Abstract
Clinical practice in the field of blood and marrow transplantation (BMT) has evolved over time, as a result of thousands of basic and clinical research studies. While it appears that scientific discovery and adaptive clinical research may be well integrated in case of BMT, there is lack of sufficient literature to definitively understand the process of translation of evidence to practice and if it may be selective . In this review, examples from BMT and other areas of medicine are used to highlight the state of and potential barriers to evidence uptake. Strategies to help improve knowledge transfer are discussed and the role of existing framework provided by Center for International Blood and Marrow Transplant Registry (CIBMTR) to monitor uptake and BMT Clinical Trials Network (BMT CTN) to enhance translation of evidence into practice is highlighted.
Keywords: Clinical trials, blood and marrow transplantation, practice patterns, evidence uptake, dissemination and implementation research
Introduction
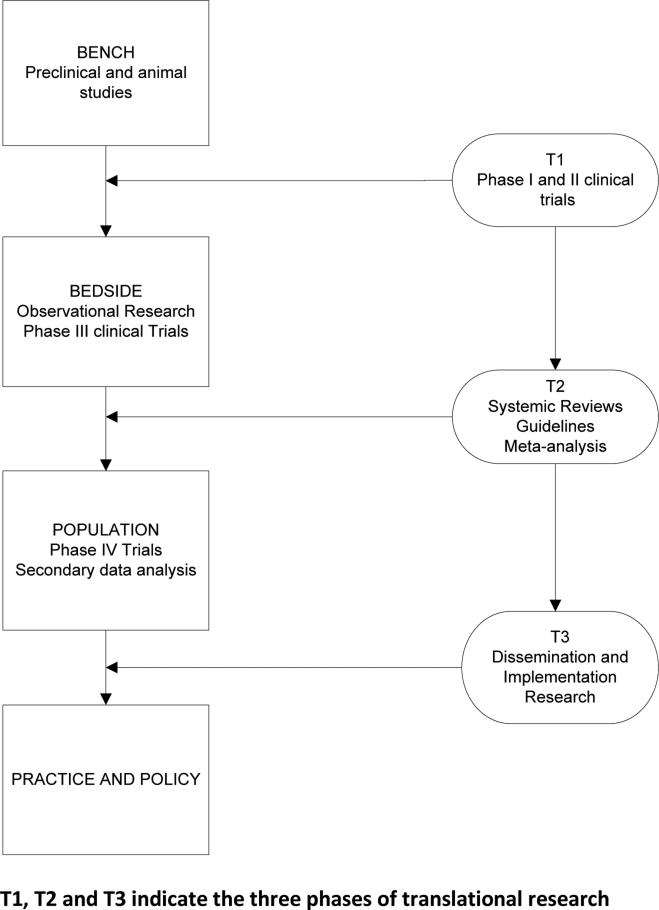
Clinical science advances by discovering new treatments and proving that they are better than what is currently available. Adoptions of results from clinical trials is unpredictable, and there are conflicting reports about whether the publication of results of randomized clinical trials (RCTs), even in high impact scientific journals, is enough to change practice patterns. Translation of research evidence into clinical practice requires a series of steps to help move knowledge gained from basic laboratory and clinical investigation to its application in clinical and community settings- the so-called ‘discovery-delivery continuum’. (Figure 1) One of the prerequisites for optimum dissemination and implementation of an intervention is that it be useful, appealing, and relevant to those who would apply it. There is no doubt that “if we want to advance evidence-based practice, we need more practice-based evidence.”1 Practice based evidence to meet the needs of all stakeholders in the process does not come solely from artificially controlled research, but is developed by synthesis of various evidence sources.
Figure 1.
Phases of translational research.
The field of blood and marrow transplantation (BMT) has evolved from bench to bedside, moving from the original studies in rodents and other primates in the early 1950s, first human transplants in 1957 to the current state of the science with haploidentical and cord blood transplants and genetically engineered immunotherapy. Although well-conducted, rigorous, multi-center RCTs in BMT may indicate scientific progress, they are resource intensive, expensive, difficult and time-consuming to conduct. The effort to conduct the trials may be wasted if they do not ultimately change clinical practice and advance the science which is not limited to academic centers alone. A survey in 2012 showed that least 20% of the BMT programs were not associated with a teaching hospital and only 44% programs were affiliated with a National Cancer Institute Comprehensive Cancer Center.2
This review aims to describe the current state of evidence uptake in the field of BMT. Since few studies assessing the process of dissemination and implementation of research findings to clinical practice have been conducted in BMT, a review of findings from other fields including oncology, critical care medicine and cardiology is presented. Challenges in the area of knowledge transfer that may be relevant to the practice of BMT are described. This review addresses three major questions relevant to evidence uptake in BMT: ‘How representative are trial patients of the entire patient population, and are the trial results generalizable to similar patients treated off protocol?’ ‘What is the impact of clinical trials on clinical practice patterns?’ ‘Are investigative sites/ researchers who help generate the evidence more likely to implement results of trials than non-investigative sites?’ For each of these questions, we review the theoretical principles behind the question, summarize relevant literature in other fields of medicine, and conclude with a discussion of relevance in the context of BMT. The review concludes by summarizing the steps which can help bridge the gap between evidence and practice in BMT.
How representative are trial patients of the entire patient population, and are the trial results generalizable to similar patients treated off protocol?
Research is important for advancing the practice of medicine, and RCTs are regarded as the gold standard in clinical research. In general, RCTs use rigorous experimental design with randomization to compare two or more treatment approaches in a selected group of patients. However, the process of applying the evidence generated from the RCTs to clinical care may highlight some limitations of clinical trials.3
It is not clear how representative trial participants are of ‘real world’ patients and whether results of the trials are applicable to individual patients seen in practice. If the general population of patients differs from trial participants, or the results of the treatment differ when given outside the trial, then the conclusions of clinical trials are not generalizable. The deviation from generalizability often starts with restrictive inclusion and exclusion criteria for the trial which gives rise to selection bias. Usually patients that are enrolled on trials are younger, with fewer comorbidities and a better performance status though they may or may not have a higher risk disease.4, 5 Patients from rural areas, from racial/ ethnic minorities such as African-Americans and Hispanics, and those with lower socioeconomic status are usually under-represented in trial populations due to a variety of reasons.6 It is possible, that better outcomes in patients enrolled in clinical trials compared to non-trial participants that have been reported by several studies are because of the recruitment of a relatively good prognostic group. 7-9 Absence of strategies to control for the potential confounding factors in the studies that compared trial and non-trial participants led Peppercorn et al to report that there is insufficient evidence to conclude there is a trial effect on outcomes.10 Some other studies and another large review also suggest that the outcomes of patients who participate in trials are comparable to similar patients treated in a similar fashion off-trial.10-13
In the last decade, many large trials performed by the BMT Clinical Trials Network (BMT CTN) represent landmark advances in the field of BMT. One of such studies: BMT CTN 0201 evaluated the efficacy of peripheral blood (PB) vs. bone marrow (BM) for unrelated donor (URD) transplants.14 We recently conducted a study comparing the outcomes of patients who were treated on this multicenter randomized study with other patients who appeared eligible and were treated in a similar fashion but off-study, using data from Center for International Blood and Marrow Transplant Registry (CIBMTR).15 In a pattern somewhat different from general oncology trials and studies in other areas of medicine, the trial participants and non-participants were found to be comparable in age, race/ ethnicity, disease distribution and comorbidities. Differences were seen in disease risk, performance status and conditioning regimen intensity. Survival was found to be comparable between study participants and non-participants, suggesting no trial effect. It is possible that one of the reasons we did not find any difference between the trial participants and non-participants may be that all BMT patients are highly selected regardless of trial participation. They undergo care and intensive follow-up in specialized settings with similar practice standards regulated by unique quality management systems like FACT (Foundation for accreditation of cellular therapy) in USA and Joint Accreditation Committee International Society for Cellular Therapy and the European Group for Blood and Marrow Transplantation (JACIE) in Europe. Implementation of standard operating procedures, definition of optimum infrastructure, equipment, release of products or services, responsibilities, training of personnel, acceptable criteria for admission and discharge etc. for accreditation can help improve outcomes of HCT in programs which adhere to the standards.16 However, whether accreditation by itself can help minimize the gap in outcomes between trial participants and non-participants will have to be confirmed by studies with this specific hypothesis. Such studies may also provide insight into whether patients treated at academic study centers have similar outcomes as those treated in a similar fashion at fully accredited non-study centers or community practices.
What is the impact of clinical trials on clinical practice patterns?
Delayed ‘knowledge transfer’ or ‘diffusion of innovation’ is one of the barriers to widespread practice of true evidence-based medicine. Failure to change practice according to the evidence provided by these trials can be expensive and harmful since it may perpetuate overutilization of ineffective care and underuse of beneficial interventions.
Similar to the controversial findings about the impact of trial participation on success of treatment, there is conflicting evidence about the impact of trial results on clinical practice. While some investigators have shown that clinical trial results leads to changes in practice, others do not support this finding.17-22 Even studies that have shown some impact on practice patterns emphasize the need for a better understanding of barriers to evidence uptake and strategies to augment the impact of clinical trials on clinical practice. Changing practice in response to evidence entails both adoption of positive experimental results and not adopting negative experimental results.
Adoption and diffusion of evidence in BMT has occurred not only in response to large clinical trials but also well conducted observational studies using registry data. Some important examples include the use of a prophylactic/ pre-emptive strategy for cytomegalovirus, ursodiol for prevention of sinusoidal obstruction syndrome, use of BM instead of PB as a graft source for HCT in aplastic anemia, cautious use of antithymocyte globulin with reduced intensity transplants in case of patients at high relapse risk and reduced intensity conditioning for patients ineligible for myeloablative transplants. There is also evidence that the practice in BMT has changed in response to negative studies. After publicity surrounding discovery of fraud in earlier publications and three RCTs in 1999 reporting negative results, there was an abrupt drop in the use of autologous HCT for breast cancer. Median time for hospitals to abandon this procedure was 15 months after the first publications raised questions about the procedure.23
More recently, there has been interest in evaluating the impact of BMT CTN protocol 0201 (PB stem cells versus BM from URD) on clinical practice. Results from the original study were presented at the plenary session of the 2011 American Society of Hematology (ASH) meetings and published in the New England Journal of Medicine in October 2012. A preliminary analysis of CIBMTR data for URD transplants for acute leukemia, myelodysplasia, chronic myeloid or myelomonocytic leukemia or myelofibrosis showed that the rate of using BM for URD HCT was 25% in 2010, as well as in 2013. (Personal communication, Dr. Mary Horowitz, CIBMTR) This is also supported by the data for type of grafts in each fiscal year from 2009 to 2014 from National Marrow Donor Program (NMDP) coordinating center as reported in table 1, kindly provided by Dr. Dennis Confer (Chief Medical Officer, NMDP). Based on the above information, it appears that the use of PB from URD continues to be preferred despite results from the RCT showing more chronic GVHD with PB than BM grafts. An ongoing detailed analysis using the CIBMTR data will provide more information on this topic. There are other large HCT studies whose results should have impacted practice patterns such as (1) the Cancer and Leukemia Group B (CALGB) 100104 trial showing improved survival with lenalidomide maintenance after autologous HCT for multiple myeloma; (2) BMT CTN 0102, showing similar outcomes between tandem autologous (standard of care) vs. autologous/allogeneic HCT (experimental arm) for multiple myeloma; and (3) BMT CTN 0501, showing similar outcomes between single vs. double cord in pediatric HCT.24-26 These multi-center trials were conducted over four to six years involving hundreds of patients and millions of dollars. The results of these studies call for adopting a practice with clinical benefit or abandoning practices with lack of clear-cut benefit, against the prevalent paradigm. Examining the trends in practice subsequent to the publication of the above studies may help decide if these studies were worth the investment, as would be reflected by change in practice patterns based on the trial results. It would also help trigger further analysis if we found that one or more of these studies resulted in no/minimal change in practice.
Table 1.
Proportion of graft source for non- cord blood unrelated donor hematopoietic cell transplantation (data obtained from Dr. Dennis Confer, National Marrow Donor Program).
| Fiscal Year | Bone marrow grafts | Peripheral Blood grafts |
|---|---|---|
| 2009 | 949 (25%) | 2815 (75%) |
| 2010 | 960 (24%) | 3115 (76%) |
| 2011 | 1035 (24%) | 3358 (76%) |
| 2012 | 1150 (25%) | 3492 (75%) |
| 2013 | 1292 (25%) | 3889 (75%) |
| 2014 | 1225 (23%) | 4068 (77%) |
Are investigative sites/ researchers more likely to implement results of trials than non-investigative sites?
One would expect that centers or investigators that conduct research and generate evidence would be the first to translate the knowledge into practice. Unfortunately, here again there is a conflict of findings. Some investigators report that adherence to guidelines or use of modalities used in trials was higher in practitioners who participated in those specific trials.27, 28 However, it is not clear if greater adherence to guidelines or more use of evidence based medicine translates into better overall outcomes of patients treated by investigators or institutions that take part in trials, in contrast to the patients treated elsewhere.29 Alternatively, there are others who have reported that being an investigative site involved in the trial does not lead to a difference in adoption of results.18, 30 This may be due to the scientific factors such as competing trials or designing new studies to improve prior results or ‘non-scientific’ factors such as financial barriers, inertia in changing current practice, logistic difficulties in adopting new information or patient demand/ expectations that may override the knowledge and awareness of published results.
This is an important question to ask in the current times when the practice of BMT is not solely restricted to academic centers, but is also present at programs that are purely clinical, akin to community practices in other areas of medicine. Even though the practices are highly regulated and usually follow standards of care, developing those standards would be subject to physicians’ biases and interpretation of literature. There may also be some differences in practice patterns between an academic and a community BMT practice which would influence the uptake of results from RCTs and possibly the outcomes.
While center effect is usually analyzed in studies conducted by BMT CTN, it is limited to the participating academic centers. The analysis that evaluated the impact of negative trials of autologous HCT for breast cancer showed that the rate of abandonment of the procedure was comparable between teaching hospitals and hospitals that participated in the phase III trials and nonteaching, nonparticipating hospitals.23
Barriers and facilitators to change
Three main factors have been shown to influence whether or not results from research studies change practice. They include attributes of the evidence, barriers and facilitators to practice change, and effectiveness of dissemination and implementation strategies.31 The relationship between perception of benefit with the innovation, the characteristics of the individuals who will adopt the innovation (ranging from innovators and early adopters to late adopters and laggards) and contextual/ systemic factors may also influence the diffusion of innovations.32 Research findings that require complex changes in clinical practice, changes in organization of care or may be contrary to prevalent values or beliefs may be more difficult to translate into a change. Sometimes, evidence offered by RCTs may not be applicable to ‘real-world’ patients because of selection bias in enrolling patients on studies or changes in techniques and technologies,33 thus rendering the results not applicable to most patients. In BMT, it is also possible that with the rapid advancement of the field, the findings from RCTs may not remain relevant to practice anymore by the time they are published. Table 2 outlines other barriers to application of clinical evidence.
Table 2.
Barriers to uptake of evidence.
| Intervention/ study design based | Use of surrogate end-points which may not be clinically relevant |
| Inconsistent clinical trial results | |
| Complex or costly intervention only available in academic centers or requiring high level of staff expertise | |
| Results not applicable to the general population because of selection bias in the trials | |
| Clinician based | Lack of knowledge of evidence |
| Lack of motivation/ Clinical inertia to change practice | |
| Disagreement with results | |
| Beliefs about lack of feasibility of the intervention | |
| Patient based | Preference/ expectations/ knowledge |
| System based | Intervention with difficult logistics |
| Payer influence/ lack of reimbursement | |
| Lack of incentive to change | |
| Limited availability of resources/ organizational support |
To illustrate these concepts in the context of BMT, we outline some hypotheses in the context of barriers for translation of evidence to practice for why the proportion of URD recipients given BM instead of PB has not increased yet:
-
a)
Intervention/ study design based barriers: The primary outcome measure i.e. survival was comparable between the two arms.
-
b)
System based barriers: Changing back to the practice of bone marrow harvests is complicated, has implications for use of hospital resources (operating room time etc.) and needs organizational support from multiple departments.
-
c)
Clinician and systems based barriers: The deleterious effects (i.e. chronic GVHD) after use of PB for transplant occur late, often after the patient has left the transplantation center, whereas the beneficial effects of earlier engraftment occur early.
-
d)
Clinician based barriers: There is evidence that other less complex approaches may achieve the same gains as the study intervention. Use of antithymocyte globulin that lowers the incidence of GVHD in matched unrelated HCT may decrease the risks of increased chronic GVHD seen with PB grafts in the trial making physicians use PB grafts still as their choice for URD transplants.34
-
e)
Intervention/ study design based barriers: Current transplant protocols may specify PB. Changing to BM requires protocol changes and new historical controls.
Additionally, it is helpful to identify characteristics of studies that were successfully disseminated and implemented rapidly. For example, Giordano et al have described the possible reasons for rapid practice change following presentation of the results of CALGB 9344 about use of taxanes for breast cancer. Positive factors include intensive media coverage, enthusiastic support by key oncologist leaders, credibility of the data coming from a rigorous multi-center randomized trial, and dissemination by pharmaceutical representatives.35 A similar example in BMT is the relatively rapid and complete abandonment of autologous HCT for breast cancer followed the negative studies by various investigators including the Philadelphia BMT group 36 , likely due to the following factors:
-
a)
There was an underlying skepticism about the positive results reported in the early trials, which were later proven to be falsified.
-
b)
All randomized studies showed a clear-cut lack of benefit for survival (which was the primary end-point) with autologous HCT.
-
c)
Results of the trials were publicized by lay media even prior to the ASCO meeting in 1999 (NBC news ran a story on March 9, 1999) which helped promote general awareness of the study results.
-
d)
Payers welcomed the trial results since they had been forced by public pressure, lawsuits and legislation to pay for the procedure despite the absence of clinical evidence.
Closing the gap between research and practice
Identifying the barriers to uptake of evidence is the first step towards improving knowledge transfer. The second step is to overcome these barriers and may include addressing the knowledge deficits, changing behaviors and developing multifaceted implementation schemes. Successful adoption of a new intervention requires a comprehensive and collaborative approach involving clinicians, researchers, patients and the health care system itself.
In BMT, many passive knowledge transfer strategies are used to communicate clinical trial results, though their impact on practice change has not been formally tested. Some of these tools include traditional didactics through continuing medical education lectures and clinical practice guidelines/ systematic evidence based reviews developed by American Society of Blood and Marrow Transplantation (ASBMT) that are periodically updated and are readily accessible online. However, in other fields of medicine, these tools are not effective by themselves, since lack of knowledge is not usually a primary determinant of gaps between research and practice.37
Efforts targeting individual and organizational behavior are more effective in changing practice patterns in other areas of medicine. Strategies such as use of media to influence different stakeholders, quality improvement programs, incentives and disincentives, and use of technology such as computerized reminders or ‘e-health’ approaches have modest efficacy, but they have not been applied or tested in the field of BMT. 38, 39 Engaging local opinion leaders to communicate the message has been shown to be a very effective strategy to change practice.40 This may be easily applicable to BMT. Berwick has summarized steps to increase the acceptance and diffusion of innovations within the framework of organizations which can be modified and be incorporated in the clinical trial design itself.32 These include finding sound innovations, supporting innovators and early adopters; increasing communication channels, allowing the change to be adapted locally if needed and lead by example. Of course, these strategies would have to be customized to specific barriers to be able to help implement evidence based practice in BMT.
In today's health care environment, one of the major contextual barriers to adoption of evidence includes issues related to the health care delivery system i.e. costs, insurance coverage and access to treatment. The Coverage with Evidence Development program is an initiative by the Centers for Medicare and Medicaid Services to use its power as a public insurer in promoting efforts for improving the adoption of evidence on critical clinical questions.41 In BMT, this program allows elderly patients who are Medicare beneficiaries with myelodysplastic syndromes and may not have been included in trials routinely, to get coverage while further evidence is developed in a study specifically evaluating this group. Results from such a study would be directly applicable to the older group of patients without any need for extrapolation from studies on younger patients and, therefore, may have a higher chance for being adopted.
Role of phase IV studies, clinical registries, population databases and qualitative research methods
As detailed in Figure 1, phase IV trials are an important component of the translational pathway and diffusion research. Phase IV trials are usually larger than phase III studies since they are population-based and designed to answer questions about effectiveness and “real world” utilization of interventions. They often make use of clinical registries and administrative databases and offer valuable information about the risks of adverse events in the general population which may be dissimilar from the trial population.42, 43 Administrative databases such as Surveillance, Epidemiology and End Results-Medicare (SEER-Medicare) also offer unique opportunities for examining the generalizability of trial results as well as impact on practice patterns following presentation or publication of large trials.35 44Combining SEER-Medicare with other commercial claims databases like MarketScan or Optum may allow evaluations of patterns of treatment before and after an intervention trial in a broader population. For example, a pharmacy claims data could be used to study if there is an increase in the use of lenalidomide for maintenance after autologous HCT in multiple myeloma since the results from two large studies were published in 2012.26, 45
A valuable resource for conducting studies to look at effectiveness rather than efficacy in BMT is the large clinical database maintained by CIBMTR. The registry function of CIBMTR provides a unique framework for monitoring uptake of new evidence and facilitating phase IV monitoring of new management strategies in BMT. Howard et al used patient-level data from 15,847 transplants reported to the CIBMTR between 1994 and 2005 to evaluate trends in autologous HCT for breast cancer. Another example of utilizing the CIBMTR database is the phase IV study, described earlier, assessing the population based effectiveness of BMT CTN 0201 study where we compared patients receiving PB vs. BM as graft source for unrelated donor transplants using the data from CIBMTR with trial participants.
Studies conducted using these databases can help us measure the practice change. Such studies can set the stage for dissemination and implementation research which includes identifying, understanding, and overcoming barriers to the adaptation (according to the local environment), adoption and integration of evidence based interventions and guidelines. This is especially relevant for interventions that have been shown to be efficacious, but uptake to date has been limited or significantly delayed. As a next step, qualitative research methods such as surveys, semi-structured interviews or focus groups with stakeholders can be used to help understand better the barriers to uptake of evidence e.g. whether they are related to the attributes of the evidence itself or other issues. They can help collect rich data to understand behavioral and attitudinal barriers that may be difficult to capture quantitatively.46
Enhancing clinical trial design and expanding research networks to improve knowledge transfer
It is important for researchers to consider the issues of dissemination and implementation during the development of the clinical trial itself. Discussion regarding generalizability of results has been included as quality indicator for RCT reporting within the CONSORT guidelines.47 Expanding the eligibility criteria for studies may allow for broader generalizations as well as increase access to trials. 48 However, this strategy would have to be considered on a case by case basis, as it may introduce heterogeneity and would be difficult to advocate for in the current times with increasing emphasis on individualizing treatment based on specific markers. 49 Knowing that simple interventions may lead to practice change more quickly than equally effective complex treatments may help investigators during the design stage of trials, especially if there is a choice between strategies of varying complexity. End-points are critically important. Use of surrogate end points including progression free survival may not be enough to convince many to change their practice. Studies using patient centered end-points can help influence practitioners more if positive results are observed. Such trials may be more labor intensive and expensive, but if they are more likely to inform practice, they may be worth the investment. A similar argument can be offered for pragmatic clinical trials to follow explanatory clinical trials to address the issue of selection bias and confounding with traditional RCTs. Even though they are expensive to conduct, they may help answer practical questions about the risks, benefits, and costs of an intervention as they would occur in routine clinical practice.50
Community-based participatory research (CBPR) is a collaborative research approach that brings together the communities affected by the issue being studied, representatives of organizations, and researchers in all aspects of the research process.51 It is an important tool to help increase the value of studies for both investigators and the patients being studied since it utilizes qualitative methods such as structured interviews and focus groups to identify and study research questions that are important to patients.52 While this has been used successfully in studies for diabetes and asthma control, it is not clear, if BMT as a highly specialized practice would lend itself to direct use of CBPR. The conceptual framework of CBPR still offers an attractive opportunity to help integrate the knowledge, perceptions and goals of practitioners from both academic and non-academic environments while designing multi-center clinical studies for BMT patients.
Expanding the clinical research network has been proposed as an important step towards increasing evidence uptake, especially if they involve community as well as academic centers.53 BMT CTN is a clinical research network in the field of BMT. It was established in October 2001 to conduct large multi-institutional clinical trials to address important issues in HCT and identify the best possible treatment approaches. This network has been instrumental in having 100 transplant centers with different characteristics and volumes as core and affiliate centers across the nation participate in the clinical trials related to HCT since its inception. Despite this, the scope of BMT CTN in enrolling patients from community programs is somewhat limited, especially, as compared to that of the cooperative group oncology trials which include researchers, cancer centers, and community physicians throughout the United States, Canada, and Europe. The trials conducted by BMT CTN are selected through a rigorous process of review by experts in the field including both researchers and clinicians. A prioritization process during the review helps select the most important trials—those that would really impact clinical practice and change the standard of care. In the current era of limited financial resources, it may also be helpful to have an outline of efforts for the dissemination and implementation created upfront to help make sure that the dollars spent on conducting these trials do not go waste. Table 3 presents a summary of approaches to enhance evidence based practice in BMT.
Table 3.
Approaches to enhance evidence based practice in BMT.
| Development of a broad outlook and need to synthesize different sources of evidence (RCTs, high quality observational studies, expert opinions) to understand the best clinical practice |
| Incorporating likelihood of adoption of the intervention into determination of impact if the primary endpoint is met |
| Further expansion of BMT CTN to include centers of different characteristics to help increase generalizability |
| Conducting effectiveness studies to evaluate the impact of therapies in the real world using CIBMTR database |
| Effective knowledge transfer strategies including use of lay media, traditional didactics at annual BMT meetings, development of quality improvement metrics and use of local pinion leaders |
| Understanding barriers and facilitators to optimum implementation of study results and plan to address them upfront in future studies |
Abbreviations: RCT, Randomized clinical trial; BMT CTN, Blood and Marrow Transplantation Clinical Trials Network; CIBMTR, Center for International Blood and Marrow transplant registry
Conclusions
In summary, it appears that the gap between knowledge and practice in BMT is similar to other areas of medicine, or in fact, may be better due to features unique to the practice of BMT. Until such information is definitively available, we have to rely on studies in other areas of medicine, where translation of research results into practice has been shown to be unpredictable and complicated, and multiple barriers to the process of knowledge transfer have been identified. In the current era, when informed decisions must be made in the setting of shrinking research funding, there is need for careful selection of high priority studies that will advance the field of BMT, which is highly specialized. Although there is scarce data to back it up, it would be safe to say that consideration of internal and external validity during clinical trial design phase, being able to measure change in practice using various data sources and having upfront plans for rapid dissemination and implementation of the study results will help improve translation of evidence to practice in BMT. The culmination of a study with presentation of its research findings at a meeting or its publication into a scientific journal should be seen as a beginning of the road to evidence based practice.
Practice Points.
Translation of research evidence into clinical practice is unpredictable, complex and inconsistent.
Administrative databases and registries can be used to monitor uptake of evidence.
Synthesis of information from various evidence sources can help determine relative efficacies and applicability of new therapies to individual patients.
There is a need for incorporation of principles of knowledge transfer into clinical trial design (e.g. use of definitive end-points, consideration of external validity and broader applicability of results).
It is important to consider a plan for dissemination of study results and/or clinical guidelines using multi-faceted interventions at the outset.
Research Agenda.
Need for high quality, methodologically robust studies to assess the impact of hematopoietic cell transplantation at academic, research intensive centers vs. others.
Assessing the generalizability of trial results to patients treated in a similar fashion (whether eligible or ineligible) and at centers other than study centers to establish the effectiveness (as opposed to efficacy) of interventions.
Examining the impact of evidence based reviews/ guidelines developed by ASBMT panel members based on synthesis of literature on practice patterns.
Application of qualitative research methods to understand the barriers in adoption of study results for the studies that do not change practice.
Acknowledgements
Special thanks to Dr. Stephanie J. Lee, Dr. Mary Horowitz and Dr. Dennis Confer for their valuable guidance and input.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
There are no potential conflicts of interest.
References
- 1.Green LW, Glasgow RE. Evaluating the Relevance, Generalization, and Applicability of Research: Issues in External Validation and Translation Methodology. Evaluation & the Health Professions. 2006;29(1):126–153. doi: 10.1177/0163278705284445. [DOI] [PubMed] [Google Scholar]
- 2.Majhail N. [February 27, 2015];CIBMTR Slides and Reports. http://www.cibmtr.org/ReferenceCenter/SlidesReports/Documents/CIBMTR_Center_Survey_Report.pdf.
- 3.Naylor CD. Grey zones of clinical practice: some limits to evidence-based medicine. The Lancet. 1995;345(8953):840–842. doi: 10.1016/s0140-6736(95)92969-x. [DOI] [PubMed] [Google Scholar]
- 4.Elting LS, Cooksley C, Bekele BN, Frumovitz M, Avritscher EB, Sun C, et al. Generalizability of cancer clinical trial results: prognostic differences between participants and nonparticipants. Cancer. 2006;106(11):2452–8. doi: 10.1002/cncr.21907. [DOI] [PubMed] [Google Scholar]
- 5.van de Water W, Kiderlen M, Bastiaannet E, Siesling S, Westendorp RGJ, van de Velde CJH, et al. External Validity of a Trial Comprised of Elderly Patients With Hormone Receptor–Positive Breast Cancer. J Natl Cancer Inst. 2014;106(4) doi: 10.1093/jnci/dju051. [DOI] [PubMed] [Google Scholar]
- 6.Ford JG, Howerton MW, Lai GY, Gary TL, Bolen S, Gibbons MC, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: A systematic review. Cancer. 2008;112(2):228–242. doi: 10.1002/cncr.23157. [DOI] [PubMed] [Google Scholar]
- 7.Braunholtz DA, Edwards SJ, Lilford RJ. Are randomized clinical trials good for us (in the short term)? Evidence for a “trial effect”. J Clin Epidemiol. 2001;54(3):217–24. doi: 10.1016/s0895-4356(00)00305-x. [DOI] [PubMed] [Google Scholar]
- 8.Davis S, Wright PW, Schulman SF, Hill LD, Pinkham RD, Johnson LP, et al. Participants in prospective, randomized clinical trials for resected non-small cell lung cancer have improved survival compared with nonparticipants in such trials. Cancer. 1985;56(7):1710–8. doi: 10.1002/1097-0142(19851001)56:7<1710::aid-cncr2820560741>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 9.Fossa SD, Skovlund E. Selection of patients may limit the generalizability of results from cancer trials. Acta Oncol. 2002;41(2):131–7. doi: 10.1080/028418602753669490. [DOI] [PubMed] [Google Scholar]
- 10.Peppercorn JM, Weeks JC, Cook EF, Joffe S. Comparison of outcomes in cancer patients treated within and outside clinical trials: conceptual framework and structured review. Lancet. 2004;363(9405):263–70. doi: 10.1016/S0140-6736(03)15383-4. [DOI] [PubMed] [Google Scholar]
- 11.Mengis C, Aebi S, Tobler A, Dahler W, Fey MF. Assessment of differences in patient populations selected for excluded from participation in clinical phase III acute myelogenous leukemia trials. J Clin Oncol. 2003;21(21):3933–9. doi: 10.1200/JCO.2003.03.186. [DOI] [PubMed] [Google Scholar]
- 12.Stiller CA, Benjamin S, Cartwright RA, Clough JV, Gorst DW, Kroll ME, et al. Patterns of care and survival for adolescents and young adults with acute leukaemia--a population-based study. Br J Cancer. 1999;79(3-4):658–65. doi: 10.1038/sj.bjc.6690104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vist GE, Bryant D, Somerville L, Birminghem T, Oxman AD. Outcomes of patients who participate in randomized controlled trials compared to similar patients receiving similar interventions who do not participate. Cochrane Database Syst Rev. 2008;(3):MR000009. doi: 10.1002/14651858.MR000009.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anasetti C, Logan BR, Lee SJ, Waller EK, Weisdorf DJ, Wingard JR, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. The New England journal of medicine. 2012;367(16):1487–96. doi: 10.1056/NEJMoa1203517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khera N, Majhail NS, Brazauskas R, Wang Z, Aljurf M, Akpek G, et al. American Society of Hematology Meeting 2014. San Francisco: 2014. Do Hematopoietic Cell Transplant Patients Treated on a Clinical Trial Do Better? Comparison of Characteristics and Outcomes of Patients Enrolled Versus Not Enrolled on Blood and Marrow Transplant Clinical Trials Network (BMT-CTN) 0201 Trial. pp. 209–209. [Google Scholar]
- 16.Gratwohl A, Brand R, McGrath E, van Biezen A, Sureda A, Ljungman P. Use of the quality management system “JACIE” and outcome after hematopoietic stem cell transplantation. 2014;99 doi: 10.3324/haematol.2013.096461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamas GA, Pfeffer MA, Hamm P, Wertheimer J, Rouleau JL, Braunwald E. Do the results of randomized clinical trials of cardiovascular drugs influence medical practice? The SAVE Investigators. The New England journal of medicine. 1992;327(4):241–7. doi: 10.1056/NEJM199207233270405. [DOI] [PubMed] [Google Scholar]
- 18.Omoigui NA, Silver MJ, Rybicki LA, Rosenthal M, Berdan LG, Pieper K, et al. Influence of a Randomized Clinical Trial on Practice by Participating Investigators: Lessons From the Coronary Angioplasty Versus Excisional Atherectomy Trial (CAVEAT) fn1. Journal of the American College of Cardiology. 1998;31(2):265–272. doi: 10.1016/s0735-1097(97)00498-1. [DOI] [PubMed] [Google Scholar]
- 19.McKenna CJ, Codd MB, McCann HA, Sugrue DD. International trials and national practice: A questionnaire survey of current physician practice in the treatment of acute myocardial infarction. Ir J Med Sci. 1996;165(3):157–158. doi: 10.1007/BF02940240. [DOI] [PubMed] [Google Scholar]
- 20.Yen TWF, Kuerer HM, Ottesen RA, Rouse L, Niland JC, Edge SB, et al. Impact of Randomized Clinical Trial Results in the National Comprehensive Cancer Network on the Use of Tamoxifen After Breast Surgery for Ductal Carcinoma in Situ. Journal of Clinical Oncology. 2007;25(22):3251–3258. doi: 10.1200/JCO.2006.10.2699. [DOI] [PubMed] [Google Scholar]
- 21.Ketley D, Woods KL. Impact of clinical trials on clinical practice: example of thrombolysis for acute myocardial infarction. The Lancet. 1993;342(8876):891–894. doi: 10.1016/0140-6736(93)91945-i. [DOI] [PubMed] [Google Scholar]
- 22.Stafford RS, Furberg CD, Finkelstein SN, Cockburn IM, Alehegn T, Ma J. Impact of clinical trial results on national trends in alpha-blocker prescribing, 1996-2002. JAMA : the journal of the American Medical Association. 2004;291(1):54–62. doi: 10.1001/jama.291.1.54. [DOI] [PubMed] [Google Scholar]
- 23.Howard DH, Kenline C, Lazarus HM, LeMaistre CF, Maziarz RT, McCarthy PL, Jr, et al. Abandonment of High-Dose Chemotherapy/Hematopoietic Cell Transplants for Breast Cancer Following Negative Trial Results. Health Services Research. 2011;46(6pt1):1762–1777. doi: 10.1111/j.1475-6773.2011.01296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krishnan A, Pasquini MC, Logan B, Stadtmauer EA, Vesole DH, Alyea E, 3rd, et al. Autologous haemopoietic stem-cell transplantation followed by allogeneic or autologous haemopoietic stem-cell transplantation in patients with multiple myeloma (BMT CTN 0102): a phase 3 biological assignment trial. Lancet Oncol. 2011;12(13):1195–203. doi: 10.1016/S1470-2045(11)70243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wagner JE, Eapen M, Carter S, Wang Y, Schultz KR, Wall DA, et al. One-Unit versus Two-Unit Cord-Blood Transplantation for Hematologic Cancers. New England Journal of Medicine. 2014;371(18):1685–1694. doi: 10.1056/NEJMoa1405584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCarthy PL, Owzar K, Hofmeister CC, Hurd DD, Hassoun H, Richardson PG, et al. Lenalidomide after stem-cell transplantation for multiple myeloma. The New England journal of medicine. 2012;366(19):1770–81. doi: 10.1056/NEJMoa1114083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meineche-Schmidt V, Hvenegaard A, Juhl HH. Participation in a clinical trial influences the future management of patients with gastro-oesophageal reflux disease in general practice. Aliment Pharmacol Ther. 2006;24(7):1117–25. doi: 10.1111/j.1365-2036.2006.03046.x. [DOI] [PubMed] [Google Scholar]
- 28.Das D, Ishaq S, Harrison R, Kosuri K, Harper E, deCaestecker J, et al. Management of Barrett's Esophagus in the UK: Overtreated and Underbiopsied but Improved by the Introduction of a National Randomized Trial. Am J Gastroenterol. 2008;103(5):1079–1089. doi: 10.1111/j.1572-0241.2008.01790.x. [DOI] [PubMed] [Google Scholar]
- 29.Clarke M, Loudon K. Effects on patients of their healthcare practitioner's or institution's participation in clinical trials: a systematic review. Trials. 2011;12:16. doi: 10.1186/1745-6215-12-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Majumdar SR, Chang WC, Armstrong PW. Do the investigative sites that take part in a positive clinical trial translate that evidence into practice? Am J Med. 2002;113(2):140–5. doi: 10.1016/s0002-9343(02)01166-x. [DOI] [PubMed] [Google Scholar]
- 31.Grol R, Grimshaw J. From best evidence to best practice: effective implementation of change in patients’ care. Lancet. 2003;362(9391):1225–30. doi: 10.1016/S0140-6736(03)14546-1. [DOI] [PubMed] [Google Scholar]
- 32.Berwick DM. Disseminating innovations in health care. JAMA. 2003;289(15):1969–75. doi: 10.1001/jama.289.15.1969. [DOI] [PubMed] [Google Scholar]
- 33.Gale RP, Eapen M, Logan B, Zhang MJ, Lazarus HM. Are there roles for observational database studies and structured quantification of expert opinion to answer therapy controversies in transplants? Bone marrow transplantation. 2009;43(6):435–46. doi: 10.1038/bmt.2008.447. [DOI] [PubMed] [Google Scholar]
- 34.Finke J, Bethge WA, Schmoor C, Ottinger HD, Stelljes M, Zander AR, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009;10(9):855–64. doi: 10.1016/S1470-2045(09)70225-6. [DOI] [PubMed] [Google Scholar]
- 35.Giordano SH, Duan Z, Kuo YF, Hortobagyi GN, Freeman J, Goodwin JS. Impact of a scientific presentation on community treatment patterns for primary breast cancer. J Natl Cancer Inst. 2006;98(6):382–8. doi: 10.1093/jnci/djj090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stadtmauer EA, O'Neill A, Goldstein LJ, Crilley PA, Mangan KF, Ingle JN, et al. Conventional-Dose Chemotherapy Compared with High-Dose Chemotherapy plus Autologous Hematopoietic Stem-Cell Transplantation for Metastatic Breast Cancer. New England Journal of Medicine. 2000;342(15):1069–1076. doi: 10.1056/NEJM200004133421501. [DOI] [PubMed] [Google Scholar]
- 37.Grimshaw JM, Thomas RE, MacLennan G, Fraser C, Ramsay CR, Vale L, et al. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technol Assess. 2004;8(6):iii–iv. 1–72. doi: 10.3310/hta8060. [DOI] [PubMed] [Google Scholar]
- 38.Pronovost P, Needham D, Berenholtz S, Sinopoli D, Chu H, Cosgrove S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. The New England journal of medicine. 2006;355(26):2725–32. doi: 10.1056/NEJMoa061115. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen HB, Lynch EL, Mou JA, Lyon K, Wittlake WA, Corbett SW. The utility of a quality improvement bundle in bridging the gap between research and standard care in the management of severe sepsis and septic shock in the emergency department. Acad Emerg Med. 2007;14(11):1079–86. doi: 10.1197/j.aem.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 40.Soumerai SB, McLaughlin TJ, Gurwitz JH, Guadagnoli E, Hauptman PJ, Borbas C, et al. Effect of local medical opinion leaders on quality of care for acute myocardial infarction: a randomized controlled trial. JAMA. 1998;279(17):1358–63. doi: 10.1001/jama.279.17.1358. [DOI] [PubMed] [Google Scholar]
- 41.Pearson SD, Miller FG, Emanuel EJ. Medicare's requirement for research participation as a condition of coverage: Is it ethical? JAMA. 2006;296(8):988–991. doi: 10.1001/jama.296.8.988. [DOI] [PubMed] [Google Scholar]
- 42.Abraham NS, Hartman C, Richardson P, Castillo D, Street RL, Naik AD. Risk of Lower and Upper Gastrointestinal Bleeding, Transfusions, and Hospitalizations With Complex Antithrombotic Therapy in Elderly Patients. Circulation. 2013;128(17):1869–1877. doi: 10.1161/CIRCULATIONAHA.113.004747. [DOI] [PubMed] [Google Scholar]
- 43.Laterre PF, Nelson DR, Macias W, Abraham E, Sashegyi A, Williams MD, et al. International integrated database for the evaluation of severe sepsis and drotrecogin alfa (activated) therapy: 28-day survival and safety. J Crit Care. 2007;22(2):142–52. doi: 10.1016/j.jcrc.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 44.Lamont EB, Schilsky RL, He Y, Muss H, Cohen HJ, Hurria A, et al. Generalizability of Trial Results to Elderly Medicare Patients With Advanced Solid Tumors (Alliance 70802). J Natl Cancer Inst. 2015;107(1) doi: 10.1093/jnci/dju336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Attal M, Lauwers-Cances V, Marit G, Caillot D, Moreau P, Facon T, et al. Lenalidomide Maintenance after Stem-Cell Transplantation for Multiple Myeloma. New England Journal of Medicine. 2012;366(19):1782–1791. doi: 10.1056/NEJMoa1114138. [DOI] [PubMed] [Google Scholar]
- 46.Creswell J PC V. Designing and conducting mixed methods research. 2nd Edition Sage Publications; Thousand Oaks, CA: 2011. [Google Scholar]
- 47.Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet. 2001;357(9263):1191–4. [PubMed] [Google Scholar]
- 48.George SL. Reducing patient eligibility criteria in cancer clinical trials. Journal of Clinical Oncology. 1996;14(4):1364–70. doi: 10.1200/JCO.1996.14.4.1364. [DOI] [PubMed] [Google Scholar]
- 49.Unger JM, Barlow WE, Martin DP, Ramsey SD, LeBlanc M, Etzioni R, et al. Comparison of Survival Outcomes Among Cancer Patients Treated In and Out of Clinical Trials. J Natl Cancer Inst. 2014;106(3) doi: 10.1093/jnci/dju002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tunis SR, Stryer DB, Clancy CM. Practical clinical trials: Increasing the value of clinical research for decision making in clinical and health policy. JAMA. 2003;290(12):1624–1632. doi: 10.1001/jama.290.12.1624. [DOI] [PubMed] [Google Scholar]
- 51.Viswanathan M, Ammerman A, Eng E, Garlehner G, Lohr KN, Griffith D, et al. Community-Based Participatory Research: Assessing the Evidence: Summary. AHRQ Evidence Report Summaries. Rockville (MD): Agency for Healthcare Research and Quality (US); 1998-2005. 2004 [PMC free article] [PubMed] [Google Scholar]
- 52.O'Toole TP, Aaron KF, Chin MH, Horowitz C, Tyson F. Community-based Participatory Research. J Gen Intern Med. 2003;18(7):592–594. doi: 10.1046/j.1525-1497.2003.30416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kahn JM. Disseminating clinical trial results in critical care. Crit Care Med. 2009;37(1 Suppl):S147–53. doi: 10.1097/CCM.0b013e3181920fa3. [DOI] [PubMed] [Google Scholar]