Summary
The purpose of this study was to establish the self-motion perception threshold, in roll, in the visual-vestibular interaction (VVI) state, creating an oculogyral illusion, and to compare this threshold to the self-motion perception threshold in darkness. A further aim was to investigate the dynamics of the threshold at a low frequency range (0.1–1 Hz) of sinusoidal rotation.
Seven healthy subjects were tested. A motion platform was used to generate motion. Single cycles of sinusoidal acceleration at four frequencies (0.1, 0.2, 0.5 and 1 Hz) were used as motion stimuli. To avoid otolith stimulation, subjects were rotated about a vertical axis in supine position. To evoke an oculogyral illusion subjects were instructed to fixate their gaze on a cross-shaped object aligned with their head, which rotated with them.
The results show a lowering of the self-motion perception threshold in the VVI state, significant for the frequencies 0.1 and 0.2 Hz (p<0.05). In all the subjects, visual fixation on the cross evoked an oculogyral illusion. The threshold in both tested conditions was frequency dependent: it decreased with increasing frequency values. However, this effect was consistently stronger in darkness across all frequencies (p<0.05).
In conclusion, the application of sinusoidal rotation during roll at low frequencies in the VVI condition evokes oculogyral illusion. This interaction lowers the self-motion perception threshold compared to that measured during rotation in darkness. This testing method could be of practical benefit in clinical application for revealing brain dysfunction involving integrative mechanisms of perception.
Keywords: interaction, perception, roll, self-motion, threshold, visual-vestibular
Introduction
The perception of self-motion is based on integrated information from several sensory systems, although the vestibular and the visual ones contribute the most. The vestibular detection threshold is higher than the visual one especially at low frequencies of sinusoidal rotation, due to the high-pass characteristics of the vestibular end organ (Kolev et al., 1996; Grabherr et al., 2008). In our daily life both signals usually interact in a way that improves our self-motion perception, lowering the threshold for its detection. However, there exist situations in which the environment creates sensory conflict, e.g. when one is sitting inside a moving vehicle and watching an object that is stationary with respect to oneself. Even so, the functional organization of our brain system is such that this conflict situation actually helps us to assess whether we are moving or not. This has been demonstrated by a number of experiments, including our earlier ones (Kolev et al., 1996; Benson et al., 1989; Doty, 1969; Clark and Stewart, 1968; Nijhoff and Roggeveen, 1956; Hallpike and Hood, 1953; Graybiel et al., 1948). These experiments involved measurement of the detection threshold for self-motion when a subject is rotated in yaw while fixing his gaze on an object aligned with his head, which moves together with him. This is a condition of visual-vestibular sensory conflict. While the vestibular receptors send information for rotation, the information from the visual system is consistent with absence of motion. The currently accepted hypothesis explaining the mechanism underlying the lowering of the threshold seen in these studies is that of the perception of apparent motion of the object in the visual field associated with angular acceleration of the body, a phenomenon known as oculogyral illusion (Graybiel and Hupp, 1946).
There are situations in which individuals (e.g. aircraft pilots or astronauts during flight) are rotated in roll only. And in some situations during roll rotation, the orientation of the head and the body with respect to the gravitational vertical is changed. In such a condition, there is not only canal stimulation, but also otolith stimulation. Therefore, the afferentation of both vestibular receptors (semicircular canal and otolith) is integrated in the process of perception of self-motion. In this condition, cues from another sensory system, i.e. the somatosensory system, are also integrated into this process.
To understand the mechanisms underlying the perception of self-motion at threshold level, it is important to clarify how visual afferentation interacts with the vertical vestibular canal cues in the detection of self-motion. Therefore, the purpose of this study was: i) to establish perception thresholds for self-motion, in roll, at low frequencies (0.1, 0.2, 0.5, and 1 Hz), in a state of visual-vestibular interaction (VVI), without concomitant otolith and somatosensory stimulation; ii) to compare these thresholds to those obtained in total darkness when the vestibular canal system alone is stimulated; and iii) to investigate whether the effect is frequency-dependent.
Materials and methods
Subjects
Seven healthy subjects (39±12 years, 3 females and 4 males; 5 right-handed and 2 left-handed) were recruited to participate in this study. Before being included in the study, all the subjects were required to complete a detailed vestibular diagnostic clinical examination to confirm that they had normal vestibular function. The vestibular screening examination consisted of caloric electronystagmography, Dix-Hallpike testing, angular vestibulo-ocular reflex (VOR) evoked by rotation, and posture control measures. Furthermore, a short health history questionnaire was administered; subjects were asked to indicate any known history of dizziness or vertigo, back/neck problems, and cardiovascular, neurological and other physical problems. They were also asked about their susceptibility to motion sickness. Informed consent was obtained from all the subjects prior to their participation in the study. The study was approved by the local ethics committee and was performed at the Massachusetts Eye and Ear Infirmary in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Apparatus and motion stimuli
A motion platform (MOOG 6DOF2000E, Moog Inc., NY) was used to generate motion. Single cycles of sinusoidal acceleration [a (t) = A sin (2πft) = A sin (2πt/T)] were used, where A is the acceleration amplitude and f is the frequency, which is the inverse of the period (and duration) of the stimulation (T = 1/f). Since the motion began at zero velocity, integration of the acceleration yields an oscillatory velocity, v (t) = AT/(2π) [1- cos (2πt/T)], and a lateral displacement Δ p (t) = AT/(2π) [t - T/(2π) sin (2πt/T)]. Therefore, both the peak velocity (vmax = AT/π) and the total lateral displacement (Δ p = AT2/2π) were proportional to the peak acceleration (A). These motion profiles were chosen because they contain no discontinuities in acceleration, velocity or position, and because they were successfully utilized in our previous study quantifying perceptual detection thresholds for yaw rotation in the VVI state as a function of frequency (Kolev and Nicoucar, 2014).
Visual stimuli
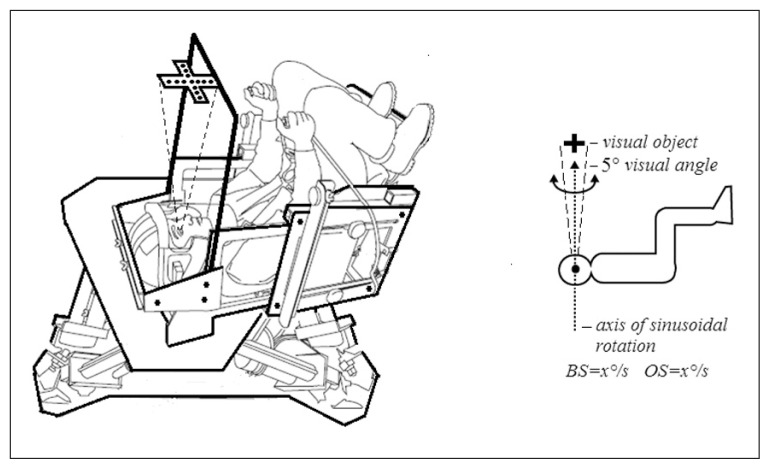
Subjects were exposed to visual stimulation while seated in a chair. We used a cross constructed of 17 light-emitting diodes (LEDs) which was fixed 60 cm in front of them at eye level creating a visual angle of 5 degrees so as to primarily engage foveal vision. The rotation axis fell between the eyes and passed through the center of the cross (Fig. 1). The cross, which rotated with the chair, stayed in fixed alignment with the subject’s head and subjects were asked to look at the cross only during motion. The brightness of the cross, which remained constant throughout the experiment, was just enough to allow it to be detected in darkness without illuminating the surrounding area. Testing was randomized and thresholds were measured in the presence of the illuminated cross and in complete darkness.
Figure 1.
Schematic drawing of the experimental set-up: Moog platform, subject being tested, and visual stimulus. Schematic drawing of the stimulus used.
Abbreviations: VEST=vestibular stimulus, OS=object in space.
Experimental procedures
Subjects were seated in supine position in a horizontally positioned chair with a five-point harness and rotated in roll about an earth-vertical axis, both in darkness and under visual stimulation. The subject’s head was held in place by an adjustable helmet, and was carefully positioned relative to the axis of rotation using external landmarks. To minimize the influence of non-vestibular motion direction cues, trials were performed in the dark in a light-tight room. All skin surfaces expect the face were covered (long sleeves, light gloves) and a visor attached to the helmet surrounded the face. Earplugs reduced external noise by about 20 dB and the remaining auditory motion cues were masked by white noise (circa 60 dB). Tactile cues were distributed as evenly as possible using padding.
Thresholds for self-motion perception under visual stimulation and in darkness were measured at four different frequencies: 0.1, 0.2, 0.5, and 1 Hz. Each frequency was tested in a block of contiguous trials. These four blocks of trials were separated by short breaks. The order of blocks was randomized across the subjects.
The subjects were rotated in roll in two visual conditions randomized between them: i) in total darkness; ii) while fixating their gaze on the illuminated cross (of LEDs), which was aligned with midline of the subject’s head and rotated together with him/her (Fig. 1); the subjects were rotated either clockwise or counterclockwise. A brief, low-pitch “warning” tone was delivered 2 s before the onset of each motion stimulus. At the end of each trial a brief high-pitch sound was emitted to indicate that the subject needed to respond. The subjects were instructed to push the button in their left hand if they had perceived a counterclockwise rotation, or the one in their right hand if they had perceived a clockwise rotation. If the subjects were uncertain of the direction of motion, they were instructed to make their best guess and press one of the two buttons accordingly. Before each test session a few supra-threshold practice trials were performed to establish that the subject understood the task and to minimize training effects. The button pushes were noted by the experimenter and recorded via computer. An adaptive two-alternative categorical forced-choice procedure (Treutwein, 1995; Leek, 2001) was used in all conditions. For this procedure, thresholds were measured using a 3-down, 1-up staircase paradigm (Kolev and Nicoucar, 2014; Leek, 2001), where 3-down means that the subject has to correctly detect the direction of motion for three motion stimuli in a row in order for the acceleration level to be reduced, and 1-up means that the acceleration level is increased every time the subject makes a mistake. This 3-down, 1-up paradigm targets a threshold at which the subject correctly detects motion 79.4% of the time (Leek, 2001), which we accepted as our threshold criterion. Typically, trials began well above threshold (starting values were 5.1 deg s−1 for condition 1 HZ, 10.2 deg s−1 for 0.5 Hz, 8.8 deg s−1 for 0.2 Hz, and 4.1 deg s−1 for 0.1 Hz). Testing continued until each test demonstrated nine direction reversals in the adaptive track: five minimum and four maximum direction reversals. Minimum reversals occur when the subject makes an error and the stimulus level goes up. Maximum reversals occur when the subject correctly detects motion at a given acceleration level three times in row immediately after incorrectly detecting motion on the previous trial. Threshold was defined as the mean of the last two – one minimum and one maximum – reversals.
The significance of the change in self-motion perception threshold was analyzed with two-way repeated measures ANOVA (SigmaPlot for Windows 11.0, SysStat Soft Inc., Tulsa, OK) with two within-subject factors: ‘visual signal’ (darkness and cross visual fixation) and ‘frequency’ (0.1, 0.2, 0.5 and 1 Hz). For post-hoc analysis we applied the Newman-Keuls test. The level of significance was fixed at p<0.05.
Results
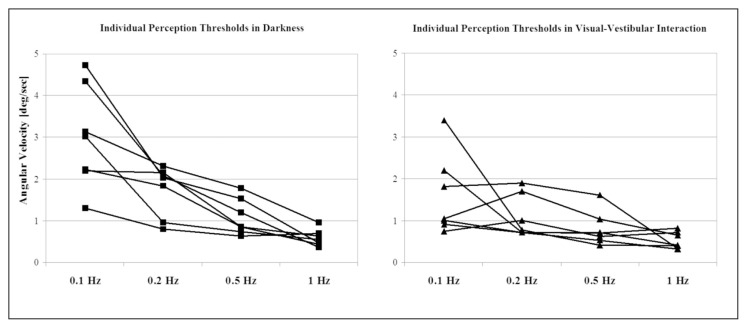
The individual data referring to the two visual conditions – with visual and without visual fixation – are presented in figure 2. Figure 3 shows the mean values and SEM, and the statistical significance of the results. The two-way repeated measures ANOVA showed a significant effect of the factor ‘frequency’ (F = 11.43, p<0.001), and of the interaction ‘visual signal’ x ‘frequency’ (F = 2.86, p<0.05). These results indicate a dependence of the frequency values on the kind of ‘visual signal’ – visual fixation or darkness.
Figure 2.
The individual self-motion perception threshold data of all the subjects.
The subjects were tested in roll during vertical axis sinusoidal acceleration, both in darkness and in the visual-vestibular interaction condition (visual fixation on the cross). The trials were performed at four stimulation frequencies 0.1, 0.2, 0.5 and 1 Hz.
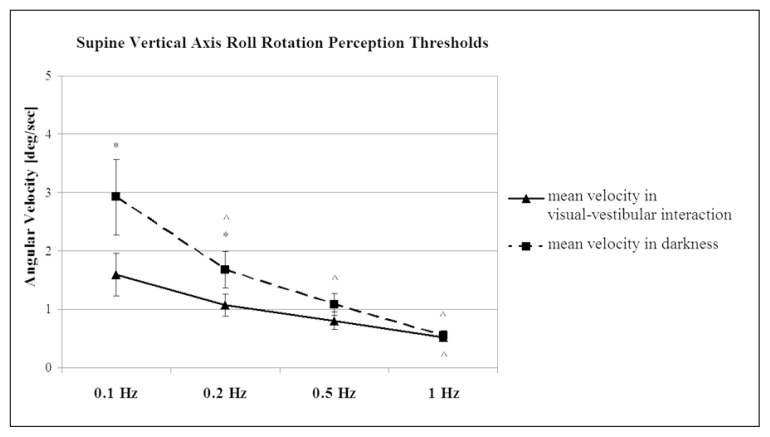
Figure 3.
Plot of mean velocity ± SEM values of self-motion per-ception threshold tested during sinusoidal acceleration in the visual-vestibular interaction condition (visual fixation on the cross) and in darkness.
The trials were performed at four stimulation frequencies 0.1, 0.2, 0.5 and 1 Hz. * Significant difference between both conditions (p<0.05); ^ significant difference between the frequencies used (p<0.05).
The self-motion perception thresholds measured during rotation with visual fixation on a cross constructed of LEDs were significantly lower than those measured during rotation in darkness (Newman-Keuls test, p<0.05) for the lowest frequencies: 0.1 Hz and 0.2 Hz (Fig. 3). This threshold reduction was almost twofold at the frequency of 0.1 Hz. The threshold reductions at the higher frequencies (0.5 Hz and 1 Hz) did not reach the level of significance. The thresholds in the visual fixation condition were dependent on the stimulus frequency, increasing when the frequency decreased (Fig. 3). This increase was most pronounced at 0.1 Hz. There was a significant difference between thresholds at the frequencies 0.1 Hz and 1 Hz (Newman-Keuls test, p<0.05). Differences were also found between the other frequencies, but these did not reach significant values (Fig. 3). The variability between subjects decreased with increasing frequency. It was highest at 0.1 Hz, as shown in figure 2.
All the subjects reported oculogyral illusions during rotation with visual fixation on the cross. They described that initially upon acceleration during the sinusoidal rotation they perceived that the cross rotated slightly with respect to them, showing a small angular displacement. The direction of the cross displacement coincided with the direction of rotation of the subjects. Upon deceleration the cross rotated again slightly but this time in the direction opposite to that of their own rotation. The perceived cross rotation corresponds to the eye movements caused by the VOR which has a lower threshold compared to the threshold for the perception for self-motion, as shown by Seemungal et al. (2004).
Self-motion perception thresholds in total darkness also showed frequency dependence, being highest at 0.1 Hz and lowest at 1 Hz. The threshold was significantly higher at 0.1 Hz than at the three other frequencies: 0.2, 0.5, and 1 Hz (Newman-Keuls test, p<0.05) (Fig. 3). The between-subjects variability decreased with increases in the frequency. At the lowest frequencies (0.1 Hz and 0.2 Hz), the variability of the self-motion perception threshold was higher than that at the same frequencies in the visual fixation condition as shown in figure 2.
Discussion
The present study shows the thresholds for self-motion perception in roll during sinusoidal head-centered rotation at low frequencies, ranging from 0.1 to 1 Hz, with and without visual fixation and with the subject in supine head and body position during which the head does not change its orientation with respect to the gravitational vertical. The study revealed that self-motion perception thresholds are frequency-dependent under both visual conditions: they decrease as the frequency increases. This effect is more pronounced in the condition of total darkness. When subjects fixate their gaze on a cross-shaped object that rotates with them and is aligned with their head, i.e., when oculogyral illusions are created, the thresholds for perception of self-motion are lower than with the same type of rotation in total darkness. In other words, under conflicting conditions, VVI facilitates the perception of self-motion in roll.
It is interesting to compare the vestibular thresholds for self-rotation in roll and yaw because of the different patterns of occurrence and use of roll-only stimulations and yaw-only stimulations in everyday activities. Head motion in yaw is more natural; it is also utilized more frequently in daily activities to obtain information about the environment.
The findings reported in previous studies comparing self-motion perception thresholds during rotation in roll and yaw in total darkness are inconsistent. While some authors found no difference in perceptual thresholds during Z and X axis rotation (Clark and Stewart, 1970), others reported significantly higher thresholds for the X axis (Benson et al. 1989; Meiry, 1965) during rotation about a vertical axis. Comparison of perceptual thresholds for self-motion in darkness during sinusoidal roll rotation (those found in the present study) with those recorded during sinusoidal yaw rotation in an earlier study (Kolev and Nicoucar, 2014) reveals that the threshold was higher during X axis rotation at the low frequency of 0.1 Hz.
The lowering of the perceptual thresholds under different VVI conditions was established during yaw vertical axis sinusoidal rotation in earlier experiments (Kolev et al., 1996; Benson et al., 1989; Doty, 1969; Clark and Stewart, 1968; Nijhoff and Roggeveen, 1956; Hallpike and Hood, 1953; Graybiel et al., 1948; Kolev and Nicoucar, 2014).
It is generally accepted that two main mechanisms explain the lower threshold for self-motion perception when a subject is rotated while viewing a head-fixed target (Carriot et al., 2011). One mechanism is visual suppression of the VOR through target fixation. To suppress the VOR the central nervous system must encode a predictive eye pursuit command (Barnes, 1988; Barnes and Eason, 1988; Whiteside et al., 1965). The other mechanism is retinal slip of the target following eye drift caused by incomplete suppression of the VOR (Carriot et al., 2011). These mechanisms have been discussed in detail for yaw rotation; it should be noted, however, that there are some differences in roll. The VOR evoked in yaw is horizontal – along the left-right axis –, while in roll it is torsional. However, there is no torsional pursuit. Therefore, the VOR in this case cannot be cancelled by a pursuit mechanism. In order to make the subject’s brain apply retinal slip detection mechanisms, a cross was used as the visual target. If the target had been a LED spot of light, as in previous experiments (Kolev et al., 1996; Kolev and Nicoucar, 2014), then retinal slip would have been impossible –the effect would have mainly concerned the level of alertness: the level of attention would have been higher. By using a head-centered cross, through the center of which the X axis of rotation passes, we induced the subject’s brain to utilize the retinal slip mechanism. When interpreting the results of the two thresholds – in roll and in yaw – in VVI, we have to consider that the VOR under the two test conditions involves different extraocular muscles innervated by different ocular motor nerves and controlled by their respective motor nuclei (Luxon, 1984; Leigh and Zee, 2006).
To be able to compare roll with yaw rotation perception thresholds, the latter obtained from previous experiments, we tested our subjects in a supine-seated position (Fig. 1), i.e. the same position used in earlier studies on perceptual thresholds during sinusoidal yaw rotation (Kolev et al., 1996; Kolev and Nicoucar, 2014). Interestingly, comparison of thresholds in VVI in roll (from the present study) and in yaw (from previous ones) showed differences: the thresholds in roll were increased at the higher frequencies: 0.5 and 1 Hz. The difference between Z and X axis perceptual thresholds could be explained either by a sensitivity difference at canal level, or it could be a result of a mechanism of amplification of central structures – the functioning of different neural networks controlling perception in each axis of rotation (Vasudevan and Bastian, 2010). It is also possible that mechanisms at both levels – canal and central nervous system – coexist.
An additional somatosensory signal which needs to be considered when seeking to explain threshold lowering is that caused by centrifugal force in the legs during rotation.
It is also necessary to mention an interesting recent finding (Kolev and Georgieva-Zhostova, 2014) which supports the somatosensory hypothesis as an explanation for the perceptual threshold difference in roll (present results) compared with yaw rotation (Kolev and Nicoucar, 2014): a difference in self-motion illusions (evoked by caloric vestibular stimulation) between vertical and supine body position, while the subject’s head remains unchanged with respect to the gravity vector (i.e. the vestibular afferentation remains the same) (Kolev and Georgieva-Zhostova, 2014).
Finally, it is important to note that the phenomenon described – the lowering of the perceptual threshold for self-motion in visual-vestibular conflict conditions –could potentially be of practical benefit in the clinic. It could be used as a test for a variety of early stages of brain dysfunction affecting areas involved in the integrative mechanisms of perception. It could also detect discrete sensory and/or motor lesions of peripheral localization. Moreover, outside of the clinic it could be used to select optimal candidates for certain professions in which correct perception of self-motion is critical, for instance, aircraft pilots or astronauts, and operators of military or civil moving platforms.
In conclusion, the present study shows that sinusoidal roll rotation in supine position at low frequencies (0.1–1 Hz) induces a VVI when a subject fixates his/her gaze on a cross-shaped object that, aligned with his/her head, rotates together with him/her and creates an oculogyral illusion. This interaction lowers the perceptual threshold for self-motion compared to the same threshold during rotation in darkness. The effect is frequency-dependent in both conditions but this dependence is more pronounced in darkness.
Acknowledgments
The author wishes to thank Dr Daniel M. Merfeld, Director of Jenks Vestibular Physiology Laboratory, MEEI, Otolaryngology and Laryngology, Harvard Medical School, Boston, MA, USA, all the personnel of the laboratory, and especially Dr Keyvan Nicoucar for helping to perform this study. The study was supported in part by ONR Global grant (N00014-06-1-4030) to O.I. Kolev.
References
- Barnes GR. Head-eye co-ordination: visual and nonvisual mechanisms of vestibulo-ocular reflex slow-phase modification. Prog Brain Res. 1988;76:319–328. doi: 10.1016/s0079-6123(08)64519-7. [DOI] [PubMed] [Google Scholar]
- Barnes GR, Eason RD. Effects of visual and non-visual mechanisms on the vestibulo-ocular reflex during pseudo-random head movements in man. J Physiol. 1988;395:383–400. doi: 10.1113/jphysiol.1988.sp016925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson AJ, Hutt EC, Brown SF. Thresholds for the perception of whole body angular movement about a vertical axis. Aviat Space Environ Med. 1989;60:205–213. [PubMed] [Google Scholar]
- Clark B, Stewart JD. Comparison of sensitivity for the perception of bodily rotation and the oculogyral illusion. Perception & Psychophysics. 1968;3:253–256. [Google Scholar]
- Clark B, Stewart JD. Thresholds for the perception of angular acceleration about the three major body axes. Acta Otolaryngol. 1970;69:231–238. doi: 10.3109/00016487009123358. [DOI] [PubMed] [Google Scholar]
- Carriot J, Bryan A, DiZio P, et al. The oculogyral illusion: retinal and oculomotor factors. Exp Brain Res. 2011;209:415–423. doi: 10.1007/s00221-011-2567-5. [DOI] [PubMed] [Google Scholar]
- Doty RL. Effect of duration of stimulus presentation on the angular acceleration threshold. J Exp Psychol. 1969;80:317–321. doi: 10.1037/h0027240. [DOI] [PubMed] [Google Scholar]
- Grabherr L, Nicoucar K, Mast FW, et al. Vestibular thresholds for yaw rotation about an earth-vertical axis as a function of frequency. Exp Brain Res. 2008;186:677–681. doi: 10.1007/s00221-008-1350-8. [DOI] [PubMed] [Google Scholar]
- Graybiel A, Kerr WA, Bartley SH. Stimulus thresholds of the semicircular canals as a function of angular acceleration. Am J Psychol. 1948;61:21–36. [PubMed] [Google Scholar]
- Graybiel A, Hupp DI. The oculogyral illusion; a form of apparent motion which may be observed following stimulation of the semicircular canals. J Aviat Med. 1946;17:3–27. [PubMed] [Google Scholar]
- Hallpike CS, Hood JD. The speed of the of the slow component of the ocular nystagmus induced by angular acceleration of the head: its experimental determination and application to the physical theory of the cupular mechanism. Proc R Soc Lond B Biol Sci. 1953;141:216–230. doi: 10.1098/rspb.1953.0038. [DOI] [PubMed] [Google Scholar]
- Kolev OI, Georgieva-Zhostova S. Illusory self-motion perception evoked by caloric vestibular stimulation in sitting versus supine body positions. Behav Brain Res. 2014;272:150–155. doi: 10.1016/j.bbr.2014.06.051. [DOI] [PubMed] [Google Scholar]
- Kolev O, Mergner T, Kimmig H, et al. Detection thresholds for object motion and self-motion during vestibular and visuo-oculomotor stimulation. Brain Res Bull. 1996;40:451–457. doi: 10.1016/0361-9230(96)00141-4. [DOI] [PubMed] [Google Scholar]
- Kolev OI, Nicoucar K. Flash induced afterimage versus single spot visual object influence on visual-vestibular interaction in detection threshold for self-motion perception. Neurosci Lett. 2014;564:43–47. doi: 10.1016/j.neulet.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Leek MR. Adaptive procedures in psychophysical research. Percept Psychophys. 2001;63:1279–1292. doi: 10.3758/bf03194543. [DOI] [PubMed] [Google Scholar]
- Luxon LM. The anatomy and physiology of the vestibular system. In: Dix MR, Hood JD, editors. Vertigo. London: John Wiley & Sons Ltd; 1984. pp. 1–36. [Google Scholar]
- Meiry JL. Vestibular system and human dynamic space orientation. Cambridge: M.I.T; 1965. Report T-65-1. [PubMed] [Google Scholar]
- Leigh RJ, Zee DS. The Neurology of Eye Movements. New York: Oxford University Press; 2006. [Google Scholar]
- Nijhoff P, Roggeveen LJ. The normal and pathological thresholds of the perception of angular accelerations for the optogyral illusion and the turning sensation. Acta Otolaryngol. 1956;46:533–541. doi: 10.3109/00016485609139009. [DOI] [PubMed] [Google Scholar]
- Seemungal BM, Gunaratne IA, Fleming IO, et al. Perceptual and nystagmic thresholds of vestibular function in yaw. J Vestib Res. 2004;14:461–466. [PubMed] [Google Scholar]
- Treutwein B. Adaptive psychophysical procedures. Vision Res. 1995;35:2503–2522. [PubMed] [Google Scholar]
- Vasudevan EV, Bastian AJ. Split-belt treadmill adaptation shows different functional networks for fast and slow human walking. J Neurophysiol. 2010;103:183–191. doi: 10.1152/jn.00501.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside TC, Graybiel A, Niven JI. Visual illusions of movement. Brain. 1965;88:193–210. doi: 10.1093/brain/88.1.193. [DOI] [PubMed] [Google Scholar]