Abstract
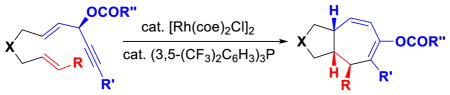
The first rhodium-catalyzed intramolecular [5 + 2] cycloaddition of 3-acyloxy-1,4-enyne and alkene was developed. The cycloaddition is highly diastereoselective in most cases. Various cis-fused bicyclo[5.3.0]decadienes were prepared stereoselectively. The chirality in the propargylic ester starting materials could be transferred to the bicyclic products with high efficiency. Electron-deficient phosphine ligand greatly facilitated the cycloaddition. Up to three new stereogenic centers could be generated. The resulting diene in the products could be hydrolyzed to enones, which allowed the introduction of more functional groups to the seven-membered ring.
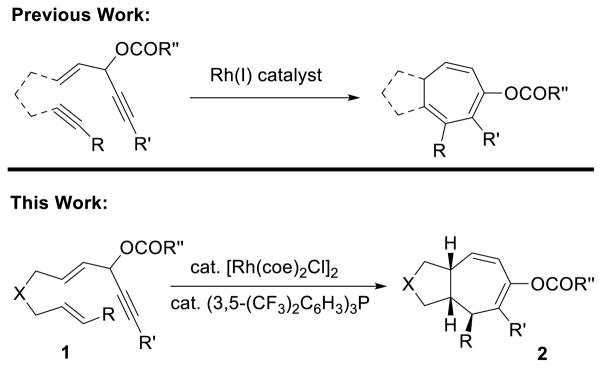
The importance of seven-membered ring in organic synthesis1 continues to stimulate the development of novel and efficient stereoselective methods.2 Cycloaddition is one of the most efficient means to achieve high atom- and step-economic efficiency.3 An important breakthrough in this area is the [5+2] cycloaddition of vinylcyclopropane (VCP) with alkyne, which can be catalyzed by Rh,4 Ru,5 Ni,6 or Fe7 for the formation of cycloheptadienes.8,9 The [5+2] cycloaddition of VCP with less reactive alkene may generate multiple new stereogenic centers. However, it proved to be a much more challenging process and has only been realized by research groups of Wender10 and Yu11 using Rh catalysts.12 We recently found that 3-acyloxy-1,4-enyne (ACE) could also serve as a 5-carbon component for Rh-catalyzed [5+2] cycloaddition with alkynes for the synthesis of cycloheptatrienes (Scheme 1).13 A 1,2-acyloxy migration was accompanied with this [5+2] cycloaddition.14 We herein report the first Rh-catalyzed highly stereoselective intramolecular [5+2] cycloaddition of ACEs and alkene for the synthesis of bi-cyclo[5.3.0]decane carbon skeletons, which are present in numerous bioactive sesquiterpenes and diterpenes (Scheme 1).1 This new reaction complements existing methods for the preparation of highly functionalized seven-membered rings.
Scheme 1. Rh-Catalyzed [5+2] Cycloadditions of ACE.
No desired cycloaddition product was observed when substrate 1a was treated with cationic rhodium catalyst (entry 1, Table 1). The addition of various ligands did not result in any cycloaddition product. Gratifyingly, a 63% yield of [5+2] cycloaddition product 2a was obtained in the presence of Wilkinson’s catalyst at 80 °C (entry 2). Other neutral Rh(I) complexes such as [Rh(cod)Cl]2 or [Rh(CO)2Cl]2 provided either no product or lower yield in the absence of any phosphine ligand (entries 3 and 4). We then examined the effect of phosphine ligands. While the addition of the (C6F5)3P ligand shut down the reaction (entry 5), the (p-CF3C6H4)3P ligand was beneficial (entry 6). Finally, the combination of [Rh(cod)Cl]2 and [3,5-(CF3)2C6H3]3P ligand afforded the desired cycloaddition product in 80% isolated yield (entry 7). Lower yields were obtained in other solvents or by running the reaction at lower temperatures (entries 8 and 9). Switching the metal complex to [Rh(coe)2Cl]2 slightly improved the yield (entry 10).
Table 1.
Screening of Catalysts and Conditions for 1a
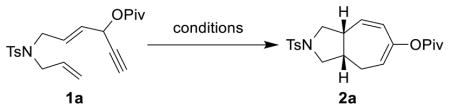
| |||
|---|---|---|---|
| entry | catalyst and ligands | conditions | yielda |
| 1 | [Rh(cod)2]BF4 (10 mol %) | DCE, 8 h, rt or 80 °C | 0 |
| 2 | Rh(PPh3)3Cl (10 mol %) | DCE, 10 h 80 °C, | 63% |
| 3 | [Rh(cod)Cl]2 (5 mol %) | DCE, 8 h, 80 °C, | 0 |
| 4 | [Rh(CO)2Cl]2 (5 mol %) | DCE, 8 h, 80 °C | 32% |
| 5 | [Rh(cod)Cl]2 (5 mol %), (C6F5)3P (30 mol%) | DCE, 20 h, 80 °C | 0 |
| 6 | [Rh(cod)Cl]2 (5 mol %), (p-CF3C6H4)3 P (30 mol%) | DCE, 20 h, 80 °C | 50% |
| 7 | [Rh(cod)Cl]2 (5 mol %), [3,5-(CF3)2C6H3]3P (30 mol%) | DCE, 20 h, 80 °C | 91% (80%)b |
| 8 | [Rh(cod)Cl]2 (5 mol %), [3,5-(CF3)2C6H3]3P (30 mol%) | CHCl3, 20 h, 50 °C | 34% |
| 9 | [Rh(cod)Cl]2 (5 mol %), [3,5-(CF3)2C6H3]3P (30 mol%) | CH2Cl2, 20 h, 50 °C | 31% |
| 10 | [Rh(coe)2Cl]2 (5 mol %), [3,5-(CF3)2C6H3]3P (30 mol%) | DCE, 20 h, 80 °C | (82%)b |
Yields were calculated based on 1H NMR using internal standard.
Isolated yield.
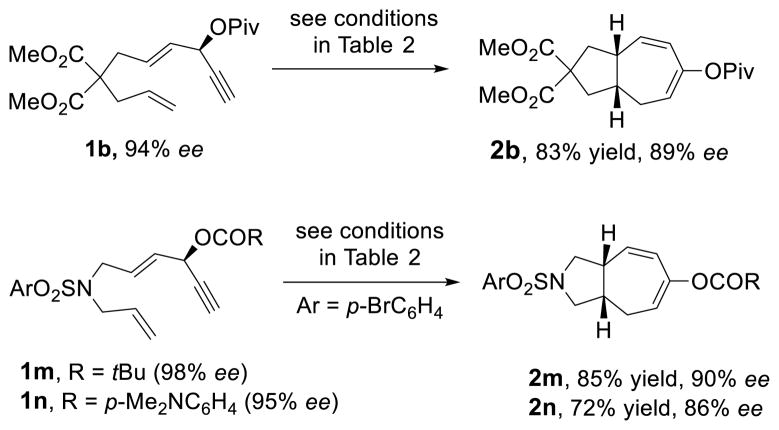
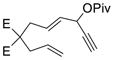
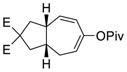
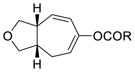
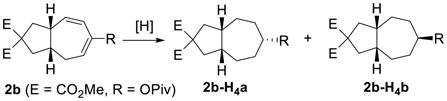
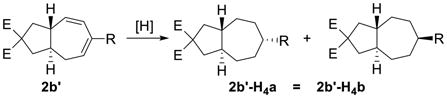
Having the optimized conditions in hand, we next investigated the scope of this [5+2] cycloaddition with alkenes (Table 2). Cycloaddition product 2b with an all carbon linker was isolated in a 82% yield. The diene moiety in product 2b could be completely reduced to afford a mixture of two diastereomeric isomers in a 1:1 ratio as shown in eq. 1. For trans-fused cycloaddition product 2b’, only one stereoisomer would be expected after hydrogenation as shown in eq. 2. The relative stereochemistry of bicyclic compound 2b was therefore assigned as the cis-configuration.10
Table 2.
Scope of Rh(I)-Catalyzed [5+2] Cycloaddition of ACE with Mono-substituted Alkenea
| entry | substrate | product | yieldb |
|---|---|---|---|
| 1 |
 1b (E = CO2Me) |
 2b |
82% |
|
| |||
| 2 |
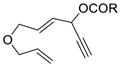 1c (R = tBu) |
 2c |
62% |
| 3 | 1d (R = p-Me2NC6H4) | 2d | 67% |
|
| |||
| 4 |
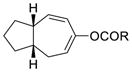
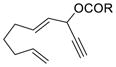 1e (R = tBu) |
 2e |
56% |
| 5 | 1f (R = p-Me2NC6H4) | 2f | 61% |
|
| |||
| 6 |
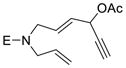 1g (E = p-BrC6H4SO2) |
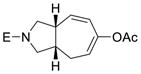 2g |
84% |
|
| |||
| 7 |
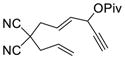 1h |
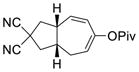 2h |
83% |
|
| |||
| 8 |
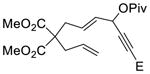 1i (E = CO2Et) |
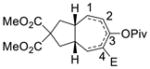 2i (14:1)c |
76% |
| 9 | 1j (E = C(O)nC4H9) | 2j (5:1)c | 73% |
| 10d | 1k (E = Br) | 2k (13:1)c | 53% |
| 11 |
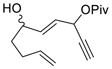 1l (dr = 1:1) |
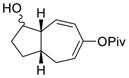 2l (dr = 1.5:1) |
71% |
Condition: [Rh(coe)2Cl]2 (5 mol %), [3,5-(CF3)2C6H3]3P (30 mol%), DCE, 80 °C, 20 h.
All yields were isolated yields. Dr > 20:1 unless noted otherwise.
The ratio of 1,3-diene and 2,4-diene.
[Rh(cod)2Cl]2 was employed as the catalyst.
 |
(1) |
 |
(2) |
The yield of cycloaddition product was slightly increased when the pivalate in 1c was changed to para-dimethylamino-benzoate in 1d (entry 3).15 Similarly, the yield of product 2f was slightly higher than that of product 2e (entries 4 and 5) but lower than 2b, presumably due to the Thorpe-Ingold effect.16 The yield of cycloaddition for acetate substrate 1g with a sulfonamide tether (entry 6) was as good as the corresponding pivalate substrate 1a in Table 1. Substrate 1h with a bis-nitrile tether also worked well (entry 7).
Propargylic esters with an internal alkyne generally prefer to undergo 1,3-acyloxy migration to form allene intermediates in the presence of rhodium(I) catalysts.17 An electron-withdrawing group such as ester, ketone or halogen on the alkyne may switch the regioselectivity back to 1,2-acyloxy migration. This was demonstrated by the successful Rh-catalyzed [5+2] cycloadditions of this type of electron-deficient ACEs and alkynes.13 We were pleased to find that ACEs in substrates 1i, 1j, and 1k also underwent [5+2] cycloadditions with the tethered alkenes (entries 8–10). A small amount of isomeric diene products was observed in these cases. We also found that a free hydroxyl group could be tolerated in the tether region (entry 11).
Catalytic asymmetric [5+2] cycloaddition of vinylcyclopropanes with alkenes or alkynes is a very challenging reaction.18 Highly enantioselective processes only appeared recently using chiral phosphine ligands.10b,19 Inspired by Toste’s work on Au-catalyzed Rautenstrauch rearrangement20 of enantio-enriched ACEs to cyclopentenones21 through a center-to-helix-to-center chirality transfer mechanism,22 we demonstrated that it was also possible to transfer the chirality from ACEs to bicyclic products in Rh-catalyzed intramolecular [5+2] cycloaddition of ACEs and alkynes.13c If a similar process can be realized for the cycloaddition between ACE and alkene, we will then be able to access highly valuable enantio-enriched 5–7 bicyclic products with multiple stereogenic centers from readily available starting materials. Substrates 1b, 1m, and 1n were easily prepared with high optical purity from the corresponding propargylic alcohols (Scheme 2). The chirality in these substrates could be transferred to the cycloaddition products efficiently. The ees of bicyclic products 2b, 2m, and 2n ranged from 86% to 90%. Only one diastereomer was observed in all three cases. The absolute and relative stereochemistry of product 2n was confirmed by X-ray analysis (CCDC977964).
Scheme 2. Transfer of Chirality.
It has been shown that Rh-catalyzed [5+2] cycloaddition of VCPs and alkenes works with substrates bearing a 1,1-disubstituted olefin but not a 1,2-disubstituted olefin as the 2-carbon component.10a,18 In our case, no reaction occurred for substrate 3a, while a complex mixture was observed for substrate 3b (Scheme 3). Interestingly, substrates with a styrene moiety participated in the cycloaddition.23 Bicyclic compounds with three new stereogenic centers were prepared diastereoselectively in all three cases (dr > 20:1). The relative stereochemistry of the three stereogenic centers was assigned based on nOe analysis.
Scheme 3. Cycloaddition of ACE with Substituted Alkene.
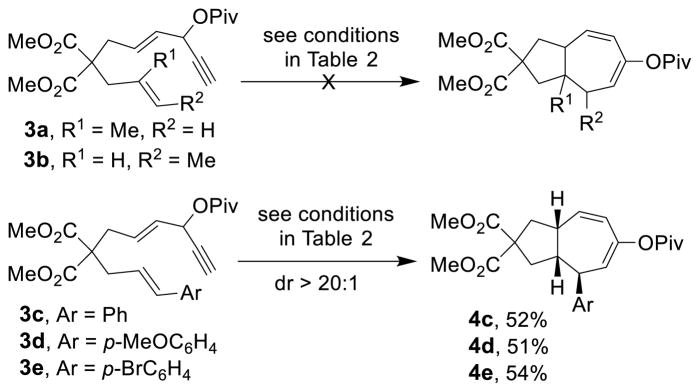
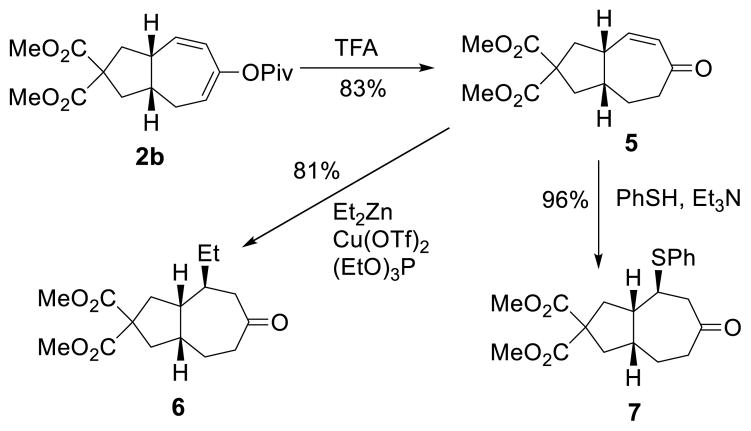
Although only styrene-type disubstituted alkenes participated in the cycloaddition reaction, the diene moiety in all products is essentially a masked enone, which may allow for the introduction of diverse functional groups and substituents to the seven-membered ring. Indeed, the pivalate group in cycloaddition product 2b was hydrolyzed to afford enone 5, which could undergo diastereoselective conjugate addition with carbon or sulfur nucleophiles to yield substituted bicyclo[5.3.0]decanes 6 and 7, respectively (Scheme 4).
Scheme 4. Functionalization of Cycloaddition Product.
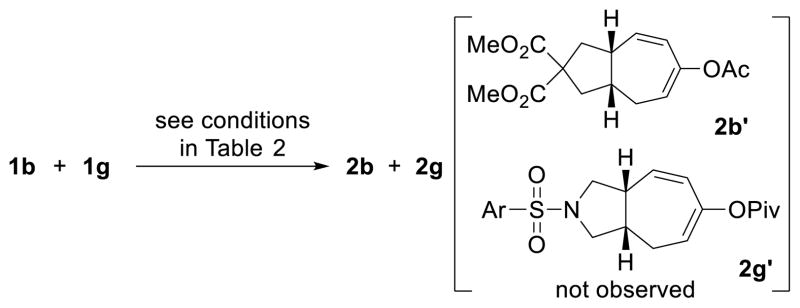
We also found that no cross-over product 2b’ or 2g’ was formed under standard conditions when a mixture of compounds 1b and 1g was employed as the substrate in a ratio of 1:1. This suggests that the acyloxy group does not completely dissociate from the enyne substrate.
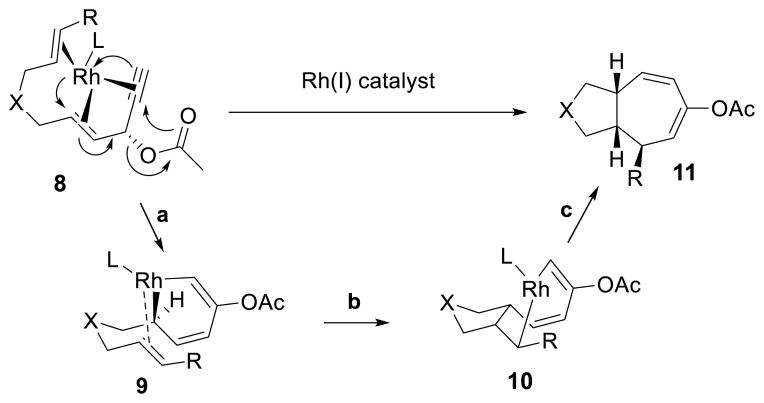
The mechanism for the Rh-catalyzed [5+2] cycloaddition of ACEs with alkenes is proposed in Scheme 6. Recent DFT calculations suggested that coordination of the Rh catalyst anti to the acyloxy group on the ACE was preferred as shown in complex 8.24 The Rh catalyst can then promote 1,2-acyloxy migration and oxidative cyclization to form metallacyclohexadiene intermediate 9. Insertion of the tethered alkene to this metallacycle may afford intermediate 10. Reductive elimination of metallacyclooctadiene 10 can then produce cycloheptadiene product 11. This mechanism is consistent with the observed absolute stereochemistry of propargylic ester 1n and product 2n, which was confirmed by X-ray structure.
Scheme 6. Proposed Mechanism.
In summary, we have demonstrated for the first time that ACEs can serve as the 5-carbon synthon in Rh-catalyzed intra-molecular [5+2] cycloadditions with less reactive alkenes. The chirality transfer from readily available optically pure propargylic esters to cycloaddition products was also shown to be feasible for this new [5+2] cycloaddition. The 2-carbon alkene component could be either a terminal or a 1,2-disubstituted alkene with an aryl substituent, which produced products with three new stereogenic centers.
Supplementary Material
Scheme 5. Cross-over Experiment.
Acknowledgments
We thank the NIH (R01GM088285), NSF (CHE-1464754) and the University of Wisconsin for funding. L. C. thanks the Chinese Scholarship Council for financial support.
Footnotes
Detailed experimental procedures, characterization data, and spectra (1H, 13C NMR, IR, HRMS and X-ray). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.a) Battiste MA, Pelphrey PM, Wright DL. Chem Eur J. 2006;12:3438. doi: 10.1002/chem.200501083. [DOI] [PubMed] [Google Scholar]; b) Butenschön H. Angew Chem Int Ed. 2008;47:5287. doi: 10.1002/anie.200801738. [DOI] [PubMed] [Google Scholar]; c) Foley DA, Maguire AR. Tetrahedron. 2010;66:1131. [Google Scholar]
- 2.a) Harmata M. Chem Commun. 2010;46:8886. doi: 10.1039/c0cc03620j. [DOI] [PubMed] [Google Scholar]; b) Harmata M. Chem Commun. 2010;46:8904. doi: 10.1039/c0cc03621h. [DOI] [PubMed] [Google Scholar]; c) Lohse AG, Hsung RP. Chem Eur J. 2011;17:3812. doi: 10.1002/chem.201100260. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Pellissier H. Adv Synth Catal. 2011;353:189. [Google Scholar]; e) Ylijoki KEO, Stryker JM. Chem Rev. 2013;113:2244. doi: 10.1021/cr300087g. [DOI] [PubMed] [Google Scholar]
- 3.a) Trost BM. Science. 1991;254:1471. doi: 10.1126/science.1962206. [DOI] [PubMed] [Google Scholar]; b) Trost BM. Angew Chem Int Ed. 1995;34:259. [Google Scholar]; c) Wender PA, Verma VA, Paxton TJ, Pillow TH. Acc Chem Res. 2008;41:40. doi: 10.1021/ar700155p. [DOI] [PubMed] [Google Scholar]; d) Wender PA, Miller BL. Nature. 2009;460:197. doi: 10.1038/460197a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.a) Wender PA, Takahashi H, Witulski B. J Am Chem Soc. 1995;117:4720. [Google Scholar]; b) Wender PA, Rieck H, Fuji M. J Am Chem Soc. 1998;120:10976. [Google Scholar]; c) Wender PA, Barzilay CM, Dyckman AJ. J Am Chem Soc. 2001;123:179. doi: 10.1021/ja0021159. [DOI] [PubMed] [Google Scholar]
- 5.a) Trost BM, Toste FD, Shen H. J Am Chem Soc. 2000;122:2379. [Google Scholar]; b) Trost BM, Shen HC. Angew Chem Int Ed. 2001;40:2313. [PubMed] [Google Scholar]; c) Trost BM, Shen HC, Horne DB, Toste FD, Steinmetz BG, Koradin C. Chem Eur J. 2005;11:2577. doi: 10.1002/chem.200401065. [DOI] [PubMed] [Google Scholar]
- 6.Zuo G, Louie J. J Am Chem Soc. 2005;127:5798. doi: 10.1021/ja043253r. [DOI] [PubMed] [Google Scholar]
- 7.Fürstner A, Majima K, Martin R, Krause H, Kattnig E, Goddard R, Lehmann CW. J Am Chem Soc. 2008;130:1992. doi: 10.1021/ja0777180. [DOI] [PubMed] [Google Scholar]
- 8.For computational studies on [5+2] cycloadditions involving VCPs, see: Yu ZX, Wender PA, Houk KN. J Am Chem Soc. 2004;126:9154. doi: 10.1021/ja048739m.Yu ZX, Cheong PHY, Liu P, Legault CY, Wender PA, Houk KN. J Am Chem Soc. 2008;130:2378. doi: 10.1021/ja076444d.Liu P, Cheong PHY, Yu ZX, Wender PA, Houk KN. Angew Chem Int Ed. 2008;47:3939. doi: 10.1002/anie.200800420.Liu P, Sirois LE, Cheong PHY, Yu ZX, Hartung IV, Rieck H, Wender PA, Houk KN. J Am Chem Soc. 2010;132:10127. doi: 10.1021/ja103253d.Xu X, Liu P, Lesser A, Sirois LE, Wender PA, Houk KN. J Am Chem Soc. 2012;134:11012. doi: 10.1021/ja3041724.Hong X, Liu P, Houk KN. J Am Chem Soc. 2013;135:1456. doi: 10.1021/ja309873z.Hong X, Trost BM, Houk KN. J Am Chem Soc. 2013;135:6588. doi: 10.1021/ja4012657.
- 9.For selected synthetic applications of Rh-catalyzed [5+2] cycloadditions, see: Wender PA, Fuji M, Husfeld CO, Love JA. Org Lett. 1999;1:137.Wender PA, Zhang L. Org Lett. 2000;2:2323. doi: 10.1021/ol006085q.Wender PA, Bi FC, Brodney MA, Gosselin F. Org Lett. 2001;3:2105. doi: 10.1021/ol0160699.Wender PA, Gamber GG, Scanio MJC. Angew Chem Int Ed. 2001;40:3895. doi: 10.1002/1521-3773(20011015)40:20<3895::AID-ANIE3895>3.0.CO;2-#.Ashfeld BL, Martin SF. Org Lett. 2005;7:4535. doi: 10.1021/ol051945u.Ashfeld BL, Martin SF. Tetrahedron. 2006;62:10497.Trost BM, Hu Y, Horne DB. J Am Chem Soc. 2007;129:11781. doi: 10.1021/ja073272b.Trost BM, Waser J, Meyer A. J Am Chem Soc. 2007;129:14556. doi: 10.1021/ja076165q.Trost BM, Waser J, Meyer A. J Am Chem Soc. 2008;130:16424. doi: 10.1021/ja806724x.Jiao L, Yuan C, Yu ZX. J Am Chem Soc. 2008;130:4421. doi: 10.1021/ja7100449.Wender PA, Stemmler RT, Sirois LE. J Am Chem Soc. 2010;132:2532. doi: 10.1021/ja910696x.Trost BM, Nguyen HM, Koradin C. Tetrahedron Lett. 2010;51:6232. doi: 10.1016/j.tetlet.2010.09.042.For Rh-catalyzed intramolecular [5+2] cycloaddition of allenyl cyclopropanes with alkynes, see: Inagaki F, Sugikubo K, Miyashita Y, Mukai C. Angew Chem Int Ed. 2010;49:2206. doi: 10.1002/anie.200906994.For related Rh-catalyzed hetero-[5+2] cycloadditions, see: Wender PA, Pedersen TM, Scanio MJC. J Am Chem Soc. 2002;124:15154. doi: 10.1021/ja0285013.Feng JJ, Zhang J. J Am Chem Soc. 2011;133:7304. doi: 10.1021/ja2014604.Feng JJ, Lin TY, Wu HH, Zhang J. J Am Chem Soc. 2015;137:3787. doi: 10.1021/jacs.5b01305.
- 10.a) Wender PA, Husfeld CO, Langkopf E, Love JA. J Am Chem Soc. 1998;120:1940. [Google Scholar]; b) Wender PA, Haustedt LO, Lim J, Love JA, Williams TJ, Yoon JY. J Am Chem Soc. 2006;128:6302. doi: 10.1021/ja058590u. [DOI] [PubMed] [Google Scholar]
- 11.Jiao L, Ye S, Yu ZX. J Am Chem Soc. 2008;130:7178. doi: 10.1021/ja8008715. [DOI] [PubMed] [Google Scholar]
- 12.For Rh-catalyzed [5+2] cycloaddition of VCPs with allenes, see: Wender PA, Glorius F, Husfeld CO, Langkopf E, Love JA. J Am Chem Soc. 1999;121:5348.Wegner HA, de Meijere A, Wender PA. J Am Chem Soc. 2005;127:6530. doi: 10.1021/ja043671w.
- 13.a) Shu XZ, Huang S, Shu D, Guzei IA, Tang W. Angew Chem Int Ed. 2011;50:8153. doi: 10.1002/anie.201103136. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Shu XZ, Li X, Shu D, Huang S, Schienebeck CM, Zhou X, Robichaux PJ, Tang W. J Am Chem Soc. 2012;134:5211. doi: 10.1021/ja2109097. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Shu XZ, Schienebeck CM, Song W, Guzei IA, Tang W. Angew Chem Int Ed. 2013;52:13601. doi: 10.1002/anie.201306919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shu XZ, Shu D, Schienebeck CM, Tang W. Chem Soc Rev. 2012;41:7698. doi: 10.1039/c2cs35235d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.a) Schienebeck CM, Robichaux PJ, Li X, Chen L, Tang W. Chem Commun. 2013;49:2616. doi: 10.1039/c3cc40634b. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Schienebeck CM, Song W, Smits AM, Tang W. Synthesis. 2015;47:1076. doi: 10.1055/s-0034-1380160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ingold CK, Thorpe JF. J Chem Soc Trans. 1915;107:1080. [Google Scholar]
- 17.a) Li X, Zhang M, Shu D, Robichaux PJ, Huang S, Tang W. Angew Chem Int Ed. 2011;50:10421. doi: 10.1002/anie.201104861. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Shu D, Li X, Zhang M, Robichaux PJ, Tang W. Angew Chem Int Ed. 2011;50:1346. doi: 10.1002/anie.201006881. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Shu D, Li X, Zhang M, Robichaux PJ, Guzei IA, Tang W. J Org Chem. 2012;77:6463. doi: 10.1021/jo300973r. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Li X, Huang S, Schienebeck CM, Shu D, Tang W. Org Lett. 2012;14:1584. doi: 10.1021/ol300330t. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Huang S, Li X, Lin CL, Guzei IA, Tang W. Chem Commun. 2012;48:2204. doi: 10.1039/c2cc17406e. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Fukuyama T, Ohta Y, Brancour C, Miyagawa K, Ryu I, Dhimane AL, Fensterbank L, Malacria M. Chem Eur J. 2012;18:7243. doi: 10.1002/chem.201200045. [DOI] [PubMed] [Google Scholar]
- 18.Wender PA, Husfeld CO, Langkopf E, Love JA, Pleuss N. Tetrahedron. 1998;54:7203. [Google Scholar]
- 19.Shintani R, Nakatsu H, Takatsu K, Hayashi T. Chem Eur J. 200915:8692. doi: 10.1002/chem.200901463. [DOI] [PubMed] [Google Scholar]
- 20.Rautenstrauch V. J Org Chem. 198449:950. [Google Scholar]
- 21.Shi X, Gorin DJ, Toste FD. J Am Chem Soc. 2005;127:5802. doi: 10.1021/ja051689g. [DOI] [PubMed] [Google Scholar]
- 22.Faza ON, Lopez CS, Alvarez R, de Lera AR. J Am Chem Soc. 2006;128:2434. doi: 10.1021/ja057127e. [DOI] [PubMed] [Google Scholar]
- 23.For discussions on the effect of conjugation in Rh-catalyzed cycloadditions, see: Croatt MP, Wender PA. Eur J Org Chem. 2010;19
- 24.Xu X, Liu P, Shu XZ, Tang W, Houk KN. J Am Chem Soc. 2013;135:9271. doi: 10.1021/ja4036785. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.