Abstract
Follicular CD4+ T helper (Tfh) cells are critical for the generation of humoral immune responses to pathogenic infections, providing help for B cell development, survival, and affinity maturation of antibodies. Although CD4+ Tfh cells are reported to accumulate in HIV or SIV infection, here we found that germinal center (GC) Tfh cells, defined here as CXCR5+PD-1HIGHCD4+ T cells, did not consistently accumulate in chronically SIV-infected rhesus macaques compared with those infected with less pathogenic SHIV, vaccinated and SIVmac-challenged, or SIVmac-infected Mamu-A*01+ macaques, all of which are associated with some control of virus replication and slower disease progression. Interestingly, CXCR5+PD-1HIGH Tfh cells in lymphoid tissues were eventually depleted in macaques with AIDS compared to the other cohorts. Chronic activation and proliferation of CXCR5+PD-1HIGH Tfh were increased, yet PD-L2 expression was downregulated on B cells, possibly resulting in GC Tfh cell apoptosis. Together, these findings suggest changes in CXCR5+PD-1HIGH Tfh cells in lymph nodes correlate with immune control during infection, and their loss or dysregulation contribute to impairment of B cell responses and progression to AIDS.
Keywords: Follicular CD4 T helper cells, B cell, SIV, HIV, Germinal center, Immunity
Introduction
CD4+ Follicular helper T (Tfh) cells are specialized in promoting antigen-specific B cell immunity. Absence of Tfh cells lead to B-cell apoptosis, thereby preventing final B cell maturation and effective humoral immune responses (1). Mature Tfh cells residing in germinal centers (GC) of organized lymphoid tissue express CXCR5, PD-1HIGH, SLAM-associated protein (SAP), GL7, ICOS and IL-21, and interact with GC B cells to provide direct help for B cell development and maturation into antigen-specific, high-affinity, memory B cells or antibody secreting plasma cells (2–5). Moreover, PD-1 is highly expressed on GC Tfh cells which are almost exclusively distributed in GC of follicles (6), and plays an important role in regulation and survival of GC B cells through interaction with its ligands PD-L1 and PD-L2 (7).
Tfh cells thus promote potent primary antibody responses and are critical for B cell responses in viral infections. However, vigorous follicular hyperplasia and dysplasia, and massive losses of CD4 T cell in lymph nodes are both hallmarks of chronic HIV/SIV infection. Eventually, there is also generalized lymphoid damage characterized by a reduction in GC size and number, which is accompanied by fibrosis, and follicular involution with nearly complete destruction of the fibroblastic reticular cell network (8, 9). These features have been shown to gradually result in an inability to mediate antibody production and generate effective antigen-specific T cell responses in late stages of infection, contributing to AIDS (10–12). It is reported that CXCR5+ Tfh in lymph nodes cells accumulate in SIV/HIV infection (13–15), so these Tfh cells are thus thought to be dysregulated due to infection, resulting in inadequate B-cell help and subsequent impairment of B cell responses (16, 17). However, other reports have described significant depletion of circulating CXCR5+ Tfh cells in HIV/SIV infection (18), but by definition, functional Tfh cells are restricted to organized lymphoid tissues. Thus, using only CD4 and CXCR5 expression to define Tfh cells may lead to confusion.(18)
Here, we examined the distribution of CXCR5+PD-1HIGHCD4 T cells, which are primarily localized in germinal centers of organized lymphoid tissues, which defines them as mature GC Tfh cells, and evaluated their changes in cohorts SIVmac infected, vaccinated and controlling macaques, as well as cohorts infected with less pathogenic SHIVs. The results demonstrate CXCR5+PD-1HIGH Tfh cells were indeed increased in lymph nodes of animals infected with pathogenic SIV, but not in macaques with some level of viral control. Notably however, CXCR5+PD-1HIGH Tfh cells were depleted in lymph nodes of animals with AIDS, accompanied by increased cellular activation, proliferation, and apoptosis. These findings suggest persistent viral replication initially induces pathologic expansion of, but ultimate depletion of CXCR5+PD-1HIGH Tfh cells in AIDS. The dysfunction or loss of Tfh cells likely contributes to the failure of effective humoral immune responses and disease progression in HIV/SIV infection.
Materials and Methods
Ethics statement
All animals in this study were housed at the Tulane National Primate Research Center in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care International standards. All studies were reviewed and approved by the Tulane University Institutional Animal Care and Use Committee. Animal housing and studies were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (NIH, AAALAC #000594) and with the recommendations of the Weatherall report; “The use of non-human primates in research”. All clinical procedures were carried out under the direction of a laboratory animal veterinarian. All procedures were performed under anesthesia using ketamine, and all efforts were made to minimize stress, improve housing conditions, and to provide enrichment opportunities (e.g., objects to manipulate in cage, varied food supplements, foraging and task-oriented feeding methods, interaction with caregivers and research staff).
Animals and virus
Tissues from a total of 191 adult Indian-origin rhesus macaques (Macaca mulatta; RMs) were utilized to examine Tfh cells in lymph nodes. Of these, 69 were uninfected controls, and others were infected with SIVmac251 and examined in acute (n=22), or chronic infection with either no overt signs of disease (chronic asymptomatic (n=29) or AIDS (n=19) as Mamu-A*01, B*08 and B*17 MHC allele-negative Indian-origin RMs (normal progressors); or Mamu-A*01+ expressing RMs (n=9), or animals that became infected with SIVmac251 despite vaccination with various gag/pol/env vaccines (n=12). Finally, 31 animals infected with SHIVsf162P3 or RT-SHIVsf162P3 only were examined. Blood from three animals was prospectively monitored at different time points post SIV infection. Blood, spleen, lymph nodes and intestinal tissues were collected at necropsy from uninfected controls, or in acute (7–28 days), chronic asymptomatic infection (SIV infection more than 3 months) or AIDS animals with defined opportunistic infections and/or neoplasm/lymphoma, processed into single cell suspensions, and analyzed by flow cytometry. Numbers of animals and tissues used for individual experiments are provided in the figure legends.
Tissue collection and phenotyping
Flow cytometry for surface and intracellular staining was performed using standard protocols (19). Cells were stained with: CD3 (SP34), CD4 (SK3), CD8 (SK1), CD20 (2H7), IL-21 (3AS-N2)(all from BD Biosciences Pharmingen, San Diego, CA), CXCR5 (MU5UBEE, eBioscience), PD-1 (EH12.2H7, BioLegend), PD-L1 (29E.2A3, BioLegend), PD-L2 (24F.10C12, BioLegend), HLA-DR (Immu-357, Beckman Coulter, Brea, CA), Ki67 (B56), Annexin V, and LIVE/DEAD Fixable Aqua Dead Cell Stain Kit (Invitrogen, Grand Island, NY). For intracellular IL-21 detection, lymphocytes (106) from lymph nodes were stimulated in vitro for 4 hours with 0.1μM phorbol 12-myristate-13-acetate (PMA) and, 0.5μg/ml ionomycin (Sigma-Aldrich, St. Louis, MO) in presence of 5μg/ml Brefeldin A. Cells were then stained for their surface markers, or further examined by intracellular molecules (IL-21). Isotype-matched controls were included in all experiments. Samples were resuspended in BD Stabilizing Fixative (BD Biosciences) and acquired on a FACS FORTESSA (Becton Dickinson, San Jose, CA). Data were analyzed with Flowjo software (Tree Star, Ashland, OR).
Multi-color confocal microscopy analysis and immunohistochemistry
Lymph nodes were obtained from rhesus macaques within 30 min of necropsy. Tissues were then processed and stained as previously described (20). In brief, tissues were embedded and snap frozen in optimum cutting temperature compound (OCT) and 7 um frozen sections were stained using unconjugated primary antibodies including CD3, CD20, PD-1, and p53 (1C12, Cell Signaling Tech., MA), followed by appropriate secondary antibodies conjugated to the fluorescent dyes Alexa 488 (green), Alexa 568 (red) or Alexa 633 (blue) (Molecular Probes, Eugene, OR). Confocal microscopy was performed using a Leica TCS SP2 confocal microscope equipped with three lasers (Leica Microsystems, Exton, PA). Individual optical slices representing 0.2 um and 32 to 62 optical slices were collected at 512 × 512 pixel resolution. NIH Image (version 1.63, Bethesda, MD) and Adobe Photoshop CS5 (San Jose, CA) were used to assign colors to the channels collected. To detect apoptotic cells in lymph nodes, paraffin-embedded sections were deparaffinized, and antigens were unmasked using high-temperature antigen retrieval by heating slides in a steam bath chamber (Flavor Scenter Steamer Plus; Black and Decker, Hunt Valley, MD) with 0.01 M citrate buffer pH 6.0 for 20 minutes. Slides were then cooled, washed twice in phosphate-buffered saline (PBS), and blocked with peroxidase blocking reagent (Dako, Glostrup, Denmark) for 10 minutes, washed again in PBS, and further blocked with serum-free protein block (Dako) for 30 minutes. Sections were then incubated with the anti-p53 Ab for 1 hour at room temperature, washed (PBS), and developed using a Vectastain ABC peroxidase kit (Vector Laboratories, Burlingame, CA) and 3,3-diaminobenzidine DAB (Biocare Medical, Concord, CA).
Quantitative image analysis was performed on 10 randomly acquired images of germinal center follicles from each lymph node (3 uninfected and 3 SIVmac-infected AIDS animals). PD-1+ or CD20+ fluorescence signals in germinal centers were quantified by automated inForm Image Analysis Software.
Autologous lymph node CD4+ T cell and B cell co-cultures
To assess the functional capacity of GC Tfh to interact with B cells and support antibody secretion, PD-1HIGHCD4+ T cells and B cells were sorted from mesenteric lymph nodes in uninfected macaques or animals in asymptomatic stage of infection with a FACS Aria (Becton Dickinson Biosciences), and assessed as >95% pure by flow cytometry. Purified B cells (CD20+, 105 cells/well) were cultured either in media alone or with the same number of purified autologous PD-1HIGHCD4+ T cells in triplicate in 96-well round bottom plates. Supernatants were collected after 11 days and analyzed for IgG levels using isotype-specific Abs and an ELISA (Life Diagnostics, PA). To evaluate the effects of PD-1 and PD-L1/PD-L2 interactions between GC Tfh cells and B cells on IgG secretion, anti-PD-1 (10ug/ml), PD-L1 (10ug/ml), PD-L2 (10ug/ml) or isotype control antibodies were added to co-cultures on day 1, and levels of IgG were detected in supernatants after 11 days culture. To observe the effects of blocking antibodies on cell survival, anti-PD-L2 or control Ab were added to co-cultures, and cells were collected at day 2, 4, 6, 8, 11, and viable CD4+ T and B cells were determined by a combination of trypan blue staining and flow cytometry and expressed as absolute numbers of viable cells.
Statistics
Statistical analyses were performed by non-parametric Mann-Whitney t test (two tailed) using GraphPad Prism 4.0 (GraphPad Software, SanDiego, CA). Significant statistic differences are indicated. Asterisks denote p values (*p<0.05, **p<0.01, or ***p<0.001). The data are presented as the mean +/− s.e.m.
Results
Changes of CXCR5+PD-1HIGH Tfh cells in lymph nodes of chronically SIV-infected macaques
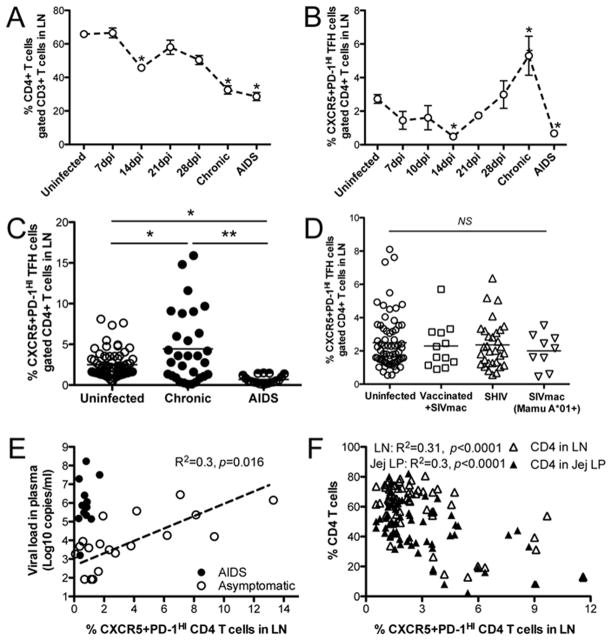
Tfh cells are a heterogeneous population of CD4+ T cells distributed in both follicular and extrafollicular regions of lymph nodes. Although CXCR5 expression is widely used to define Tfh cells (18, 20, 21), CD4+CXCR5+ Tfh cells consist of both of PD-1INT and PD-1HIGH CD4 T cell populations, yet the latter are almost exclusively found within follicular germinal centers. Therefore, PD-1HIGH CD4 T cells expressing CXCR5 represent the more functionally relevant Tfh cells that reside in germinal centers (6, 22). To monitor the effects of SIV infection on CXCR5+PD-1HIGH Tfh cells in lymph nodes, we first examined changes in total CD4+ T cells in LNs after SIV infection and found that CD4+ T cells were significantly decreased in acute (14dpi, 48.8% ± 1.7, n=11), chronic, asymptomatic SIV infection (32.54% ± 2.5, n=28), and in macaques with AIDS (28.57% ± 2.42, n=15), to as low as ~5% of total CD3+ T lymphocytes in some AIDS animals compared to uninfected controls, which contained ~60–70% CD4+ T cells (Fig. 1A). In comparison, no significant reductions of total CD4+ T cells were observed in animals with that had been vaccinated and protected (60.1% ± 2.8, n=12), or infected with less pathogenic SHIV’s (68.31% ± 1.27, n=31), or even compared to pathogenic SIV-infected Mamu A*01+ macaques (51.74% ± 3.32, n=10).
Figure 1. Changes of total CD4+ T and CXCR5+PD-1HIGH Tfh cell subsets in lymph nodes (LNs) of uninfected and SHIV/SIV-infected macaques.
(A) Dynamics of changes in total CD4+ T cells from LNs after pathogenic SIVmac251 infection (7 dpi, n=6; 14 dpi, n=10; 21 dpi, n=3; 28 dpi, n=3) and chronically SIV-infected cohorts; (B) Dynamics of CXCR5+PD-1HIGH Tfh cells from LNs during pathogenic SIVmac infection (7 dpi, n=6; 10dpi, n=3; 14 dpi, n=7; 21 dpi, n=3; 28 dpi, n=3), compared with uninfected controls; (C) changes of CXCR5+PD-1HIGH Tfh cells in LNs from uninfected (n=69), no vaccination and A*01/B*08/B*17 negative in asymptomatic chronic (n=29) and AIDS (n=19) animals; (D) Frequencies of CXCR5+PD-1HIGH Tfh cells from LNs of uninfected (n=69), and chronic cohorts (vaccinated then infected with SIVmac, n=12; SHIV infected, n=31; or SIVmac-infected animals expressing Mamu A*01+, n=9). (E) Correlation of plasma viral loads with changes of Tfh cells in lymph nodes in macaques (asymptomatic, n=19; AIDS, n=13). (F) Correlation of Tfh cell in lymph nodes with CD4 T cells in lymph nodes or jejunum lamina propria in macaques (uninfected, n=46; asymptomatic, n=19). *p<0.05, or **p<0.001 (two tailed Mann-Whitney t test). The chronic cohorts represent chronic asymptomatic infection (SIV infection more than 3 months) or AIDS animals as defined opportunistic infections and/or neoplasm/lymphoma unless otherwise noted. NS, not significant. Data are presented as means ± s.e.m (A–B).
The dynamics of CXCR5+PD-1HIGH Tfh cells in lymph nodes were then examined throughout SIV infection. In general, GC Tfh cells were significantly depleted at 14 dpi, but gradually accumulated in chronic, asymptomatic stages of infection, yet were again depleted in terminal stages of disease (Fig. 1B). Specifically, total CXCR5+PD-1HIGH CD4+ T (Tfh) cells increased in chronically SIVmac251-infected animals that were negative for MHC I alleles associated with some degree of protection (A*01, B*08, and B*17) compared with uninfected controls (p<0.042), yet they were significantly reduced in animals that progressed to AIDS (Fig. 1C).
To seek correlations between changes in CXCR5+PD-1HIGH Tfh cells and levels of viral control, we compared the frequency of these cells in chronically SIV-infected animals (having no known protective alleles) with vaccinated animals later challenged and infected with SIVmac251, animals infected with less pathogenic SHIVsf162P3, and SIVmac251-infected Mamu-A*01+ macaques. In contrast to progressors, CXCR5+PD-1HIGH Tfh cells were relatively maintained, with neither significant expansions nor losses when compared to uninfected controls (Fig. 1D). Further, Tfh cells in lymph nodes accumulated with increased plasma viral loads in chronically SIV-infected macaques (p=0.016), except in animals with AIDS who maintained higher viral loads in plasma, but had fewer Tfh cells in lymph nodes (Fig. 1E). Interestingly, percentages of Tfh cells inversely correlated with CD4 T cells both in lymph nodes and intestinal jejunum lamina propria during SIV infection (except animals in AIDS) (Fig. 1F), suggesting persistent SIV infection promotes Tfh cell expansion and CD4 T-cell depletion in chronic stage.
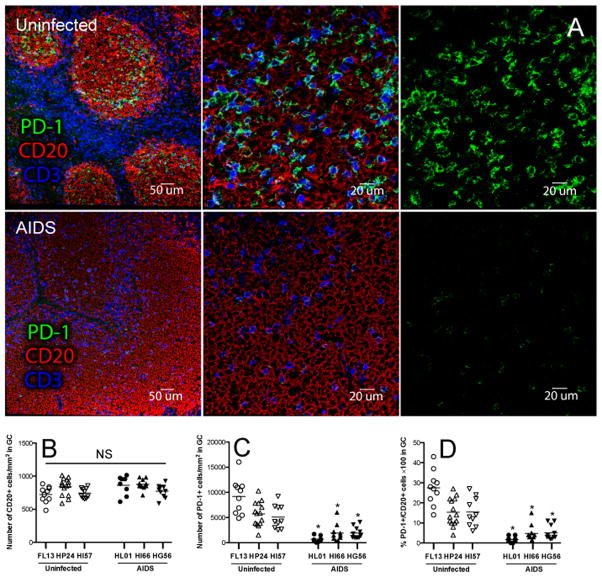
Since we observed depletion of CXCR5+PD-1HIGH Tfh cells in animals that progressed to AIDS by flow cytometry, we performed quantitative image analysis to further confirm that absolute numbers of PD-1+ Tfh cells in follicles of lymph nodes were depleted in macaques with AIDS compared to uninfected controls. Figure 3 shows that follicular PD-1+ Tfh cells were significantly depleted in macaques with AIDS, compared to naïve controls (p<0.0001), despite no significant reductions in total CD20+ follicular B cells (Figs. 2A–2D).
Figure 3. Activation, proliferation, and apoptosis of Tfh cells in lymph nodes in SIV-infected macaques.
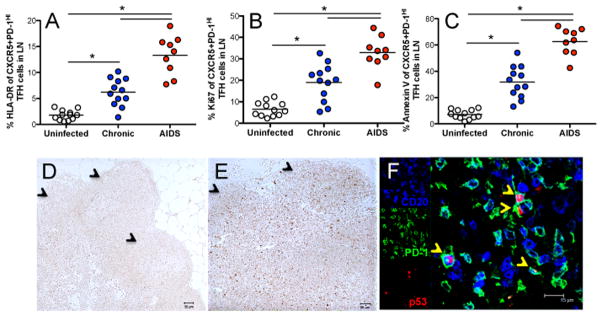
(A–C) Activation (HLA-DR+), turnover (Ki67+), and apoptosis (Annexin V) of CXCR5+PD-1HIGH Tfh cells in uninfected (n=12), asymptomatic chronic cohorts (n=12) and macaques with AIDS (n=9). *p<0.001; (D and E) DNA damage and apoptosis-associated p53 expression in lymph node of uninfected (D) and chronically SIV-infected (E) macaques. (F) Co-expression of p53 (red) on PD-1+ cells (green) and B cells (CD20, blue) in lymph node follicles.
Figure 2. Comparison of PD-1+ cells in follicular germinal centers (GC) of lymph nodes between uninfected and AIDS animals.
(A) Confocal microscopy of PD-1+ cells in GC of lymph nodes in uninfected (top panels) and AIDS animals (bottom panels). CD3, blue; PD-1, green; CD20, red. (B–D) Statistical analysis of total numbers of CD20+B cells per mm2 (B), PD-1+ cells per mm2 (C), and PD-1+ cells per 100 B cells (D) within follicular GC of lymph nodes from individual animals (uninfected, n=3; AIDS, n=3). *p<0.001, determined by two-tailed Mann-Whitney t test.
Apoptosis of CXCR5+PD-1HIGH Tfh cells in chronic SIV infection
To explore the mechanisms of CXCR5+PD-1HIGH Tfh depletion in animals with AIDS, we examined their activation, turnover and apoptosis in lymph nodes in SIV infection. CXCR5+PD-1HIGH Tfh cells had higher levels of activation (HLA-DR+) and proliferation (Ki-67+) in chronically SIV-infected animals, and even higher levels in cohorts with AIDS, compared with uninfected animals (Figs. 3A and 3B). To quantify apoptosis in Tfh subsets, we examined levels of annexin V and p53, the latter accumulates in response to DNA damage, and when in excess, is associated with apoptosis in HIV infection (23–25). Tfh cells from animals with AIDS displayed the highest levels of annexin V, followed by chronically SIV-infected macaques, with the lowest levels detected in uninfected controls (Fig. 3C). In chronically SIV-infected macaques, levels of p53 were highly expressed in lymph node compartments, including both follicular and paracortical regions, compared to uninfected controls (Figs. 3D and 3E). Confocal microscopy and image analysis further confirmed p53 was mostly co-expressed on CD4+ PD-1+ cells specifically residing within follicles of chronically SIV-infected animals (Fig. 3F). Combined these results suggest the depletion of CXCR5+PD-1HIGH Tfh cells in AIDS is attributed to their higher states of activation, turnover and apoptosis in persistent, chronic infection.
Impairment of functional B cell responses by dysregulated or/and losses of CXCR5+PD-1HIGH Tfh cells
As indicated by the accumulation of CXCR5+PD-1HIGH Tfh cells in normal progressors, and an absence of significant expansions in cohorts with some level of protective immunity, we hypothesized the expanded CXCR5+PD-1HIGH Tfh cells may be dysfunctional and incapable of inducing effective antibody responses. Evidence indicates high expression of PD-1 on CXCR5+PD-1HIGH Tfh cells is critical for regulating GC formation, and promoting B cell maturation and antibody production (7, 26). To further elucidate the role of PD-1 and PD-L1/L2 interactions on antibody production between CXCR5+PD-1HIGH Tfh cells and B-cells in lymph nodes, we performed in vitro blockade experiments with anti-PD-1, anti-PD-L1 or anti-PD-L2 antibodies (Ab) in autologous lymph node B and GC Tfh cell co-cultures for up to 11 days. We previously showed that B cells alone could constitutively produce low levels of IgG, yet autologous Tfh and B cell co-cultures result in significantly higher levels of IgG production (6). Interestingly, both PD-1 and PD-L2 blockade significantly reduced IgG production (Fig. 4A). Blockade of PD-L2 blockade was also associated with a loss of CD4+ T cell and B cell viability, which may be the result of apoptosis of both T and B-cells (Fig. 4B). This suggests the PD-1/PD-L2 axis is involved in homeostasis, survival and interaction of Tfh and B cells in lymph nodes. However, PD-L1 blockade has minimal effects on antibody production, which may be due to inadequate IL-21 production (16). In SIV-infected macaques, PD-L1 expression was significantly increased on B cells in both asymptomatic infection and AIDS (Fig. 4C). In contrast, PD-L2 was significantly downregulated on B cells in chronic SIV infection, and further reduced in animals with AIDS (Fig. 4D), which may be a mechanism behind the decreased survival of T/B cells in germinal centers. Finally, IL-21 production was significantly reduced in both non-Tfh (PD-1INT) and PD-1HIGH Tfh cells in chronic infection (Fig. 4E). Finally, impairment of Tfh cells was verified by the fact that IgG production was reduced in co-cultured supernatants of Tfh/B cells from SIV-infected animals (Fig. 4F). Combined, various factors, such as increased PD-L1, but decreased PD-L2 expression on B cells, accompanied by IL-21 deficiency, may all contribute to the eventual dysfunction/loss of GC Tfh in HIV infection and AIDS, and may play a major role in the diminished help for humoral immune responses in HIV infection.
Figure 4. Effects of PD-1/PD-L1/PD-L2 blockade on antibody production, T/B cell survival, and IL-21 production of PD-1HIGH Tfh cells from chronically SIV-infected macaques.
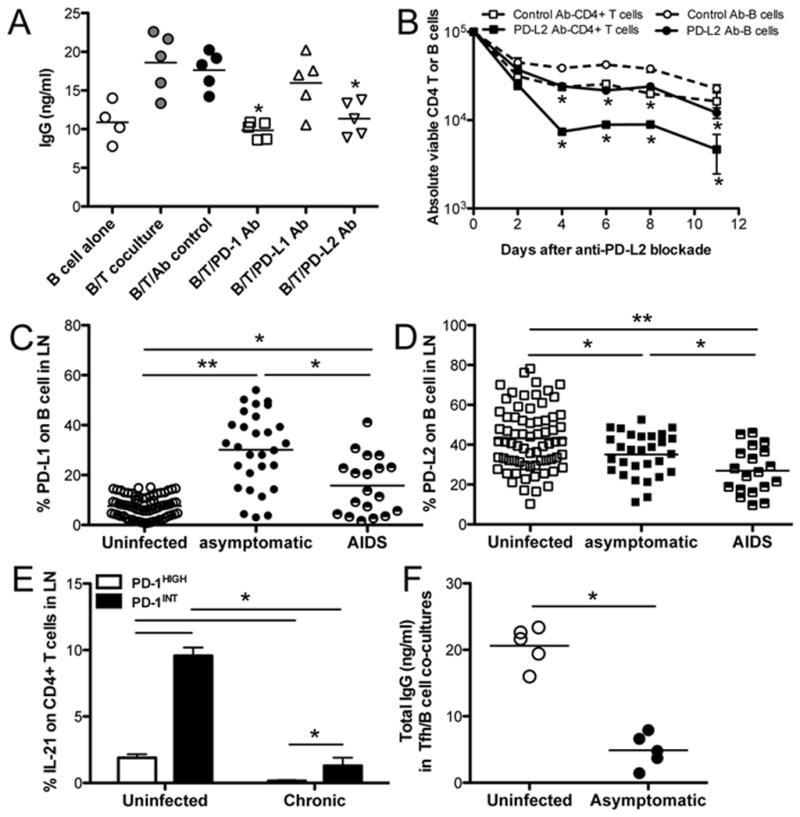
(A) IgG production in autologous B cell and PD-1HIGH Tfh cell co-cultures after PD-1, PD-L1 or PD-L2 blockade. Supernatants were collected 11 days after culture and IgG levels detected by ELISA. Data show results from 5 uninfected animals. (B) Survival of lymph node-derived B cells or CD4+ T cells in co-culture after PD-L2 blockade in vitro. (C and D), Expression of PD-L1 or PD-L2 on B cells in lymph nodes (uninfected, n=69, asymptomatic chronic, n=29; AIDS, n=19); (E) IL-21 production from both PD-1HIGH and PD-1INT CD4+ T cells in lymph nodes of uninfected controls and chronically SIVmac251-infected macaques (uninfected, n=5; chronic, n=14). IL-21-producing cells were detected by intracellular staining after LN-derived lymphocytes were stimulated by mitogen. (F) Levels of IgG in supernatants of autologous Tfh/B cell co-cultures; lymph node cells were derived from macaques in the asymptomatic stage of infection. *p<0.05, **p<0.001, determined by two-tailed Mann-Whitney t test. Data are presented as mean ± s.e.m (B and E).
Discussion
T follicular helper (Tfh) cells play a prominent role in the generation of humoral immune responses. Here we compared changes of CXCR5+PD-1HIGH Tfh cells in cohorts of macaques at different states of infection or immunity, and investigated the mechanisms behind immunologic abnormalities in SIV-infected animal cohorts. The results indicate that the frequencies of CXCR5+PD-1HIGH Tfh cells increase in chronic SIV infection, but these cells are dysfunctional. Eventually, CXCR5+PD-1HIGH Tfh cells are depleted in animals that progress to AIDS, yet are maintained in controllers or animals with some level of protection. Moreover, impairment of B-cell immunity may be associated with this dysfunction/loss of Tfh cells, which correlates with aberrant PD-L1/L2 expression on B cells during SIV infection. Finally, the eventual depletion of CXCR5+PD-1HIGH Tfh cells is associated with their apoptosis. These studies have important implications for therapeutic interventions aimed at inhibiting persistent viral replication by ART, and potentially for boosting B cell and immunoglobulin responses in HIV infection by immunotherapy.
GC Tfh cells specifically reside in follicular germinal centers, which are the crucial niche for the optimal expansion and survival of activated T cells, and B-cell development and maturation (4, 27, 28). GC Tfh cells are predominantly distributed in the follicles of the GALT and other organized lymphoid tissues (spleen, lymph nodes). Tfh cells represent a heterogeneous cell population in lymph nodes (29–32) but mature GC Tfh cells highly express CXCR5, PD-1, ICOS, and produce IL-21 (6, 33). Previous studies have reported Tfh cells in lymph nodes are expanded in SIV or HIV infected individuals (14, 15). Other studies indicate “circulating” Tfh cells are significantly depleted during HIV infection (34). However, some of the discrepancies may be lie in the variable ways in which Tfh cells are defined, and/or variation in stages of infection or immune responses. Here we compared CXCR5+PD-1HIGH Tfh cells from several animals with different backgrounds and inoculations (vaccination, MHC alleles etc.), and observed expansion of CXCR5+PD-1HIGH Tfh cells in animals infected with pathogenic SIVmac (asymptomatic chronic), whereas little to no changes were observed in these cells in animals inoculated with less pathogenic viruses or controlling infection compared to uninfected macaques (Figs. 1B–1D). Since certain MHC class I alleles are associated with better control of infection (Mamu-A*01/B*08/B*17 in macaques and HLA-B27/B57 in humans) (35), we also compared CXCR5+PD-1HIGH Tfh cells in SIV-infected Mamu-A*01+ macaques and found minimal changes in chronic infection (Fig. 1D). Comparing normal and SIV-infected macaques, we did not find consistent expansions of CXCR5+PD-1HIGH Tfh in all SIV-infected macaques, but we did observe significant losses of CXCR5+PD-1HIGH Tfh in animals with AIDS (with AIDS-defining opportunistic infections or neoplasms) (Figs. 1B and 1C). Further, the frequencies of Tfh cells inversely correlated with CD4 T cells in both lymph nodes and jejunum lamina propria during persistent and asymptomatic infection (Fig. 1F). In chronic SIV/HIV infection, marked lymphoid follicular hyperplasia is followed by severe architectural damage to lymph node characterized by fibrosis and follicular involution (36, 37). Thus CXCR5+PD-1HIGH Tfh cells may undergo apoptosis due to excessive and persistent chronic activation and proliferation in persistent, uncontrolled SIV infection (Figs. 3A–3C), inevitably resulting in their loss in terminal AIDS (Figs. 1B and 1C). Other studies have shown that direct infection of Tfh is prominent in pathogenic SIV infection of macaques, but rare/absent in non-pathogenic SIV infection of natural hosts such as Sooty mangabeys (16, 17). Thus, changes in CXCR5+PD-1HIGH Tfh cells in lymph nodes due to direct, or indirect effects of infection may correlate with levels of immunity and disease progression in HIV/SIV infection, which may be responsible for defective host responses in HIV infection.
PD-1 is highly expressed on CXCR5+PD-1HIGH Tfh cells, presumably related to its interaction with its ligands PD-L1 or PD-L2 expressed by GC B cells, which may contribute to their regulation and maturation (6, 7). However, PD-1 has also been described as a potent inhibitory receptor for CD8+ T cells, and is associated with CD8+ T-cell “exhaustion” and/or as a negative regulator of T cell function (20, 38). As shown in Fig. 4A, constitutive and high expression of PD-1 on CXCR5+PD-1HIGH Tfh cells in uninfected animals promotes IgG production, and these effects are markedly reduced by PD-1 or PD-L2 blockade, suggesting PD-1 regulates B-cell function and survival in GC via the PD-1/PD-L2 axis. However, recent reports indicate that engagement of PD-1 on Tfh cells inhibits IL-21 production resulting in inadequate B-cell help indirectly through the PD-1/PD-L1 pathway in HIV infection (16), which is supported by the decreased functional capacity of Tfh cells from infected macaques (Figs. 4C, 4E and 4F). Thus, dysregulation in HIV infection, such as PD-L1 upregulation yet PD-L2 downregulation as well as IL-21 deficiency, may be a cause of the functional impairment of B cells in chronic infection. Combined, these factors undoubtedly result in impairments of B-cell function and antibody production in chronic HIV/SIV infection
In summary, CXCR5+PD-1HIGH Tfh cells are expanded in animals with chronic, asymptomatic stages of progressive SIV infection, yet depleted in animals that have progressed to AIDS. However, there are minimal changes in CXCR5+PD-1HIGH Tfh phenotype or distribution in animals showing some level of protective immunity, or spontaneous viral control. Persistent high level viral replication in progressors may thus promote higher and sustained levels of activation, proliferation, and apoptosis of CXCR5+PD-1HIGH Tfh cells, resulting in their eventual depletion in animals with AIDS. Dysfunction and/or loss of CXCR5+PD-1HIGH Tfh cells, combining with marked PD-L1 upregulation and PD-L2 downregulation on B cells, is likely a fundamental contributor to the impairment of functional B-cell responses, and the impaired antibody responses in HIV/SIV infections.
Acknowledgments
We thank Julie Bruhn and Calvin Lanclos for flow cytometry support and Megan Gardner, Meagan Watkins and Maury Duplantis for technical support.
Footnotes
Author Contributions: H.X. performed and analyzed most of the experiments and wrote the manuscript; X.W. and N.M. performed flow cytometry and confocal microscopy; H.X., A.A.L. and R.S.V. designed the experiments and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
References
- 1.Reinhardt RL, Liang HE, Locksley RM. Cytokine-secreting follicular T cells shape the antibody repertoire. Nature immunology. 2009;10:385–393. doi: 10.1038/ni.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.King C, Sprent J. Emerging cellular networks for regulation of T follicular helper cells. Trends Immunol. 2012;33:59–65. doi: 10.1016/j.it.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, Dent AL, Craft J, Crotty S. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 5.Zotos D, Coquet JM, Zhang Y, Light A, D’Costa K, Kallies A, Corcoran LM, Godfrey DI, Toellner KM, Smyth MJ, Nutt SL, Tarlinton DM. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. The Journal of experimental medicine. 2010;207:365–378. doi: 10.1084/jem.20091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu H, Wang X, Lackner AA, Veazey RS. PD-1(HIGH) Follicular CD4 T Helper Cell Subsets Residing in Lymph Node Germinal Centers Correlate with B Cell Maturation and IgG Production in Rhesus Macaques. Frontiers in immunology. 2014;5:85. doi: 10.3389/fimmu.2014.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Good-Jacobson KL, Szumilas CG, Chen L, Sharpe AH, Tomayko MM, Shlomchik MJ. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nature immunology. 2010;11:535–542. doi: 10.1038/ni.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chalifoux LV, King NW, Letvin NL. Morphologic changes in lymph nodes of macaques with an immunodeficiency syndrome. Lab Invest. 1984;51:22–26. [PubMed] [Google Scholar]
- 9.Zeng M, Haase AT, Schacker TW. Lymphoid tissue structure and HIV-1 infection: life or death for T cells. Trends Immunol. 2012;33:306–314. doi: 10.1016/j.it.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Schacker TW, Nguyen PL, Martinez E, Reilly C, Gatell JM, Horban A, Bakowska E, Berzins B, van Leeuwen R, Wolinsky S, Haase AT, Murphy RL. Persistent abnormalities in lymphoid tissues of human immunodeficiency virus-infected patients successfully treated with highly active antiretroviral therapy. The Journal of infectious diseases. 2002;186:1092–1097. doi: 10.1086/343802. [DOI] [PubMed] [Google Scholar]
- 11.Fauci AS. Multifactorial nature of human immunodeficiency virus disease: implications for therapy. Science. 1993;262:1011–1018. doi: 10.1126/science.8235617. [DOI] [PubMed] [Google Scholar]
- 12.Hong JJ, Amancha PK, Rogers KA, Courtney CL, Havenar-Daughton C, Crotty S, Ansari AA, Villinger F. Early lymphoid responses and germinal center formation correlate with lower viral load set points and better prognosis of simian immunodeficiency virus infection. J Immunol. 2014;193:797–806. doi: 10.4049/jimmunol.1400749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cubas R, Perreau M. The dysfunction of T follicular helper cells. Current opinion in HIV and AIDS. 2014 doi: 10.1097/COH.0000000000000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petrovas C, Yamamoto T, Gerner MY, Boswell KL, Wloka K, Smith EC, Ambrozak DR, Sandler NG, Timmer KJ, Sun X, Pan L, Poholek A, Rao SS, Brenchley JM, Alam SM, Tomaras GD, Roederer M, Douek DC, Seder RA, Germain RN, Haddad EK, Koup RA. CD4 T follicular helper cell dynamics during SIV infection. The Journal of clinical investigation. 2012;122:3281–3294. doi: 10.1172/JCI63039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindqvist M, van Lunzen J, Soghoian DZ, Kuhl BD, Ranasinghe S, Kranias G, Flanders MD, Cutler S, Yudanin N, Muller MI, Davis I, Farber D, Hartjen P, Haag F, Alter G, Schulze Zur Wiesch J, Streeck H. Expansion of HIV-specific T follicular helper cells in chronic HIV infection. The Journal of clinical investigation. 2012;122:3271–3280. doi: 10.1172/JCI64314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cubas RA, Mudd JC, Savoye AL, Perreau M, van Grevenynghe J, Metcalf T, Connick E, Meditz A, Freeman GJ, Abesada-Terk G, Jr, Jacobson JM, Brooks AD, Crotty S, Estes JD, Pantaleo G, Lederman MM, Haddad EK. Inadequate T follicular cell help impairs B cell immunity during HIV infection. Nature medicine. 2013;19:494–499. doi: 10.1038/nm.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brenchley JM, Vinton C, Tabb B, Hao XP, Connick E, Paiardini M, Lifson JD, Silvestri G, Estes JD. Differential infection patterns of CD4+ T cells and lymphoid tissue viral burden distinguish progressive and nonprogressive lentiviral infections. Blood. 2012;120:4172–4181. doi: 10.1182/blood-2012-06-437608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Locci M, Havenar-Daughton C, Landais E, Wu J, Kroenke MA, Arlehamn CL, Su LF, Cubas R, Davis MM, Sette A, Haddad EK, Poignard P, Crotty S. Human circulating PD-(+)1CXCR3(−)CXCR5(+) memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity. 2013;39:758–769. doi: 10.1016/j.immuni.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu H, Wang X, Liu DX, Moroney-Rasmussen T, Lackner AA, Veazey RS. IL-17-producing innate lymphoid cells are restricted to mucosal tissues and are depleted in SIV-infected macaques. Mucosal immunology. 2012;5:658–669. doi: 10.1038/mi.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu H, Wang X, Pahar B, Moroney-Rasmussen T, Alvarez X, Lackner AA, Veazey RS. Increased B7-H1 expression on dendritic cells correlates with programmed death 1 expression on T cells in simian immunodeficiency virus-infected macaques and may contribute to T cell dysfunction and disease progression. J Immunol. 2010;185:7340–7348. doi: 10.4049/jimmunol.1001642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fairfax KC, Everts B, Amiel E, Smith AM, Schramm G, Haas H, Randolph GJ, Taylor JJ, Pearce EJ. IL-4-secreting secondary T follicular helper (Tfh) cells arise from memory T cells, not persisting Tfh cells, through a B cell-dependent mechanism. J Immunol. 2015;194:2999–3010. doi: 10.4049/jimmunol.1401225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mylvaganam GH, Velu V, Hong JJ, Sadagopal S, Kwa S, Basu R, Lawson B, Villinger F, Amara RR. Diminished Viral Control during Simian Immunodeficiency Virus Infection Is Associated with Aberrant PD-1hi CD4 T Cell Enrichment in the Lymphoid Follicles of the Rectal Mucosa. J Immunol. 2014 doi: 10.4049/jimmunol.1401222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suzuki K, Dashzeveg N, Lu ZG, Taira N, Miki Y, Yoshida K. Programmed cell death 6, a novel p53-responsive gene, targets to the nucleus in the apoptotic response to DNA damage. Cancer science. 2012;103:1788–1794. doi: 10.1111/j.1349-7006.2012.02362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weige CC, Allred KF, Armstrong CM, Allred CD. P53 mediates estradiol induced activation of apoptosis and DNA repair in non-malignant colonocytes. The Journal of steroid biochemistry and molecular biology. 2012;128:113–120. doi: 10.1016/j.jsbmb.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Gougeon ML, Piacentini M. New insights on the role of apoptosis and autophagy in HIV pathogenesis. Apoptosis. 2009;14:501–508. doi: 10.1007/s10495-009-0314-1. [DOI] [PubMed] [Google Scholar]
- 26.Kawamoto S, Tran TH, Maruya M, Suzuki K, Doi Y, Tsutsui Y, Kato LM, Fagarasan S. The inhibitory receptor PD-1 regulates IgA selection and bacterial composition in the gut. Science. 2012;336:485–489. doi: 10.1126/science.1217718. [DOI] [PubMed] [Google Scholar]
- 27.MacLennan IC. Germinal centers. Annu Rev Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 28.Yu D, Vinuesa CG. The elusive identity of T follicular helper cells. Trends Immunol. 2010;31:377–383. doi: 10.1016/j.it.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Shulman Z, Gitlin AD, Targ S, Jankovic M, Pasqual G, Nussenzweig MC, Victora GD. T follicular helper cell dynamics in germinal centers. Science. 2013;341:673–677. doi: 10.1126/science.1241680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu H, Li X, Liu D, Li J, Zhang X, Chen X, Hou S, Peng L, Xu C, Liu W, Zhang L, Qi H. Follicular T-helper cell recruitment governed by bystander B cells and ICOS-driven motility. Nature. 2013;496:523–527. doi: 10.1038/nature12058. [DOI] [PubMed] [Google Scholar]
- 31.Linterman MA, Liston A, Vinuesa CG. T-follicular helper cell differentiation and the co-option of this pathway by non-helper cells. Immunological reviews. 2012;247:143–159. doi: 10.1111/j.1600-065X.2012.01121.x. [DOI] [PubMed] [Google Scholar]
- 32.Barnett LG, Simkins HM, Barnett BE, Korn LL, Johnson AL, Wherry EJ, Wu GF, Laufer TM. B cell antigen presentation in the initiation of follicular helper T cell and germinal center differentiation. J Immunol. 2014;192:3607–3617. doi: 10.4049/jimmunol.1301284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang C, Hillsamer P, Kim CH. Phenotype, effector function, and tissue localization of PD-1-expressing human follicular helper T cell subsets. BMC immunology. 2011;12:53. doi: 10.1186/1471-2172-12-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boswell KL, Paris R, Boritz E, Ambrozak D, Yamamoto T, Darko S, Wloka K, Wheatley A, Narpala S, McDermott A, Roederer M, Haubrich R, Connors M, Ake J, Douek DC, Kim J, Petrovas C, Koup RA. Loss of circulating CD4 T cells with B cell helper function during chronic HIV infection. PLoS Pathog. 2014;10:e1003853. doi: 10.1371/journal.ppat.1003853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goulder PJ, Watkins DI. Impact of MHC class I diversity on immune control of immunodeficiency virus replication. Nat Rev Immunol. 2008;8:619–630. doi: 10.1038/nri2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schacker TW, Brenchley JM, Beilman GJ, Reilly C, Pambuccian SE, Taylor J, Skarda D, Larson M, Douek DC, Haase AT. Lymphatic tissue fibrosis is associated with reduced numbers of naive CD4+ T cells in human immunodeficiency virus type 1 infection. Clin Vaccine Immunol. 2006;13:556–560. doi: 10.1128/CVI.13.5.556-560.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Estes JD. Pathobiology of HIV/SIV-associated changes in secondary lymphoid tissues. Immunological reviews. 2013;254:65–77. doi: 10.1111/imr.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nature immunology. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]