Abstract
Sensitized recipients with pre-transplant donor-specific antibodies (DSA) are at higher risk for antibody-mediated rejection (AMR) than non-sensitized recipients, yet little is known about the properties of memory B cells that are central to the recall alloantibody responses. Using cell enrichment and MHC Class I tetramers, C57BL/6 mice sensitized with BALB/c splenocytes were shown to harbor H-2Kd-specific IgG+ memory B cells with a post-GC phenotype (CD73+CD273+CD38hiCD138−GL7−). These memory B cells adoptively transferred in naïve mice without memory T cells recapitulated class-switched recall alloantibody responses. During recall, memory H-2Kd-specific B cells preferentially differentiated into antibody-secreting cells (ASCs), whereas in primary response, H-2Kd-specific B cells differentiated into germinal center (GC) cells. Finally, our studies revealed that despite fundamental differences in alloreactive B cell fates in sensitized versus naive recipients, CTLA-4Ig was unexpectedly effective at constraining B cell responses and heart allograft rejection in sensitized recipients.
Introduction
Desensitization protocols with intravenous immunoglobulin (IVIG) in combination with plasmapheresis, rituximab, bortezomib, rabbit antithymocyte globulin and edulizumab are being used in sensitized recipients to reduce pre-transplant DSA and inhibit the increase in DSA post-transplantation (1, 2) (3). Despite these efforts, the rates of antibody-mediated rejection in sensitized patients remain significantly higher compared to non-sensitized recipients (4) (5), and there continues to be a need for better desensitization and immunosuppressive strategies.
Long-lived plasma cells and quiescent memory B cells confer memory in sensitized individuals (6). Plasma cells reside in specialized niches in the bone marrow, secondary lymphoid organs and inflammatory sites, and are responsible for maintaining elevated DSA levels. In contrast, memory B cells remain quiescent in the absence of antigen but are responsible for the faster, more vigorous and class-switched antibody response upon antigen re-exposure. Plasma cells have been extensively investigated (7, 8), while there is limited information on memory B cells in the setting of allograft transplantation. Recent breakthroughs in tracking rare endogenous B cells to model antigens have identified new features of memory B cells including their heterogeneity (9–17). In this study, we used Class I MHC tetramers (18) to identify endogenous memory alloreactive B cells, track their fate after heart allograft transplantation, and define their susceptibility to continuous CTLA-4Ig therapy.
Materials and Methods
Animals and Tetramers
4–6 weeks old C57BL/6 and BALB/c mice were purchased from Harlan Sprague Dawley. Congenic Igha mice, B6.Cg-Gpi1a Thy1a Igha/J, were purchased from Jackson Laboratory (Bar Harbor, ME). Mice sensitized with BALB/c spleen or hearts, were injected 500 µg of CTLA4-Ig (Nulojix; Bristol-Myers Squibb) per mouse, intraperitoneally, on day −2, 0 and 2 and then twice per week until the end of the experiment. H2Kd-biotin monomers, H2Kd tetramers, loaded with the SYIPSAEKI peptide from malaria Plasmodium berghei, were conjugated with PE or APC (NIH Tetramer Core Facility, Atlanta, GA).
Statistics
Data are presented as mean ± SEM. Student’s t test (unpaired) was used when two groups were compared or one-way ANOVA when there were ≥2 groups. Two-way ANOVA was used when there were ≥2 variables, followed by Turkey test to compare pairs of means. Survival curves were compared using Mantel-Cox test. All analyses were performed using GraphPad Prism. P values ≤ 0.05 were considered statistically significant.
Study approval
All animal experiments were performed under protocols approved by the Institutional Animal Care and Use Committee (IACUC) of University of Chicago.
Results and Discussion
Recall antibody response is B cell intrinsic
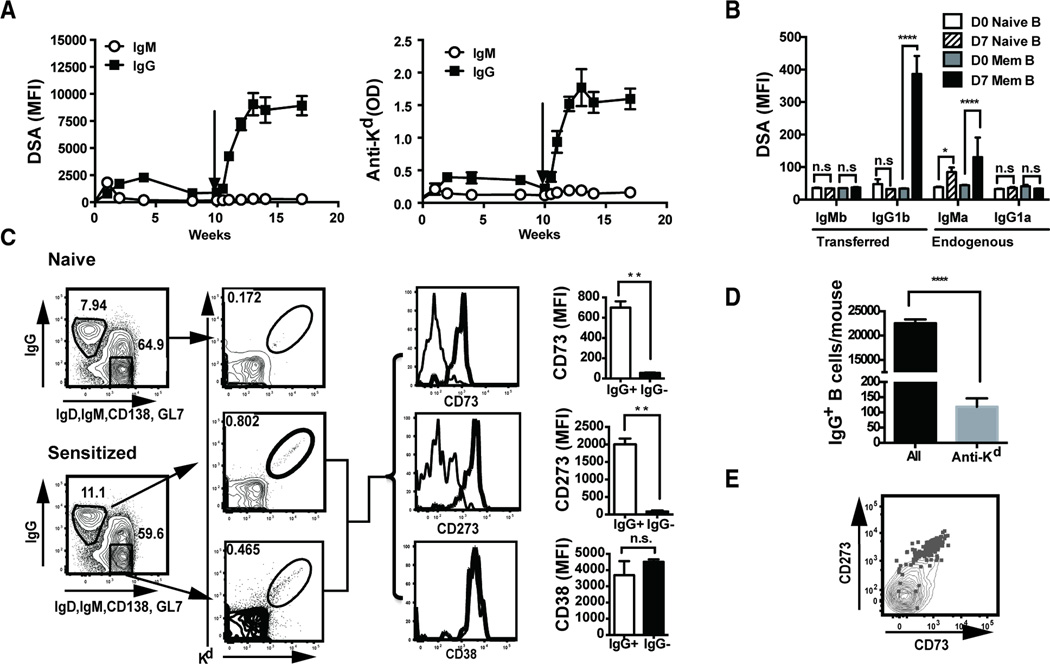
Specific sensitization to HLA antigens can be induced by blood transfusion, pregnancies and previous allografts, as well as through cross-reactivity to environmental antigens or microbes. For our studies, we sensitized C57BL/6 mice with a single s.c. injection of BALB/c splenocytes, and elicited a recall response with a second injection 10 weeks later (Fig 1A). The primary donor-specific and anti-H2Kd IgM response peaked on day 7, while the IgG response peaked at 4 weeks post-sensitization and gradually returned towards baseline by 8–10 weeks post-immunization (Fig 1A). The recall donor-specific and anti-H2Kd antibody response was predominantly IgG, detected by day 7 post-immunization and peaked at titers that were significantly higher than the primary response (Fig 1A).
Fig 1.
The recall alloantibody response is B cell intrinsic. (A) Antibody responses following primary and secondary immunization with BALB/c spleen cells (s.c.). Donor specific antibodies (DSA) were measured by flow cytometry (mean fluorescence intensity (MFI)) and anti-Kd antibodies by ELISA. N=5 mice per experiment, repeated twice. (B) Adoptive transfer to demonstrate memory B cell responses. C57BL/6 mice (IgHb) were immunized with BALB/c splenocytes (s.c.), and ≥10 weeks later, mice were sacrificed and B cells were purified and adoptively transferred ((20 × 106) into congenic IgHa recipients. One day after transfer, IgHa recipient mice were immunized with BALB/c splenocytes, and 7 days later, donor specific IgMb and IgG1b produced by transferred B cells, and IgMa and IgG1a produced by recipient B cells, were quantified by flow cytometry. N=2–3/group, repeated 2 times. (C) Gating strategy and percentage of IgG+ B cells and H-2Kd-binding IgG+ B cells from naïve or sensitized mice (left two panels), and phenotype of memory H-2Kd-binding IgG+ versus IgG− B cells. Representative histograms and MFI for CD73, CD38 and CD273 expression are presented (right two panels). N=3–4/group, repeated 3 times. (D) Total number of IgG memory and H-2Kd-binding IgG+ cells per sensitized or naïve mouse (pooled spleen plus 6 lymph nodes). (E) Co-expression of CD273 and CD73 on memory H-2Kd-binding IgG+ B cells.
To investigate whether the features of the vigorous class-switched recall response were due to memory B cells that did not require the presence of memory T cells, we transferred purified B cells from sensitized or naive C57BL/6 mice (IgHb) into naïve syngeneic recipients (IgHa). This model avoids homeostatic proliferation, yet allows antibodies secreted by transferred or recipient B cells to be identified by their IgHb and IgHa allotype, respectively. One day after transfer we sensitized with BALB/c splenocytes, and 7 days later we observed a significant increase in BALB/c-specific IgG1a that was absent in recipients receiving B cells from naïve mice (Fig 1B). These observations confirmed that memory B cells in the presence of naive T cells were capable of generating a rapid class-switched alloantibody response. An endogenous B cell IgMa response was observed in mice receiving memory B cells, suggesting a lack of interference by the BALB/c-specific IgG. In mice receiving naïve B cells, no IgG1a responses were observed, and the observed BALB/c-specific IgMa response confirmed the efficacy of the immunization.
Phenotype and fate of endogenous memory alloreactive B cells
Low numbers and lack of specific cell surface markers have made the study of endogenous antigen-specific memory B cells in mice challenging, however, recently described enrichment protocols have allowed these low-frequency B cells to be identified (10, 15). Memory B cells can express IgM or IgG, but because of higher affinity for antigen, memory IgG+ B cells were shown to dominate the recall response when circulating antigen-specific IgG was present (10). Because low levels circulating IgG alloantibodies are present in our sensitized mice, so we focused our efforts on characterizing the IgG+ memory alloreactive B cells. Memory IgG+ B cells from mice sensitized with BALB/c splenocytes ≥10 weeks prior were enriched using FITC-conjugated anti-IgG followed by anti-FITC magnetic bead capture. Very few IgG+ cells were detected in the flow through (Supplemental Fig 1), indicating efficient enrichment with this approach. Alloreactive B cells within the enriched IgG+ population were then identified as double-positive for PE- or APC-conjugated H-2Kd tetramers (Fig 1C). Approximately 100–200 quiescent (GL7−CD138−) H-2Kd-reactive IgG+ B cells were recovered from each sensitized mouse, representing approximately 0.5% of total IgG+ expressing B cells (Fig 1D).
Shlomchik and colleagues (9, 12, 13) reported that memory B cells can be subdivided into at least five phenotypic subsets based on the expression of CD273 (PD-L2), CD80 and CD73. Of these, the CD80+CD273+ subset was most “memory-like” and dominated the recall response by preferentially differentiating into antibody secreting cells (ASCs), whereas the CD273−CD80− subset was most “naive-like” and differentiated into GC cells. We determined the phenotype of the H-2Kd-reactive IgG+ B cells as quiescent CD38+, CD73+ and CD273+ (Fig 1C) and CD80low (data not shown). In contrast, the IgG− (predominantly IgM) population was CD273low/− and CD73−, while there were too few H-2Kd-reactive IgG+ B cells in naïve mice for reliable assessment (Fig 1C). The expression of CD73 marks post-GC memory cells (12, 13, 15), while the single-positive expression of CD273 is consistent with an “intermediate” memory phenotype (13) (9). We speculate that differences in the long-lived memory B cell phenotypes following allo-sensitization compared to immunization with NP-CGG in alum, arose from different innate inflammatory signals during the primary antigen encounter. Supporting this is the observation by Katsuri et al. (19) that innate signals triggered by Toll-like receptors affected the persistence of GC and plasma cell responses, and the programming towards B cell memory. Whether this “intermediate” memory B cell phenotype induced by donor splenocytes (DSC) is preserved following other different clinically-relevant routes of sensitization, such as pregnancy or organ transplantation, is not known.
Differentiation of endogenous memory alloreactive B cells after allogeneic heart transplantation
Recent studies on memory B cells using model antigens and adjuvants have identified two possible fate trajectories of memory B cells following antigen reencounter. Most studies in mice and humans show that class-switched memory B cells preferentially differentiate into plasma cells whereas unswitched (IgM+) memory B cells preferentially differentiate into GC cells (9–11, 20). However others suggest that IgG+ memory B cells retain the potential to differentiate into GCs and undergo further BCR-diversification (21). We previously reported that BALB/c heart transplantation into naïve C57BL/6 recipients induced a preferential differentiation of Kd-specific B cells into GC cells (18). We here report that when BALB/c hearts were transplanted into sensitized C57BL/6 mice, minimal differentiation of Kd-specific memory B cells into GL7+Fas+ GC B cells was observed at both early (7 days) or late (14–15 days) times post-transplant (Fig 2A–B).
Fig 2.
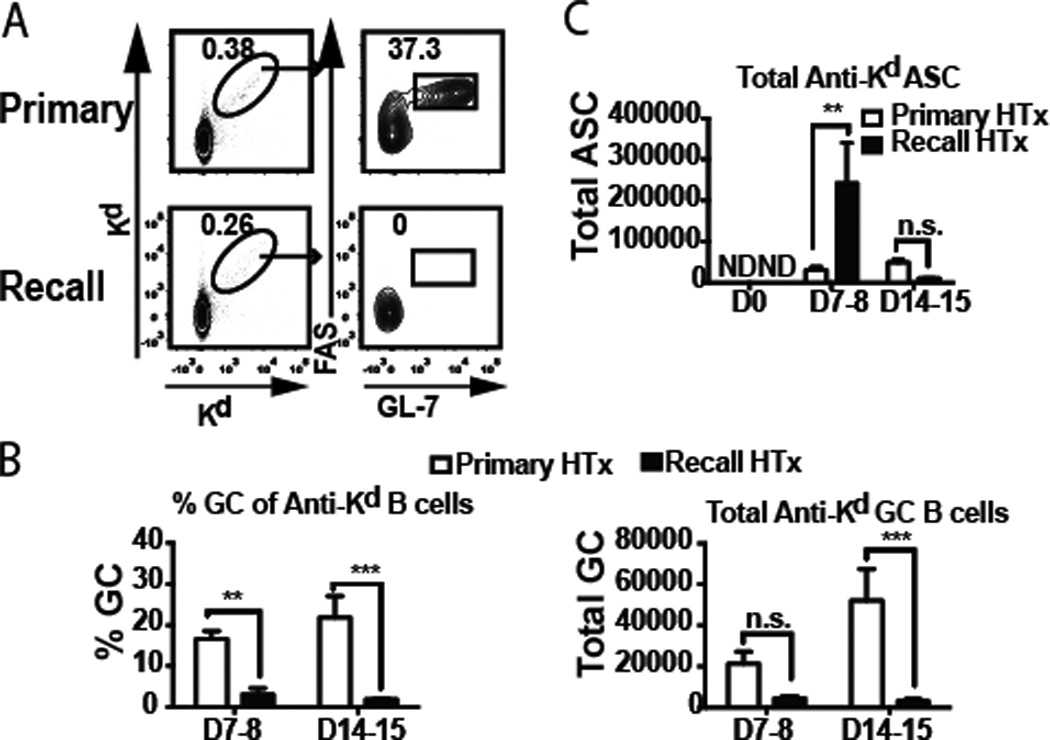
Germinal center (GC) responses predominate during a primary response whereas ASC responses predominate during secondary response. (A) Flow cytometry analysis of pre-gated B220+IgDlow B cells binding to APC- and PE-conjugated H2Kd tetramers, and expressing a GC phenotype (Fas+GL7+). (B) Percentage and total numbers of H-2Kd-binding GC B cells, and (C) total number of H-2Kd-specific IgG secreting ASCs per mouse (one spleen plus 6 lymph nodes) from naïve or sensitized recipients, and analyzed on the indicated days post-heart transplant. N=3–5/group, repeated twice; N.D. not detected.
We next investigated whether Kd-specific memory B cells differentiated preferentially into antibody secreting cells (ASCs) using a Kd-specific enzyme-linked immunospot (ELISPOT) assay. An increase in the frequency of ASCs from pooled spleen and lymph node cells was observed on day 7 post-transplant in sensitized recipients, and this response was significantly more vigorous than in naïve recipients (Fig 2C). Thus similar to memory B cells generated to model antigens adjuvanted in alum or complete Freund’s adjuvant (9, 10, 13, 17), and in contrast to those generated with monophosphoryl lipid A adjuvant (21), memory alloreactive B cells generated by DSC immunization almost exclusively differentiated into ASC following allogeneic heart transplantation. Interestingly the generation of ASCs to the allograft was unexpectedly short-lived and no longer detectable by day 14 post-transplant. While it is possible that a subset of ASC may have migrated into the BM, we were unable to reliably detect them (data not shown). Similar transient ASC responses have been described for recall B cell responses in humans (22, 23), and rapid clearance of antigen by IgG produced during the recall response as well as the short-lived lifespan of ASC have been proposed to explain the rapid decline of the ASC response (23). In our studies, allografts are not removed after rejection so it is unlikely that clearance of alloantigen causes the rapid abrogation of ASC response, and the signals controlling the kinetics of recall ASC response warrants further investigation.
CTLA-4Ig inhibits memory alloreactive B cell responses to heart allografts
The correlation between pre-transplant or de novo alloantibody responses, AMR and poor graft outcomes suggests a need for therapies that inhibit the B cell recall response. Belatacept, a high affinity CTLA-4Ig, as continuous dosing regimen has been approved for the prophylaxis of rejection in kidney transplant recipients (24, 25). We tested whether CTLA-4Ig in a continuous regimen (500 µg/mouse; day −2 and 0 and then 2 times/week till sacrifice), could modulate the recall B cell response to BALB/c hearts in DSC-sensitized recipients (Fig 3). CTLA-4Ig was unexpectedly efficacious, inhibiting both recall ASC and DSA responses in sensitized recipients, whereas control Fc-Ig1 did not (Fig 3A–B; Supplemental Fig 2). Immunohistochemistry confirmed an absence of C4d deposition in the graft at day 7 post-transplantation (Fig 3C). Coincident with this control of recall antibody responses, continuous CTLA-4Ig treatment prolonged the survival of heart allografts in sensitized recipients; with the majority (7 of 11) of the allografts still beating on day 30 post-transplantation (Fig 3D). Thus recall alloantibody responses are unexpectedly dependent on CD28-CD80/CD86 interactions, and can be controlled by CTLA-4Ig. However, we cannot exclude the possibility that sensitization through routes that elicit more vigorous alloreactive responses may result in memory B cells that are less sensitive to CTLA-4Ig.
Fig 3.
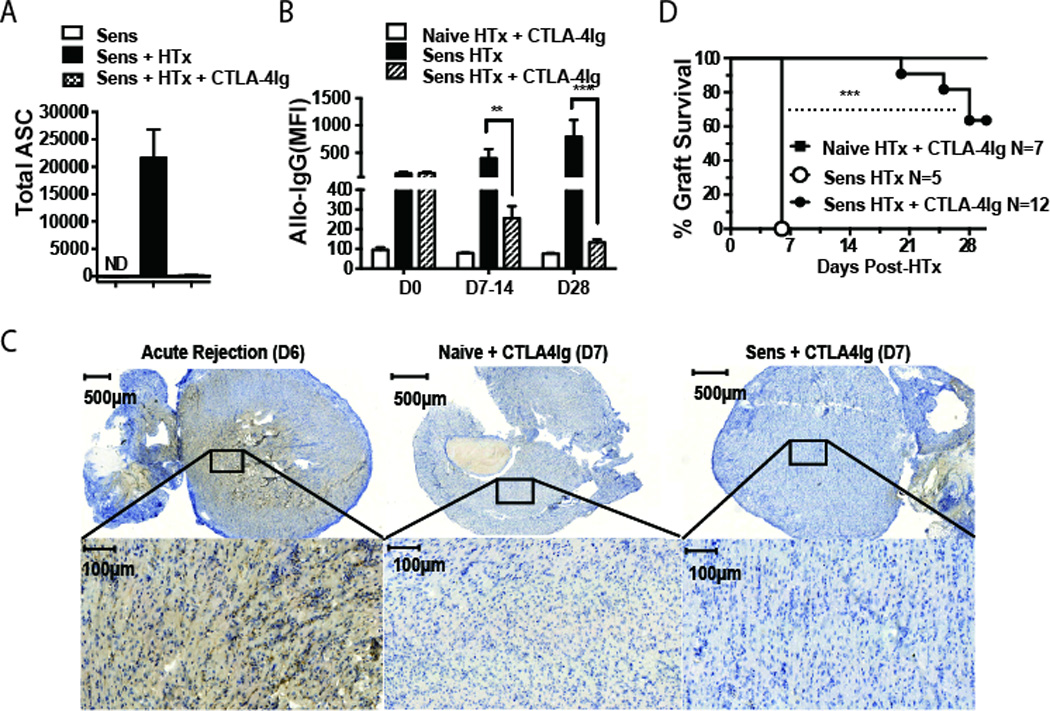
Continuous CTLA-4Ig inhibits the recall antibody response and prolongs allograft survival in sensitized recipients. (A) Spleen and lymph nodes were harvested from sensitized mice, prior to or on day 7 post-transplantation, and the total number of H-2Kd-specific ASC per mouse was quantified by H-2Kd-IgG ELISPOT. (B) Donor-specific IgG production was quantified by flow cytometry, and (C) C4d deposition in allografts on day 7 post-transplant was assessed by immunohistochemistry. N=2–3/group, repeated twice. (D) BALB/c allograft survival in sensitized C57BL/6 recipients.
The efficacy of CTLA-4Ig in sensitized mice contrasts with reports that CTLA-4Ig is only effective at inhibiting naïve but not memory T cell responses because of redundancy of co-stimulatory molecules on memory T cells (26, 27). However, those studies were based on the transient administration of CTLA-4Ig to induce tolerance, whereas our treatment protocol involves a continuous administration of CTLA-4Ig. The inhibition of memory as well as ongoing (18) B cell responses by CTLA-4Ig may explain the clinical observation of DSA being significantly lower in transplant patients on Belatacept compared to those on calcineurin-inhibitors, despite higher rates of acute rejection (28). Recently, CTLA-4Ig has been shown to be expressed on both Tfh and regulatory Tfh cells and to be dominantly critical for constraining B cell responses (29, 30). In light of these observations, it is reassuring that the outcome of CTLA-4Ig treatment is the inhibition of B cell responses. Finally, our results together with the observation that the majority of memory CD4 cells in humans retain CD28 expression (31) provide a rationale for testing whether Belatacept can inhibit recall antibody responses in humans.
Supplementary Material
Acknowledgement
We are grateful to Dr. Wink Baldwin, Cleveland Clinic, for his generous assistance with the C4d immunohistochemistry
This work was supported in part by grants (1R01AI110513, P01AI097113) from the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health. MHC Class I tetramers were provided by the NIH Tetramer Core Facility (contract HHSN272201300006C).
References
- 1.Montgomery RA, Cozzi E, West LJ, Warren DS. Humoral immunity and antibody-mediated rejection in solid organ transplantation. Semin Immunol. 2011;23:224–234. doi: 10.1016/j.smim.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 2.Huber L, Lachmann N, Durr M, Matz M, Liefeldt L, Neumayer HH, Schonemann C, Budde K. Identification and therapeutic management of highly sensitized patients undergoing renal transplantation. Drugs. 2012;72:1335–1354. doi: 10.2165/11631110-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 3.Burton SA, Amir N, Asbury A, Lange A, Hardinger KL. Treatment of antibody-mediated rejection in renal transplant patients: a clinical practice survey. Clin Transplant. 2014 doi: 10.1111/ctr.12491. [DOI] [PubMed] [Google Scholar]
- 4.Haririan A, Nogueira J, Kukuruga D, Schweitzer E, Hess J, Gurk-Turner C, Jacobs S, Drachenberg C, Bartlett S, Cooper M. Positive cross-match living donor kidney transplantation: longer-term outcomes. Am J Transplant. 2009;9:536–542. doi: 10.1111/j.1600-6143.2008.02524.x. [DOI] [PubMed] [Google Scholar]
- 5.Riella LV, Safa K, Yagan J, Lee B, Azzi J, Najafian N, Abdi R, Milford E, Mah H, Gabardi S, Malek S, Tullius SG, Magee C, Chandraker A. Long-term outcomes of kidney transplantation across a positive complement-dependent cytotoxicity crossmatch. Transplantation. 2014;97:1247–1252. doi: 10.1097/01.TP.0000442782.98131.7c. [DOI] [PubMed] [Google Scholar]
- 6.Chong AS, Sciammas R. Memory B cells in transplantation. Transplantation. 2015;99:21–28. doi: 10.1097/TP.0000000000000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perry DK, Burns JM, Pollinger HS, Amiot BP, Gloor JM, Gores GJ, Stegall MD. Proteasome inhibition causes apoptosis of normal human plasma cells preventing alloantibody production. Am J Transplant. 2009;9:201–209. doi: 10.1111/j.1600-6143.2008.02461.x. [DOI] [PubMed] [Google Scholar]
- 8.Sadaka B, Alloway RR, Shields AR, Schmidt NM, Woodle ES. Proteasome inhibition for antibody-mediated allograft rejection. Semin Hematol. 2012;49:263–269. doi: 10.1053/j.seminhematol.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Zuccarino-Catania GV, Sadanand S, Weisel FJ, Tomayko MM, Meng H, Kleinstein SH, Good-Jacobson KL, Shlomchik MJ. CD80 and PD-L2 define functionally distinct memory B cell subsets that are independent of antibody isotype. Nature immunology. 2014;15:631–637. doi: 10.1038/ni.2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pape KA, Taylor JJ, Maul RW, Gearhart PJ, Jenkins MK. Different B cell populations mediate early and late memory during an endogenous immune response. Science. 2011;331:1203–1207. doi: 10.1126/science.1201730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dogan I, Bertocci B, Vilmont V, Delbos F, Megret J, Storck S, Reynaud CA, Weill JC. Multiple layers of B cell memory with different effector functions. Nature immunology. 2009;10:1292–1299. doi: 10.1038/ni.1814. [DOI] [PubMed] [Google Scholar]
- 12.Anderson SM, Tomayko MM, Ahuja A, Haberman AM, Shlomchik MJ. New markers for murine memory B cells that define mutated and unmutated subsets. The Journal of experimental medicine. 2007;204:2103–2114. doi: 10.1084/jem.20062571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomayko MM, Steinel NC, Anderson SM, Shlomchik MJ. Cutting edge: Hierarchy of maturity of murine memory B cell subsets. Journal of immunology. 2010;185:7146–7150. doi: 10.4049/jimmunol.1002163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Good-Jacobson KL, Shlomchik MJ. Plasticity and heterogeneity in the generation of memory B cells and long-lived plasma cells: the influence of germinal center interactions and dynamics. Journal of immunology. 2010;185:3117–3125. doi: 10.4049/jimmunol.1001155. [DOI] [PubMed] [Google Scholar]
- 15.Taylor JJ, Pape KA, Jenkins MK. A germinal center-independent pathway generates unswitched memory B cells early in the primary response. The Journal of experimental medicine. 2012;209:597–606. doi: 10.1084/jem.20111696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaji T, Ishige A, Hikida M, Taka J, Hijikata A, Kubo M, Nagashima T, Takahashi Y, Kurosaki T, Okada M, Ohara O, Rajewsky K, Takemori T. Distinct cellular pathways select germline-encoded and somatically mutated antibodies into immunological memory. The Journal of experimental medicine. 2012;209:2079–2097. doi: 10.1084/jem.20120127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kometani K, Nakagawa R, Shinnakasu R, Kaji T, Rybouchkin A, Moriyama S, Furukawa K, Koseki H, Takemori T, Kurosaki T. Repression of the transcription factor Bach2 contributes to predisposition of IgG1 memory B cells toward plasma cell differentiation. Immunity. 2013;39:136–147. doi: 10.1016/j.immuni.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 18.Chen J, Yin H, Xu J, Wang Q, Edelblum KL, Sciammas R, Chong AS. Reversing endogenous alloreactive B cell GC responses with anti-CD154 or CTLA-4Ig. Am J Transplant. 2013;13:2280–2292. doi: 10.1111/ajt.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, Ravindran R, Stewart S, Alam M, Kwissa M, Villinger F, Murthy N, Steel J, Jacob J, Hogan RJ, Garcia-Sastre A, Compans R, Pulendran B. Programming the magnitude and persistence of antibody responses with innate immunity. Nature. 2011;470:543–547. doi: 10.1038/nature09737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seifert M, Przekopowitz M, Taudien S, Lollies A, Ronge V, Drees B, Lindemann M, Hillen U, Engler H, Singer BB, Kuppers R. Functional capacities of human IgM memory B cells in early inflammatory responses and secondary germinal center reactions. Proc Natl Acad Sci U S A. 2015;112:E546–E555. doi: 10.1073/pnas.1416276112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McHeyzer-Williams LJ, Milpied PJ, Okitsu SL, McHeyzer-Williams MG. Class-switched memory B cells remodel BCRs within secondary germinal centers. Nature immunology. 2015;16:296–305. doi: 10.1038/ni.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, Zheng NY, Mays I, Garman L, Helms C, James J, Air GM, Capra JD, Ahmed R, Wilson PC. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453:667–671. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fink K. Origin and Function of Circulating Plasmablasts during Acute Viral Infections. Frontiers in immunology. 2012;3:78. doi: 10.3389/fimmu.2012.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Archdeacon P, Dixon C, Belen O, Albrecht R, Meyer J. Summary of the US FDA approval of belatacept. Am J Transplant. 2012;12:554–562. doi: 10.1111/j.1600-6143.2011.03976.x. [DOI] [PubMed] [Google Scholar]
- 25.Vincenti F, Blancho G, Durrbach A, Friend P, Grinyo J, Halloran PF, Klempnauer J, Lang P, Larsen CP, Muhlbacher F, Nashan B, Soulillou JP, Vanrenterghem Y, Wekerle T, Agarwal M, Gujrathi S, Shen J, Shi R, Townsend R, Charpentier B. Five-year safety and efficacy of belatacept in renal transplantation. J Am Soc Nephrol. 2010;21:1587–1596. doi: 10.1681/ASN.2009111109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valujskikh A. The challenge of inhibiting alloreactive T-cell memory. Am J Transplant. 2006;6:647–651. doi: 10.1111/j.1600-6143.2005.01215.x. [DOI] [PubMed] [Google Scholar]
- 27.Page AJ, Ford ML, Kirk AD. Memory T-cell-specific therapeutics in organ transplantation. Curr Opin Organ Transplant. 2009;14:643–649. doi: 10.1097/MOT.0b013e328332bd4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masson P, Henderson L, Chapman JR, Craig JC, Webster AC. Belatacept for kidney transplant recipients. Cochrane Database Syst Rev. 2014;11:CD010699. doi: 10.1002/14651858.CD010699.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wing JB, Ise W, Kurosaki T, Sakaguchi S. Regulatory T Cells Control Antigen-Specific Expansion of Tfh Cell Number and Humoral Immune Responses via the Coreceptor CTLA-4. Immunity. 2014;41:1013–1025. doi: 10.1016/j.immuni.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Sage PT, Paterson AM, Lovitch SB, Sharpe AH. The coinhibitory receptor ctla-4 controls B cell responses by modulating T follicular helper, T follicular regulatory, and T regulatory cells. Immunity. 2014;41:1026–1039. doi: 10.1016/j.immuni.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thome JJ, Yudanin N, Ohmura Y, Kubota M, Grinshpun B, Sathaliyawala T, Kato T, Lerner H, Shen Y, Farber DL. Spatial map of human T cell compartmentalization and maintenance over decades of life. Cell. 2014;159:814–828. doi: 10.1016/j.cell.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.