Abstract
Vitamin D deficiency is associated with adverse health outcomes, including impaired bone growth, gingival inflammation and increased risk for autoimmune disease, but the relationship between vitamin D deficiency rickets in childhood and long‐term health has not been studied. In this study, we assessed the effect of early vitamin D deficiency on growth, bone density, dental health and immune function in later childhood to determine if children previously diagnosed with rickets were at greater risk of adverse health outcomes compared with healthy children. We measured serum 25‐hydroxyvitamin D, calcium, parathyroid hormone, bone mineral density, anthropometric measures, dietary habits, dental health, general health history, and markers of inflammation in 14 previously diagnosed rickets case children at Children's Hospital Oakland Research Center. We compared the findings in the rickets cases with 11 healthy children selected from the population of CHO staff families. Fourteen mothers of the rickets cases, five siblings of the rickets cases, and seven mothers of healthy children also participated. Children diagnosed with vitamin D deficiency rickets had a greater risk of fracture, greater prevalence of asthma, and more dental enamel defects compared with healthy children. Given the widespread actions of vitamin D, it is likely that early‐life vitamin D deficiency may increase the risk of disease later in childhood. Further assessment of the long‐term health effects of early deficiency is necessary to make appropriate dietary recommendations for infants at risk of deficiency.
Keywords: rickets, vitamin D, bone mineral density, dental health, gingival infection, bone fracture
Introduction
Vitamin D deficiency (VDD) in children causes rickets, which is characterised by inadequate mineralisation of bones. Severe VDD is typically defined in children with a 25‐hydroxy vitamin D (25(OH)D) status of <10 ng mL−1. Common clinical findings include bone abnormalities, delayed growth or motor development and seizures (Nield et al. 2006). Biochemical findings include hypocalcemia, low‐circulating 25(OH)D, hypophosphatemia, elevated serum alkaline phosphatase and hyperparathyroidism (Weisberg et al. 2004). Nutritional rickets is rare in the United States; however, reports published over the last two decades have documented an apparent resurgence of VDD in infants and young children (Kreiter et al. 2000; Shah et al. 2000; Biser‐Rohrbaugh & Hadley‐Miller 2001; Tomashek et al. 2001; Mylott et al. 2004; Lazol et al. 2008). The Children's Hospital of Oakland documented over 80 cases in children aged 2 months to 5 years from 2000 to 2009 (Fung et al. 2009). This increase in prevalence of rickets may be due in part to environmental factors such as higher prevalence of breastfeeding without vitamin D supplementation, and decreased sun exposure in women and their infants (Weisberg et al. 2004).
The current adequate intake value for vitamin D for infants up to 12 months of age is 400 IU (international units) day−1 (Institute of Medicine 2011). For exclusively breastfed infants, the American Academy of Pediatrics recommends supplemental oral intake of 400 IU per day (Wagner & Greer 2008) to maintain adequate vitamin D status. For older children and adults, dietary sources of vitamin D3 (cholecalciferol) are limited. The major source of vitamin D for most individuals is cutaneous synthesis, which varies according to season, latitude, skin pigmentation, clothing, sunscreen use and air pollution (Holick 1995).
Long‐term consequences of early VDD have not been rigorously studied. Negative associations have been seen between vitamin D status during pregnancy or early infancy and whole‐body bone mineral content (BMC) at 9 years of age (Javaid et al. 2006). Associations with respiratory health have also been documented, including an inverse association between vitamin D status and wheezing in children at 3 years and 5 years of age (Camargo et al. 2007, 2011; Devereux et al. 2007), and a greater risk of developing lower respiratory tract infections in the first year of life (Belderbos et al. 2011). The data regarding VDD and allergy are conflicting, with some studies documenting a protective effect (Erkkola et al. 2009; Litonjua 2009), and others showing an increased risk (Hypponen 2004). Early VDD has been associated with adverse long‐term effects on dental health, including delayed dentation, enamel defects, increased risk of caries and gingival inflammation (Purvis et al. 1973; Cockburn et al. 1980; Schroth et al. 2005).
This study describes an investigation of the long‐term effects of VDD rickets in children on growth, bone health, dental health and immune function. We hypothesise that VDD in early life may predispose to adverse outcomes in growth, bone formation, dental health and immune function.
Key messages.
Early‐life vitamin D deficiency may have long‐term consequences for young children including poor linear growth, increased risk for fracture, greater prevalence of asthma, and dental enamel defects.
Factors that may contribute to vitamin D deficiency include attitudes towards supplement use, lactose intolerance, and inconsistent sunscreen use.
Clinicians should consider both vitamin D status during pregnancy and infant nutrition for prevention of rickets.
Materials and methods
Participant recruitment
All children diagnosed with VDD rickets at the Children's Hospital & Research Center Oakland (CHRCO) from 2000 to 2009 were invited to participate in a Bone Health Day at the Children's Hospital Oakland Research Institute (CHORI). The mothers of the rickets cases and a sibling closest in age were also invited to participate. After the cases were enrolled, healthy age‐matched (‘control’) children (within ±2 years) and their mothers were recruited from staff at CHRCO.
This study was approved by the Institutional Review Board at CHRCO. All mothers provided informed written consent for themselves and their children. Children over the age of 7 years provided informed written assent to participate.
Study visits
Rickets case children, with their mothers and siblings, and matched healthy ‘control’ children, with their mothers, came to a single‐study visit at CHORI in Oakland, CA. The study visit consisted of six stations: (1) informed consent was obtained from the mothers of the rickets cases and healthy children; (2) height, weight and blood pressure were measured in the children and the mothers; (3) a diet, health and sun exposure questionnaire was administered to the mothers to assess their own and their child's dietary and environmental sources of vitamin D; (4) dual‐energy X‐ray absorptiometry (DXA) scan to measure BMC and density was performed on the case children, siblings and healthy children; (5) a dental exam was performed on all of the children and mothers; and (6) a non‐fasting blood sample was drawn from all of the children and mothers.
Laboratory and clinical methods
Biochemical and immune function analyses, anthropometry and bone density methods, medical history, and dental exam procedures are described in Supporting Information http://onlinelibrary.wiley.com/doi/10.1111/mcn.12187/suppinfo.
Statistical analysis
Statistical analysis was performed using SAS 9.2 (SAS Institute, Inc., Cary, NC, USA) and GraphPad Prism software (Version 6.0f for Mac, GraphPad Software, La Jolla, CA, USA). The normality of continuous variables was assessed using a Shipiro–Wilk test. Variables that were not normally distributed were analysed by non‐parametric methods. Children previously diagnosed with rickets were compared with healthy children using Wilcoxon rank‐sum comparisons. Mothers of children with rickets were compared with mothers of healthy children. Spearman correlations were calculated for associations between vitamin D status and gingival index and plaque index.
Results
Study population and subject recruitment
Of the identified eligible sample of previously diagnosed rickets cases from Oakland Children's Hospital, 41% (33/80) were unable to be contacted. Of the 47 families who were contacted, 13 declined to participate, and five were unable to attend the study visit. The participating study population included 14 children previously diagnosed with VDD rickets, or 17.5% of the original sample. All 14 mothers of the case children, as well as five siblings, also participated in the study. Eleven healthy control children and seven mothers of healthy children (some of the healthy children were siblings and thus had the same mother) participated in the study.
The rickets case children ranged in age from 1.6 to 11.5 years (mean 4.3 years) at the time of the study visit. The mean age at diagnosis for the cases was 1.7 years (range, 0.2–5.3 years). The average time since diagnosis was 2.6 years (range, 0.3–6.1 years).
There were no significant differences between rickets case children and healthy children in sex or age at the time of the study (Table 1). Ethnicity varied between the rickets and healthy groups, with a greater number of African American and Hispanic children in the rickets group and a greater number of Asian and Caucasian children in the healthy group (P = 0.02).
Table 1.
Characteristics of children enrolled in the study
|
Rickets cases (n = 14) |
Healthy controls (n = 11) |
P‐value | |
|---|---|---|---|
| Demographics and anthropometrics | |||
| Female sex, n (%) | 8 (57) | 5 (45) | – |
| Age, years | 4.3 (2.0, 5.5) | 5.5 (1.9, 7.0) | 0.32 |
| Height for age Z‐score |
−0.45 (−0.96, 0.56) (n = 12) |
0.28 (−0.45, 1.59) | 0.21 |
| Weight for age Z‐score |
−0.40 (−1.06, 0.3) (n = 12) |
0.44 (−0.58, 1.21) | 0.29 |
| Weight for height Z‐score |
0.27 (−0.95, 0.43) (n = 10) |
0.17 (−0.59, 0.93) (n = 8) |
0.95 |
| BMI Z‐score, kg m−2 |
0.12 (−0.97, 0.91) (n = 9) |
0.15 (−0.58, 0.69) (n = 8) |
0.94 |
| Race (Wh/AfAm/Lat/Asn), n | 1/9/2/2 | 4/1/1/5 | 0.02* |
| Race (AfAm/Other), n | 9/5 | 1/10 | 0.005* |
| Vitamin D and related markers | |||
| 25(OH)D, ng mL−1 | 26.5 (19.7, 38.9) | 33.3 (27.9, 36.3) (n = 10) | 0.59 |
| 25(OH)D < 30 ng mL−1, n (%) | 8 (57) | 4 (40) | 0.41* |
| 25(OH)D < 20 ng mL−1, n (%) | 4 (29) | 2 (20) | 0.63* |
| PTH, pg mL−1 |
33.0 (27.0, 40.0) (n = 11) |
32.0 (28.0, 34.0) (n = 10) |
0.52 |
| BSAP, μg L−1 |
86.0 (61.0, 117.3) (n = 13) |
68.2 (55.1, 83.3) (n = 10) |
0.06 |
| Contributors to vitamin D status | |||
| Dietary vitamin D intake, IU day−1 | 382.5 (189.0, 490.0) | 381.0 (342.0, 428.0) | 0.95 |
| Range, IU day−1 | 90–1315 | 142–790 | – |
| Dietary calcium intake, mg day−1 | 676.5 (340.0, 1117.0) | 966.0 (558.0, 1065.0) | 0.43 |
| Range, mg day−1 | 223–3202 | 366–1764 | – |
| Supplement use, n (%) | 3 (21) | 9 (82) | 0.003* |
| Lactose intolerance, n (%) | 2 (14) | 0 (0) | 0.19* |
| Sunscreen use, n (%) | 2 (14) | 10 (91) | 0.0001* |
| Time spent outdoors, h month−1 |
60 (30, 150) (n = 13) |
35 (30, 45) (n = 10) |
0.18 |
BMI, body mass index; BSAP, bone‐specific alkaline phosphatase; IU, international units; PTH, parathyroid hormone. Data are presented as median (25th/75th percentile) unless otherwise indicated. P‐values are determined by Wilcoxon rank‐sum test or by chi‐squared test (*). Dietary intakes of vitamin D and calcium were estimated from modified food frequency questionnaires of vitamin D and calcium‐containing foods. Calculations based on a comparison of rickets (n = 14) and controls (n = 11), unless otherwise noted.
Predictors of vitamin D status
Vitamin D status at the time of the study visit did not differ between rickets cases and healthy children (Table 1). When all study participants were considered, the mothers of the rickets cases had the lowest vitamin D levels (P < 0.05), with nearly 70% of this group having a 25(OH)D < 20 ng mL−1 (Fig. 1).
Figure 1.
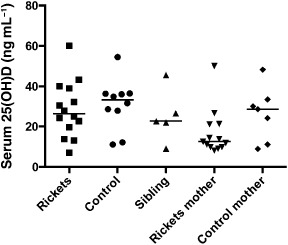
Vitamin D status did not differ between rickets cases and healthy children, and was the lowest overall in the mothers of the rickets cases (P < 0.05). Median values are indicated by a horizontal bar. Serum 25(OH)D was measured at the time of the study by radioimmunoassay.
Ethnicity was defined as one of four categories: white, black, Hispanic or Asian. Among the rickets cases and healthy children, 25(OH)D did not vary by ethnicity (P = 0.33).
Twenty‐five percent (6/25) of all study subjects had serum 25‐hydroxyvitamin D < 20 ng mL−1 at the time of the study visit, but vitamin D status and dietary intakes of vitamin D and calcium did not differ between rickets cases and healthy children (Table 1). Oral multivitamin supplement use was greater in the healthy children than in rickets cases (Table 1). There was a trend towards greater prevalence of lactose intolerance in the rickets cases, but this observation did not meet statistical significance (Table 1). Self‐reported sunscreen use was greater in the healthy children compared with rickets cases (Table 1). Estimated time spent outdoors (h month−1; an estimate of sun exposure) did not differ between rickets cases and healthy children (Table 1).
Indicators of calcium metabolism, bone health and growth
Clinically relevant elevation in parathyroid hormone levels was not seen in the children (Table 1). There were no significant differences in bone‐specific alkaline phosphatase among the children (Table 1).
There were no differences in the DXA Z‐score measurements of whole‐body BMD (bone mineral density) or AP spine BMD between rickets cases and healthy children (Table 2). There was a significant group difference in lifetime history of fracture, with five rickets cases having fractures compared with no fractures in the healthy children (Table 2). Of the five rickets cases with fractures, four experienced one or more fractures (at the same time) at or before diagnosis (case 4, tibia; case 7, tibia and fibula; case 10, radius and ulna; case 14, right clavicle) while one of these cases (case 14) suffered a second fracture at the same site 2 years after rickets diagnosis. The fifth rickets case with a fracture (case 8, tibia) suffered the fracture one year after diagnosis.
Table 2.
Clinical outcomes measured in the follow‐up study
| Rickets cases | Healthy controls | P‐value | |
|---|---|---|---|
| Bone markers | |||
| n | 14 | 11 | – |
| Whole body aBMD, score | −0.5 ± 1.3 | −1.2 ± 0.7 | 0.22 |
| AP spine aBMD, score | 0.8 ± 2.1 | −0.1 ± 0.9 | 0.31 |
| History of fracture, n (%) | 5 (35.7) | 0 (0) | 0.03* |
| Dental health | |||
| n | 14 | 11 | – |
| Baby teeth, n | 14.5 ± 6.1 | 14.5 ± 6.3 | 0.96 |
| Adult teeth, n | 3.5 ± 6.5 | 4.5 ± 7.0 | 1 |
| Total teeth, n | 18.0 ± 4.8 | 19.0 ± 6.0 | 0.40 |
| Number of fillings, n | 1.0 ± 2.4 | 0.45 ± 0.82 | 0.86 |
| Teeth with filling, % | 5.4 ± 13.1% | 2.0 ± 3.6% | 0.88 |
| Teeth with decay, n | 0.6 ± 0.9 | 0.27 ± 0.65 | 0.30 |
| Teeth with decay, % | 4.5 ± 7.5 | 1.3 ± 3.1 | 0.25 |
| Teeth with enamel defects, n | 4.8 ± 5.7 | 1.8 ± 2.8 | 0.04 |
| Gingival index † |
0.40 ± 0.40 (n = 5) |
0.26 ± 0.14 (n = 4) |
0.90 |
| Plaque index † |
0.57 ± 0.45 (n = 5) |
0.38 ± 0.26 (n = 4) |
0.46 |
| Plasma inflammatory markers | |||
| n | 11 | 10 | – |
| IL‐6, pg mL−1 | 6.3 (0.2, 12.5) | 19.6 (8.3, 40.0) | 0.019 |
| IL‐10, pg mL−1 | 8.2 (1.6, 12.8) | 8.2 (7.0, 11.0) | 0.65 |
| IL‐17, pg mL−1 | 3.5 (1.7, 11.6) | 8.4 (5.9, 24.0) | 0.13 |
| TNF‐α, pg mL−1 | 8.7 (5.2, 17.2) | 9.8 (8.2, 10.4) | 0.81 |
| Eotaxin, pg mL−1 | 71.9 (46.3, 87.1) | 93.1 (64.3, 127.7) | 0.11 |
| IP‐10, pg mL−1 | 148.2 (100.9, 168.2) | 134.2 (117.3, 216.9) | 0.50 |
| Regulatory T‐cells (defined as % CD25 + CD127lo of CD4+) |
8.4 (7.0, 10.4) (n = 12) |
9.1 (8.1, 10.2) (n = 8) |
0.59 |
aBMD, areal bone mineral density; AP, anterior‐posterior. Data are presented as mean ± SD or median (IQR). P‐values are determined by Wilcoxon rank‐sum test or by chi‐squared test (*) comparing rickets to control children. †Gingival index and plaque index were only calculated in subjects with noted gingival inflammation or plaque, n = 9 children. Number of individuals included in mean value noted in column for indices means.
To evaluate growth, we compared height and weight Z‐scores for the rickets cases and healthy children. The rickets cases did not differ significantly from the healthy children in the Z‐scores for height‐for‐age, weight‐for‐age, weight‐for‐height or body mass index (Table 1).
Clinical outcomes
Asthma
Whereas 7 out of 14 (50%) of the rickets children had been diagnosed with asthma, none of the healthy children had an asthma diagnosis (P = 0.006). This difference remained significant when we considered only those children greater than 3 years old, the age at which asthma is commonly diagnosed (P = 0.021). Additionally, 40% of the siblings of rickets cases (2/5) had been diagnosed with asthma.
Dental health
We did not find any difference between the rickets cases and healthy children in the number of baby or adult teeth, percentage of teeth with fillings or percentage of teeth with decay. Similarly, plaque index and gingival index did not differ between the groups. The rickets case children had a greater amount of enamel defects than the healthy children (P = 0.04, Table 2).
Immune function
There was no difference in the percentage of regulatory T‐cells in peripheral blood between healthy children and rickets cases (Table 2). Among the children (rickets cases, healthy children, siblings), the number of regulatory T‐cells in whole blood correlated positively with serum 25(OH)D (P = 0.009). Of the measured inflammatory markers in plasma, only IL‐6 was significantly different between the groups, being lower in the rickets cases than healthy children (P = 0.019; Table 2).
Discussion
In this study, we found no difference in current vitamin D status between children previously treated for VDD rickets and healthy children. The mothers of the rickets cases had the lowest mean serum 25(OH)D overall, which is likely due to their low dietary intake of vitamin D‐containing foods, but as mothers of cases were more likely to be of African ancestry, lower cutaneous synthesis could also be a factor. None of the mothers in our sample met the current recommended dietary allowance for vitamin D for pregnant or non‐pregnant women 19–50 years old, 600 IU day−1 (Institute of Medicine 2011); all had intakes of less than 400 IU day−1. Since a newborn's vitamin D status is completely dependent on maternal vitamin D stores and exclusively breastfed infants depend entirely on maternal stores for dietary vitamin D, any successful intervention to prevent VDD in infants would need to consider maternal diet as well as sun exposure.
Compared with the healthy children, the rickets cases had a greater proportion of Latinos and African Americans. This is consistent with published studies that documented a greater risk of rickets among children of African American ancestry (Kreiter et al. 2000; Mylott et al. 2004; Weisberg et al. 2004) and more VDD among black mothers than their white counterparts (Bodnar et al. 2007). Of the 200 black pregnant women studied at delivery in Pittsburgh, 29.2% had a 25(OH)D < 37.5 nmol L−1 (15 ng mL−1) and 54.1% had a 25(OH)D between 37.5 and 80 nmol L−1 (15–32 ng mL−1, despite 90% of women reporting prenatal vitamin use (Bodnar et al. 2007). Among the black neonates in the same study, 45.6% had a 25(OH)D < 37.5 nmol L−1 and 46.8% had a 25(OH)D between 37.5 and 80 nmol L−1 at birth. Infant cord 25(OH)D correlated strongly with maternal serum 25(OH)D before delivery (r = 0.89). A limitation of our study is that controls were not randomly selected from the community served by Oakland Children's Hospital, thus limiting our ability to assess the association of demographic characteristics with risk of rickets.
Several factors potentially contribute to insufficient intake of vitamin D and thus lead to VDD rickets. For example, attitudes towards supplement use could affect vitamin D intake, and concerns relating to lactose intolerance may contribute to lower vitamin D intake as the primary food sources of vitamin D for most Americans are fortified dairy products (milk and some yogurts). In our study population, there was a lower reported use of nutritional supplements in the rickets children compared with the healthy children, and a non‐significant trend of more lactose intolerance among the rickets cases. Thus, better education of parents on sources of dietary vitamin D could decrease the risk of VDD rickets.
Within our study population, there was no significant group difference in the Z‐scores for measures of age‐specific height and weight. Similarly, we observed no difference in whole‐body or spine bone mineral density Z‐scores between rickets cases and healthy children at the time of the study. Data regarding long‐term effects of early VDD on bone density are conflicting. In one study, low 25(OH)D in late pregnancy was associated with lower whole‐body BMC in the offspring at 9 years of age (Javaid et al. 2006). In a retrospective cohort study, Caucasian girls who had been supplemented with vitamin D during the first year of life had greater areal bone mineral density measurements of the radial metaphysis, femoral neck and femoral trochanter sites at 8 years of age compared with un‐supplemented girls (Zamora et al. 1999). However, another trial of vitamin D supplementation of 500 or 1000 IU per day in preterm infants demonstrated that supplementation in infancy does not appear to make a difference in BMC at 9 years old (Backström et al. 1999). This latter study implies that children may compensate for early nutritional deficiencies if given proper nutrition during growth.
A clinically relevant finding in our study is the greater rate of fractures among the rickets cases compared with the healthy children. Although we did not demonstrate any differences in bone mineral density Z‐scores between rickets cases and healthy children, the difference in fracture rates between the groups suggests a detrimental functional effect of early VDD, and is consistent with previous observations (Sonneville et al. 2012).
Of particular interest is whether early VDD predisposes to immune dysfunction in later life. One possible consequence of such an effect is an increased risk of asthma. In our study population, we found a significantly greater rate of asthma diagnosis among the rickets cases. Since our controls were not selected as a representative community sample, we cannot directly infer an increased risk of asthma based on previous VDD in the cases. However, a number of studies have documented such a positive association between poor maternal vitamin D status during pregnancy and later risk of asthma (Erkkola et al. 2009) or wheezing (Camargo et al. 2007, 2011; Devereux et al. 2007) in their children. Such findings are not universal, however, as a Finnish birth cohort study demonstrated a positive association between asthma risk at 31 years of age and vitamin D supplementation in infancy (Hypponen 2004). Another study showed that asthma risk at 9 years was greater among offspring of women with serum 25(OH)D > 75 nmol L−1 (30 ng mL−1) during late pregnancy compared with children whose mothers had a serum 25(OH)D < 30 nmol L−1 (12 ng mL−1) during pregnancy (Gale et al. 2008).
This paradoxical relationship between vitamin D status and asthma risk may be a result of the dual effects of vitamin D on the innate and adaptive components of the immune system. Vitamin D is necessary for production of cathelicidin, an antimicrobial peptide that plays a role in pulmonary mucosal innate immunity, particularly protection against respiratory infection (Gombart 2009). Vitamin D also plays a role in the adaptive immune response; it promotes expression of IL‐10 (a regulatory cytokine) and a shift towards Th2‐mediated T‐cell responses (e.g. IL‐4) such as those which occur in allergic asthma.
Regulatory T‐cells are a subset of CD4+ T‐lymphocytes that can down‐regulate immune responses mediated by other subsets of T‐lymphocytes. Vitamin D has been shown to promote the development of this T‐cell phenotype in vitro (Jeffery et al. 2009). Having a low percentage of regulatory T‐cells has been associated with autoimmune disease and inflammation. Early VDD has been shown to be a risk factor for development of autoimmune diseases later in life, including type 1 diabetes (Eurodiab 1999; Hypponen et al. 2001; Zipitis & Akobeng 2008). In this study, there was no difference in the proportion of regulatory T‐cells in the controls compared with rickets cases. VDD could increase the risk of asthma by impairing innate immunity and thus increasing severity of certain respiratory infections early in infancy, while better vitamin D status could have unpredictable effects on adaptive immunity related to allergic asthma, either enhancing Th2 responses that underlie allergic responses, or enhancing regulatory responses that could control allergic responses.
Rickets was associated with defects in dental enamel in the present study, which is consistent with previous observations that one of the signs of rickets in children is hypoplasia of dental enamel, leading to a greater susceptibility to dental caries (Wharton & Bishop 2003). Enamel formation begins in utero and is complete before eruption of the teeth. In one study of 112 infants treated for neonatal tetany in Edinburgh, 56% had severe enamel defects, implying an association between tetany (often a consequence of severe VDD caused by maternal deficiency during pregnancy) and enamel defects (Purvis et al. 1973). Furthermore, children whose mothers received 100 000 IU ergocalciferol at diagnosis of pregnancy and again in the third trimester had significantly earlier eruption times of the first tooth than children of non‐supplemented mothers (Schroth et al. 2005). VDD has been associated with greater risk of periodontal disease (Weisberg et al. 2004; Dietrich et al. 2005), and is likely the cause of increased risk of periodontal disease and caries in children with VDD rickets. While we did not observe different rates of periodontal disease in the present study, it is possible that the relatively long follow‐up period after rickets diagnosis for some cases may have decreased our ability to identify such an association, presuming that initial treatment and ongoing attention to vitamin D nutrition for rickets cases may have resolved some transient effects of vitamin D deficiency, such as gingival inflammation, particularly in cases with long follow‐up periods. Adequate vitamin D status is necessary for adequate innate immunity, particularly expression of antimicrobial peptides such as cathelicidin (Liu et al. 2006; Gombart 2009), which promotes oral health.
While the principal strength of this study is its identification and follow‐up of rickets cases, an unfortunate shortcoming is the small sample size. Despite the sizable pool of potential follow‐up study participants, only a small number of families decided to participate in the study. We believe our inability to reconnect with previous patients may be due to the mobile nature of this patient population. Furthermore, the selection of the healthy control children from the population of hospital staff may have limited generalisability of the data because this population is likely different from the rickets cases in ways not directly evaluated in this study. Another challenge to interpretation of the data is the heterogeneous time since rickets diagnosis and age at diagnosis for our cohort of patients. Early exposure to VDD may contribute to later risk of disease (dental, immune function) or negatively impact growth and bone development. However, it is likely that the timing of this exposure is important to the long‐term effects. Given this, future studies are needed to determine whether VDD at certain time points during development is more detrimental.
Source of funding
This project was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF‐CTSI Grant Number UL1 TR000004. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Contributions
MZ participated in the Bone Health Days, performed immune function laboratory analyses, performed data analyses, wrote the manuscript and approved the final manuscript as submitted.
MR designed the dental data collection protocol, performed the dental exams, consulted on data analysis, critically reviewed the manuscript and approved the final manuscript as submitted.
SB conceptualised the study design, participated in the Bone Health Days, consented subjects, and reviewed and approved the final manuscript as submitted.
CS contributed to study design, supervised immune function laboratory analyses, reviewed and revised the manuscript, and approved the final manuscript as submitted.
JK conceptualised and oversaw study design, participated in the Bone Health Days, and reviewed and approved the final manuscript as submitted.
EF conceptualised and designed the study, coordinated and supervised the data collection at the Bone Health Days, consented subjects, performed the DXA exams and data analysis, critically reviewed the manuscript, and approved the final manuscript as submitted.
Supporting information
Appendix S1. Supplemental Methods.
Supporting info item
Acknowledgements
We would like to acknowledge Dr. Steven Silverstein, DDS and Dr. Preeti Prakesh, DDS for their assistance in developing and conducting the dental exam protocols. We would like to thank Annie Higa, Betty Flores, PNP, Nancy Sweeters, PNP, Lisa Lavrisha, PNP, Lisa Calvelli, Whitney Dwyer and Carol Manzor for their assistance with subject recruitment, study visits, phlebotomy, sample preparation and shipping, and data management.
Zerofsky, M. , Ryder, M. , Bhatia, S. , Stephensen, C. B. , King, J. , and Fung, E. B. (2016) Effects of early vitamin D deficiency rickets on bone and dental health, growth and immunity. Maternal & Child Nutrition, 12: 898–907. doi: 10.1111/mcn.12187.
References
- Backström M.C., Mäki R., Kuusela A.L., Sievänen H., Koivisto A.M., Koskinen M. et al (1999) The long‐term effect of early mineral, vitamin D, and breast milk intake on bone mineral status in 9‐ to 11‐year‐old children born prematurely. Journal of Pediatric Gastroenterology and Nutrition 29 (5), 575–582. [DOI] [PubMed] [Google Scholar]
- Belderbos M.E., Houben M.L., Wilbrink B., Lentjes E., Bloemen E.M., Kimpen J.L.L. et al (2011) Cord blood vitamin D deficiency is associated with respiratory syncytial virus bronchiolitis. Pediatrics 127 (6), e1513–e1520. [DOI] [PubMed] [Google Scholar]
- Biser‐Rohrbaugh A. & Hadley‐Miller N. (2001) Vitamin D deficiency in breast‐fed toddlers. Journal of Pediatric Orthopedics 21 (4), 508–511. [PubMed] [Google Scholar]
- Bodnar L., Simhan H., Powers R., Frank M., Cooperstein E. & Roberts J. (2007) High prevalence of vitamin D insufficiency in black and white pregnant women residing in the northern United States and their neonates. The Journal of Nutrition 137 (2), 447–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo C.A., Rifas‐Shiman S.L., Litonjua A.A., Rich‐Edwards J.W., Weiss S.T., Gold D.R. et al (2007) Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 years of age. The American Journal of Clinical Nutrition 85 (3), 788–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo C.A., Ingham T., Wickens K., Thadhani R., Silvers K.M., Epton M.J. et al (2011) Cord‐blood 25‐hydroxyvitamin D levels and risk of respiratory infection, wheezing, and asthma. Pediatrics 127 (1), e180–e187. [DOI] [PubMed] [Google Scholar]
- Cockburn F., Belton N.R., Purvis R.J., Giles M.M., Brown J.K., Turner T.L. et al (1980) Maternal vitamin D intake and mineral metabolism in mothers and their newborn infants. British Medical Journal 281 (6232), 11–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux G., Litonjua A.A., Turner S.W., Craig L.C.A., McNeill G., Martindale S. et al (2007) Maternal vitamin D intake during pregnancy and early childhood wheezing. The American Journal of Clinical Nutrition 85 (3), 853–859. [DOI] [PubMed] [Google Scholar]
- Dietrich T., Nunn M., Dawson‐Hughes B. & Bischoff‐Ferrari H.A. (2005) Association between serum concentrations of 25‐hydroxyvitamin D and gingival inflammation. The American Journal of Clinical Nutrition 82 (3), 575–580. [DOI] [PubMed] [Google Scholar]
- Erkkola M., Kaila M., Nwaru B.I., Kronberg‐Kippilä C., Ahonen S., Nevalainen J. et al (2009) Maternal vitamin D intake during pregnancy is inversely associated with asthma and allergic rhinitis in 5‐year‐old children. Clinical and Experimental Allergy: Journal of the British Society for Allergy and Clinical Immunology 39 (6), 875–882. [DOI] [PubMed] [Google Scholar]
- Eurodiab (1999) Vitamin D supplement in early childhood and risk for Type I (insulin‐dependent) diabetes mellitus. The EURODIAB Substudy 2 Study Group. Diabetologia 42 (1), 51–54. [DOI] [PubMed] [Google Scholar]
- Fung E.B., Gariepy C., King J.C. & Bhatia S. (2009) Low 25(OH)D not consistent predictor of nutritional rickets. Pediatrics 124 (3), e362–e370.19661054 [Google Scholar]
- Gale C.R., Robinson S.M., Harvey N.C., Javaid M.K., Jiang B., Martyn C.N. et al (2008) Maternal vitamin D status during pregnancy and child outcomes. European Journal of Clinical Nutrition 62 (1), 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombart A.F. (2009) The vitamin D–antimicrobial peptide pathway and its role in protection against infection. Future Microbiology 4 (9), 1151–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick M.F. (1995) Environmental factors that influence the cutaneous production of vitamin D. The American Journal of Clinical Nutrition 61 (3 Suppl.), 638S–645S. [DOI] [PubMed] [Google Scholar]
- Hypponen E. (2004) Infant vitamin D supplementation and allergic conditions in adulthood: Northern Finland birth cohort 1966. Annals of the New York Academy of Sciences 1037 (1), 84–95. [DOI] [PubMed] [Google Scholar]
- Hypponen E., Laara E., Reunanen A., Jarvelin M. & Virtanen S. (2001) Intake of vitamin D and risk of type 1 diabetes: a birth‐cohort study. Lancet 358 (9292), 1500–1503. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine (2011) Dietary Reference Intakes for Calcium and Vitamin D, National Academy Press: Washington, D.C. [Google Scholar]
- Javaid M.K., Crozier S.R., Harvey N.C., Gale C.R., Dennison E.M., Boucher B.J. et al (2006) Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet 367 (9504), 36–43. [DOI] [PubMed] [Google Scholar]
- Jeffery L.E., Burke F., Mura M., Zheng Y., Qureshi O.S., Hewison M. et al (2009) 1,25‐Dihydroxyvitamin D3 and IL‐2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA‐4 and FoxP3. Journal of Immunology (Baltimore, MD: 1950) 183 (9), 5458–5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiter S.R., Schwartz R.P., Kirkman H.N. Jr, Charlton P.A., Calikoglu A.S. & Davenport M.L. (2000) Nutritional rickets in African American breast‐fed infants. The Journal of Pediatrics 137 (2), 153–157. [DOI] [PubMed] [Google Scholar]
- Lazol J.P., Cakan N. & Kamat D. (2008) 10‐year case review of nutritional rickets in children's hospital of Michigan. Clinical Pediatrics 47 (4), 379–384. [DOI] [PubMed] [Google Scholar]
- Litonjua A.A. (2009) Childhood asthma may be a consequence of vitamin D deficiency. Current Opinion in Allergy and Clinical Immunology 9 (3), 202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Stenger S., Li H., Wenzel L., Tan B., Krutzik S. et al (2006) Toll‐like receptor triggering of a vitamin D‐mediated human antimicrobial response. Science 311 (5768), 1770–1773. [DOI] [PubMed] [Google Scholar]
- Mylott B., Kump T., Bolton M. & Greenbaum L. (2004) Rickets in the dairy state. WMJ 103 (5), 84–87. [PubMed] [Google Scholar]
- Nield L.S., Mahajan P., Joshi A. & Kamat D. (2006) Rickets: not a disease of the past. American Family Physician 74 (4), 619–626. [PubMed] [Google Scholar]
- Purvis R.J., Barrie W.J., MacKay G.S., Wilkinson E.M., Cockburn F. & Belton N.R. (1973) Enamel hypoplasia of the teeth associated with neonatal tetany: a manifestation of maternal vitamin‐D deficiency. Lancet 2 (7833), 811–814. [DOI] [PubMed] [Google Scholar]
- Schroth R.J., Smith P.J., Whalen J.C., Lekic C. & Moffatt M.E.K. (2005) Prevalence of caries among preschool‐aged children in a northern Manitoba community. Journal (Canadian Dental Association) 71 (1), 27–27f. [PubMed] [Google Scholar]
- Shah M., Salhab N., Patterson D. & Seikaly M. (2000) Nutritional rickets still afflict children in north Texas. Texas Medicine 96 (6), 64–68. [PubMed] [Google Scholar]
- Sonneville K.R., Gordon C.M., Kocher M.S., Pierce L.M., Ramappa A. & Field A.E. (2012) Vitamin D, calcium, and dairy intakes and stress fractures among female adolescents. Archives of Pediatrics and Adolescent Medicine 166 (7), 595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomashek K.M., Nesby S., Scanlon K.S., Cogswell M.E., Powell K.E., Parashar U.D. et al (2001) Nutritional rickets in Georgia. Pediatrics 107 (4), E45. [DOI] [PubMed] [Google Scholar]
- Wagner C.L. & Greer F.R. (2008) Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics 122 (5), 1142–1152. [DOI] [PubMed] [Google Scholar]
- Weisberg P., Scanlon K., Li R. & Cogswell M. (2004) Nutritional rickets among children in the United States: review of cases reported between 1986 and 2003. The American Journal of Clinical Nutrition 80 (6 Suppl.), 1697S–1705. [DOI] [PubMed] [Google Scholar]
- Wharton B. & Bishop N. (2003) Rickets. Lancet 362 (9393), 1389–1400. [DOI] [PubMed] [Google Scholar]
- Zamora S.A., Rizzoli R., Belli D.C., Slosman D.O. & Bonjour J.P. (1999) Vitamin D supplementation during infancy is associated with higher bone mineral mass in prepubertal girls. Journal of Clinical Endocrinology & Metabolism 84 (12), 4541–4544. [DOI] [PubMed] [Google Scholar]
- Zipitis C.S. & Akobeng A.K. (2008) Vitamin D supplementation in early childhood and risk of type 1 diabetes: a systematic review and meta‐analysis. Archives of Disease in Childhood 93 (6), 512–517. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supplemental Methods.
Supporting info item


