Abstract
Over the last 40 years, we have learnt a great deal about the Ras onco-proteins. These intracellular molecular switches are essential for the function of a variety of physiological processes, including signal transduction cascades responsible for cell growth and proliferation. Molecular simulations and free energy calculations have played an essential role in elucidating the conformational dynamics and energetics underlying the GTP hydrolysis reaction catalysed by Ras. Here we present an overview of the main lessons from molecular simulations on the GTPase reaction and conformational dynamics of this important anti-cancer drug target. In the first part, we summarise insights from quantum mechanical and combined quantum mechanical/molecular mechanical simulations as well as other free energy methods and highlight consensus viewpoints as well as remaining controversies. The second part provides a very brief overview of new insights emerging from large-scale molecular dynamics simulations. We conclude with a perspective regarding future studies of Ras where computational approaches will likely play an active role.
Keywords: GTP hydrolysis, conformational dynamics, quantum mechanics, molecular mechanics, molecular dynamics
1. Introduction
Phosphoryl ( ) transfer reactions catalysed by G-proteins, kinases and phosphatases are critical for many biological processes, including those that control cell signalling and metabolic pathways. A detailed characterisation of the hydrolysis reaction is essential to understand how these biological enzymes work, and to develop therapeutic agents against them when they malfunction. Therefore, considerable effort over several decades has been made towards deciphering the precise chemical steps and energetics involved in the cleavage of the phosphoester bond and release of the monophosphate product. These efforts included computational approaches [1–10] in addition to, and often surpassing the resolution of, crystallographic [11 – 16] and other spectroscopic methods.[17–20] The number of reports that have utilised simulations as the primary tool to elucidate phosphate hydrolysis reactions is enormous (e.g. [9,21–23]). In fact, our current knowledge about the reaction mechanisms of GTPases and ATPases would probably have not been possible without an active and critical contribution from various types of molecular simulations.
Among the many G-proteins,[24–27] kinases [28–30] or phosphatases [31] that have been studied computationally, the GTP hydrolysing Ras protein has attracted arguably the most sustained attention.[32-34] This is because (i) Ras is essential in the normal human physiology, (ii) its malfunction is associated with many diseases [35-38] and (iii) Ras is the prototype for a large superfamily of small G-proteins.[36,39]
Ras is a membrane-associated intracellular switch that controls cell proliferation, differentiation and apoptosis. [40,41] It acts as a molecular switch by cycling between GDP-bound ‘off’ and GTP-bound ‘on’ conformational states.[27,42–44] Since the intrinsic GTPase activity of Ras is extremely slow (kcat = 0.028 min−1 [45]), efficient cycling requires the activity of guanine nucleotide activating proteins (GAPs) [46,47] and guanine nucleotide exchange factors.[44] Defective cycling and subsequent up-regulation of Ras-GTP levels due to somatic or germline mutation is associated with about a quarter of all human tumours and a variety of developmental disorders.[35,36,48-51] In particular, mutations that interfere with the ability of Ras to hydrolyse GTP by blocking its intrinsic GTPase activity or GAP action can result in constitutive signalling and development of cancer.[38,41]
A number of research groups in the fields of computational chemistry and biology have invested considerable time and resources on Ras.[4,5,7 –10,22,23,52–65] The primary tools employed towards characterising the dynamics and chemical reactions in Ras proteins include molecular dynamics (MD) simulations [53–62,64] and free energy and ab initio methods such as quantum mechanics (QM) and quantum mechanical/molecular mechanical (QM/MM) simulations.[2,4–7,9,10,23,52,65–72] In a recent review,[32] we have summarised the key contributions of MD to the study of normal and aberrant Ras function in solution and in its physiological setting of lipid membranes.[32]
The current review is divided in two parts. The first and comparatively more detailed part focuses on lessons from QM and QM/MM or related methods that have played a critical role in addressing the central question of how the Ras GTPase reaction works. The second part provides a brief overview of some new insights emerging from the study of large-scale Ras dynamics by MD simulations. We conclude with a perspective in future applications of molecular simulations in Ras research. We note that our goal here is not to provide a complete account of the large body of work in the field but rather to highlight some of the key conclusions from, and issues yet to be resolved by, QM and molecular simulations.
2. Key players in the Ras-catalysed GTP hydrolysis reaction
Crystallographic and mutagenesis studies identified a number of residues in the active site of Ras that directly or indirectly participate in GTP hydrolysis. These include Gln61, Lys16, Thr35 and Asp57 as well as the conserved Mg2+ ion [12,13,73,74] (see Figure 1). Additional insights into the Ras GTPase reaction emerged from crystal structures solved in the presence of aluminium or magnesium trifluoride, which emulate the hydrolysed γ-phosphate before dissociation and thus model the transition state in the hydrolysis reaction.[15,73,75] These crystallographic studies proposed a somewhat conflicting role for some of the active site residues in hydrolysing GTP. For instance, some studies implied a direct role of Gln61 in proton abstraction [12,13] while another study on a related GTPase, transducin, suggested the γ-phosphate as the ‘ultimate base’.[73] Similarly, alternative mechanisms were proposed for the hydrolysis reaction, including direct attack by the nucleophilic water molecule (W in Figure 1) [73] or a concerted proton shuttle mechanism involving the nucleophilic water molecule, Gln61 and Gln63.[73] These observations inspired numerous QM- and/or QM/MM-type computational studies aimed at elucidating the detailed chemical steps and the roles of the critical residues in the Ras GTPase reaction. Below we review the major conclusions derived from these calculations with the view of documenting consensuses that have been achieved as well as highlighting the remaining issues that continue to generate controversy.
Figure 1.
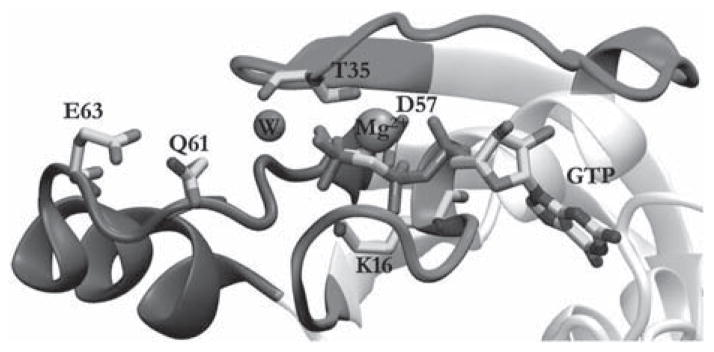
The active site structure of Ras. Several residues that have roles in catalysis are highlighted, as are the bound GTP, the catalytic water molecule and Mg2+ ion. The rest of the structure, which has been extensively discussed in other reviews (e.g. Ref. [32]), is omitted for clarity.
2.1 The role of Gln61
Earlier mutagenesis studies had shown that mutation of Gln61 to 17 different amino acids affect the GTPase reaction of Ras,[74,76] with the exception of Glu and Pro. [76] In contrast, substitution by non-natural Gln-homologues showed no change in the intrinsic or GAP-accelerated GTP hydrolysis.[74] One of the first simulation studies of Ras which has probed the role of Gln61 [7] used the empirical valence bond (EVB)/free energy perturbation (FEP) approach developed by Arieh Warshel. [77] The study found that the activation barrier for proton abstraction by Gln61 was about 30 kcal/mol, substantially higher than the 23 kcal/mol estimated from transition state theory.[45] Moreover, the GlnH+–OH− ion pair resulting from proton abstraction by Gln61 was less stable in the protein environment than in water.[7] Therefore, the authors concluded that Gln61 is unlikely to directly act as the general base for the GTPase reaction. Additional studies by the same group led to a similar conclusion.[9,65] These studies thus casted doubt on Gln61 as the general base hypothesis, though they did not rule out a more active role for Gln61 in the GAP-assisted GTP hydrolysis by Ras.[7]
In contrast, QM/MM calculations by Burt and colleagues [4,5,72] assigned a more direct role for Gln61 both in the Ras- and Ras–GAP-catalysed GTP hydrolysis reactions. According to these studies, the reaction proceeds via a two-step dissociative mechanism. The first step involves an almost complete separation of the γ-phosphate from the rest of the substrate, which they argued is plausible given the relatively low activation energy they have calculated (16.7 and 4.4 kcal/mol for Ras and Ras–GAP, respectively). The resulting GDP molecule and metaphosphate anion are stabilised by hydrogen bonding with Gln61, which in turn is stabilised by hydrogen bonding with the lytic H2O. The product is formed in the second step, and involves proton transfer mediated by two water molecules and Gln61. A more recent QM/MM study [22] on the entire Ras–GAP complex also showed an active role for Gln61 but through a somewhat different mechanism. This study proposed a mechanism in which a proton is transferred from the attacking water molecule to a second water molecule, followed by transfer of a different proton from the second water molecule to the GTP, with the resulting transient OH− and H3O− ions stabilised by Gln61.[22] This mechanism also suggested an explanation for the observed differences in the GTPase reaction of Q61A and Q61E mutants,[76] with the latter increasing the rate due to a more efficient stabilisation of the negatively charged glutamate by the hydronium ion. Such multi-water paths are consistent with results from kinetic isotope effect experiments on the nucleotidyl transfer reaction catalysed by nucleic acid polymerases.[78]
2.2 GTP as the general base
Given the conflicting results mentioned above, it is important to ask: If not Gln61 what then is the general base? To address this question, several alternative sources of electron for proton abstraction have been examined, [7,9] including the possibility that water molecules in the active site (other than the lytic water molecule) may serve as the base. For instance, Schweins et al. [9] calculated the activation energy for proton transfer between two crystal water molecules found in the active site.[13,14] They obtained very large activation energy for this process (68 kcal/mol), which indicated that this mechanism might be unlikely. After similarly ruling out a number of other alternative mechanisms, the γ-phosphate of the GTP substrate itself appeared to be a more likely candidate as the general base.[9] Calculations with the same EVB/FEP method mentioned above yielded activation energy of 24 kcal/mol for this process,[9] which is in good agreement with the 23 kcal/mol [45] obtained from transition state theory. In addition, a linear relationship was found between the rate of the GTPase reaction and the pKa of the bound GTP,[65,69,70,79] and subsequent studies from the same group provided additional support for a substrate-assisted GTP hydrolysis reaction.[65]
However, other related studies, such as the ab initio MD simulations by Cavalli and Carloni [2] on Cdc42/Cdc42GAP complex, supported the notion that Gln61–not GTP – is the base. In fact, the issue is far from settled and new evidence for and against the substrate-assisted mechanism continue to appear (see summary in Figure 2). Arguing for the former, Prasad et al. [80] suggested that ‘current studies that challenge the phosphate as a base mechanism’ did not base their conclusions on careful analysis or interpretation. An example of the latter is the recent study by Martin-Garcia et al. [22], which provided new evidence for Gln61 acting as the general base. Some earlier studies have also raised questions about the ability of the substrate to act as its own base. For instance, according to Maegley et al. [63], the GTP cannot act as the base because having the proton on the leaving γ-phosphate would be anti-catalytic in the dissociative transition state, which is observed in solution and thought to be applicable in Ras as well. Instead, the authors proposed a mechanism in which the amide of Gly13 forms a hydrogen bond with the β-γ-bridge oxygen of GTP. However, whether the Ras-catalysed GTPase reaction proceeds via a fully associative, dissociative or a concerted transition state continues to be debated.[4,81–83] For example, some studies found that Pγ–O(Pβ) breakage occurs at the second step after proton transfer has occurred from the lytic water molecule to the γ-phosphate,[52] while others reported that the Pγ–O(Pβ) breakage is the first step of the hydrolysis mechanism, thereby lowering the activation barrier for the overall reaction.[4,5,72]
Figure 2.
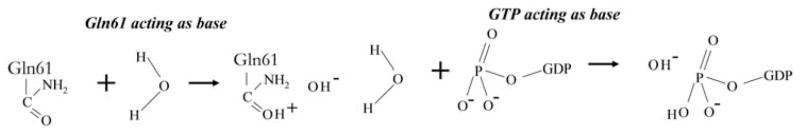
Ras-catalysed GTP hydrolysis. Shown here is a schematic illustration of the two competing hypotheses of proton abstraction by the side of Gln61 (left) and the GTP substrate (right).
2.3 Lys16 and proton relay
As mentioned earlier, Lys16 forms hydrogen bonds with the γ- and β-oxygen atoms in the crystal structure of the GTP- (or analogue-) bound Ras.[12] Inspired by this observation, Futatsugi et al. [66] examined the role of Lys16 in the GTPase reaction using gas phase ab initio molecular orbital calculations. In these calculations the hydroxyl group of Thr35 and Ser17 was approximated by water molecule and the α-phosphate of GTP by a hydrogen atom.[66] They found a one-step proton relay mechanism in which (i) a proton from Lys16 is transferred to the γ-oxygen, (ii) during the GTPase reaction this same proton returns back to Lys16, (iii) this promotes the transfer of a proton from the lytic water molecule to the γ-phosphate and (iv) the proton from Lys16 transfers to the β-phosphate upon production of GDP. It was suggested that the very high activation energy (40 kcal/mol) calculated for this reaction might be lowered by GAP-assisted reorganisation of Gln61 and the attacking water molecule. To our knowledge, there are no reports that disproved or corroborated this mechanism, apart from those questioning the gas phase calculations due to the ostensible instability of the protonated Lys16 under such an environment.[52]
2.4 The role of Mg2+
The importance of the magnesium ion in the GTPase reaction of Ras is apparent from the crystal structure [85] and biochemical experiments [84] as well as from simulations.[8,23] A series of recent reports by Gerwert and colleagues [23,68,86] have provided arguably some of the most detailed insights into the role of Mg2+ in Ras- and Ras·GAP-catalysed GTP hydrolysis reactions. (Although their work goes far beyond the role of Mg2+ and spans many years, [18–20] here we limit our discussion to the most recent ones that highlight the role of Mg2+.) They have shown that the coordination of Mg2+ with GTP changes from tri-dentate (simultaneous interaction with the α-, β- and γ-phosphates) in water to bi-dentate in Ras (with the β- and γ-phosphate).[68,86] As a consequence, conformation of the non-bridging oxygen atoms of the β and γ-phosphates changes from staggered in water to the high-energy eclipsed form in Ras. The arginine finger of GAP also induces an eclipsed position of the β- and α-phosphates.[68] As a result, the Pγ–O(Pβ) bond elongates and is thus primed for cleavage. This is accompanied by changes in charge distribution, with the γ-phosphate becoming more positive and hence ‘facilitating the positioning of the negatively charged oxygen of the attacking water’.[68] Another important observation was that Mg2+ serves as a temporary storage for electrons transferred from the γ-phosphate before hydrolysis, which it returns back to the β-phosphate after the formation of GDP.[23] These results thus show not only how Ras and GAP prime the substrate for hydrolysis by altering the coordination of Mg2+ and GTP, but also how the ion plays a key role during the reaction by acting as a temporary storage of charge.
Furthermore, in a similar study using time-resolved Fourier transform infrared spectroscopy and molecular simulations, Kotting et al. [19] investigated the hydrolysis reaction of the intermediate bound to the Ras–GAP complex. They found that in the intermediate, the γ-phosphate adopts an eclipsed conformation with respect to the β-phosphate of GDP.[19] Importantly, Ras in this intermediate state was found to be in the ‘on state’. [20] A follow-up study by Xia et al. [86] showed the similarity between the hydrogen bonding network of the educt and the states, providing (indirect) support for the ‘on’ status of the -bound Ras.
2.5 The role of GAP
GAP enhances the rate of GTP hydrolysis by Ras by 105-fold.[46,47] Determination of the Ras–GAP X-ray structure bound to the transition state analogue GDP·AlF3 [15] was a major breakthrough in our understanding of the role of GAPs in phosphoryl transfer reactions. For instance, it was immediately apparent from the structure that Arg789 of GAP is poised to directly affect the hydrolysis reaction. Subsequent simulations provided the details needed to develop a better understanding of the mechanism by which GAP induces conformational change and/or directly participates in the Ras-catalysed hydrolysis reaction. The first computational study to tackle this issue was carried out by the Warshel group using their EVB/FEP method.[52] They calculated the difference in the activation energy of the Ras GTPase reaction with and without GAP and found a 7 kcal/mol lower barrier in the latter, which is remarkably close to the experimental value of 6.5 kcal/mol. To probe the source of the reduction in the activation energy, the authors estimated the contribution of electrostatics due to Arg789 of GAP by calculating the activation energy when the side chain is stripped off its charges.[52] They found that the barrier for the mutant is higher than the wild type by 4.0 kcal/mol, very close to the experimental value of 4.5 kcal/mol for R789A mutant.[11] This was taken as confirming the electrostatic origin of the enhanced catalysis in the presence of GAP, and is consistent with the findings of Heesen et al. [71] who showed that the positive charge of Arg789 anchored GAP in the binding niche. Similarly, calculations with a non-polar Gln61 (with its charges striped off) resulted in a small (1–2 kcal/mol) and large (9–10 kcal/mol) increase in the activation barrier when the calculations were performed on Ras and on Ras coordinates from Ras–GAP complex, respectively.[52] This suggested that one of the reasons for the ability of GAP to enhance the GTPase reaction might be its ability to induce conformational changes on Ras to reposition key residues, such as Gln61, to attain a catalytic conformation. Other calculations, such as MD simulations,[87] arrived at more or less similar conclusions: the Arg789 finger intrudes into the active site of Ras and participates in the reorganisation of charges and side chains important for the catalytic reaction.[52,71,87] In sum, therefore, there appears to be a general consensus on the role and mechanism of action of GAP in the Ras-catalysed GTPase reaction, which is in sharp contrast with the continuing debate about the role of Gln61 of Ras.
3. Large-scale dynamics in Ras proteins
Alongside the detailed QM-based studies discussed above, MM-based MD simulation approaches have played a vital role in Ras research. Whereas QM methods are suitable for studying reaction mechanisms in great detail, they are too expensive to be used for sampling large phase spaces accessible to dynamic biomolecules. MD, on the other hand, is suitable for sampling configurational space but is too crude to study detailed reaction mechanisms. The two methods can be used in combination, such as in QM/MM, and when used judiciously, the two approaches can independently yield complementary results. For instance, consistent with the results from QM and free energy calculations discussed above, MD has shown that Arg789 of GAP forms stable hydrogen bonds with the γ- and α-phosphate oxygen atoms of Ras, suggesting that it plays a direct role in activating the catalytic water molecule by polarising the γ-phosphate.[87] That said, arguably the most important contribution of MD was to provide atomically detailed insights into the large-scale dynamics of Ras in solution and membrane environments. Below we provide a brief overview of these insights based mostly on publications from our laboratory. We refer the reader to our recent review on this topic for a more detailed discussion.[32]
3.1 Dynamics and long-range coupled motions
One of the key areas in which MD can play (and has played) an indispensable role is in providing insights into the possible sources of functional diversity among the homologous N-, H- and K-Ras proteins. These isoforms share a nearly identical catalytic domain (amino acids 1–166) yet have non-redundant biological functions [88,89] and differentially contribute to tumour formation.[36] This is perhaps due partly to differences in dynamics and sequence diversity at their C-terminus (residues 167–185/186). For instance, MD studies have documented differences in the dynamics of H- and K-Ras[54,90] and wild-type and oncogenic H-Ras.[54,67,90] Differential coupled motions linking the conserved nucleotide binding and the more variable membrane-interacting regions were observed.[54,57] Moreover, predictions from MD that Ras is an allosteric enzyme [54,55,57,58,114] have been confirmed by crystallography and biochemical techniques. [91] These motional differences can be exploited for the development of selective allosteric inhibitors to block aberrant Ras function.[59,92] Although to date there are no effective small molecule inhibitors that directly and selectively target a Ras isoform, the prevailing view that Ras is undruggable is now changing. This is due in large measure to an increased appreciation of the role of dynamics [93–95] in which MD simulations have played a leading role. For instance, aided by extensive MD simulations, we were able to identify four allosteric ligand binding sites on Ras, [59] others used fragment screening, NMR and X-ray crystallographic approaches to find ligands that bind to some of these sites or their vicinity with micro-molar affinity.[96–104] Moreover, guided by MD simulations, we have shown for the first time that nucleotide exchange factors are required for oncogenic Ras signalling and that inhibiting nucleotide exchange is a valid approach to abrogating the function of oncogenic mutant Ras.[105] This finding has the potential to change the paradigm of Ras drug discovery and may lead to the first anti-cancer therapeutic that directly targets Ras in an isoform selective manner.
3.2 Membrane binding and assembly
Another key contribution of MD in the study of Ras proteins involves the structure and energetics of membrane binding and assembly (reviewed in Ref. [32]). For instance, MD simulations have predicted significant differences in the membrane binding behaviour of H- and K-Ras [55,106] proteins and yielded atomically detailed structures for the isolated lipid-modified membrane-binding motif of each Ras isoform.[34,56,60] These are important because differential membrane-organisation arising in part (but not exclusively) from distinct lipid-modification has been shown to partly explain functional diversity among Ras proteins.[116] Moreover, MD was instrumental in the discovery of activation state-dependent membrane reorientation of the Ras catalytic domain,[33,55] a finding subsequently supported by biophysical experimental studies.[109,110] In addition, an interesting recent study has combined MD with spectroscopic analysis and proposed that N-Ras forms a dimer upon binding to a palmitoyl oleoyl phosphatidylcholine (POPC) bilayer.[112] The free energy profile for the transfer of Ras peptides from water to bilayer has also been calculated using the MD-based Adaptive Biasing Force method.[53,115,117] Finally, coarse-grained MD simulations were used to investigate the mechanism of cluster formation and lateral distribution of Ras in membrane domains.[61,62,108,111,113] These large-scale simulations illuminated the physical basis for the distinct clustering behaviour and non-overlapping distribution of different Ras proteins in membrane domains. For example, the nature of the lipid modification was found to play a key role in the lateral organisation, [61] while cholesterol modulates lipid domain stability and thereby the stability of Ras nanoclusters.[62] The simulations also provided new insights into how oligomerisation of surface-bound lipidated proteins can induce or stabilise membrane curvature.[61,111]
4. Conclusion and perspective
We have discussed some of the key contributions of detailed ab initio QM and combined QM/MM calculations to the study of Ras and its GTPase activity. Together with a variety of experimental methods,[12–15,17,18,46,47,84,85] these calculations have elucidated the chemical steps involved in the intrinsic and GAP-facilitated hydrolysis of GTP by Ras. [4,5,7,9,10,22,23,52,65,68,71,83,87] The accumulating simulation data provided a clear picture on the catalytic role of a number of residues in the Ras active site. Moreover, the significant contribution of the Ras-bound Mg2+ and the arginine finger of GAP in the catalytic reaction are now well established. However, there are areas of continued controversy. In particular, there exist reports supporting the competing hypotheses of GTP as the general base and the side chain of Gln61 as the general base. It is intriguing that the ever-growing body of computational data paints a conflicting picture about the exact mechanistic role of Gln61 given, as discussed in Section 2, its requirement for normal Ras function and the fact that mutations at this site render Ras constitutively active. Interestingly, recent reports suggest that the orientation of Gln61 can be allosterically modulated by conformational changes in distal sites.[91] Future studies that take such long-range allosteric effects into account may provide a more coherent picture of the role of Gln61.
Similarly, MD simulations have contributed immensely to the study of Ras proteins. For instance, they provided the initial clues about the allosteric nature of Ras, [54,93] and highlighted the role of conformational selection in its function.[114] They predicted isoform-specific membrane binding and assembly processes that have the potential to decode, at least in part, the source of functional diversity among Ras proteins. These insights have led us to propose the potential druggability of Ras at the time when most have given up on this major anti-cancer drug target.[93] Aided in part by the confluence of other factors, such as the reaffirmation by large-scale genomic studies that K-Ras is the main culprit in many forms of cancers, Ras is now back in the forefront of the search for new cancer medications. In the last 2 years, many compounds have been discovered that directly bind Ras, although their potential as drug leads is yet to be determined. We believe the search for Ras inhibitors will continue and molecular simulations will play a vital role in this endeavour.
Acknowledgments
P.P. is supported by a postdoctoral training fellowship by the Computational Cancer Biology Training Program (CCBTP) from the Cancer Prevention and Research Institute of Texas (CPRIT) (Grant No. RP101489). We thank the Texas Advanced Computing Center (TACC) for computational resources. This work was supported in part by grant from the National Institutes of Health General Medical Sciences (Grant No. R01GM100078).
References
- 1.Åqvist J, Kolmodin K, Florian J, Warshel A. Mechanistic alternatives in phosphate monoester hydrolysis: What conclusions can be drawn from available experimental data? Chem Biol. 1999;6:R71–R80. doi: 10.1016/S1074-5521(99)89003-6. [DOI] [PubMed] [Google Scholar]
- 2.Cavalli A, Carloni P. Enzymatic GTP hydrolysis: insights from an ab initio molecular dynamics study. J Am Chem Soc. 2002;124:3763–3768. doi: 10.1021/ja015821y. [DOI] [PubMed] [Google Scholar]
- 3.Florián J, Warshel A. Phosphate ester hydrolysis in aqueous solution: associative versus dissociative mechanisms. J Phys Chem B. 1998;102:719–734. [Google Scholar]
- 4.Grigorenko BL, Nemukhin AV, Shadrina MS, Topol IA, Burt SK. Mechanisms of guanosine triphosphate hydrolysis by Ras and Ras–GAP proteins as rationalized by ab initio QM/MM simulations. Proteins Struct Funct Bioinf. 2007;66:456–466. doi: 10.1002/prot.21228. [DOI] [PubMed] [Google Scholar]
- 5.Grigorenko BL, Nemukhin AV, Topol IA, Cachau RE, Burt SK. QM/MM modeling the Ras–GAP catalyzed hydrolysis of guanosine triphosphate. Proteins Struct Funct Bioinf. 2005;60:495–503. doi: 10.1002/prot.20472. [DOI] [PubMed] [Google Scholar]
- 6.Grigorenko BL, Rogov AV, Nemukhin AV. Mechanism of triphosphate hydrolysis in aqueous solution: QM/MM simulations in water clusters. J Phys Chem B. 2006;110:4407–4412. doi: 10.1021/jp056395w. [DOI] [PubMed] [Google Scholar]
- 7.Langen R, Schweins T, Warshel A. On the mechanism of guanosine triphosphate hydrolysis in ras p21 proteins. Biochemistry. 1992;31:8691–8696. doi: 10.1021/bi00152a002. [DOI] [PubMed] [Google Scholar]
- 8.Lu Q, Nassar N, Wang J. A mechanism of catalyzed GTP hydrolysis by Ras protein through magnesium ion. Chem Phys Lett. 2011;516:233–238. [Google Scholar]
- 9.Schweins T, Langen R, Warshel A. Why have mutagenesis studies not located the general base in ras p21. Nat Struct Biol. 1994;1:476–484. doi: 10.1038/nsb0794-476. [DOI] [PubMed] [Google Scholar]
- 10.Shurki A, Warshel A. Why does the Ras switch ‘break’ by oncogenic mutations? Proteins. 2004;55:1–10. doi: 10.1002/prot.20004. [DOI] [PubMed] [Google Scholar]
- 11.Ahmadian MR, Stege P, Scheffzek K, Wittinghofer A. Confirmation of the arginine-finger hypothesis for the GAP-stimulated GTP-hydrolysis reaction of Ras. Nat Struct Mol Biol. 1997;4:686–689. doi: 10.1038/nsb0997-686. [DOI] [PubMed] [Google Scholar]
- 12.Krengel U, Schlichting I, Scherer A, Schumann R, Frech M, John J, Kabsch W, Pai EF, Wittinghofer A. Three-dimensional structures of H-ras p21 mutants: molecular basis for their inability to function as signal switch molecules. Cell. 1990;62:539–548. doi: 10.1016/0092-8674(90)90018-a. [DOI] [PubMed] [Google Scholar]
- 13.Pai EF, Krengel U, Petsko GA, Goody RS, Kabsch W, Wittinghofer A. Refined crystal structure of the triphosphate conformation of H-ras p21 at 1.35 A resolution: implications for the mechanism of GTP hydrolysis. EMBO J. 1990;9:2351–2359. doi: 10.1002/j.1460-2075.1990.tb07409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prive GG, Milburn MV, Tong L, de Vos AM, Yamaizumi Z, Nishimura S, Kim SH. X-ray crystal structures of transforming p21 ras mutants suggest a transition-state stabilization mechanism for GTP hydrolysis. Proc Natl Acad Sci USA. 1992;89:3649–3653. doi: 10.1073/pnas.89.8.3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scheffzek K, Ahmadian MR, Kabsch W, Wiesmüller L, Lautwein A, Schmitz F, Wittinghofer A. The Ras–RasGAP complex: structural basis for GTPase activation and its loss in oncogenic Ras mutants. Science. 1997;277:333–339. doi: 10.1126/science.277.5324.333. [DOI] [PubMed] [Google Scholar]
- 16.Milburn MV, Tong L, deVos AM, Brunger A, Yamaizumi Z, Nishimura S, Kim SH. Molecular switch for signal transduction: structural differences between active and inactive forms of protooncogenic ras proteins. Science. 1990;247:939–945. doi: 10.1126/science.2406906. [DOI] [PubMed] [Google Scholar]
- 17.Allin C, Ahmadian MR, Wittinghofer A, Gerwert K. Monitoring the GAP catalyzed H-Ras GTPase reaction at atomic resolution in real time. Proc Natl Acad Sci USA. 2001;98:7754–7759. doi: 10.1073/pnas.131549798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cepus V, Scheidig AJ, Goody RS, Gerwert K. Time-resolved FTIR studies of the GTPase reaction of H-ras p21 reveal a key role for the beta-phosphate. Biochemistry. 1998;37:10263–10271. doi: 10.1021/bi973183j. [DOI] [PubMed] [Google Scholar]
- 19.Kotting C, Blessenohl M, Suveyzdis Y, Goody RS, Wittinghofer A, Gerwert K. A phosphoryl transfer intermediate in the GTPase reaction of Ras in complex with its GTPase-activating protein. Proc Natl Acad Sci USA. 2006;103:13911–13916. doi: 10.1073/pnas.0604128103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kotting C, Kallenbach A, Suveyzdis Y, Wittinghofer A, Gerwert K. The GAP arginine finger movement into the catalytic site of Ras increases the activation entropy. Proc Natl Acad Sci USA. 2008;105:6260–6265. doi: 10.1073/pnas.0712095105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li G, Zhang XC. GTP hydrolysis mechanism of Ras-like GTPases. J Mol Biol. 2004;340:921–932. doi: 10.1016/j.jmb.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 22.Martin-Garcia F, Mendieta-Moreno JI, Lopez-Vinas E, Gomez-Puertas P, Mendieta J. The role of Gln61 in HRas GTP hydrolysis: a quantum mechanics/molecular mechanics study. Biophys J. 2012;102:152–157. doi: 10.1016/j.bpj.2011.11.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rudack T, Xia F, Schlitter J, Kotting C, Gerwert K. The role of magnesium for geometry and charge in GTP hydrolysis, revealed by quantum mechanics/molecular mechanics simulations. Biophys J. 2012;103:293–302. doi: 10.1016/j.bpj.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kötting C, Gerwert K. The dynamics of the catalytic site in small GTPases, variations on a common motif. FEBS Lett. 2013;587:2025–2027. doi: 10.1016/j.febslet.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 25.McCudden CR, Hains MD, Kimple RJ, Siderovski DP, Willard FS. G-protein signaling: back to the future. Cell Mol Life Sci. 2005;62:551–577. doi: 10.1007/s00018-004-4462-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cherfils J, Zeghouf M. Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol Rev. 2013;93:269–309. doi: 10.1152/physrev.00003.2012. [DOI] [PubMed] [Google Scholar]
- 27.Wittinghofer A, Vetter IR. Structure–function relationships of the G domain, a canonical switch motif. Annu Rev Biochem. 2011;80:943–971. doi: 10.1146/annurev-biochem-062708-134043. [DOI] [PubMed] [Google Scholar]
- 28.Helms V, McCammon JA. Kinase conformations: a computational study of the effect of ligand binding. Protein Sci. 1997;6:2336–2343. doi: 10.1002/pro.5560061106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barkho S, Pierce LC, McGlone ML, Li S, Woods VL, Jr, Walker RC, Adams JA, Jennings PA. Distal loop flexibility of a regulatory domain modulates dynamics and activity of C-terminal SRC kinase (csk) PLoS Comput Biol. 2013;9:e1003188. doi: 10.1371/journal.pcbi.1003188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caballero J, Alzate-Morales JH. Molecular dynamics of protein kinase-inhibitor complexes: a valid structural information. Curr Pharm Des. 2012;18:2946–2963. doi: 10.2174/138161212800672705. [DOI] [PubMed] [Google Scholar]
- 31.Duarte F, Amrein BA, Kamerlin SC. Modeling catalytic promiscuity in the alkaline phosphatase superfamily. Phys Chem Chem Phys. 2013;15:11160–11177. doi: 10.1039/c3cp51179k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prakash P, Gorfe AA. Lessons from computer simulations of Ras proteins in solution and in membrane. Biochim Biophys Acta Gen Subj. 2013;1830:5211–5218. doi: 10.1016/j.bbagen.2013.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abankwa D, Hanzel-Bayer M, Ariotti N, Plowman SJ, Gorfe AA, Parton RG, McCammon JA, Hancock JF. A novel switch region regulates H-ras membrane orientation and signal output. EMBO J. 2008;27:727–735. doi: 10.1038/emboj.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gorfe AA, Babakhani A, McCammon JA. H-ras protein in a bilayer: interaction and structure perturbation. J Am Chem Soc. 2007;129:12280–12286. doi: 10.1021/ja073949v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.di Magliano MP, Logsdon CD. Roles for KRAS in pancreatic tumor development and progression. Gastroenterology. 2013;144:1220–1229. doi: 10.1053/j.gastro.2013.01.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cox AD, Der CJ. Ras history: the saga continues. Small GTPases. 2010;1:2–27. doi: 10.4161/sgtp.1.1.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karnoub AE, Weinberg RA. Ras oncogenes: split personalities. Nat Rev Mol Cell Biol. 2008;9:517–531. doi: 10.1038/nrm2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malumbres M, Barbacid M. RAS oncogenes: the first 30 years. Nat Rev Cancer. 2003;3:459–465. doi: 10.1038/nrc1097. [DOI] [PubMed] [Google Scholar]
- 39.Baines AT, Xu D, Der CJ. Inhibition of ras for cancer treatment: the search continues. Future Med Chem. 2011;3:1787–1808. doi: 10.4155/fmc.11.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barbacid M. Ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- 41.Bos JL. ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- 42.Bourne HR, Sanders DA, McCormick F. The GTPase superfamily: a conserved switch for diverse cell functions. Nature. 1990;348:125–132. doi: 10.1038/348125a0. [DOI] [PubMed] [Google Scholar]
- 43.Sprang SR. G proteins, effectors and GAPs: structure and mechanism. Curr Opin Struct Biol. 1997;7:849–856. doi: 10.1016/s0959-440x(97)80157-1. [DOI] [PubMed] [Google Scholar]
- 44.Vetter IR, Wittinghofer A. The guanine nucleotide-binding switch in three dimensions. Science. 2001;294:1299–1304. doi: 10.1126/science.1062023. [DOI] [PubMed] [Google Scholar]
- 45.Temeles GL, Gibbs JB, D’Alonzo JS, Sigal IS, Scolnick EM. Yeast and mammalian ras proteins have conserved biochemical properties. Nature. 1985;313:700–703. doi: 10.1038/313700a0. [DOI] [PubMed] [Google Scholar]
- 46.Gideon P, John J, Frech M, Lautwein A, Clark R, Scheffler JE, Wittinghofer A. Mutational and kinetic analyses of the GTPase-activating protein (GAP)-p21 interaction: the C-terminal domain of GAP is not sufficient for full activity. Mol Cell Biol. 1992;12:2050–2056. doi: 10.1128/mcb.12.5.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eccleston JF, Moore KJ, Morgan L, Skinner RH, Lowe PN. Kinetics of interaction between normal and proline 12 Ras and the GTPase-activating proteins, p120-GAP and neurofibromin. The significance of the intrinsic GTPase rate in determining the transforming ability of ras. J Biol Chem. 1993;268:27012–27019. [PubMed] [Google Scholar]
- 48.Gripp KW, Lin AE. Costello syndrome: a Ras/mitogen activated protein kinase pathway syndrome (rasopathy) resulting from HRAS germline mutations. Genet Med. 2012;14:285–292. doi: 10.1038/gim.0b013e31822dd91f. [DOI] [PubMed] [Google Scholar]
- 49.Prior IA, Lewis PD, Mattos C. A comprehensive survey of Ras mutations in cancer. Cancer Res. 2012;72:2457–2467. doi: 10.1158/0008-5472.CAN-11-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schubbert S, Shannon K, Bollag G. Hyperactive Ras in developmental disorders and cancer. Nat Rev Cancer. 2007;7:295–308. doi: 10.1038/nrc2109. [DOI] [PubMed] [Google Scholar]
- 51.Schubbert S, Zenker M, Rowe SL, Boll S, Klein C, Bollag G, van der Burgt I, Musante L, Kalscheuer V, Wehner LE, Nguyen H, West B, Zhang KY, Sistermans E, Rauch A, Niemeyer CM, Shannon K, Kratz CP. Germline KRAS mutations cause Noonan syndrome. Nat Genet. 2006;38:331–336. doi: 10.1038/ng1748. [DOI] [PubMed] [Google Scholar]
- 52.Glennon TM, Villa J, Warshel A. How does GAP catalyze the GTPase reaction of Ras? A computer simulation study. Biochemistry. 2000;39:9641–9651. doi: 10.1021/bi000640e. [DOI] [PubMed] [Google Scholar]
- 53.Gorfe AA, Babakhani A, McCammon JA. Free energy profile of H-ras membrane anchor upon membrane insertion. Angew Chem Int Ed. 2007;46:8234–8237. doi: 10.1002/anie.200702379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gorfe AA, Grant BJ, McCammon JA. Mapping the nucleotide and isoform-dependent structural and dynamical features of Ras proteins. Structure. 2008;16:885–896. doi: 10.1016/j.str.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gorfe AA, Hanzal-Bayer M, Abankwa D, Hancock JF, McCammon JA. Structure and dynamics of the full-length lipid-modified H-Ras protein in a 1,2-dimyristoylglycero-3-phosphocholine bilayer. J Med Chem. 2007;50:674–684. doi: 10.1021/jm061053f. [DOI] [PubMed] [Google Scholar]
- 56.Gorfe AA, Pellarin R, Caflisch A. Membrane localization and flexibility of a lipidated ras peptide studied by molecular dynamics simulations. J Am Chem Soc. 2004;126:15277–15286. doi: 10.1021/ja046607n. [DOI] [PubMed] [Google Scholar]
- 57.Grant BJ, Gorfe AA, McCammon JA. Ras conformational switching: simulating nucleotide-dependent conformational transitions with accelerated molecular dynamics. PLoS Comput Biol. 2009;5:e1000325. doi: 10.1371/journal.pcbi.1000325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grant BJ, Gorfe AA, McCammon JA. Large conformational changes in proteins: signaling and other functions. Curr Opin Struct Biol. 2010;20:142–147. doi: 10.1016/j.sbi.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grant BJ, Lukman S, Hocker HJ, Sayyah J, Brown JH, McCammon JA, Gorfe AA. Novel allosteric sites on Ras for lead generation. PLoS One. 2011;6:e25711. doi: 10.1371/journal.pone.0025711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Janosi L, Gorfe AA. Segregation of negatively charged phospholipids by the polycationic and farnesylated membrane anchor of Kras. Biophys J. 2010;99:3666–3674. doi: 10.1016/j.bpj.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Janosi L, Li Z, Hancock JF, Gorfe AA. Organization, dynamics, and segregation of Ras nanoclusters in membrane domains. Proc Natl Acad Sci USA. 2012;109:8097–8102. doi: 10.1073/pnas.1200773109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li Z, Janosi L, Gorfe AA. Formation and domain partitioning of H-ras peptide nanoclusters: effects of peptide concentration and lipid composition. J Am Chem Soc. 2012;134:17278–17285. doi: 10.1021/ja307716z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maegley KA, Admiraal SJ, Herschlag D. Ras-catalyzed hydrolysis of GTP: a new perspective from model studies. Proc Natl Acad Sci USA. 1996;93:8160–8166. doi: 10.1073/pnas.93.16.8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Prakash P, Sayyed-Ahmad A, Gorfe AA. The role of conserved waters in conformational transitions of Q61H K-ras. PLoS Comput Biol. 2012;8:e1002394. doi: 10.1371/journal.pcbi.1002394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schweins T, Warshel A. Mechanistic analysis of the observed linear free energy relationships in p21ras and related systems. Biochemistry. 1996;35:14232–14243. doi: 10.1021/bi961119g. [DOI] [PubMed] [Google Scholar]
- 66.Futatsugi N, Hata M, Hoshino T, Tsuda M. Ab initio study of the role of lysine 16 for the molecular switching mechanism of Ras protein p21. Biophys J. 1999;77:3287–3292. doi: 10.1016/S0006-3495(99)77159-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Futatsugi N, Tsuda M. Molecular dynamics simulations of Gly-12 → Val mutant of p21(ras): dynamic inhibition mechanism. Biophys J. 2001;81:3483–3488. doi: 10.1016/S0006-3495(01)75979-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rudack T, Xia F, Schlitter J, Kötting C, Gerwert K. Ras and GTPase-activating protein (GAP) drive GTP into a precatalytic state as revealed by combining FTIR and biomolecular simulations. Proc Natl Acad Sci USA. 2012;109:15295–15300. doi: 10.1073/pnas.1204333109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schweins T, Geyer M, Kalbitzer HR, Wittinghofer A, Warshel A. Linear free energy relationships in the intrinsic and GTPase activating protein-stimulated guanosine 5′-triphosphate hydrolysis of p21ras. Biochemistry. 1996;35:14225–14231. doi: 10.1021/bi961118o. [DOI] [PubMed] [Google Scholar]
- 70.Schweins T, Geyer M, Scheffzek K, Warshel A, Kalbitzer HR, Wittinghofer A. Substrate-assisted catalysis as a mechanism for GTP hydrolysis of p21ras and other GTP-binding proteins. Nat Struct Biol. 1995;2:36–44. doi: 10.1038/nsb0195-36. [DOI] [PubMed] [Google Scholar]
- 71.te Heesen H, Gerwert K, Schlitter J. Role of the arginine finger in Ras·RasGAP revealed by QM/MM calculations. FEBS Lett. 2007;581:5677–5684. doi: 10.1016/j.febslet.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 72.Topol IA, Cachau RE, Nemukhin AV, Grigorenko BL, Burt SK. Quantum chemical modeling of the GTP hydrolysis by the RAS–GAP protein complex. Biochim Biophys Acta Proteins Proteomic. 2004;1700:125–136. doi: 10.1016/j.bbapap.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 73.Sondek J, Lambright DG, Noel JP, Hamm HE, Sigler PB. GTPase mechanism of Gproteins from the 1.7-A crystal structure of transducin [alpha] – GDP AIF-4. Nature. 1994;372:276–279. doi: 10.1038/372276a0. [DOI] [PubMed] [Google Scholar]
- 74.Chung HH, Benson DR, Schultz PG. Probing the structure and mechanism of Ras protein with an expanded genetic code. Science. 1993;259:806–809. doi: 10.1126/science.8430333. [DOI] [PubMed] [Google Scholar]
- 75.Graham DL, Lowe PN, Grime GW, Marsh M, Rittinger K, Smerdon SJ, Gamblin SJ, Eccleston JF. MgF(3)(−) as a transition state analog of phosphoryl transfer. Chem Biol. 2002;9:375–381. doi: 10.1016/s1074-5521(02)00112-6. [DOI] [PubMed] [Google Scholar]
- 76.Der CJ, Finkel T, Cooper GM. Biological and biochemical properties of human rasH genes mutated at codon 61. Cell. 1986;44:167–176. doi: 10.1016/0092-8674(86)90495-2. [DOI] [PubMed] [Google Scholar]
- 77.Warshel A, Sussman F, Hwang JK. Evaluation of catalytic free energies in genetically modified proteins. J Mol Biol. 1988;201:139–159. doi: 10.1016/0022-2836(88)90445-7. [DOI] [PubMed] [Google Scholar]
- 78.Castro C, Smidansky E, Maksimchuk KR, Arnold JJ, Korneeva VS, Götte M, Konigsberg W, Cameron CE. Two proton transfers in the transition state for nucleotidyl transfer catalyzed by RNA- and DNA-dependent RNA and DNA polymerases. Proc Natl Acad Sci USA. 2007;104:4267–4272. doi: 10.1073/pnas.0608952104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Straub JE, Karplus M. The interpretation of site-directed mutagenesis experiments by linear free energy relations. Protein Eng. 1990;3:673–675. doi: 10.1093/protein/3.8.673. [DOI] [PubMed] [Google Scholar]
- 80.Prasad BR, Plotnikov NV, Warshel A. Addressing open questions about Phosphate hydrolysis pathways by careful free energy mapping. J Phys Chem B. 2012;117:153–163. doi: 10.1021/jp309778n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Klähn M, Rosta E, Warshel A. On the mechanism of hydrolysis of phosphate monoesters dianions in solutions and proteins. J Am Chem Soc. 2006;128:15310–15323. doi: 10.1021/ja065470t. [DOI] [PubMed] [Google Scholar]
- 82.Kamerlin SCL, Florián J, Warshel A. Associative versus dissociative mechanisms of phosphate monoester hydrolysis: on the interpretation of activation entropies. Chem Phys Chem. 2008;9:1767–1773. doi: 10.1002/cphc.200800356. [DOI] [PubMed] [Google Scholar]
- 83.Klahn M, Schlitter J, Gerwert K. Theoretical IR spectroscopy based on QM/MM calculations provides changes in charge distribution, bond lengths, and bond angles of the GTP ligand induced by the Ras-protein. Biophys J. 2005;88:3829–3844. doi: 10.1529/biophysj.104.058644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rohrer M, Prisner TF, Brugmann O, Kass H, Spoerner M, Wittinghofer A, Kalbitzer HR. Structure of the metal–water complex in Ras × GDP studied by high-field EPR spectroscopy and 31P NMR spectroscopy. Biochemistry. 2001;40:1884–1889. doi: 10.1021/bi002164y. [DOI] [PubMed] [Google Scholar]
- 85.Schweins T, Scheffzek K, Aßheuer R, Wittinghofer A. The role of the metal ion in the p21ras catalysed GTP-hydrolysis: Mn2+ versus Mg2+ J Mol Biol. 1997;266:847–856. doi: 10.1006/jmbi.1996.0814. [DOI] [PubMed] [Google Scholar]
- 86.Xia F, Rudack T, Cui Q, Kotting C, Gerwert K. Detailed structure of the H2PO4(−)-guanosine diphosphate intermediate in Ras–GAP decoded from FTIR experiments by biomolecular simulations. J Am Chem Soc. 2012;134:20041–20044. doi: 10.1021/ja310496e. [DOI] [PubMed] [Google Scholar]
- 87.Resat H, Straatsma TP, Dixon DA, Miller JH. The arginine finger of RasGAP helps Gln-61 align the nucleophilic water in GAP-stimulated hydrolysis of GTP. Proc Natl Acad Sci USA. 2001;98:6033–6038. doi: 10.1073/pnas.091506998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wolfman A. Ras isoform-specific signaling: location, location, location. Sci Signal. 2001;2001:pe2. doi: 10.1126/stke.2001.96.pe2. [DOI] [PubMed] [Google Scholar]
- 89.Ehrhardt A, David MD, Ehrhardt GR, Schrader JW. Distinct mechanisms determine the patterns of differential activation of H-Ras, N-Ras, K-Ras 4B, and M-Ras by receptors for growth factors or antigen. Mol Cell Biol. 2004;24:6311–6323. doi: 10.1128/MCB.24.14.6311-6323.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lukman S, Grant BJ, Gorfe AA, Grant GH, McCammon JA. The distinct conformational dynamics of K-Ras and H-Ras A59G. PLoS Comput Biol. 2010;6:e1000922. doi: 10.1371/journal.pcbi.1000922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Buhrman G, Holzapfel G, Fetics S, Mattos C. Allosteric modulation of Ras positions Q61 for a direct role in catalysis. Proc Natl Acad Sci USA. 2010;107:4931–4936. doi: 10.1073/pnas.0912226107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Buhrman G, O’Connor C, Zerbe B, Kearney BM, Napoleon R, Kovrigina EA, Vajda S, Kozakov D, Kovrigin EL, Mattos C. Analysis of binding site hot spots on the surface of ras GTPase. J Mol Biol. 2011;413:773–789. doi: 10.1016/j.jmb.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gorfe AA. Mechanisms of allostery and membrane attachment in Ras GTPases: implications for anti-cancer drug discovery. Curr Med Chem. 2010;17:1–9. doi: 10.2174/092986710789957832. [DOI] [PubMed] [Google Scholar]
- 94.Zheng X, Gan L, Wang E, Wang J. Pocket-based drug design: exploring pocket space. AAPS J. 2013;15:228–241. doi: 10.1208/s12248-012-9426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zheng X, Liu Z, Li D, Wang E, Wang J. Rational drug design: the search for Ras protein hydrolysis intermediate conformation inhibitors with both affinity and specificity. Curr Pharm Des. 2013;19:2246–2258. doi: 10.2174/1381612811319120012. [DOI] [PubMed] [Google Scholar]
- 96.Maurer T, Garrenton LS, Oh A, Pitts K, Anderson DJ, Skelton NJ, Fauber BP, Pan B, Malek S, Stokoe D, Ludlam MJ, Bowman KK, Wu J, Giannetti AM, Starovasnik MA, Mellman I, Jackson PK, Rudolph J, Wang W, Fang G. Small-molecule ligands bind to a distinct pocket in Ras and inhibit SOS-mediated nucleotide exchange activity. Proc Natl Acad Sci USA. 2012;109:5299–5304. doi: 10.1073/pnas.1116510109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rosnizeck IC, Spoerner M, Harsch T, Kreitner S, Filchtinski D, Herrmann C, Engel D, Konig B, Kalbitzer HR. Metal-bis(2-picolyl)amine complexes as state 1(T) inhibitors of activated Ras protein. Angew Chem Int Ed Engl. 2012;51:10647–10651. doi: 10.1002/anie.201204148. [DOI] [PubMed] [Google Scholar]
- 98.Shima F, Yoshikawa Y, Ye M, Araki M, Matsumoto S, Liao J, Hu L, Sugimoto T, Ijiri Y, Takeda A, Nishiyama Y, Sato C, Muraoka S, Tamura A, Osoda T, Tsuda K, Miyakawa T, Fukunishi H, Shimada J, Kumasaka T, Yamamoto M, Kataoka T. In silico discovery of small-molecule Ras inhibitors that display antitumor activity by blocking the Ras–effector interaction. Proc Natl Acad Sci USA. 2013;110:8182–8187. doi: 10.1073/pnas.1217730110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim RK, Suh Y, Lim EJ, Yoo KC, Lee GH, Cui YH, Son A, Hwang E, Uddin N, Yi JM, Kang SG, Lee SJ. A novel 2-pyrone derivative, BHP, impedes oncogenic KRAS-driven malignant progression in breast cancer. Cancer Lett. 2013;337:49–57. doi: 10.1016/j.canlet.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 100.Meierhofer T, Rosnizeck IC, Graf T, Reiss K, König B, Kalbitzer HR, Spoerner M. Cu2+-cyclen as probe to identify conformational states in guanine nucleotide binding proteins. J Am Chem Soc. 2011;133:2048–2051. doi: 10.1021/ja108779j. [DOI] [PubMed] [Google Scholar]
- 101.Ostrem JM, Peters U, Sos ML, Wells JA, Shokat KM. K-Ras (G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature. 2013 doi: 10.1038/nature12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Patgiri A, Yadav KK, Arora PS, Bar-Sagi D. An orthosteric inhibitor of the Ras–Sos interaction. Nat Chem Biol. 2011;7:585–587. doi: 10.1038/nchembio.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rosnizeck IC, Graf T, Spoerner M, Tränkle J, Filchtinski D, Herrmann C, Gremer L, Vetter IR, Wittinghofer A, König B, Kalbitzer HR. Stabilizing a weak binding state for effectors in the human ras protein by cyclen complexes. Angew Chem Int Ed. 2010;49:3830–3833. doi: 10.1002/anie.200907002. [DOI] [PubMed] [Google Scholar]
- 104.Sun Q, Burke JP, Phan J, Burns MC, Olejniczak ET, Waterson AG, Lee T, Rossanese OW, Fesik SW. Discovery of small molecules that bind to K-Ras and inhibit Sos-mediated activation. Angew Chem Int Ed Engl. 2012;51:6140–6143. doi: 10.1002/anie.201201358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hocker HJ, Cho K-J, Chen C-YK, Rambahal N, Sagineedu SR, Shaari K, Stanslas J, Hancock JF, Gorfe AA. Andrographolide derivative inhibit guanine nucleotide exchange and abrogate oncogenic Ras function. Proc Natl Acad Sci USA. 2013;110:10201–10206. doi: 10.1073/pnas.1300016110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Abankwa D, Gorfe AA, Hancock JF. Ras nanoclusters: molecular structure and assembly. Semin Cell Dev Biol. 2007;18:599–607. doi: 10.1016/j.semcdb.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Harding A, Hancock JF. Ras nanoclusters: combining digital and analog signaling. Cell Cycle. 2008;7:127–134. doi: 10.4161/cc.7.2.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.de Jong DH, Lopez CA, Marrink SJ. Molecular view on protein sorting into liquid-ordered membrane domains mediated by gangliosides and lipid anchors. Faraday Discuss. 2013;161:347–363. doi: 10.1039/c2fd20086d. [DOI] [PubMed] [Google Scholar]
- 109.Mazhab-Jafari MT, Marshall CB, Stathopulos PB, Kobashigawa Y, Stambolic V, Kay LE, Inagaki F, Ikura M. Membrane-dependent modulation of the mTOR activator Rheb: NMR observations of a GTPase tethered to a lipid-bilayer nanodisc. J Am Chem Soc. 2013;135:3367–3370. doi: 10.1021/ja312508w. [DOI] [PubMed] [Google Scholar]
- 110.Kapoor S, Triola G, Vetter IR, Erlkamp M, Waldmann H, Winter R. Revealing conformational substates of lipidated N-Ras protein by pressure modulation. Proc Natl Acad Sci USA. 2012;109:460–465. doi: 10.1073/pnas.1110553109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li Z, Gorfe AA. Deformation of a two-domain lipid bilayer due to asymmetric insertion of lipid-modified ras peptides. Soft Matter. 2013;9:11249–11256. doi: 10.1039/C3SM51388B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Guldenhaupt J, Rudack T, Bachler P, Mann D, Triola G, Waldmann H, Kotting C, Gerwert K. N-Ras forms dimers at POPC membranes. Biophys J. 2012;103:1585–1593. doi: 10.1016/j.bpj.2012.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li H, Gorfe AA. Aggregation of lipid-anchored full-length H-Ras in lipid bilayers: simulations with the MARTINI force field. PLoS One. 2013;8:e71018. doi: 10.1371/journal.pone.0071018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Grant BJ, McCammon JA, Gorfe AA. Conformational selection in G-proteins: lessons from Ras and Rho. Biophys J. 2010;99:L87–L89. doi: 10.1016/j.bpj.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gorfe AA, McCammon JA. Similar membrane affinity of mono-and Di-S-acylated ras membrane anchors: a new twist in the role of protein lipidation. J Am Chem Soc. 2008;130:12624–12625. doi: 10.1021/ja805110q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Abankwa D, Gorfe AA, Inder K, Hancock JF. Ras membrane orientation and nanodomain localization generate isoform diversity. Proc Natl Acad Sci USA. 2010;107:1130–1135. doi: 10.1073/pnas.0903907107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gorfe AA, Baron R, McCammon JA. Water-membrane partition thermodynamics of an amphiphilic lipopeptide: an enthalpy-driven hydrophobic effect. Biophys J. 2008;95:3269–3277. doi: 10.1529/biophysj.108.136481. [DOI] [PMC free article] [PubMed] [Google Scholar]


