the following categories are prohibited in competition: S6. Stimulants S7. Narcotics S8. Cannabinoids S9. Glucocorticoids |
|
Substances prohibited in particular sports P1. Alcohol P2. Beta-blockers |
Conversely, the IPC does take into account and prohibit the practice of voluntary induction of AD. It is interesting to note that the IPC expressly prohibits this practice not so much because it is unacceptable ethically as because it is extremely dangerous for health (Bhambhani, 2002). Thus, although athletes not taking performance-enhancing drugs were not committing a formal offence (only an ethical one), the IPC’s goal was primarily to protect the health of athletes (Legg and Mason, 1998). In particular, Art. 1, Chapter 4.3 of the IPC Handbook defines AD and boosting as follows: “Persons with cervical or high thoracic spinal injuries can suffer from an abnormal sympathetic reflex called Autonomic Dysreflexia. This reflex is caused by painful stimuli to the lower part of the body, particularly distension or irritation of the urinary bladder. The symptoms of dysreflexia are a rapid rise in blood pressure, headache, sweating, skin blotchiness and gooseflesh. In serious cases, confusion, cerebral hemorrhage and even death can occur. This reflex may happen spontaneously or may be deliberately caused (“Boosting”). As this is a health hazard, the IPC forbids athletes to compete in a hazardous dysreflexic state”.
According to the Handbook, the syndrome is considered to be present when the systolic BP is 180 mmHg or higher. An athlete exhibiting the symptoms will be re-examined 10 minutes after the initial detection. If his/her BP remains unchanged, the athlete will be excluded from the competition. The Handbook also explicitly prohibits any attempt to self-induce AD, stating that an athlete involved in such attempts will be excluded from the competition regardless of his/her systolic BP reading. In addition, a report on the voluntary attempt to induce AD will be provided to the Legal and Ethics Committee of the IPC for further investigation in relation to the non-compliance with ethical and legal principles by the athlete and/or his support staff. The Handbook also specifies that athletes with SCI at T6 or higher who are hypertensive must document the pre-existence of this condition, providing clinical charts predating the competition in which they are to participate. These medical records should provide data about the level of resting BP over a period of at least 14 days prior to the competition, and should indicate the athlete’s current treatment (Blauwet et al., 2013).
Treatment and prevention
As general remark, it should be pointed out that since it can sometimes be difficult to identify the trigger of an acute increase in BP, affected subjects require immediate medical care (Elliott and Krassioukov, 2006). The Consortium for Spinal Cord Medicine recommends that if non-pharmacological measures are ineffective and arterial BP remains 150 mmHg or higher, pharmacological management should be undertaken.
The Consortium does not identify any particular medication for management of AD. Several drugs (e.g. nifedipine, nitrates, captopril, terzaosin, prazosin, phenoxybenamine, prostaglandin E2, sildenafil) have been proposed for the treatment of episodes of AD (Blackmer, 2003; Naftchi and Richardson, 1997). Antihypertensive drugs with rapid onset and short duration of action should be preferred in the management of acute episodes (Blackmer, 2003).
The first step to prevent the practice of boosting is to raise awareness and increase knowledge of it, and particularly of its dangerous effects on health (Blauwet et al., 2013). All athletes with SCI, their technical and medical staff and the Paralympic sporting committees should be aware of the causes that trigger AD. The national governments and sports federations should develop written warnings and educational initiatives to draw attention to the effects of boosting (Milligan et al., 2012; Fraser, 2004). This material should be developed in an interactive style and in different languages. Such information campaigns should target, in particular, countries where awareness is low, and specific sports in which the incidence of boosting is likely to be high. Furthermore, they should start at junior level. Coaches and trainers should be educated about AD and convey the information to athletes (Bhambhani, 2002; Schmid et al., 2001).
A further approach to prevention is related to the psychological dimension. Individuals with SCI are under an enormous amount of stress, to which they typically respond with attempts to cope, which may or may not be adaptive in reducing their stress levels. Coping resources and strategies (especially engagement coping) are reliably linked to adaptation to SCI. In particular, engagement coping positively influences psychosocial adaptation even when coping resources are mostly absent (Livneh and Martz, 2014). Resilience may be looked on as an important factor of engagement coping. A recent study of quadriplegic wheelchair rugby players showed that “the development of resilience is a multifactorial process involving pre-existing factors and pre-adversity experiences, disturbance/disturbing emotions, various types and sources of social support, special opportunities and experiences, various behavioral and cognitive coping strategies, motivation to adapt to changes, and learned attributes or gains from the resilience process” (Machida et al., 2013). In this framework, psychological interventions aimed at reinforcing effective coping strategies and resilience should be considered in order to support athletes in their efforts to adapt to their new lifestyle, and promoting fair and safe practising of sporting activities is part of this.
Discussion
No sporting activity may be considered free from the desire to force the body by drugs, substances or other methods (Lippi et al., 2010). This desire is shared by both able-bodied and disabled athletes (Legg and Mason, 1998).
Three main categories of drugs are used by athletes: i) nutritional supplements, ergogenic substances or techniques aimed at improving performance; ii) medical drugs (i.e., testosterone, anabolic steroids, stimulants such as amphetamines, and peptide hormones such as growth hormone and erythropoietin); these are substances usually given to treat specific medical conditions, but in this case are used in ways that do not comply with conventional clinical practice; iii) uncontrolled substances sold on the open market for stimulant use. This last category includes illegal substances or drugs that are usually taken at very high and uncontrolled doses. This behavior results in changes in an athlete’s mood and self-perception (Santamaria et al., 2013).
Furthermore, in a subject affected by SCI, several organs and systems, including the cardiovascular system, are no longer under the control of the ANS. This leads to a critical discrepancy between the level of physical activity and the resources of the body, which results in low BP and, in turn, in reduced endurance and performance (Mills and Krassioukov, 2011). Athletes with SCI adopt a specific practice to overcome this: voluntary induction of AD (Ashley et al., 1993). As already mentioned, boosting is a means of improving performance, and can thus be compared to the doping methods banned by the WADA, yet it is not prohibited by this body (Bhambhani et al., 2012). As a consequence, an athlete self-inducing AD will not be subjected to any penalty, since he has not committed any offense. Only the IPC expressly prohibits the use of boosting, because it is extremely dangerous for health (Bhambhani, 2002). Finally, AD is clearly considered a prohibited manipulation by the doping guidelines of the IPC.
Unfortunately, boosting is a very fast-spreading phenomenon about which still little is known. Boosting has been shown to confer an up to 9.7% improvement in race time (Bhambhani et al., 2012). On the other hand, it can cause serious complications due to the fact that it causes a dramatic increase in BP (Schmid et al., 2001). Governments and sports authorities should organize information campaigns targeting both technical and medical staff, and focusing in particular on youth sectors. Indeed, the first step towards preventing this practice is to improve knowledge of it, and particularly of its deleterious effects on health (Bhambhani et al., 2012; Van de Vliet, 2012).
Autonomic dysreflexia is an unpredictable risk, mainly due to hypertension, which can occur in SCI subjects both at rest and, in particular, during physical activity. Testing for the presence of AD in Paralympic athletes with SCI prior to competition has now been carried out at three major international Paralympic competitions. Testing was conducted on athletes from the relevant sport classes: athletics (wheelchair racing classes T51/T52/T53) and handcycling (H1). There have been no positive tests thus far; no athletes have been withdrawn from competition due to the presence of AD (Blauwet et al., 2013). Given the limited data, it is not yet possible to evaluate the real extent of the phenomenon, although the data currently available may represent the starting point for developing studies aiming to get to the heart of this question (Lippi et al., 2010; Fraser, 2004).
The fact that about 40% of Paralympians with a high-level SCI are uninformed about the nature and risks of boosting underlines the need for educational programs aimed at improving knowledge and awareness of the phenomenon in athletes and in the general population (Bhambhani et al., 2010; Ashley et al., 1993; Krassioukov, 2012).
References
- Ashley EA, Laskin JJ, Olenik LM, et al. Evidence of autonomic dysreflexia during functional electrical stimulation in individuals with spinal cord injuries. Paraplegia. 1993;31:593–605. doi: 10.1038/sc.1993.95. [DOI] [PubMed] [Google Scholar]
- Bhambhani Y. Physiology of wheelchair racing in athletes with spinal cord injury. Sports Med. 2002;32:23–51. doi: 10.2165/00007256-200232010-00002. [DOI] [PubMed] [Google Scholar]
- Bhambhani Y, Mactavish J, Warren S, et al. Boosting in athletes with high-level spinal cord injury: knowledge, incidence and attitudes of athletes in paralympic sport. Disabil Rehabil. 2010;32:2172–2190. doi: 10.3109/09638288.2010.505678. [DOI] [PubMed] [Google Scholar]
- Bhambhani Y, Forbes S, Forbes J, et al. Physiologic responses of competitive Canadian cross-country skiers with disabilities. Clin J Sport Med. 2012;22:31–38. doi: 10.1097/JSM.0b013e3182432f0c. [DOI] [PubMed] [Google Scholar]
- Blackmer J. Rehabilitation medicine: 1. Autonomic dysreflexia. CMAJ. 2003;169:931–935. [PMC free article] [PubMed] [Google Scholar]
- Blauwet CA, Benjamin-Laing H, Stomphorst J, et al. Testing for boosting at the Paralympic games: policies, results and future directions. Br J Sports Med. 2013;47:832–837. doi: 10.1136/bjsports-2012-092103. [DOI] [PubMed] [Google Scholar]
- Elliott S, Krassioukov A. Malignant autonomic dysreflexia in spinal cord injured men. Spinal Cord. 2006;44:386–392. doi: 10.1038/sj.sc.3101847. [DOI] [PubMed] [Google Scholar]
- Fraser AD. Doping control from a global and national perspective. Ther Drug Monit. 2004;26:171–174. doi: 10.1097/00007691-200404000-00015. [DOI] [PubMed] [Google Scholar]
- Harris P. Self-induced autonomic dysreflexia (‘boosting’) practised by some tetraplegic athletes to enhance their athletic performance. Paraplegia. 1994;32:289–291. doi: 10.1038/sc.1994.50. [DOI] [PubMed] [Google Scholar]
- Karlsson AK. Autonomic dysreflexia. Spinal Cord. 1999;37:383–391. doi: 10.1038/sj.sc.3100867. [DOI] [PubMed] [Google Scholar]
- Krassioukov A. Autonomic dysreflexia: current evidence related to unstable arterial blood pressure control among athletes with spinal cord injury. Clin J Sport Med. 2012;22:39–45. doi: 10.1097/JSM.0b013e3182420699. [DOI] [PubMed] [Google Scholar]
- Krassioukov A, West C. The role of autonomic function on sport performance in athletes with spinal cord injury. PM R. 2014;6(8 Suppl):S58–S65. doi: 10.1016/j.pmrj.2014.05.023. [DOI] [PubMed] [Google Scholar]
- Legg D, Mason DS. Autonomic dysreflexia in wheelchair sport: a new game in the legal arena? Marquette Sports Law Review. 1998;8:225–237. [Google Scholar]
- Lippi G, Longo UG, Maffulli N. Genetics and sports. Br Med Bull. 2010;93:27–47. doi: 10.1093/bmb/ldp007. [DOI] [PubMed] [Google Scholar]
- Livneh H, Martz E. Coping strategies and resources as predictors of psychosocial adaptation among people with spinal cord injury. Rehabil Psychol. 2014;59:329–339. doi: 10.1037/a0036733. [DOI] [PubMed] [Google Scholar]
- Machida M, Irwin B, Feltz D. Resilience in competitive athletes with spinal cord injury: the role of sport participation. Qual Health Res. 2013;23:1054–1065. doi: 10.1177/1049732313493673. [DOI] [PubMed] [Google Scholar]
- Marsh DR, Weaver LC. Autonomic dysreflexia, induced by noxious or innocuous stimulation, does not depend on changes in dorsal horn substance p. J Neurotrauma. 2004;21:817–828. doi: 10.1089/0897715041269605. [DOI] [PubMed] [Google Scholar]
- Milligan J, Lee J, McMillan C, et al. Autonomic dysreflexia: recognizing a common serious condition in patients with spinal cord injury. Can Fam Physician. 2012;58:831–835. [PMC free article] [PubMed] [Google Scholar]
- Mills PB, Krassioukov A. Autonomic function as a missing piece of the classification of paralympic athletes with spinal cord injury. Spinal Cord. 2011;49:768–776. doi: 10.1038/sc.2011.2. [DOI] [PubMed] [Google Scholar]
- Moreno MA, Zamunér AR, Paris JV, et al. Effects of wheelchair sports on respiratory muscle strength and thoracic mobility of individuals with spinal cord injury. Am J Phys Med Rehabil. 91:470–477. doi: 10.1097/PHM.0b013e3182adcb0. [DOI] [PubMed] [Google Scholar]
- Morente-Sánchez J, Zabala M. Doping in sport: a review of elite athletes’ attitudes, beliefs, and knowledge. Sports Med. 2013;43:395–411. doi: 10.1007/s40279-013-0037-x. [DOI] [PubMed] [Google Scholar]
- Naftchi NE, Richardson JS. Autonomic dysreflexia: pharmacological management of hypertensive crises in spinal cord injured patients. J Spinal Cord Med. 1997;20:355–360. [PubMed] [Google Scholar]
- Santamaria S, Ascione A, Tafuri D, et al. Gene doping: biomedical and laws aspects of genetic modification of athletes. Medicina Sportiva. 2013;17:193–199. [Google Scholar]
- Shepard RJ. Boosting of performance in the athlete with high-level spinal injury. Adapted Physical Activity Quarterly. 2003;20:103–117. [Google Scholar]
- Schmid A, Schmidt-Trucksäss A, Huonker M, et al. Catecholamines response of high performance wheelchair athletes at rest and during exercise with autonomic dysreflexia. Int J Sports Med. 2001;22:2–7. doi: 10.1055/s-2001-11330. [DOI] [PubMed] [Google Scholar]
- Thumbikat P, Tophill PR. Autonomic dysreflexia. J R Soc Med. 2003;96:618–619. doi: 10.1258/jrsm.96.12.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Vliet P. Antidoping in paralympic sport. Clin J Sport Med. 2012;22:21–25. doi: 10.1097/JSM.0b013e31824206af. [DOI] [PubMed] [Google Scholar]
- Webborn AD. “Boosting” performance in disability sport. Br J Sports Med. 1999;33:74–75. [PMC free article] [PubMed] [Google Scholar]
- Webborn N, Van de Vliet P. Paralympic medicine. Lancet. 2012;380:65–71. doi: 10.1016/S0140-6736(12)60831-9. [DOI] [PubMed] [Google Scholar]