Abstract
Background:
Heart rate and heart rate variability, markers of cardiac autonomic function, have been linked with cardiovascular disease. We investigated whether heart rate and heart rate variability are associated with functional status in older adults, independent of cardiovascular disease.
Methods:
We obtained data from the Prospective Study of Pravastatin in the Elderly at Risk (PROSPER). A total of 5042 participants were included in the present study, and mean follow-up was 3.2 years. Heart rate and heart rate variability were derived from baseline 10-second electrocardiograms. Heart rate variability was defined as the standard deviation of normal-to-normal RR intervals (SDNN). Functional status in basic (ADL) and instrumental (IADL) activities of daily living was measured using Barthel and Lawton scales, at baseline and during follow-up.
Results:
The mean age of the study population was 75.3 years. At baseline, higher heart rate was associated with worse ADL and IADL, and lower SDNN was related to worse IADL (all p values < 0.05). Participants in the highest tertile of heart rate (range 71–117 beats/min) had a 1.79-fold (95% confidence interval [CI] 1.45–2.22) and 1.35-fold (95% CI 1.12–1.63) higher risk of decline in ADL and IADL, respectively (p for trend < 0.001 and 0.001, respectively). Participants in the lowest tertile of SDNN (range 1.70–13.30 ms) had 1.21-fold (95% CI 1.00–1.46) and 1.25-fold (95% CI 1.05–1.48) higher risk of decline in ADL and IADL, respectively (both p for trends < 0.05). All associations were independent of sex, medications, cardiovascular risk factors and comorbidities.
Interpretation:
Higher resting heart rate and lower heart rate variability were associated with worse functional status and with higher risk of future functional decline in older adults, independent of cardiovascular disease. This study provides insight into the role of cardiac autonomic function in the development of functional decline.
Elevated heart rate and reduced heart rate variability — the beat-to-beat variation in heart rate intervals — both reflect an altered balance of the autonomic nervous system tone characterized by increased sympathetic and/or decreased parasympathetic activity.1–3 Sympathetic overactivity has been linked to a procoagulant state and also to risk factors for atherosclerosis, including metabolic syndrome, obesity and subclinical inflammation.2–4 Moreover, increased heart rate is related to atherosclerosis, not only as an epiphenomenon of sympathetic overactivity, but also through hemodynamic mechanisms, such as high pulsatile shear stress, which leads to endothelial dysfunction.5
Atherosclerosis has been linked to increased risk of functional decline in older people via cardiovascular events.6 As the world population is aging, the burden of functional disability is expected to increase.6 It has been hypothesized that heart rate and heart rate variability are markers of frailty, an increased vulnerability to stressors and functional decline.7 However, the direct link between these 2 parameters and risk of functional decline has not been fully established, and it is uncertain whether this association is independent of cardiovascular comorbidities.
In this study, we examined whether heart rate and heart rate variability were cross-sectionally and longitudinally associated with functional status in older adults at high risk of cardiovascular disease, independent of cardiovascular risk factors and comorbidities.
Methods
Study design and participants
The data in this study were obtained from the Prospective Study of Pravastatin in the Elderly at Risk (PROSPER), a randomized controlled trial on the effect of pravastatin in a cohort of older men and women (70–82 yr) with pre-existing vascular disease or risk factors thereof. A total of 5804 individuals were recruited from 3 collaborating centres in Ireland, Scotland and the Netherlands. Details of study design, population recruitment and characteristics have been previously reported.8,9 Exclusion criteria included physical or mental inability to attend clinic visits, poor cognitive function at baseline (Mini Mental State Examination score < 24), advanced heart failure (New York Heart Association functional class III or IV), electrocardiographic (ECG) evidence of atrial fibrillation or other major arrhythmias and implanted cardiac pacemakers. Participants were followed up for a mean of 3.2 years.
From the original population, we excluded 150 participants with missing heart rate and/or heart rate variability measurements at baseline, 489 participants with cardiac rhythm not generated by sinoatrial node and 123 participants with missing data on functional status at baseline or during follow-up. We included participants from both the pravastatin and placebo arms because the PROSPER study group had previously shown that pravastatin did not affect functional status during follow-up.9 Hence, 5042 participants were included in the present study.
The PROSPER study complied with the Declaration of Helsinki and was approved by the medical ethics committees of the 3 centres. All participants provided written informed consent.
Measurement of heart rate and heart rate variability
We measured resting heart rate and heart rate variability from a 10-second, 12-lead ECG, recorded in the morning of the first enrolment visit to limit circadian variability. All ECGs were transmitted electronically for storage at the University of Glasgow ECG Core Laboratory based at Glasgow Royal Infirmary, Scotland, and interpreted using the same software.10
We computed the standard deviation of normal-to-normal RR intervals (SDNN), one of the most frequently used and easily calculated indices of heart rate variability, by deriving it from normal-to-normal RR intervals.11 Normal-to-normal RR intervals were defined as the time between 2 successive normally conducted QRS complexes.
Functional status
Functional status was assessed using 2 questionnaires: the Barthel Index12 and the Lawton Instrumental Activities of Daily Living Scale (IADL).13 The Barthel Index measures performance in basic activities of daily living (ADL) and consists of 10 items: fecal continence, urinary continence, grooming, toilet use, feeding, transfers (e.g., from chair to bed), walking, dressing, climbing stairs and bathing. The Lawton IADL evaluates more complex instrumental activities and includes 7 items: doing housework, taking medication as prescribed, managing money, shopping, using a phone or other forms of communication, using technology and taking transportation within the community. Scores for ADLs and IADLs range from 0 to 20 and from 0 to 14, respectively, with higher scores indicating higher independence and better functional status. Functional status using the 2 questionnaires was measured at baseline; after 9, 18 and 30 months; and at the end of the study, which varied between 36 and 42 months. Based on changes in functional status scores during follow-up, participants were classified as either declining or not declining in ADL and IADL.
Statistical analysis
We used SPSS version 20 for all the analyses. We reported baseline characteristics of participants as number of participants (percentage) for categorical variables and as mean (standard deviation) for continuous variables. We tested differences in baseline characteristics first across heart rate tertiles and then across SDNN tertiles, using analysis of variance for continuous variables and χ2 test for categorical variables.
Linear regression analyses tested the cross-sectional associations of heart rate and SDNN with functional status. Dependent variables were the scores on each of the 2 functional status tests. We computed p values for trend using tertiles of heart rate and SDNN.
We performed binary logistic regression analyses to investigate longitudinal associations of heart rate and SDNN with risk of decline in functional status. Independent variables were heart rate and SDNN. The outcome variable was the risk of declining in each of the functional status tests. We calculated odds ratios (ORs) and 95% confidence intervals (CIs) in tertiles of heart rate and SDNN, respectively. The reference categories were the lowest tertile of heart rate and the highest tertile of SDNN. We calculated p values for trend using tertiles of heart rate and SDNN.
We performed all cross-sectional and longitudinal analyses in 2 steps. In the first step, analyses were adjusted for age, sex, country of enrolment and education (minimally adjusted model). In the second step, we further adjusted for cardiovascular risk factors (smoking status, body mass index [BMI], history of hypertension, history of diabetes mellitus), cardiovascular morbidities (history of myocardial infarction, history of stroke or transient ischemic attack, history of claudication), use of medications (diuretics, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, β-blockers, calcium channel blockers, nitrates, acetylsalicylic acid, anticoagulants) and statin treatment group. In the longitudinal analyses we also adjusted for baseline functional status (fully adjusted model).
To test whether the association of heart rate and SDNN with functional status is independent of β-blocker use, we repeated the longitudinal analyses after exclusion of participants taking β-blockers. Furthermore, we repeated the longitudinal analyses after stratifying the participants by sex, history of hypertension, history of vascular diseases, use of β-blockers, calcium channel blockers or statin treatment to explore the potential modifying effect of these covariates. We computed interaction terms by multiplying heart rate and SDNN, as continuous variables, per these covariates.
To explore the influence of vascular events on the longitudinal associations, we performed sensitivity analyses from which we excluded the following: 1) participants with incident stroke, 2) participants with incident coronary events and 3) participants who were admitted to hospital for heart failure during follow-up. Furthermore, to check whether the longitudinal associations are affected by baseline functional status or by duration of follow-up, we performed sensitivity analyses including only 1) participants with maximum functional status at baseline and 2) participants who completed 36 months of follow-up.
To check whether the association between SDNN and functional status is independent of heart rate, we repeated the analyses after standardizing SDNN for heart rate (dividing SDNN by heart rate).14
Finally, we repeated the longitudinal analyses by dividing the participants in the lowest tertile of heart rate into 2 groups of participants with a heart rate of less than 50 beats/min and participants with a heart rate of 50–60 beats/min.
Results
The mean age of the study population was 75.3 years. A total of 2619 (51.9%) participants were female (Table 1). The median resting heart rate and SDNN were 65 beats/min and 18.6 ms, respectively. Participants with a higher resting heart rate were older, were more likely to be female and current smokers, and had a higher BMI and a higher prevalence of diabetes mellitus. In contrast, participants with a lower resting heart rate used β-blockers more frequently and had a higher prevalence of myocardial infarction (all p values < 0.05) (Appendix 1, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.150462/-/DC1). Participants with lower heart rate variability as measured by SDNN had a higher BMI, a higher prevalence of diabetes mellitus and less frequently used β-blockers (all p values < 0.05) (Appendix 2, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.150462/-/DC1).
Table 1:
Characteristics of the study population at baseline, n = 5042
| Characteristic | No. (%) of participants* |
|---|---|
| Sociodemographics | |
| Age, yr, mean ± SD | 75.3 ± 3.3 |
| Female sex | 2619 (51.9) |
| Age left school, yr, mean ± SD | 15.1 ± 2.1 |
| Cardiovascular risk factors | |
| History of hypertension | 3127 (62.0) |
| History of stroke or TIA | 552 (10.9) |
| History of MI | 662 (13.1) |
| History of claudication | 336 (6.7) |
| History of diabetes mellitus | 517 (10.3) |
| Current smoking | 1334 (26.5) |
| BMI, mean ± SD | 26.8 ± 4.2 |
| Medications | |
| β-blockers | 1320 (26.2) |
| Calcium channel blockers | 1275 (25.3) |
| Statins | 2504 (49.7) |
Note: BMI = body mass index, MI = myocardial infarction, TIA = transient ischemic attack.
Unless stated otherwise.
Table 2 shows the associations of resting heart rate and SDNN with functional status at baseline. In the minimally adjusted model, participants with a higher resting heart rate had a worse performance in both functional status scales (p for trend < 0.05, for both). These associations remained significant in the fully adjusted model (p for trend < 0.05, for both). Likewise, participants with lower SDNN had a worse performance in both functional status scales in the minimally adjusted model (p for trend < 0.05, for both). After full adjustment, the same association persisted between SDNN and IADL (p for trend = 0.03). The same trend was observed between SDNN and ADL, although it did not reach significance (p for trend = 0.11).
Table 2:
Baseline functional status in tertiles of resting heart rate and SDNN
| Variable | Tertiles of heart rate and SDNN; mean (SE)* | p for trend | ||
|---|---|---|---|---|
| Low | Middle | High | ||
| Heart rate | n = 1649 | n = 1742 | n = 1651 | |
| Heart rate, beats/min, range | 34–60 | 61–70 | 71–117 | |
| ADL score | ||||
| Model 1† | 19.79 (0.02) | 19.78 (0.02) | 19.71 (0.02) | 0.004 |
| Model 2‡ | 19.27 (0.25) | 19.26 (0.25) | 19.21 (0.25) | 0.02 |
| IADL score | ||||
| Model 1 | 13.67 (0.03) | 13.62 (0.02) | 13.52 (0.03) | < 0.001 |
| Model 2 | 12.94 (0.34) | 12.89 (0.34) | 12.80 (0.33) | < 0.001 |
| SDNN | n = 1689 | n = 1670 | n = 1683 | |
| SDNN, ms, range | 1.70–13.30 | 13.40–26.50 | 26.60–422.60 | |
| ADL score | ||||
| Model 1 | 19.73 (0.02) | 19.75 (0.02) | 19.80 (0.02) | 0.01 |
| Model 2 | 19.23 (0.25) | 19.24 (0.25) | 19.27 (0.25) | 0.11 |
| IADL score | ||||
| Model 1 | 13.55 (0.03) | 13.62 (0.03) | 13.65 (0.02) | 0.004 |
| Model 2 | 12.84 (0.33) | 12.90 (0.34) | 12.91 (0.34) | 0.03 |
Note: ADL = basic activities of daily living, IADL = instrumental activities of daily living, SDNN = standard deviation of normal-to-normal RR intervals.
Unless stated otherwise.
Adjusted for country, age, sex and education.
Adjusted for country, age, sex, education, smoking, body mass index, history of hypertension, history of diabetes mellitus, history of claudication, history of myocardial infarction, history of stroke/transient ischemic attack, statin treatment, diuretics, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, β-blockers, calcium channel blockers, nitrates, acetylsalicylic acid and anticoagulants.
During a mean follow-up of 3.2 years, 779 participants (15.5%) declined in ADL score and 1128 participants (22.4%) declined in IADL score. Among the participants who declined in ADL score, 406 (52.1%) declined 1 point, 141 (18.1%) 2 points and 232 (29.8%) 3 or more points. Among the participants who declined in IADL score, 402 (35.6%) declined 1 point, 224 (19.9%) 2 points and 502 (44.5%) 3 or more points.
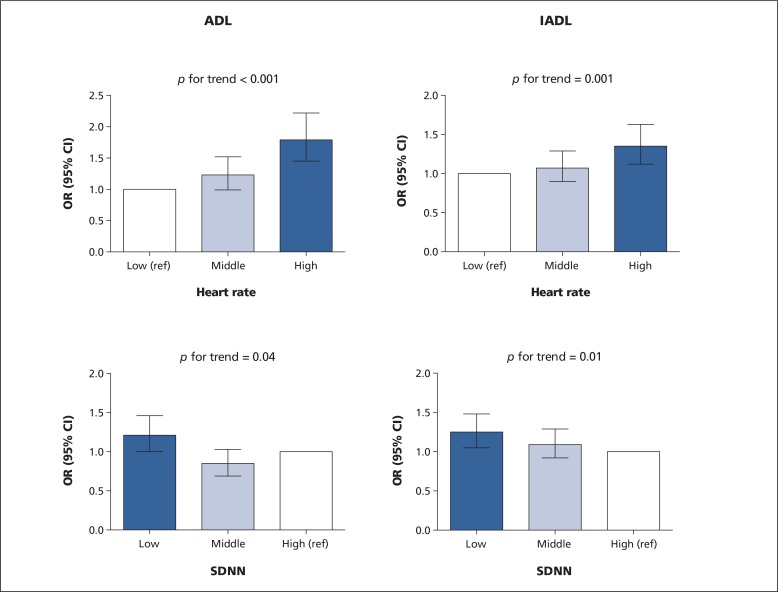
Figure 1 shows the longitudinal associations of resting heart rate and SDNN with risk of decline in functional status after full adjustment. Participants with a resting heart rate in the highest tertile had a 1.79-fold (95% CI 1.45–2.22) and a 1.35-fold (95% CI 1.12–1.63) higher risk of decline in ADL and IADL scores, respectively (p for trend < 0.001 and 0.001, respectively). Participants with SDNN in the lowest tertile had 1.21-fold (95% CI 1.00–1.46) and 1.25-fold (95% CI 1.05–1.48) higher risk of decline in ADL and IADL scores, respectively (p for trend < 0.05, for both groups). These associations were similar in the minimally adjusted model (p for trend < 0.05, for all groups) (Appendix 3, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.150462/-/DC1).
Figure 1:
Risk of decline in functional status in tertiles of resting heart rate and standard deviation of normal-to-normal RR intervals (SDNN). All analyses are adjusted for country, age, sex, education, basic activities of daily living (ADL) and instrumental activities of daily living (IADL) scores at baseline, smoking, body mass index, history of hypertension, history of diabetes mellitus, history of claudication, history of myocardial infarction, history of stroke/transient ischemic attack, statin treatment, diuretics, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, β-blockers, calcium channel blockers, nitrates, acetylsalicylic acid and anticoagulants. Range of heart rate (no. of participants) in heart rate tertiles: low 34–60 beats/min (n = 1649); middle 61–70 beats/min (n = 1742); high 71–117 beats/min (n = 1651). Range of SDNN (no. of participants) in SDNN tertiles: low 1.70–13.30 ms (n = 1689); middle 13.40–26.50 ms (n = 1670); high 26.60–422.60 ms (n = 1683). Note: CI = confidence interval, OR = odds ratio.
Table 3 shows the sensitivity analyses after exclusion of the 1320 participants receiving treatment with β-blockers. Higher resting heart rate and lower SDNN remained significantly related to a higher risk of decline for both ADL and IADL in the fully adjusted model (p for trend < 0.05, for all groups). To clarify whether cardiovascular events during follow-up might affect the longitudinal associations between resting heart rate/SDNN and risk of decline in functional status, we performed a series of sensitivity analyses after exclusion of 1) participants with incident stroke during follow-up (n = 220); 2) participants with incident coronary events during follow-up (n = 541); and 3) participants who were admitted to hospital for heart failure during follow-up (n = 196). Results did not materially change (Appendices 4, 5 and 6, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.150462/-/DC1).
Table 3:
Risk of decline in functional status in tertiles of resting heart rate and SDNN after exclusion of participants taking β-blockers
| Variable | Tertiles of heart rate and SDNN; OR (95% CI) | p for trend | ||
|---|---|---|---|---|
| Low | Middle | High | ||
| Heart rate | n = 863 | n = 1379 | n = 1480 | |
| ADL score | ||||
| Model 1* | 1 (ref) | 1.27 (0.97–1.67) | 1.95 (1.50–2.53) | < 0.001 |
| Model 2† | 1 (ref) | 1.25 (0.95–1.65) | 1.86 (1.43–2.42) | < 0.001 |
| IADL score | ||||
| Model 1 | 1 (ref) | 1.09 (0.87–1.37) | 1.46 (1.17–1.81) | < 0.001 |
| Model 2 | 1 (ref) | 1.07 (0.85–1.35) | 1.39 (1.11–1.74) | 0.002 |
| SDNN | n = 1312 | n = 1192 | n = 1218 | |
| ADL score | ||||
| Model 1 | 1.31 (1.06–1.63) | 0.85 (0.67–1.08) | 1 (ref) | 0.009 |
| Model 2 | 1.25 (1.00–1.55) | 0.82 (0.65–1.04) | 1 (ref) | 0.03 |
| IADL score | ||||
| Model 1 | 1.30 (1.07–1.58) | 1.09 (0.89–1.34) | 1 (ref) | 0.008 |
| Model 2 | 1.26 (1.03–1.53) | 1.07 (0.87–1.31) | 1 (ref) | 0.02 |
Note: ADL = basic activities of daily living, CI = confidence interval, IADL = instrumental activities of daily living, OR = odds ratio, SDNN = standard deviation of normal-to-normal RR intervals.
Adjusted for country, age, sex and education.
Adjusted for country, age, sex, education, ADL and IADL score at baseline, smoking, body mass index, history of hypertension, history of diabetes mellitus, history of claudication, history of myocardial infarction, history of stroke/transient ischemic attack, statin treatment, diuretics, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, calcium channel blockers, nitrates, acetylsalicylic acid and anticoagulants.
To explore whether poor functional status at baseline might affect the longitudinal relation between resting heart rate/SDNN and risk of decline in functional status, we performed further sensitivity analyses including only participants with maximum functional status scores at baseline (n = 4343 participants with maximum ADL score, n = 4129 participants with maximum IADL score). Results did not materially change (Appendix 7, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.150462/-/DC1).
To test whether short duration of follow-up might affect the results, we repeated the longitudinal analyses including only participants who completed 36 months of follow-up (n = 4552). The longitudinal associations between resting heart rate/SDNN and risk of decline in functional status remained significant (Appendix 8, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.150462/-/DC1.).
The associations of resting heart rate and SDNN with functional decline were not modified by sex, history of hypertension or vascular diseases, use of β-blockers, calcium channel blockers or statin treatment (p for interaction > 0.05, for all groups) (Appendix 9, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.150462/-/DC1, for heart rate; data not shown for SDNN). In an extra analysis, we tested whether the observed associations were independent of baseline cognitive function as assessed by the Mini Mental State Examination. The associations did not materially change after adjustment for baseline cognitive function (data not shown). Likewise, these associations remained unchanged when we standardized SDNN for heart rate (Appendices 10 and 11, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.150462/-/DC1). Furthermore, we observed no difference in risk of functional decline between participants with a heart rate of less than 50 beats/min (n = 284) and those with a heart rate of 50–60 beats/min (n = 1365). Participants in the highest tertile had a higher risk of functional decline compared with the participants in the group with a heart rate of 50–60 beats/min (Appendix 12, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.150462/-/DC1.).
Interpretation
In our study, higher resting heart rate and lower heart rate variability were associated with worse functional performance at baseline and with higher risk of future functional decline in older adults at high cardiovascular risk. These associations were independent of cardiovascular risk factors, cardiovascular morbidities and use of medications.
The results of our study are in line with the results of the Prevention Regimen for Effectively Avoiding Second Stroke (PRoFESS) trial, which showed that higher heart rate was related to worse functional outcomes in patients with a recurrent stroke.15 Our results are also consistent with findings from the Women’s Health and Aging Study-I (WHAS-I), which showed a cross-sectional association between lower heart rate variability and frailty in disabled older women living in the community.16 Our study extends the findings of WHAS-I to older adults at risk for cardiovascular disease with preserved functional status. Furthermore, we showed that the association of heart rate variability with functional decline was independent of sex.
Different pathophysiological mechanisms may underlie these associations. First, higher heart rate and lower heart rate variability have been consistently associated with incident cardiovascular events in previous studies.1–3 In this study, the strength of the associations between heart rate/heart rate variability and functional decline did not materially change after exclusion of participants with incident cardiovascular events. This might suggest that mechanisms other than macrovascular damage play roles in the association between heart rate/heart rate variability and functional decline. Second, lower heart rate is associated with better cardiovascular fitness, which is a protective factor for brain aging and functional decline.17 In particular, lower heart rate is related to less myocardial oxygen consumption and more prolonged time available for diastolic heart chamber filling and coronary perfusion.18 Furthermore, higher heart rate has been suggested to increase pulsatile shear stress, which leads to endothelial dysfunction and accelerated atherosclerosis.5,19 In this setting, use of ivabradine, a pure heart rate–lowering agent, in relation to cardiovascular outcomes has been tested with conflicting results.20–22 Third, heart rate and heart rate variability reflect the autonomic nervous system’s control over cardiac function. Cardiac autonomic control regulates the interaction between circulation and respiration. Higher heart rate variability in synchrony with respiration improves the efficiency of gas exchange at the level of the lung via efficient ventilation and perfusion matching.23 Furthermore, cardiac autonomic control keeps blood pressure constant within a certain range to maintain adequate perfusion to vital organs, including the brain. A preserved cardiac autonomic control buffers variations in blood pressure in response to stressors. Indeed, participants with lower heart rate variability present higher blood pressure variability in response to psychological challenge or tilt test.24,25 Higher blood pressure variability is associated with atherosclerosis26 and silent brain damage.27 Finally, the autonomic nervous system is connected to regions of the central nervous system,28,29 which are involved in mood regulation. Lower heart rate variability has been associated with depression,30,31 which is a cause of disability.6
Strengths and limitations
A strength of our study was the longitudinal design, which allowed us to show that high heart rate and low heart rate variability preceded the decline in functional status. We also showed that this association was independent of potential confounders such as vascular diseases and use of antihypertensive and cardioprotective medications. However, causality cannot be inferred given the observational nature of this study. Further strengths are the large study population of older adults and the multicentre design.
A limitation of our study was that all participants were older adults at high cardiovascular risk, which may limit the generalizability of our findings. Nevertheless, a considerable number of older adults carry high loads of cardiovascular pathologies and comorbidities. Moreover, we categorized our participants into the clinically distinguishable groups of those who declined and those who did not decline, although this categorization may result in loss of information. Another possible limitation is the use of a 10-second ECG; nonetheless, we were able to show a significant association of resting heart rate and heart rate variability with functional status even by using a short ECG recording, which is more feasible in clinical practice than longer recordings. Heart rate variability measured from standard 10-second ECG recordings correlates with heart rate variability measured from longer ECG recordings.11
Conclusion
We found that higher resting heart rate and lower heart rate variability were associated with worse functional status in older adults, independent of cardiovascular risk factors and comorbidities. This study provides insight into the role of cardiac autonomic function in the development of functional decline. Because functional disability has a long preclinical phase, it is crucial to identify potential interventions to delay it. Further research is needed to establish whether heart rate and heart rate variability are risk markers and/or potentially modifiable risk factors for functional decline. Pharmacologic and nonpharmacologic interventions (e.g., drugs with antiadrenergic properties, physical exercise, nervus vagus stimulation) aimed at modulating cardiac autonomic function may be beneficial in preserving functional status. It is well established that physical activity is a key contributor in autonomic regulation and is linked with preservation of functional status.32,33 However, future studies are needed to test the influence of physical activity on functionality through autonomic regulation in older adults.
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Contributors: Giulia Ogliari and Simin Mahinrad contributed equally to this work and both drafted the manuscript. Behnam Sabayan had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Giulia Ogliari, Simin Mahinrad and Behnam Sabayan contributed to study concept and design. All of the authors acquired, analyzed or interpreted the data. Giulia Ogliari, Simin Mahinrad, Anton de Craen and Behnam Sabayan performed the statistical analysis. Anton de Craen and Behnam Sabayan supervised. All of the authors revised the manuscript for important intellectual content. All of the authors gave final approval of the version to be published and agreed to act as guarantors of the work.
References
- 1.Fox K, Borer JS, Camm AJ, et al. Resting heart rate in cardiovascular disease. J Am Coll Cardiol 2007;50:823–30. [DOI] [PubMed] [Google Scholar]
- 2.Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J 1996;17:354–81. [PubMed] [Google Scholar]
- 3.Palatini P, Benetos A, Julius S. Impact of increased heart rate on clinical outcomes in hypertension: implications for antihypertensive drug therapy. Drugs 2006;66:133–44. [DOI] [PubMed] [Google Scholar]
- 4.Sajadieh A, Nielsen OW, Rasmussen V, et al. Increased heart rate and reduced heart-rate variability are associated with subclinical inflammation in middle-aged and elderly subjects with no apparent heart disease. Eur Heart J 2004;25:363–70. [DOI] [PubMed] [Google Scholar]
- 5.Custodis F, Schirmer SH, Baumhakel M, et al. Vascular pathophysiology in response to increased heart rate. J Am Coll Cardiol 2010;56:1973–83. [DOI] [PubMed] [Google Scholar]
- 6.Mathers C, Fat DM, Boerma J. The global burden of disease: 2004 update. Geneva: World Health Organization; 2008. [Google Scholar]
- 7.Chaves PH, Varadhan R, Lipsitz LA, et al. Physiological complexity underlying heart rate dynamics and frailty status in community-dwelling older women. J Am Geriatr Soc 2008;56:1698–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shepherd J, Blauw GJ, Murphy MB, et al. The design of a prospective study of Pravastatin in the Elderly at Risk (PROSPER). PROSPER Study Group. PROspective Study of Pravastatin in the Elderly at Risk. Am J Cardiol 1999;84:1192–7. [DOI] [PubMed] [Google Scholar]
- 9.Shepherd J, Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002;360:1623–30. [DOI] [PubMed] [Google Scholar]
- 10.Macfarlane P, Devine B, Clark E, editors. The university of Glasgow (Uni-G) ECG analysis program. Computers in Cardiology 2005;32:451–4. [Google Scholar]
- 11.Hamilton RM, McKechnie PS, Macfarlane PW. Can cardiac vagal tone be estimated from the 10-second ECG? Int J Cardiol 2004;95:109–15. [DOI] [PubMed] [Google Scholar]
- 12.Mahoney FI, Barthel DW. Functional evaluation: the Barthel index. Md State Med J 1965;14:61–5. [PubMed] [Google Scholar]
- 13.Lawton MP. The functional assessment of elderly people. J Am Geriatr Soc 1971;19:465–81. [DOI] [PubMed] [Google Scholar]
- 14.Sacha J. Why should one normalize heart rate variability with respect to average heart rate. Front Physiol 2013;4:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Böhm M, Cotton D, Foster L, et al. Impact of resting heart rate on mortality, disability and cognitive decline in patients after ischaemic stroke. Eur Heart J 2012;33:2804–12. [DOI] [PubMed] [Google Scholar]
- 16.Varadhan R, Chaves PH, Lipsitz LA, et al. Frailty and impaired cardiac autonomic control: new insights from principal components aggregation of traditional heart rate variability indices. J Gerontol A Biol Sci Med Sci 2009;64:682–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jefferson AL, Himali JJ, Beiser AS, et al. Cardiac index is associated with brain aging: the Framingham Heart Study. Circulation 2010;122:690–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nelson RR, Gobel FL, Jorgensen CR, et al. Hemodynamic predictors of myocardial oxygen consumption during static and dynamic exercise. Circulation 1974;50:1179–89. [DOI] [PubMed] [Google Scholar]
- 19.Chatzizisis YS, Coskun AU, Jonas M, et al. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J Am Coll Cardiol 2007;49:2379–93. [DOI] [PubMed] [Google Scholar]
- 20.Fox K, Ford I, Steg PG, et al. Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a randomised, double-blind, placebo-controlled trial. Lancet 2008;372:807–16. [DOI] [PubMed] [Google Scholar]
- 21.Fox K, Ford I, Steg PG, et al. Ivabradine in stable coronary artery disease without clinical heart failure. N Engl J Med 2014; 371:1091–9. [DOI] [PubMed] [Google Scholar]
- 22.Swedberg K, Komajda M, Bohm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. [published erratum Lancet 2010;376:1988] Lancet 2010;376:875–85. [DOI] [PubMed] [Google Scholar]
- 23.Yasuma F, Hayano J. Respiratory sinus arrhythmia: Why does the heartbeat synchronize with respiratory rhythm? Chest 2004; 125:683–90. [DOI] [PubMed] [Google Scholar]
- 24.Sloan RP, Demeersman RE, Shapiro PA, et al. Cardiac autonomic control is inversely related to blood pressure variability responses to psychological challenge. Am J Physiol 1997; 272: H2227–32. [DOI] [PubMed] [Google Scholar]
- 25.Sloan RP, DeMeersman RE, Shapiro PA, et al. Blood pressure variability responses to tilt are buffered by cardiac autonomic control. Am J Physiol 1997;273:H1427–31. [DOI] [PubMed] [Google Scholar]
- 26.Shintani Y, Kikuya M, Hara A, et al. Ambulatory blood pressure, blood pressure variability and the prevalence of carotid artery alteration: the Ohasama study. J Hypertens 2007;25:1704–10. [DOI] [PubMed] [Google Scholar]
- 27.Gómez-Angelats E, de La Sierra A, Sierra C, et al. Blood pressure variability and silent cerebral damage in essential hypertension. Am J Hypertens 2004;17:696–700. [DOI] [PubMed] [Google Scholar]
- 28.Castle M, Comoli E, Loewy AD. Autonomic brainstem nuclei are linked to the hippocampus. Neuroscience 2005;134:657–69. [DOI] [PubMed] [Google Scholar]
- 29.Mujica-Parodi LR, Korgaonkar M, Ravindranath B, et al. Limbic dysregulation is associated with lowered heart rate variability and increased trait anxiety in healthy adults. Hum Brain Mapp 2009; 30:47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kemp AH, Quintana DS, Gray MA, et al. Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biol Psychiatry 2010;67:1067–74. [DOI] [PubMed] [Google Scholar]
- 31.Licht CM, de Geus EJ, Zitman FG, et al. Association between major depressive disorder and heart rate variability in the Netherlands Study of Depression and Anxiety (NESDA). Arch Gen Psychiatry 2008;65:1358–67. [DOI] [PubMed] [Google Scholar]
- 32.Soares-Miranda L, Sattelmair J, Chaves P, et al. Physical activity and heart rate variability in older adults: the Cardiovascular Health Study. Circulation 2014;129:2100–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peterson MJ, Giuliani C, Morey MC, et al. Health, Aging and Body Composition Study Research Group. Physical activity as a preventative factor for frailty: the health, aging, and body composition study. J Gerontol A Biol Sci Med Sci 2009;64:61–8. [DOI] [PMC free article] [PubMed] [Google Scholar]