Abstract
Granzyme B (GzmB) has previously been shown to be critical for CD8+ T cell-mediated graft-versus-host disease (GVHD) but dispensable for GVHD mediated by CD4+ T cells. However, previous studies used high doses of CD4+ T cells in MHC-mismatched models that caused rapid and lethal GVHD. Due to the hyperacute lethality, it is possible that the role of GzmB was concealed by the system. Therefore, in this study we have titrated down the T cell dose to precisely determine the contribution of GzmB in GVHD mediated by CD4+CD25− T cells. Surprisingly, we have found that GzmB−/− CD4+CD25− T cells cause more severe GVHD compared to wild-type (WT) CD4+CD25− T cells in both MHC-matched and mismatched models. Mechanistic analyses reveal that while GzmB does not affect donor T cell engraftment, proliferation or tissue-specific migration, GzmB−/− CD4+CD25− T cells exhibit significantly enhanced expansion due to GzmB-mediated activation-induced cell death of WT CD4+CD25− T cells. As a result of enhanced expansion, GzmB−/− T cells produced higher amounts of proinflammatory cytokines (e.g., TNF-α and IFN-γ) that may contribute to the exacerbated GVHD. These results reveal that GzmB diminishes the ability of CD4+ T cells to cause acute GVHD, which contradicts its established role in CD8+ T cells. The differential roles suggest that targeting GzmB in selected T cell subsets may provide a strategy to control GVHD.
Introduction
Allogeneic hematopoietic cell transplantation (allo-HCT) is a potentially curative treatment for leukemia, lymphoma, and other hematologic diseases (1, 2). However, acute graft-versus-host disease (GVHD), a potentially life threating complication of allo-HCT, occurs in about 35% of patients receiving major histocompatibility complex (MHC)-matched transplantation (3–5). GVHD is mediated by donor-derived T cells which recognize the genetically distinct host as non-self, subsequently leading to host cell destruction (3–5). To prevent GVHD, T cell depletion may be performed to the hematopoietic graft or prophylaxis with immunosuppressive agents can be used (3–5). However, these strategies are not always successful and nearly 20% of allo-HCT patients eventually succumb to GVHD (3–5). Therefore, new therapeutic strategies for preventing GVHD are necessary if we hope to reach the curative potential of allo-HCT, which requires a better understanding of the immunobiology of GVHD.
Donor-derived CD4+ and CD8+ T cells are the major effector cells mediating GVHD (4). At the molecular level, three major pathways have been described for T cell-mediated cytotoxicity: perforin and granzymes, Fas and its ligand, and secreted cytokines (e.g., TNFα, IFNγ) (6–9). Earlier studies with MHC-mismatched models reported that the perforin/granzyme pathway was required for CD8+ but not CD4+ T cells to cause GVHD, while Fas ligand was required for CD4+ but not CD8+ T cells to cause GVHD (10, 11). As a key cytotoxic molecule, granzyme B (GzmB) deficiency was shown to alleviate CD8+ T cell-mediated GVHD but did not alter CD4+ T cell-mediated GVHD (10, 11). However, while our recent study confirmed that GzmB is an essential molecule used by CD8+ T cells to cause severe GVHD, it also raised a question about the contribution of GzmB in CD4+ T cell-mediated GVHD (12). The major issue may lie in the significantly higher GVH activity of CD4+ T cells as opposed to CD8+ T cells in the MHC-mismatched models preciously used. For example, while 1.5×106 CD8+ T cells were required to cause lethal GVHD in 4 weeks after allo-HCT, 1×105 CD4+ T cells caused rapid and lethal GVHD within 2 weeks after allo-HCT (12). Therefore, we suspected that the hyperacute GVHD caused by lethal doses of CD4+ T cells may have concealed a role of GzmB in previous studies. Based on the this notion, we have titrated down the T cell doses in this study to precisely determine the contribution of GzmB in GVHD mediated by CD4+CD25− T cells. Surprisingly, we have found that GzmB−/− CD4+CD25− T cells cause more severe GVHD compared to wild-type (WT) CD4+CD25− T cells in both MHC-matched and mismatched models. Mechanistic analyses reveal that GzmB−/− T cells exhibit significantly enhanced survival and expansion compared to WT T cells, due to GzmB-mediated activation-induced cell death of WT T cells. As a result of enhanced expansion, GzmB−/− T cells produced higher amounts of proinflammatory cytokines (e.g., TNF-α and IFN-γ) that may contribute to the exacerbated GVHD. These results reveal that GzmB diminishes the ability of CD4+ T cells to cause GVHD, which contradicts its established role in CD8+ T cells. The differential roles suggest that targeting GzmB in selected T cell subsets may provide a strategy to control GVHD.
Materials and Methods
Animals
C57BL/6 (H-2b) WT, RAG1−/− (H-2b), and 129/SvJ (H-2b) WT mice were obtained from the Jackson Laboratory. BALB/c (H-2d) mice were purchased from NCI. GzmB−/− mice in the C57BL/6 and 129/SvJ strains were developed as previously described (13–17). All mice were maintained in SPF housing, and all experiments were conducted in accordance with the animal care guidelines at Roswell Park Cancer Institute, using protocols approved by the animal studies committee.
Reagents and antibodies
Antibodies including anti-mouse CD3, CD4, CD8, CD25, H-2Kb, H-2Kd, CD16/32, IFN-γ, Ki-67, CCR9, LPAM-1, CaspGLOW fluorescein active caspase-3 staining kits, total reactive oxygen species (ROS) assay kits and Annexin V apoptosis detection kits were purchased from eBioscience. BCL-2 antibody reagent set was purchased from BD bioscience.
Donor cell preparation
Donor bone marrow (BM) cells were isolated from WT mice or RAG1−/− mice. T cell depletion (TCD) was performed with auto-MACS by using anti-CD90.2 microbeads (Miltenyi Biotec). Donor CD4+CD25− T cells (purity > 95%) were purified from the spleens by using Pan T isolation kit II combined with biotin-conjugated anti-CD8 and anti-CD25 antibodies.
MHC-mismatched and MHC-matched HCT models
For MHC-mismatched HCT, BALB/c hosts (H-2d) were irradiated with 8 Gy from a Cs-137 source. One day later, the hosts were injected intravenously with 2×106 TCD-BM cells only or combined with 2–30×104 CD4+CD25− T cells isolated from C57BL/6 (H-2b) WT or GzmB−/− mice. For MHC-matched HCT, C57BL/6 (H-2b) host mice were irradiated with 10 Gy. One day later, the host mice were injected with 7×106 BM cells alone or combined with 4×106 WT or GzmB−/− CD4+CD25− T cells isolated from 129/SvJ (H-2b) donor mice. The host mice were weighed twice every week and monitored for survival.
Clinical GVHD scoring system
The clinical GVHD manifestations are weight loss, change in posture, activity, fur texture, hair loss and in some cases diarrhea. Clinical GVHD is evaluated comprehensively with a scoring system as follows: a) Posture: normal-0, hunching at rest-1, severe hunching impairs movement-2; b) Activity: normal-0, mild decrease-1, stationary unless stimulated-2; c) Fur texture: normal-0, moderate ruffling-1, severe ruffling and poor grooming-2; d) Skin: normal-0, scaling, mild hair loss-1, areas of denuded skin-2; e) Diarrhea: no-0, mild-1, severe-2; f) Body weight loss: ≤ 5%-0, 5–15%-1, >15%-2. This scoring system is modified from a previously established protocol to assess acute GHVD (18, 19).
Histopathological analyses of GVHD target organs
Liver, lung, large and small intestines were removed, formalin-fixed, sectioned, and stained with H&E. Blinded histopathological analyses were performed and interpreted by a pathologist (J.Q). An established, a semi-quantitative scoring system was used to assess abnormalities associated with GVHD (20, 21), which designated 0 as normal, 0.5 as focal and rare, 1.0 as focal and mild, 2.0 as diffuse and mild, 3.0 as diffuse and moderate, and 4.0 as diffuse and severe.
In vivo GI tract permeability measurement
FITC-Dextran (FD4) was purchased from Sigma-Aldrich. Experimental and naïve control mice were fasted for 3 hours before administration of FD4 (60mg/100g body weight) by oral gavage. 4 hours later, blood samples were collect via eye-bleeding to prepare serum samples. Serum samples were diluted and FD4 levels were measured on a 96-well plate with serial dilution of FD4 standard (from 10 to 0.005ug/ml). FITC signals were measured by a plate reader with excitation wavelength 490 nm and emission wavelength 520 nm.
Results
GzmB is activated in CD4+CD25− T cells following allo-HCT
Several studies have examined the roles of GzmB in GVHD mediated by different T cell subsets. GzmB was shown to be critical for CD8+ T cells to cause severe GVHD (10–12), but was not required for CD4+CD25+ Treg cell-mediated suppression of GVHD (20). In addition, GzmB did not seem to impact GVHD mediated by CD4+CD25− T cells (12). However, the previous studies used lethal doses of (1–10×105) CD4+ T cells in MHC-mismatched HCT that caused rapid and uniform lethality to both groups of mice receiving WT and GzmB−/− T cells within 2 weeks. This result caused us to suspect that the rapidity and severity of CD4+ T cell-mediated GVHD in the previous studies may have concealed a contribution by GzmB. Based on this notion, we first wanted to determine whether CD4+CD25− T cells express GzmB after allo-HCT. To extend host survival long enough for evaluating GzmB expression in vivo, we injected a low dose of (2×104) CD4+CD25− T cells. While GzmB is virtually non-detectable in naïve donor CD4+CD25− T cells, substantial GzmB expression is detected in donor-derived CD4+CD25− T cells harvested from the spleens of the host mice on day 8 after allo-HCT (Figure 1). This result shows for the first time that GzmB is activated in donor-derived CD4+CD25− T cells following allo-HCT.
Figure 1. GzmB is activated in donor CD4+CD25− T cells after allo-HCT.
BALB/c (H-2d) host mice were lethally irradiated on day −1. On day 0, host mice were injected with 2×106 TCD-BM cells alone or combined with 2×104 WT or GzmB−/− CD4+CD25− T cells purified from C57BL/6 (H-2b) donor mice. On day 8, total cells harvested from the host spleens were analyzed with flow cytometry to examine GzmB expression in donor-derived T cells. Purified pre-HCT naive CD4+CD25− T cells were also examined as controls. (A) Representative dot plots are gated on donor-derived T cells (H-2Kb+CD3+CD4+). (B) Summary data of the percentages of donor CD4+CD25− T cells that are GzmB+ in the host spleens are shown with each point representing an individual mouse, with 9 mice assessed in each group.
GzmB diminishes the ability of CD4+CD25− T cells to cause lethal acute GVHD
To better determine the contribution of GzmB in GVHD mediated by CD4+CD25− T cells, we used a low dose of 5×104 cells to perform GVHD experiments. At this dose, about 60% of the host mice receiving WT T cells died, while 100% of the host mice receiving GzmB−/− T cells succumbed to acute GVHD (Figure 2A). To confirm this surprising result, we decreased the dose of CD4+CD25− T cells to 2×104 cells. Now only 1 out of 16 host mice receiving WT T cells died while nearly 50% of the host mice receiving GzmB−/− T cells succumbed to acute GVHD (Figure 2B). To test whether T cells developed de novo from donor BM cells play a role in this phenotype, we performed allo-HCT experiments that use RAG1−/− BM cells combined with WT or GzmB−/− CD4+CD25− T cells. Because RAG1−/− BM cells are not able to generate any mature T cells after transplantation, this design would rule out the effect of de novo developed T cells. As shown in Figure 2C, transplantation with RAG1−/− BM combined with WT versus GzmB−/− T cells essentially reproduced the phenotype. This result confirms that de novo developed T cells did not make a significant impact in this system, and that the observed phenotype is due to GzmB function in the transplanted mature CD4+CD25− T cells. In addition, we employed a clinical scoring system to comprehensively evaluate GVHD manifestations including body weight loss, activity, diarrhea, posture, fur and skin change. Together, these parameters indicate that GzmB−/− T cells caused more severe acute GVHD than WT T cells (Figure 2D). To further confirm this phenotype in a more clinically relevant model, we performed MHC-matched allo-HCT using 129/SvJ mice (H-2b) as donors and C57BL/6 mice (H-2b) as hosts. Although GVHD occurred in a slower manner in this MHC-matched model, GzmB−/− CD4+CD25− T cells again caused more severe GVHD compared to WT CD4+CD25− T cells as indicated by the different survival curves (Figure 2E) and overall clinical GVHD scores (Figure 2F). Taken together, these results demonstrate that GzmB activation diminishes the ability of donor CD4+CD25− T cells to cause lethal acute GVHD.
Figure 2. GzmB−/− CD4+CD25− T cells cause more severe GVHD than WT CD4+CD25− T cells.
BALB/c (H-2d) host mice were lethally irradiated on day −1. On day 0, mice were injected with 2×106 TCD-BM alone or combined with 5×104 (A) or 2×104 (B) WT or GzmB−/− CD4+CD25− T cells purified from C57BL/6 (H-2b) donor mice and survival was monitored. (C) Allo-HCT was performed as described in (B) except that 2×106 RAG1−/− BM cells were used instead of WT TCD-BM cells. (D) Shown are clinical GVHD scores for host mice on day 8 after allo-HCT in the experiment as described in (C). (E) C57BL/6 (H-2b) host mice were lethally irradiated on day −1. On day 0, host mice were injected with 7×106 BM cells alone or combined with 4×106 WT or GzmB−/− CD4+CD25− T cells isolated from 129/SvJ (H-2b) donor mice and survival was monitored. (F) Shown are clinical GVHD scores for host mice in the experiment described in (E). Summary survival results in each panel of (A,B,C,E) are combined from two independent experiments with similar results. Shown are Kaplan-Meier survival curves with Log-rank (Mantel-Cox) tests. Two-tailed t-tests were performed in (D) and Two-way-ANOVA in (F) to determine statistically significant differences.
GzmB deficiency in donor CD4+CD25− T cells does not affect donor cell engraftment
An important function of donor-derived T cells is to improve donor cell engraftment by eliminating residual host T cells and NK cells that may cause graft rejection (22, 23). Because of the small doses of donor CD4+CD25− T cells used for transplantation and the rapid lethality observed, we were concerned that host mice might have died from engraftment failure rather than acute GVHD. To address this concern, we performed flow cytometry to assess donor cell engraftment by using H-Kb as donor cell marker and H-2Kd as host cell marker. Compared to transplantation of TCD-BM only, transplantation with donor CD4+CD25− T cells significantly enhanced overall donor cell engraftment, donor T cell engraftment and donor myeloid cell engraftment (Supplemental Figure 1). However, GzmB deficiency in donor CD4+CD25− T cells did not affect donor cell engraftment. This result rules out engraftment failure as a cause of the rapid lethality observed in mice receiving GzmB−/− CD4+CD25− T cells.
GzmB−/− CD4+CD25− T cells cause more severe colon damage and bleeding
To confirm the occurrence and severity of GVHD, we performed histopathological analyses of GVHD target organs using an established semi-quantitative scoring system to assess abnormalities associated with GVHD (20, 21). As shown in Figure 3A–3B, livers show mild to severe grades of GVHD with scores ranging between from 1 and 4. Lungs show normal structure with minimal to no damage (data not shown). Mild to severe grades of GVHD were also observed in the large intestines. However, no significant differences in histopathological GVHD scores were observed between host mice receiving GzmB−/− and WT CD4+CD25− T cells (Figure 3A–3B). Interestingly, we observed highly frequent intraluminal bleeding in the colons of host mice receiving GzmB−/− CD4+CD25− T cells (Figure 3C–3D), while this type of bleeding was observed at significantly lower frequency in the host mice receiving WT CD4+CD25− T cells. Furthermore, intramural bleeding was confirmed by H&E staining of colon sections, which clearly showed red blood cells trapped out of blood vessels within the lamina propria and intraepithelial spaces (Figure 3C). Of note, these types of intestinal bleeding were not observed on day 5, but were frequently observed on day 8 after allo-HCT with the host mice receiving GzmB−/− CD4+CD25− T cells (Figure 3D). This time point closely preceded host death occurring at a significantly higher frequency for the host mice receiving GzmB−/− T cells (Figure 2). These data suggest that intestinal bleeding may contribute to the rapid death of the host mice.
Figure 3. GzmB−/− CD4+CD25− T cells cause more severe colon damage and bleeding.
(A) BALB/c host mice were lethally irradiated on day −1. On day 0, mice were injected with 2×106 TCD-BM cells alone or combined with 5×104 WT or GzmB−/− CD4+CD25− T cells isolated from C57BL/6 donor mice. On day 8, host mice were dissected to examine GVHD damage. (B) C57BL/6 (H-2b) host mice were lethally irradiated on day −1. On day 0, host mice were injected with 7×106 BM cells alone or combined with 4×106 WT or GzmB−/− CD4+CD25− T cells isolated from 129/SvJ (H-2b) donor mice, On day 25, host mice were dissected to examine GVHD damage. Liver and colon histopathological GVHD scores were acquired as described the methods. (C) Representative image of intraluminal bleeding as indicated by a black arrow pointing at a colon segment that shows internal bleeding (upper panel). Representative images of intramural bleeding with black arrows pointing at areas of trapped red blood cells within the colon wall (lower panel). (D) Summary of the occurrence of observed colon bleeding in the host mice. Shown are summary results combined from two independent experiments with similar results.
GzmB−/− CD4+CD25− T cells cause more severe GI tract permeability
Although our histopathological analyses revealed the presence of moderate to severe GVHD, the semi-quantitative scoring system did not show a significant difference between mice receiving WT and GzmB−/− T cells. To better examine the cause of the observed differences in host lethality and intestinal bleeding, we employed a more sensitive assay to assess GI tract permeability associated with GVHD damage. This assay is based on oral delivery of FITC-dextran which cannot cross the healthy GI mucosal barrier to enter circulating blood. However, FITC-dextran can be detected in peripheral blood when T cell-induced GVHD damage increases GI tract permeability (24). We performed this assay with the MHC-mismatched (C57BL/6 → BALB/c) model on day 8 after HCT, a time point when substantial GzmB activation is observed. Indeed, significantly higher levels of FITC-dextran were observed in the blood of mice receiving GzmB−/− CD4+CD25− T cells than mice receiving WT CD4+CD25− T cells (Figure 4A). We also performed this assay in the MHC-matched (129/SvJ → C57BL/6) model with more time points assessed due to the slower manner of GVHD development. As expected, similar results were observed with significantly higher levels of FITC-dextran detected in the blood of mice receiving GzmB−/− T cells (Figure 4B–4D). These results confirm that GzmB−/− CD4+CD25− T cells induce more severe GI tract damage than WT CD4+CD25− T cells, which is consistent the significantly reduced survival of host mice receiving GzmB−/− CD4+CD25− T cells.
Figure 4. GzmB−/− CD4+CD25− T cells caused more severe GI tract permeability.
(A) BALB/c host mice were lethally irradiated with on day −1. On Day 0, mice were injected with 2×106 TCD-BM cells alone or combined with 2×104 WT or GzmB−/− CD4+CD25− T cells isolated from C57BL/6 (H-2b) donor mice. (B,C,D) C57BL/6 (H-2b) host mice were lethally irradiated on day −1. On day 0, mice were injected with 7×106 BM cells alone or combined with 4×106 WT or GzmB−/− CD4+CD25− T cells isolated from 129/SvJ (H-2b) donor mice. On the indicated time after allo-HCT, FITC-dextran GI tract permeability assay was performed as described in the methods with serum samples collected via eye bleeding. Summary data are shown with 7–12 mice assessed in each group at each time point. Two-tailed t-tests were performed to determine statistically significant differences.
GzmB−/− CD4+CD25− T cells exhibit enhanced expansion after allo-HCT
To explore the mechanism underlying the enhanced GVH activity of GzmB−/− CD4+CD25− T cells, we used flow cytometry to examine T cell expansion in the host mice. In the control group that received TCD-BM only, very few donor-derived T cells were detected in the spleens within the first 3 weeks after transplantation (Figure 5A). On day 5 after HCT, GzmB−/− T cells exhibit an expansion that is more than 2-fold higher than of WT T cells in the host spleens (Figure 5A–5B). A similar trend of higher expansion was also observed for GzmB−/− T cells on day 7 and day 9 after HCT, although the differences did not reach statistical significance. We also attempted to determine donor T cell number within the mesenteric lymph nodes (MLN) and large intestines (Figure 5C–5D). We observed a trend towards increased T cell number in these tissues receiving GzmB−/− versus WT T cells. However, the observed difference did not reach statistical significance, even though the trend is consistent with significantly increased T cell expansion in host spleens receiving GzmB−/− T cells. The issue lies in the highly variable and low lymphocyte isolation yields from these tissues due to extensive and severe damage associated with lethal irradiation conditioning. In contrast, lymphocyte isolation yields from the spleens are consistent, though also low at this early stage. Nevertheless, these data demonstrate that GzmB deficiency in donor CD4+CD25− T cells increases their expansion at the early stage following allo-HCT.
Figure 5. GzmB−/− CD4+CD25− T cells exhibit enhanced expansion compared to WT cells.
BALB/c host mice were lethally irradiated on day −1. On day 0, mice were injected with 2×106 TCD-BM cells alone or combined with 5×104 WT or GzmB−/− CD4+CD25− T cells isolated from C57BL/6 donor mice. (A) On day 5 after allo-HCT, splenocytes were harvested from the host mice and analyzed by flow cytometry. Representative flow plots showing CD3 and CD4 staining of live donor cells are gated on H-2Kb+Live/Dead− cells. Summary data show the absolute total numbers of live donor T cells acquired by multiplying the percentages of H-2Kb+Live/Dead− CD3+CD4+ cells to total cell numbers harvested from the host spleens (B), large intestines (C) and MLN (D). Data are shown as mean ± SD, with 4–6 mice assessed in each group at each time point. Two-tailed t-tests were performed to determine statistically significant differences.
GzmB does not affect donor CD4+CD25− T cell proliferation or tissue-specific migration
We next examined whether donor T cell proliferation was altered by GzmB deficiency. Using a CFSE-based proliferation assay, we observed robust T cell proliferation in vivo on day 3 after HCT, as evidenced by significant CFSE dilution in donor CD4+CD25− T cells. However, GzmB deficiency in donor T cells did not impact T cell proliferation (Supplemental Figure 2A). As an alternative method, we examined Ki-67 expression levels in the donor-derived CD4+CD25− T cells. Again, no significant difference was observed between WT and GzmB−/− T cells (data not shown). These data indicate that GzmB does not affect T cell proliferation. Since we observed significantly increased damage in the large intestines of hosts receiving GzmB−/− T cells (Figures 3–4), we next examined the expression of chemokine receptors that are important in gut-specific homing for T cells, including LPAM-1 and CCR9. However, no significant difference was observed for the expression of LPAM-1 or CCR9 on WT versus GzmB−/− T cells in spleen, MLN and intraepithelial lymphocytes (IELs) in the large intestine 5 days (Supplemental Figure 2B–2C) and 8 days (data not shown) post transplantation. These results suggest that GzmB may not affect T cell trafficking and gut tissue infiltration in our models.
GzmB is involved in activation-induced cell death of donor CD4+CD25− T cells
Previous reports showed that GzmB was involved in activation-induced cell death (AICD) of CD4+ and CD8+ T cells (25, 26). Our recent study shows that GzmB contributes to AICD in donor CD8+ T cells after allo-HCT (12). Thus we hypothesized that GzmB-mediated AICD may account for the different expansion and GVH activity between WT and GzmB−/− CD4+CD25− T cells. To test this idea, we used Annexin V staining in combination with Live/Dead dye to measure donor CD4+CD25− T cell death in the host mice (Figure 6A). This staining system has been used to distinguish cells undergoing early stage of cell death (Annexin V+) versus cells that have entered the late stage of death (Live/Dead+). We chose to assess AICD on day 5 after transplantation, a time point when we observed significantly higher numbers of live T cells in host mice receiving GzmB−/− T cells. Our results show that on day 5 after HCT about 40–50% of WT donor T cells were Annexin V+ and undergoing early stage of cell death, while GzmB−/− donor T cells showed a significant decrease in the frequency of Annexin V+ cells in the spleen and MLN (Figure 6B). In an attempt to assess other molecules associated with cell death, we examined Bcl-2 expression, caspase-3 activation, and reactive oxygen species (ROS) production in donor CD4+ T cells. Bcl-2 expression was not altered between WT and GzmB−/− T cells (data not shown); nor was caspase-3 activation (Figure 6C). Interestingly, we have observed that ROS production was significantly increased in WT versus GzmB−/− T cells (Figure 6D), correlated with increased death of WT CD4+ T cells as measured by Annexin V staining (Figure 6B). Notably, this result is consistent with a recent report showing that GzmB induces cell death in an ROS-dependent but caspase-independent manner (27). Together, these data indicate that GzmB contributes to AICD of donor CD4+CD25− T cells following allo-HCT. Therefore, GzmB deficiency results in a T cell survival advantage that leads to the increased expansion of GzmB−/− CD4+CD25− T cells after allo-HCT.
Figure 6. GzmB is involved in activation-induced cell death of donor CD4+CD25− T cells.
BALB/c host mice were lethally irradiated on day −1. On day 0, mice were injected with 2×106 TCD-BM cells alone or combined with 3×105 WT or GzmB−/− CD4+CD25− T cells isolated from C57BL/6 donor mice. On day 5 after allo-HCT, total cells harvested from the host spleens and MLN were assessed by Annexin V and Live/Dead dye staining to measure cell death. (A) Representative dot plots are gated on donor T cells (H-2Kb+CD3+CD4+) with naïve healthy donor mice serving as negative controls to show background cell death. (B) The percentages of donor T cells from the spleen (left panel) and MLN (right panel) that are Annexin V+ are shown, with 5 mice in each group. Background cell death indicated by the negative control was subtracted from each sample to show cell death induced specifically by allogeneic activation. Shown are representative data from one out of five independent experiments with similar results. Activated caspase 3 (C) and ROS (D) were stained to assess cell death. Shown are representative data from one out of three independent experiments with similar results. Two-tailed t-tests were performed to determine statistically significant differences.
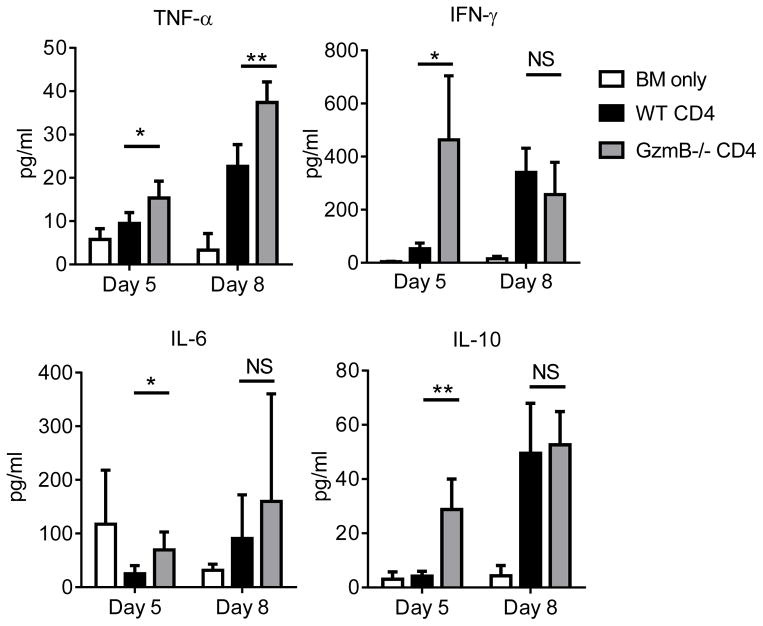
GzmB−/− CD4+CD25− T cells induce increased levels of proinflammatory cytokines
To explore the GzmB-independent mechanisms that cause the rapid and lethal GVHD, we performed Luminex assays to measure a panel of proinflammatory cytokines in the host mice. On day 5 after allo-HCT, significantly higher levels of IFN-γ were found in the peripheral blood of the hosts receiving GzmB−/− T cells compared to WT T cells (Figure 7). Intracellular IFN-γ staining did not show a significant difference between WT and GzmB−/− T cells (data not shown), suggesting that the enhanced IFN-γ levels may be due to the increased expansion of GzmB−/− T cells observed (Figure 5). Significantly higher amounts of TNF-α were also found in the hosts receiving GzmB−/− T cells. A similar pattern was also observed for IL-6 and IL-10. We reason that some of the elevated cytokines may cause an acute systemic inflammation that leads to the rapid lethality observed. For example, TNF-α in combination with IFN-γ has been shown to damage endothelial cells and contribute to vascular injury (28), which may be responsible for the frequently observed colonic bleeding in the host mice receiving GzmB−/− T cells. This observation suggests that the significantly enhanced production of proinflammatory cytokines early following allo-HCT may be responsible for different survival outcomes between hosts receiving WT and GzmB−/− CD4+CD25− T cells.
Figure 7. GzmB−/− CD4+CD25− T cells induce higher amounts of proinflammatory cytokines.
BALB/c (H-2d) host mice were lethally irradiated on day −1. On day 0, mice were injected with 2×106 TCD-BM cells alone or combined with 5×104 WT or GzmB−/− CD4+CD25− T cells isolated from C57BL/6 (H-2b) donor mice. Serum samples were collected via eye bleeding on day 5 and day 8 after allo-HCT. Serum cytokines levels were measured by luminex assays. Data are shown as mean ± SD, with 4–5 mice assessed in each group at each time point. Two-tailed t-tests were performed to determine statistically significant differences (*P<0.05; **P<0.01).
Discussion
GzmB was initially known to be a key cytotoxic molecule used only by cytotoxic lymphocytes to kill host cells infected by intracellular pathogens or transformed tumor cells. However, recent studies have revealed complex regulatory roles played by GzmB in the settings of tumor immunity, infection immunity and transplantation immunity (13, 29–31). In the setting of allo-HCT, our previous study demonstrated that GzmB is required for donor CD8+ T cells to cause severe GVHD. In the current study focusing on CD4+CD25− T cells, we have found that GzmB plays a role opposite to that in CD8+ T cells in that GzmB diminishes the GVH activity of CD4+CD25− T cells. Although an underlying mechanism involving AICD appears to be shared by these two effector T cell subsets, the opposite impacts on GVHD may be due to the fundamental difference between how CD4+ versus CD8+ T cells recognize and interact with their target cells. That is, GzmB-mediated killing requires direct contact between T cells and target cells (6, 7). MHC class I and II molecules are an essential component for the primary synapse which is required for T cell priming and activation. Later for activated T cells to execute their function, a secondary synapse may be required to mediate direct cell contact between T cells and target cells in a process that may also require MHC molecules (6, 7). Almost all tissue cells express MHC class I and are thus susceptible to contact-dependent, GzmB-mediated killing by CD8+ T cells. Therefore, loss of GzmB function in CD8+ T cells understandably decreases their GVH activity. In contrast, most normal tissue cells do not typically express MHC class II and are therefore less likely subjected to contact-dependent, GzmB-mediated killing triggered by CD4+ T cells. In this case, GzmB-mediated damage of CD4+ T cells will have a negative impact on their GVH activity which is mainly mediated through cell contact-independent proinflammatory cytokines. This mechanism could explain why loss of GzmB in CD4+CD25− T cells enhances their GVH activity.
In conclusion, the differential impacts of GzmB delivered from different T cell subsets suggest a promising strategy to separate GVHD from the desired graft-versus-tumor (GVT) effect. Based on our findings, we believe that genetically silencing GzmB expression in donor CD8+ T cells may lead to favorable outcomes. Specifically, we propose to use GzmB-silenced CD8+ T cells as a supplement to HSCs because of their enhanced GVT effect and decreased GVH activity (12). On the other hand, due to the higher GVH toxicity, CD4+ T cells could be excluded from the supplement or added at a very small dose to provide essential help. In the latter case, the added CD4+ T cells should have normal GzmB function because it reduces their GVH activity. Based on further studies, this concept may potentially result in new clinical trials that can lead to better survival and improved quality of life for allo-HCT patients with selected hematologic diseases.
Supplementary Material
Acknowledgments
We thank Kelvin Lee, Sharon Evans and Elizabeth Repasky for their helpful advice and technical assistance.
This work was supported by NIH research grant # R01CA184728 (X.C.) and a generous donation from Brendan and Elise McCarthy to the RPCI Alliance BMT Donation Fund (X.C. and P.L.M.). N.D.L. was supported by a T32 pre-doctoral training grant from NIH (CA085183).
Footnotes
Disclosure of Conflict of Interest
The authors have no potential conflict of interest to disclose.
References
- 1.Shlomchik WD. Graft-versus-host disease. Nat Rev Immunol. 2007;7:340–352. doi: 10.1038/nri2000. [DOI] [PubMed] [Google Scholar]
- 2.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 3.Welniak LA, Blazar BR, Murphy WJ. Immunobiology of allogeneic hematopoietic stem cell transplantation. Annu Rev Immunol. 2007;25:139–170. doi: 10.1146/annurev.immunol.25.022106.141606. [DOI] [PubMed] [Google Scholar]
- 4.Vogelsang GB, Lee L, Bensen-Kennedy DM. Pathogenesis and treatment of graft-versus-host disease after bone marrow transplant. Annu Rev Med. 2003;54:29–52. doi: 10.1146/annurev.med.54.101601.152339. [DOI] [PubMed] [Google Scholar]
- 5.Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nature reviews Immunology. 2012;12:443–458. doi: 10.1038/nri3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lieberman J. The ABCs of granule-mediated cytotoxicity: new weapons in the arsenal. Nature reviews Immunology. 2003;3:361–370. doi: 10.1038/nri1083. [DOI] [PubMed] [Google Scholar]
- 7.Russell JH, Ley TJ. Lymphocyte-mediated cytotoxicity. Annu Rev Immunol. 2002;20:323–370. doi: 10.1146/annurev.immunol.20.100201.131730. [DOI] [PubMed] [Google Scholar]
- 8.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nature reviews Immunology. 2006;6:836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 9.Schmaltz C, Alpdogan O, Muriglan SJ, Kappel BJ, Rotolo JA, Ricchetti ET, Greenberg AS, Willis LM, Murphy GF, Crawford JM, van den Brink MR. Donor T cell-derived TNF is required for graft-versus-host disease and graft-versus-tumor activity after bone marrow transplantation. Blood. 2003;101:2440–2445. doi: 10.1182/blood-2002-07-2109. [DOI] [PubMed] [Google Scholar]
- 10.Graubert TA, Russell JH, Ley TJ. The role of granzyme B in murine models of acute graft-versus-host disease and graft rejection. Blood. 1996;87:1232–1237. [PubMed] [Google Scholar]
- 11.Graubert TA, DiPersio JF, Russell JH, Ley TJ. Perforin/granzyme-dependent and independent mechanisms are both important for the development of graft-versus-host disease after murine bone marrow transplantation. J Clin Invest. 1997;100:904–911. doi: 10.1172/JCI119606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bian G, Ding X, Leigh ND, Tang Y, Capitano ML, Qiu J, McCarthy PL, Liu H, Cao X. Granzyme B-Mediated Damage of CD8+ T Cells Impairs Graft-versus-Tumor Effect. J Immunol. 2013;190:1341–1350. doi: 10.4049/jimmunol.1201554. [DOI] [PubMed] [Google Scholar]
- 13.Cao X, Cai SF, Fehniger TA, Song J, Collins LI, Piwnica-Worms DR, Ley TJ. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity. 2007;27:635–646. doi: 10.1016/j.immuni.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 14.Revell PA, Grossman WJ, Thomas DA, Cao X, Behl R, Ratner JA, Lu ZH, Ley TJ. Granzyme B and the downstream granzymes C and/or F are important for cytotoxic lymphocyte functions. J Immunol. 2005;174:2124–2131. doi: 10.4049/jimmunol.174.4.2124. [DOI] [PubMed] [Google Scholar]
- 15.Shresta S, Graubert TA, Thomas DA, Raptis SZ, Ley TJ. Granzyme A initiates an alternative pathway for granule-mediated apoptosis. Immunity. 1999;10:595–605. doi: 10.1016/s1074-7613(00)80059-x. [DOI] [PubMed] [Google Scholar]
- 16.Fehniger TA, Cai SF, Cao X, Bredemeyer AJ, Presti RM, French AR, Ley TJ. Acquisition of murine NK cell cytotoxicity requires the translation of a pre-existing pool of granzyme B and perforin mRNAs. Immunity. 2007;26:798–811. doi: 10.1016/j.immuni.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Kagi D, Ledermann B, Burki K, Seiler P, Odermatt B, Olsen KJ, Podack ER, Zinkernagel RM, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan DH, Anderson BE, McNiff JM, Jain D, Shlomchik MJ, Shlomchik WD. Target antigens determine graft-versus-host disease phenotype. J Immunol. 2004;173:5467–5475. doi: 10.4049/jimmunol.173.9.5467. [DOI] [PubMed] [Google Scholar]
- 19.Shi M, Adachi Y, Cui Y, Li M, Lian Z, Zhang Y, Yanai S, Shima C, Imai Y, Ikehara S. Combination of intra-bone marrow-bone marrow transplantation and subcutaneous donor splenocyte injection diminishes risk of graft-versus-host disease and enhances survival rate. Stem cells and development. 2011;20:759–768. doi: 10.1089/scd.2010.0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cai SF, Cao X, Hassan A, Fehniger TA, Ley TJ. Granzyme B is not required for regulatory T cell-mediated suppression of graft-versus-host disease. Blood. 2010;115:1669–1677. doi: 10.1182/blood-2009-07-233676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding X, Bian G, Leigh ND, Qiu J, McCarthy PL, Liu H, Aygun-Sunar S, Burdelya LG, Gudkov AV, Cao X. A TLR5 Agonist Enhances CD8+ T Cell-Mediated Graft-versus-Tumor Effect without Exacerbating Graft-versus-Host Disease. J Immunol. 2012;189 doi: 10.4049/jimmunol.1201206. published online 8 October 2012. [DOI] [PubMed] [Google Scholar]
- 22.Goerner M, Weber-Nordt R, Hoepfner S, Benner A, Luft T, Ho AD. Addition of a low fixed number of CD3+ cells to CD34-enriched allografts: effects on engraftment, graft-versus-host disease, and survival after related and unrelated peripheral stem cell transplantation. Journal of hematotherapy & stem cell research. 2003;12:309–320. doi: 10.1089/152581603322023043. [DOI] [PubMed] [Google Scholar]
- 23.Martin PJ, Akatsuka Y, Hahne M, Sale G. Involvement of donor T-cell cytotoxic effector mechanisms in preventing allogeneic marrow graft rejection. Blood. 1998;92:2177–2181. [PubMed] [Google Scholar]
- 24.Hanash AM, Dudakov JA, Hua G, O’Connor MH, Young LF, Singer NV, West ML, Jenq RR, Holland AM, Kappel LW, Ghosh A, Tsai JJ, Rao UK, Yim NL, Smith OM, Velardi E, Hawryluk EB, Murphy GF, Liu C, Fouser LA, Kolesnick R, Blazar BR, van den Brink MR. Interleukin-22 protects intestinal stem cells from immune-mediated tissue damage and regulates sensitivity to graft versus host disease. Immunity. 2012;37:339–350. doi: 10.1016/j.immuni.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Devadas S, Das J, Liu C, Zhang L, Roberts AI, Pan Z, Moore PA, Das G, Shi Y. Granzyme B is critical for T cell receptor-induced cell death of type 2 helper T cells. Immunity. 2006;25:237–247. doi: 10.1016/j.immuni.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 26.Zhang M, Park SM, Wang Y, Shah R, Liu N, Murmann AE, Wang CR, Peter ME, Ashton-Rickardt PG. Serine protease inhibitor 6 protects cytotoxic T cells from self-inflicted injury by ensuring the integrity of cytotoxic granules. Immunity. 2006;24:451–461. doi: 10.1016/j.immuni.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Jacquemin G, Margiotta D, Kasahara A, Bassoy EY, Walch M, Thiery J, Lieberman J, Martinvalet D. Granzyme B-induced mitochondrial ROS are required for apoptosis. Cell death and differentiation. 2015;22:862–874. doi: 10.1038/cdd.2014.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li JH, Pober JS. The cathepsin B death pathway contributes to TNF plus IFN-gamma-mediated human endothelial injury. J Immunol. 2005;175:1858–1866. doi: 10.4049/jimmunol.175.3.1858. [DOI] [PubMed] [Google Scholar]
- 29.Knickelbein JE, Khanna KM, Yee MB, Baty CJ, Kinchington PR, Hendricks RL. Noncytotoxic lytic granule-mediated CD8+ T cell inhibition of HSV-1 reactivation from neuronal latency. Science. 2008;322:268–271. doi: 10.1126/science.1164164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Froelich CJ, Pardo J, Simon MM. Granule-associated serine proteases: granzymes might not just be killer proteases. Trends Immunol. 2009;30:117–123. doi: 10.1016/j.it.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 31.van Dommelen SL, Sumaria N, Schreiber RD, Scalzo AA, Smyth MJ, Degli-Esposti MA. Perforin and granzymes have distinct roles in defensive immunity and immunopathology. Immunity. 2006;25:835–848. doi: 10.1016/j.immuni.2006.09.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.