A 46-year-old man was referred by an ophthalmologist to an ophthalmology clinic in a tertiary care hospital for severe bilateral corneal edema. He presented with painless, progressive loss of visual acuity over the course of three months. His ocular and family history was unremarkable, with previous 20/20 best-corrected visual acuity in each eye. The patient had a history of depression, vasculopathy (i.e., erectile dysfunction, dyslipidemia and pulmonary embolism), type 2 diabetes mellitus, osteoarthritis, irritable bowel syndrome and sleep apnea. At the time of presentation, he was taking bupropion, metformin, rosuvastatin, clomiphene, amantadine, niacin, warfarin, zopiclone and ketotifen.
At the time of his initial consultation (day 1), his best-corrected visual acuity had deteriorated to 20/60 in each eye (timeline in Appendix 1, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.140542/-/DC1). He had been prescribed fluorometholone drops four times daily by the referring ophthalmologist for inflammation; the drops were stopped one week before his visit. On slit-lamp examination, moderate bilateral corneal edema with Descemet folds was seen (Figure 1). Pachymetry and anterior segment optical coherence tomography showed increased central corneal thickness (Figure 2). Of note was the very central location of the edema in each eye and an unaffected peripheral cornea. The rest of the examination was unremarkable.
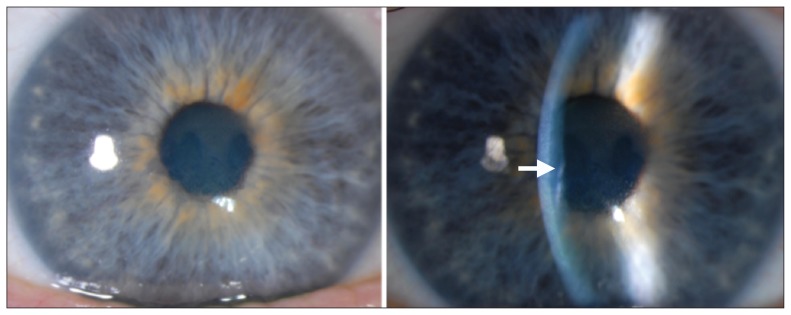
Figure 1:
Slit-lamp biomicroscopy images showing moderate central corneal edema in the right eye (also seen in the left eye) of a 46-year-old man with progressive bilateral vision loss. Descemet folds (arrow), seen as small wrinkles in the posterior cornea, suggest corneal edema.
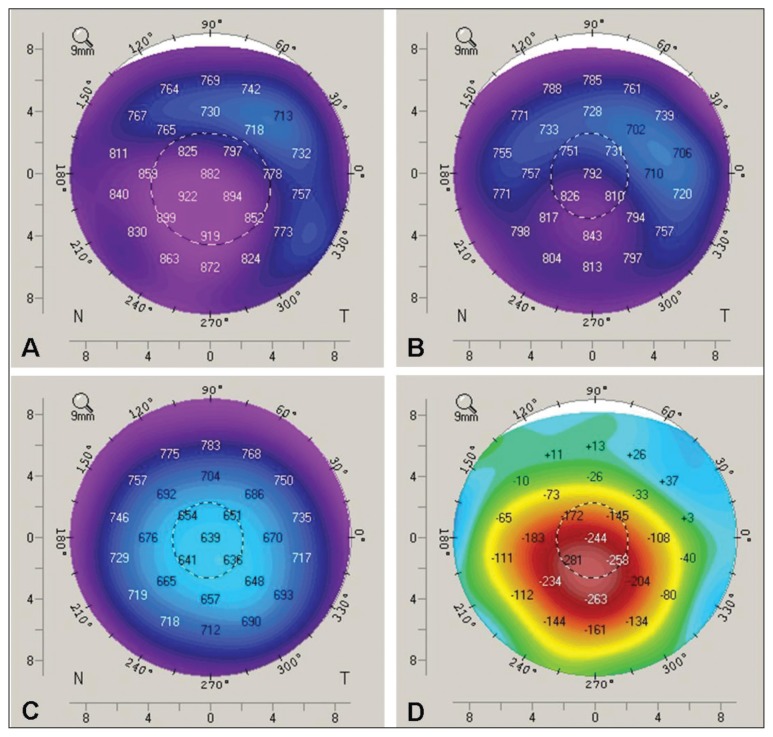
Figure 2:
Pachymetry results showing corneal thickness in the left eye (A) before, (B) one week after and (C) four months after amantadine was discontinued. The colours correspond to the range of corneal thickness. Normal cornea thickness is about 550 μm (green to blue) with the thinnest area in the centre. The purple colour centrally in A and B indicates increased corneal thickness. The images show resolving corneal edema over time after amantadine was stopped. Image D shows the difference in corneal thickness between time points A and C.
We prescribed acyclovir 400 mg orally five times daily, loteprednol 0.5% drops four times daily (a weak steroid) and sodium chloride ointment nightly for possible bilateral herpetic endotheliitis.
On day 7, the loteprednol drops were replaced by a stronger steroid, prednisolone acetate 0.5% drops four times daily, because the patient had not experienced substantial improvement in vision. On day 28, his intraocular pressure had increased, with no other important changes found on examination of his eyes. This increase was likely secondary to the use of steroid drops and was first treated with dorzolamide–timolol drops and later with travoprost brimonidine 0.2%–timolol drops (to lower intraocular pressure).
Puzzled by the etiology of this corneal edema and lack of response to treatment, we reviewed the patient’s medical and medication history. He had been taking oral amantadine for three years (200 mg/d). He had a 19-year history of treatment-resistant depression, and amantadine had been added as an augmentative agent.
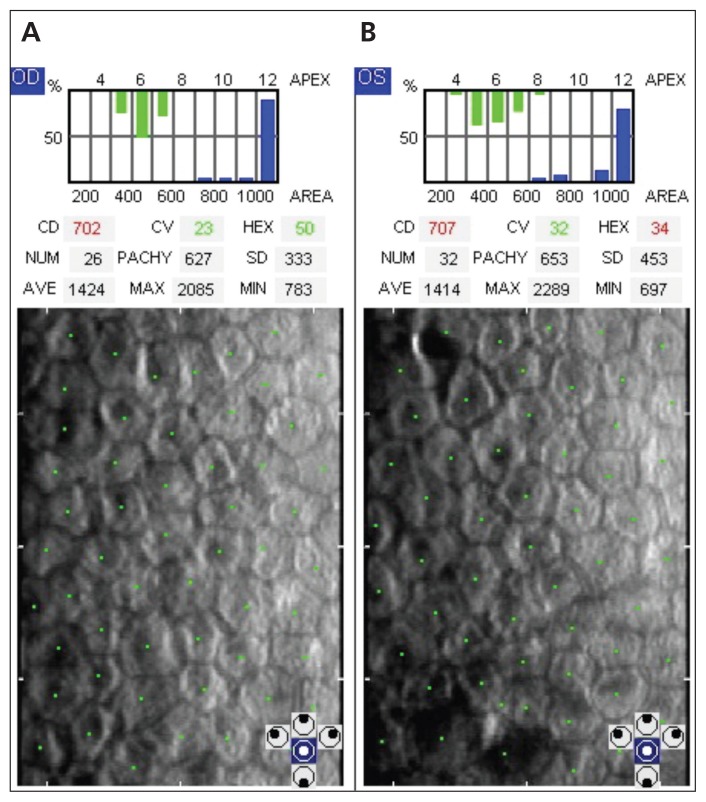
After discussion with the patient’s psychiatrist, amantadine was stopped on day 38. On day 44, corneal thickness had decreased. By day 92, the corneal edema had resolved, with improvement of best-corrected visual acuity to 20/20 in each eye and clear corneas on slit-lamp examination (Figure 3). Intraocular pressure had stabilized within the normal range by day 120. Pachymetry was repeated four months after amantadine was stopped, and corneal thickness had stabilized within the normal range (Figure 2C). Specular microscopy showed low endothelial cell density in both eyes at five-month follow-up (right eye: 702 cells/mm2; left eye: 707 cells/mm2) (Figure 4; Appendix 2, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.140542/-/DC1).
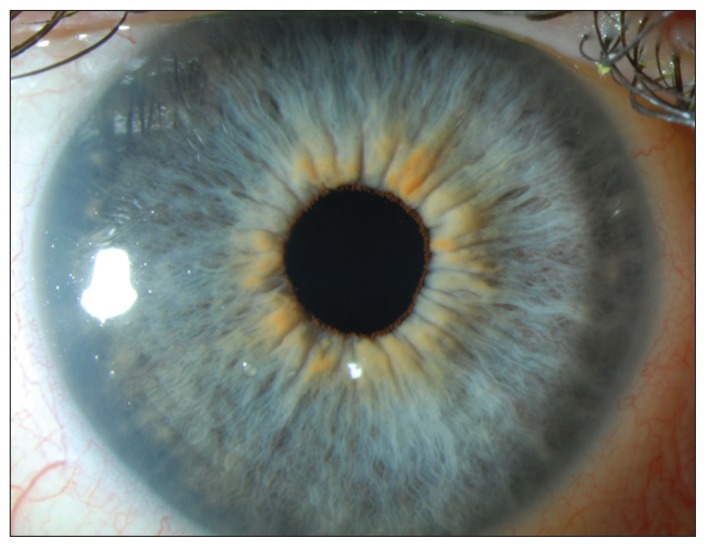
Figure 3:
Slit-lamp biomicroscopy image of the patient’s right eye showing a clear cornea, four months after discontinuation of amantadine. The bilateral edema resolved within six weeks after amantadine was stopped.
Figure 4:
Specular microscopy images showing the corneal endothelium, with each green dot corresponding to an individual cell. Decreased endothelial cell density is seen in the (A) right (702 cells/mm2) and (B) left eye (707 cells/mm2) at five-month follow-up. The normal cell density for this patient’s age group is between 2300 and 3100 cells/mm2.
Discussion
Amantadine hydrochloride is a dopaminergic agonist originally developed for prevention and treatment of influenza A.1 It has a variety of effects on the brain, influencing dopamine, monoamine oxidase and N-methyl-d-aspartate receptors, and has been investigated for several neuropsychiatric conditions.2 It is indicated as a treatment for Parkinson disease, as well as for drug-induced extrapyramidal adverse effects.1 Off-label uses include treatment of attention-deficit/hyperactivity disorder and fatigue associated with multiple sclerosis, and more recently as an augmentative agent for treatment-resistant depression.2 Ocular adverse effects are rare (< 1%) and include corneal edema,3–9 visual disturbances secondary to punctate subepithelial opacities, oculogyric crises, keratitis and mydriasis.3
Causes of corneal edema
The list of possible causes of bilateral edema is extensive. The most common cause in this patient’s age group is endothelial disorders, in particular Fuchs endothelial dystrophy. Other causes include pseudophakic bullous keratopathy, herpetic endotheliitis, trauma, and medications or toxins.5
Given the patient’s ocular history, we first suspected herpetic endotheliitis. Medications were reviewed for corneal toxicity, after the conditions failed to respond to initial steroid treatment. On review of the literature, we found that most cases of corneal edema secondary to amantadine use present with progressive visual loss within weeks to months of starting amantadine, are not dose-dependent and are reversible within weeks on discontinuation of therapy, with some exceptions.3,8 In one report, resuming amantadine use led to acute recurrence of corneal edema, which suggests a causal relation.5 Our patient had been taking amantadine for more than three years before corneal edema developed.
Interestingly, our patient was taking bupropion at the same time, another dopamine agonist associated with reversible corneal edema.10 Recently, a study from the US Food and Drug Administration suggested that 0.03% of reported adverse effects from bupropion were corneal edema and that, of those cases, amantadine was the top drug being taken concurrently (6/14 patients).10 It is possible that the two drugs may have been synergistic in causing corneal edema.
Pathophysiology
The pathophysiology of amantadine-induced corneal edema is unclear. The mechanism likely involves endothelial damage, based on specular microscopy and histopathologic findings showing loss of endothelial cells in the affected cornea reported in other cases.3,6,8 The toxic effect of amantadine on endothelium was seen in one report showing atrophy of the endothelial layer, as well as rare endothelial cells on light microscopy.3 The endothelial cell density in our patient measured at five-month follow-up was low with enlargement of remaining cells, suggesting permanent loss (Figure 4). We do not have a baseline measurement of endothelial cell density for this patient; however, the ocular and family ocular history was unremarkable, with no reason to suggest pre-existing endothelial damage.
In 2007, a database review involving 5.8 million patients taking amantadine showed that corneal edema or Fuchs endothelial corneal dystrophy was diagnosed in 0.27% of patients within two years of starting the medication, indicating a relative risk of 1.7.1 As most of these cases had been diagnosed as idiopathic corneal edema or Fuchs endothelial corneal dystrophy, a failure to associate the onset with drug use is evident, which leads us to believe that the complication may be underreported.
Initial assessment
Patients presenting to a primary care physician’s office with progressive bilateral visual loss require an initial visual assessment, including visual acuity (with and without pinhole correction), confrontational visual fields, red reflex and relative afferent pupillary defect (swinging light test) to document the patient’s baseline visual status. Bilateral visual loss can be caused by refractive error, media opacity (e.g., cornea, lens, anterior chamber, vitreous), retinal or optic nerve damage, or neurologic deficits.
Patients with corneal edema may have a loss of corneal clarity with a decreased best-corrected visual acuity that does not substantially improve with pinhole correction. Their posterior examination will be intact with a preserved red reflex and the absence of an afferent pupillary defect. Patients with unexplained bilateral visual loss that does not return to normal with pinhole correction require a thorough medication review and prompt referral to ophthalmology.
Conclusion
We highlight a rare ocular adverse effect associated with a common neuropsychiatric drug. The complication may be underreported and, if left unrecognized, may lead to irreversible endothelial damage. It is important to consider medications as part of the differential diagnosis in patients presenting with bilateral corneal edema causing progressive visual acuity loss, especially if they are taking amantadine and do not respond to medical treatments.
Key points
Bilateral corneal edema is a rare adverse effect of amantadine, a commonly prescribed neuropsychiatric drug.
Corneal edema secondary to amantadine use may have a delayed presentation; damage may be irreversible if left unrecognized.
Current medications should be carefully reviewed in patients with acute bilateral visual loss, especially in those taking amantadine.
The section Cases presents brief case reports that convey clear, practical lessons. Preference is given to common presentations of important rare conditions, and important unusual presentations of common problems. Articles start with a case presentation (500 words maximum), and a discussion of the underlying condition follows (1000 words maximum). Visual elements (e.g., tables of the differential diagnosis, clinical features or diagnostic approach) are encouraged. Consent from patients for publication of their story is a necessity. See information for authors at www.cmaj.ca.
Acknowledgements
The authors thank Bruce Jackson and Ronan Conlon for their expertise in editing the manuscript.
Footnotes
Competing interests: Kashif Baig receives grant and research support from Allergan, Bausch & Lomb, Merck and Moria; and employment, honoraria, consulting fees and travel expenses from Alcon, Allergan, Bausch & Lomb and Labtician; and is a member of an advisory panel, standing committee and board of directors of Allergan and Bausch & Lomb. No other competing interests were declared.
This article has been peer reviewed.
The authors have obtained patient consent.
Contributors: Yelin Yang and Salina Teja drafted the manuscript. Kashif Baig was responsible for patient care and contributed to revisions of the manuscript. All of the authors gave final approval of the version to be published and agreed to act as guarantors of the work.
References
- 1.French DD, Margo CE. Postmarketing surveillance of corneal edema, Fuchs dystrophy, and amantadine use in the Veterans Health Administration. Cornea 2007;26:1087–9. [DOI] [PubMed] [Google Scholar]
- 2.Stryjer R, Strous RD, Shaked G, et al. Amantadine as augmentation therapy in the management of treatment-resistant depression. Int Clin Psychopharmacol 2003;18:93–6. [DOI] [PubMed] [Google Scholar]
- 3.Jeng BH, Galor A, Lee MS, et al. Amantadine-associated corneal edema potentially irreversible even after cessation of the medication. Ophthalmology 2008;115:1540–4. [DOI] [PubMed] [Google Scholar]
- 4.Blanchard DL. Amantadine caused corneal edema. Cornea 1990;9:181. [DOI] [PubMed] [Google Scholar]
- 5.Chang KC, Kim MK, Wee WR, et al. Corneal endothelial dysfunction associated with amantadine toxicity. Cornea 2008;27:1182–5. [DOI] [PubMed] [Google Scholar]
- 6.Hotehama A, Mimura T, Kawashima H, et al. Sudden onset of amantadine-induced reversible bilateral corneal edema in an elderly patient: case report and literature review. Jpn J Ophthalmol 2011;55:71–4. [DOI] [PubMed] [Google Scholar]
- 7.Hughes B, Feiz V, Flynn SB, et al. Reversible amantadine induced corneal edema in an adolescent. Cornea 2004;23:823–4. [DOI] [PubMed] [Google Scholar]
- 8.Koenig SB, McDermott ML, Simons KB. Nonimmunologic graft failure after Descemet’s Stripping Automated Endothelial Keratoplasty (DSAEK) for presumed amantadine-induced corneal edema. Eye Contact Lens 2009;35:209–11. [DOI] [PubMed] [Google Scholar]
- 9.Kubo S, Iwatake A, Ebihara N, et al. Visual impairment in Parkinson’s disease treated with amantadine: case report and review of the literature. Parkinsonism Relat Disord 2008;14:166–9. [DOI] [PubMed] [Google Scholar]
- 10.EHealthMe study from FDA and social media reports. Available: www.ehealthme.com/print/ds16571140 (accessed 2014 Mar. 1).