Abstract
Expression of promyelocytic leukemia zinc finger (PLZF) protein directs the effector differentiation of invariant NKT (iNKT) cells and IL-4+γδNKT cells. We show here that PLZF is also required for the development and function of IL-17+γδT-cells. We observed that PLZF is expressed in fetal-derived invariant Vγ5+ and Vγ6+γδT-cells, which secrete IFN-γ and IL-17 respectively. PLZF-deficiency specifically affected the effector differentiation of Vγ6+ cells, leading to reduced numbers of mature CD27−CD44+ phenotype capable of secreting IL-17. Although PLZF was not required for Vγ5+γδT-cells to develop, when these cells were re-programmed into IL-17-secreting cells in SkinT-mutant mice, they required PLZF for their effector maturation, similarly to Vγ6+γδT-cells. The impaired effector differentiation of PLZF-deficient Vγ6+ γδT-cells was not due to increased apoptosis and it was related to reduced proliferation of immature CD27+CD44− Vγ6+γδT-cells, which was required for their differentiation into mature CD27−CD44+ IL-17-secreting cells. Thus, this work identifies that PLZF function is not restricted to NKT or IL-4+ T-cells cells but it also controls the development of IL-17+ γδT-cells.
Keywords: gamma delta T-cells, PLZF, IL-17
INTRODUCTION
Conventional T-cells leave the thymus as naïve cells. Only after recognition of their cognate antigen in the periphery they differentiate into cytokine-secreting cells belonging mainly to the Th1, Th2 and Th17 T-cell subsets. “Innate-like” T-cells acquire cytokine secreting functions within the thymus and preferentially migrate to non-lymphoid tissues, providing a first line of defense against invading pathogens (1, 2) A common feature of different subsets of innate-like T-cells within the adult T-cell compartment, including invariant NKT (iNKT) cells, γδNKT-cells (Vγ1.1+Vδ6.3+γδT-cells), and human mucosal associated invariant T (MAIT) cells, is the expression of the promyelocytic leukemia zinc finger (PLZF) protein (3–7). PLZF is considered a determinant of innate-like differentiation as it is necessary for the acquisition of effector functions in iNKT cells and γδNKT cells. PLZF-deficient iNKT cells have a naïve phenotype, preferentially secrete IL-2 and migrate to lymph-nodes (3, 4, 6). Conversely, transgenic PLZF expression during T-cell development is sufficient to provide some “innate” effector functions to conventional T-cells. These are the acquisition of a memory/activated phenotype, the ability to co-secrete IL-4 and IFN-γ and preferential migration to the liver microvasculature, unique characteristics of iNKT cells (4, 8, 9).
During fetal hematopoiesis γδT-cell subsets with innate-like properties are generated, such as invariant Vγ5+ and Vγ6+γδT-cells. Hematopoietic progenitors that enter the thymus at approximately embryonic day 14 (E14) first rearrange the Vδ1 and Vγ5 TCR loci. Successful rearrangement allows the development of dendritic epidermal T-cells (DETC), which secrete IFN-γ and migrate to the intraepithelial compartment of the skin (10, 11). In contrast, upon unproductive Vγ5 rearrangements, cells attempt to rearrange the Vγ6 loci, leading to the generation of invariant Vγ6Vδ1 γδT-cells, which secrete IL-17 and colonize the epithelium of the uterus, tongue, lungs and peritoneum. IL-17+γδT-cells are broadly identified as CD27-negative cells in peripheral tissues (12, 13) and preferentially proliferate in response to IL-7 signals, allowing their self-renewal in peripheral tissues (14). IL-17+ γδT-cells are a major source of innate IL-17 in several mucosal infection models such as Mycobacterium tuberculosis, Escherichia coli, and Listeria monocytogenes infections (15, 16). Innate IL-17 and IL-21 secretion by γδT-cells in response to IL-1 and IL-23 was shown to mediate autoimmune inflammation in an experimental autoimmune encephalomyelitis (EAE) model (17).
In this study, we have uncovered a novel function of PLZF in the development of innate-like IL-17+Vγ6+ γδT-cells. Although PLZF is expressed in both Vγ5+ and Vγ6+ fetal thymic precursors, it is specifically required for the development of Vγ6+ γδT-cells, allowing their normal maturation, expansion, and acquisition of IL-17 secretion after selection. Interestingly, despite PLZF expression in Vγ5+ γδT-cells, PLZF-deficiency didn’t affect their development or their ability to colonize the skin. The inability of PLZF-deficient Vγ6+γδT-cells to expand intrathymically was not due to increased apoptosis, but to an impairment of Vγ6+γδT-cells to proliferate and acquire a mature CD27−CD44+ phenotype and function.
MATERIAL AND METHODS
Materials and Mice
C57BL/6 mice were purchased from Jackson laboratories. PLZF-deficient (PLZF KO) mice have been described (3). Lck-Bcl2 TG mice were previously described (18). Rag2/γc-deficient mice (Rag2-gc KO) were purchased from Taconic. FVB/N mice from Taconic farms, which present the Skin1 mutation, were crossed with PLZF−/− mice to generate compound PLZF−/− Skint1-mutant mice. A similar cross was performed between FVB/N mice from Jackson labs, which does not contain the Skint1 mutation, with PLZF−/− mice to obtain PLZF−/− Skint1-wild type mice. All animals were bred in-house and surgeries performed under approved animal protocols by the NCI animal care and use committee.
Tissue dissections, antibodies and flow cytometry
Pregnant day 16 mothers were euthanized by bottled CO2, fetus were isolated and placed on ice for 30–60 minutes. Neonatal day1 pups were similarly sacrificed by hypothermia. Fetus and neonates were washed in PBS and tail samples digested for genotyping. Their thymuses extracted under a magnifier and dispersed under a 70 µm cell strainer (Falcon) to obtain single cell suspensions. γδT-cells from uterus and skin were isolated by Liberase (Roche) and DNaseI (Sigma) digestion of the tissues, wash and filtration through a 70 µm cell strainer (Falcon). Immune cells in these cell suspensions were identified by staining with an anti-CD45.2 (104) specific antibody. Antibody staining was performed for 30 min on ice and FACs analysis was done using BD LSRFortessa cell analyzer (BD Biosciences). The following antibody clones were used: Lin-biotin panel (TER-119, RB6-8C5, RA3-6B2, M1/70, 145-2C11), c-kit (2B8), CD44(IM7), CD25(PC61), TCR γδ (GL3), CD3ε (145-2C11), CD27(16.7F9/LG.7F9), Vγ5(536), CCR6(140706), CD127(A7R34), CD62L(MEL-14). Alpha-galcer-(aGC)-CD1d tetramers were obtained from the NIH tetramer core facility. Identification of Vγ6+ γδT-cells was performed by prestaining with GL3 antibody, followed by staining with the unconjugated rabbit 17D1 IgM antibody and a labeled secondary anti-IgM antibody (Jackson ImmunoResearch). When identifying Vγ6+ γδT-cells, a simultaneous staining with Vγ5-specific antibody was performed to exclude Vγ5+ cells from the analysis.
Intranuclear PLZF staining was performed after surface staining using the Foxp3 transcription factor staining buffer set (eBioscience) followed by staining with the anti-PLZF-PE antibody (Mags.21F7) or isotype control (eBiosciences). Analysis of IL-17+ γδT-cells was performed by sorting γδT-cells according to surface markers using the FACsaria cell sorter and stimulation for 4 hours with PMA plus ionomycin and Brefeldin-A, followed by surface staining, fixation and permeabilization of cells using the intracellular staining kit (BD Biosciences) and staining with the anti-IL-17a antibody (TC11-18H10.1, BioLegend). FACS was performed using FITC, PE, PERCP5.5, PEcy7, APC and Pacific Blue dyes. Dead cells were excluded by PI staining or LIVE/DEAD Fixable Dead Cell Stain Kits (Life technologies) and doublets were excluded based on FCS-W and size distribution.
BrdU labeling
Analysis of in vivo proliferation was performed by a single intraperitoneal injection of BrdU (10mg/ml) in PBS to the mother. 24hs later, mothers were sacrificed and fetuses dissected to analyze BrdU incorporation in thymic γδT-cells by FACs analysis
RT-PCR
qPCR was analyzed using SYBR green in 7900KT Fast-Real-time PCR system. The following primers were used: Zbtb16 forward, TCTGACAAAGATGGGGATGATCC, reverse, CAGTATTCCGTGCAGATGGTACAC; Bgl2 forward, GGTGGGGTCATGTGTGTGG, reverse, CGGTTCAGGTACTCAGTCATCC; Vγ6 forward, GAAGCCCGATGCATACATAC, and reverse, GGGAAATGTCTGCATCAAGC. Vγ5 forward, GAGGATCCCGCTTGGAAATGGATGAGA, reverse, CTTATGGAGATTTGTTTCAGC; Sox13 forward, TACTCACAGACTGATCCCCAG, reverse, AAGTGCAGTTTCACCCATCAC; Runx1 forward, TGAACCACTCCACTGCCTTTAA, reverse, AATGGGTGTCGCTGGGTG; Rorc forward, TGCAAGACTCATCGACAAGGC, reverse, AGCTTTTCCACATGTTGGCTG; Hprt forward, TCAGTCAACGGGGGACATAAA, reverse, GGGGCTGTACTGCTTAACCAG.
Statistics
Plotting of the data and statistic analysis was performed using the Graphpad Prism software using unpaired standard T-test to compare two populations. The significance obtained is represented in the graphs.
RESULTS
PLZF is expressed in fetal Vγ5+ and Vγ6+ γδT-cells
PLZF is required for the innate-like differentiation of iNKT and Vγ1.1Vδ6.3 γδT-cells. As fetal hematopoiesis preferentially gives rise to innate-like subsets, we assessed whether PLZF expression is also shared with other innate-like T-cells of fetal origin. To this end, we analyzed intracellular PLZF levels in different thymic subsets in embryonic day 16 (E16) and day one (d1) neonatal thymi. PLZF was present in early thymic progenitors (ETP) of fetal origin, increasing at the DN2a stage and then gradually decreasing as cells progressed towards the DN3 and DN4 stages. Analysis of PLZF mean fluorescence intensity (MFI) levels in fetal and neonatal γδT-cells identified approximate 50% lower levels in d1 neonatal cells as compared to fetal γδT-cells (Figure 1a). We next analyzed whether the presence of PLZF protein correlated with increased expression by measuring Zbtb16 (PLZF) mRNA transcripts in fetal-derived γδT-cells. We included CD4 single positive (CD4SP) thymocytes, iNKT and γδT-cells from adult thymus as reference controls for the analysis. Zbtb16 mRNA levels were high in fetal (E16) γδT-cells and were reduced eighty-fold in neonatal (d1) γδT-cells (Figure 1b), indicating that high PLZF expression is restricted to γδT-cell subsets that develop at the earliest fetal stages. The low Zbtb16 mRNA levels that we detected in adult γδT-cells may correspond to the PLZF+Vγ1.1Vδ6.3 γδT-cell subset that develops after birth (6). Interestingly, Zbtb16 mRNA levels in fetal γδT-cells were approximately four-fold higher than those of adult iNKT cells. Furthermore, the high levels of PLZF in DN2 thymocytes in the fetal thymus, which are the precursors of all T-cells and have not been committed to the γδT-cell lineage, indicated that PLZF expression occurs before γδT-cell selection.
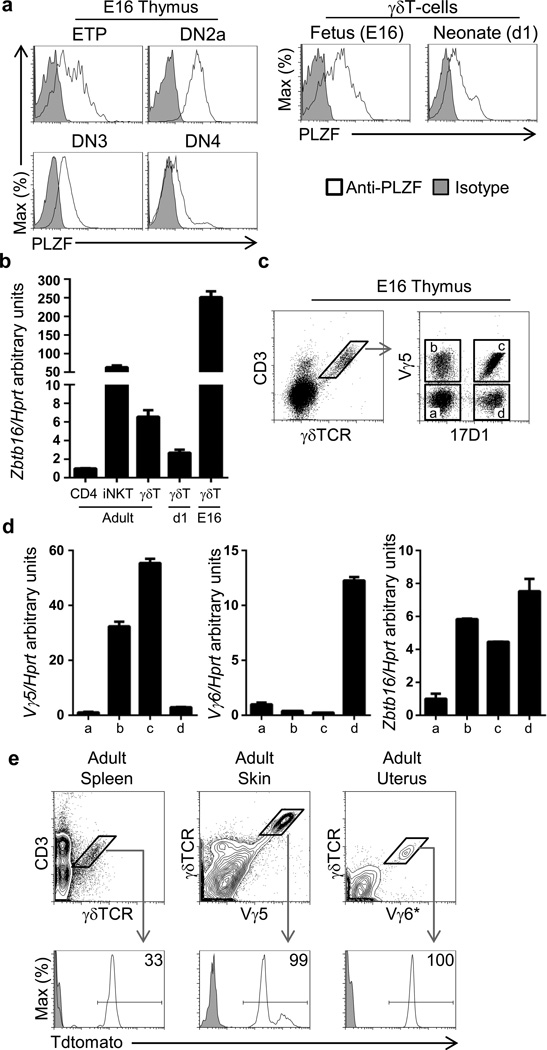
Figure 1. PLZF is expressed in fetal-derived Vγ5+ and Vγ6+ γδT-cells.
a). PLZF intracellular staining of C57BL/6 fetal embryo day 16 (E16) and neonatal d1 thymus. Double negative (DN) thymocytes: ETP (CD4−CD8−Lin−CD44+ckithiCD25−); DN2a (CD4−CD8−Lin−CD44+ckithiCD25+); DN3 (CD4−CD8−Lin−CD44−CD25+); DN4 (CD4−CD8−Lin−CD44−CD25−) and γδT-cells (GL3+, 2C11+). b) RT-PCR of sorted T-cell subsets from adult, neonatal day 1 (d1) and fetal day 16 (E16) thymus. c) Gating strategy used to sort subpopulations of γδT-cells based on Vγ5 and 17D1 staining. d) RT-PCR from from sorted γδT-cell subsets indicated in (C). e) FACs analysis of Tdtomato label in the subpopulations indicated from different tissues. The numbers indicate the percentage of events within the gate from compound PLZF-CRE x Rosa-Tdtomato mice. (a and e) are representative of three independent experiments, (b, c, and d) are representative of two independent experiments. * Vγ6+ cells are identified as CD45.2+ GL3+2C11+Vγ5−17D1+ cells.
To investigate the γδT-cell subsets that express PLZF in the fetal (E16) thymus, we set up to identify Vγ5+ and Vγ6+ γδT-cell subsets by FACs analysis. We used the 17D1 antibody that recognizes the Vγ5Vδ1 γδTCR but can also recognize the Vγ6Vδ1 γδTCR if cells are pre-stained with the anti-γδTCR GL3 antibody clone (19). In order to verify the specificity of the 17D1 antibody for Vγ6+ γδT-cells, we first performed a double staining with an anti-Vγ5 antibody and 17D1. We observed that 17D1+ cells were Vγ5+ as well as Vγ5− cells; that some Vγ5+ cells didn’t present 17D1 staining; and that many γδT-cells didn’t present Vγ5 or 17D1 staining (Figure 1c). Vγ5−17D1− γδT-cells corresponded mostly to the Vγ4 γδT-cell subset (Supplementary Figure 1). In order to clearly identify which of these γδT-cell subpopulations corresponded to the Vγ6 and Vγ5 subsets, we sorted the different γδT-cell subpopulation and analyzed Vγ5 and Vγ6 transcripts by RT-PCR. As expected, Vγ5 transcripts were present only in Vγ5+ γδT-cells irrespective of staining with the 17D1 antibody while expression of the Vγ6 TCR was restricted to Vγ5−17D1+ γδT-cells (Figure 1d), indicating that the combination of 17D1 and Vγ5 staining could be used to specifically identify Vγ6+γδT-cells. Sequence analysis of the purified Vγ6 RT-PCR product further confirmed that it corresponded to the canonical invariant Vγ6+ γδTCR (data not shown). High Zbtb16 transcript levels were found in both Vγ5+ and Vγ6+ but not in Vγ5−Vγ6− γδT-cell subsets in the E16 thymus (Figure 1d), suggesting that PLZF expression may play a role in the development of these lineages.
In order to evaluate whether Vγ5+ and Vγ6+ γδT-cells found in adult mice were exclusively derived from PLZF-expressing cells, we performed fate mapping using compound PLZF-CRE transgenic x Rosa-Tdtomato mice. In this strain, expression of CRE is controlled by the PLZF promoter, leading to the excision of a stop cassette in the Rosa promoter and to the constitutive expression of Tdtomato. Therefore, even the transient expression of PLZF leads to the permanent label of cells and their progeny. Using this fate-mapping model we observed that 100% of Vγ5+ γδT-cell in the adult skin and 100% of Vγ6+ γδT-cells in the adult uterus were derived from PLZF-expressing cells, while approximately 30% of γδT-cells found in the spleen were derived from PLZF-expressing cells (Figure 1e). This 30% Tdtomato label in adult γδT-cells is consequence of PLZF expression in hematopoietic stem cells, which also leads to a 30% Tdtomato label in CD4−CD8− double negative (DN) and CD4+CD8+ double positive (DP) thymocytes as well as in CD4 and CD8 T-cells in spleen (20) (Supplementary Figure 2).
Thus, PLZF is expressed in Vγ5+ and Vγ6+ γδT-cells in the fetal thymus.
PLZF is required for normal development of Vγ6+ but not of Vγ5+γδT-cells
Based on the data presented above, we were interested in determining whether PLZF plays similar or different roles in the development of Vγ6+ and Vγ5+ γδT-cells. PLZF-deficiency didn’t affect the number of Vγ5+ or Vγ6+γδT-cells in the fetal thymus, at the time point at which they are first selected (E16). However, by day 1 of life, the number of PLZF-deficient Vγ6+ γδT-thymocytes was reduced by approximately four-fold (Figure 2a and 2b and 2c; p<0.0001), suggesting that the amplification of Vγ6+γδT-cells between the fetal to the neonatal stage required PLZF. Analysis of Vγ6+γδT-cell numbers in lungs and utero of adult mice didn’t identify a significant reduction in PLZF-deficient mice (Figure 2d), suggesting that self renewal in peripheral tissues may compensate their impaired development. Interestingly, Vγ5+γδT-cell numbers in the fetal thymus and adult skin were not affected by PLZF deficiency (Figure 2e), strongly suggesting that PLZF specifically contributes to the development of Vγ6+ but not Vγ5+γδT cells.
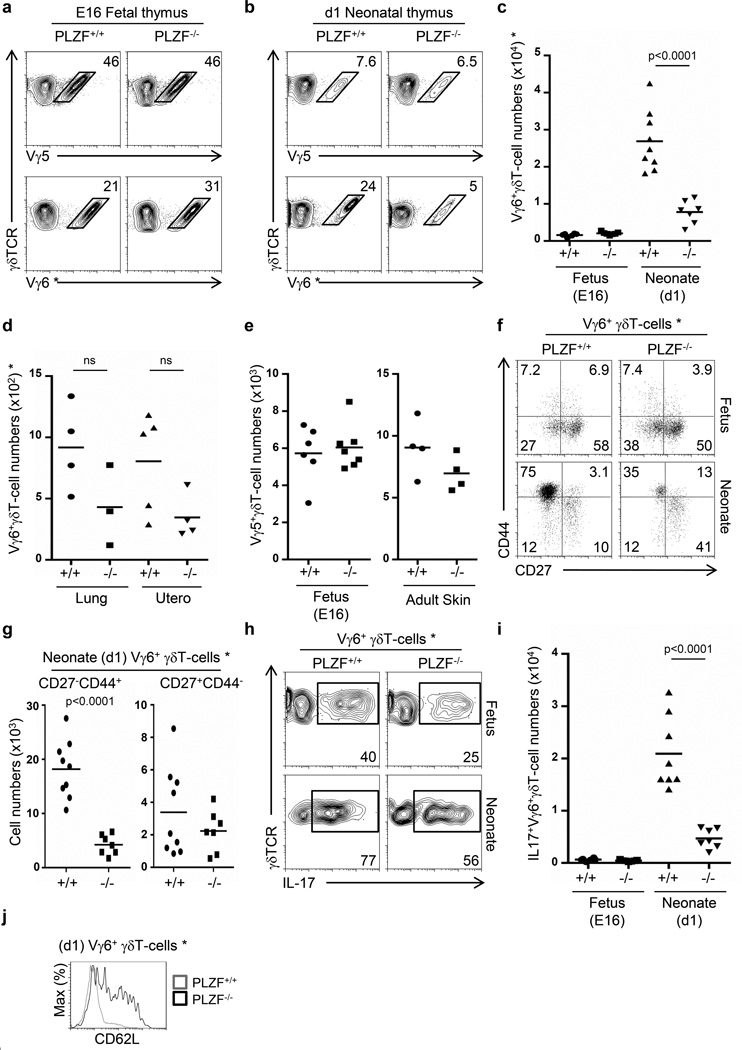
Figure 2. PLZF is required for the generation of Vγ6+ γδT-cells with a mature CD27−CD44+ phenotype and it does not affect the development of Vγ5+ γδT-cells.
FACs analysis showing the proportion of Vγ5+ and Vγ6+ γδT-cells in fetal day 16 (E16) (a) and neonatal day 1 thymus (b). Gram plots showing thymic Vγ6+γδT-cell numbers (c), and Vγ6+γδT-cell numbers in Lung and uterus (d) comparing wild type (+/+) and PLZF-deficient (−/−) cells. e) Number of Vγ5+ γδT-cells in the Fetal (E16) thymus and in adult skin. f) FACs profile of Vγ6+ γδT-cells in the fetal (E16) and neonatal (d1) thymus. g) Gram plot indicating the cell numbers of subsets of Vγ6+ γδT-cells identified in panel (f). h) FACs analysis for intracellular IL-17 staining in Vγ6+γδT-cells. i) Gram plot indicating the number of IL-17+Vγ6+γδT-cells.j) Histogram analysis of Vγ6+γδT-cells in the neonatal thymus. The numbers within the dot-plots represent the proportion of events (a, b, f, h). Data correspond to two and three independent experiments. Each dot in the gram plots represents data from a fetus or neonate and horizontal lines represent the mean value. p values corresponding to the significance using T-test are shown. * Vγ6+ cells are identified as GL3+2C11+Vγ5−17D1+ cells.
To determine whether PLZF deficiency affects the phenotype as well as the expansion of Vγ6+γδT cells, we assessed their phenotype. γδT-cells that secrete IL-17 are identified as CD27−CD44+ in the periphery, but within the fetal thymus, this subset represented less than 10% of Vγ6+γδ cells and was not affected by PLZF-deficiency (Figure 2f). Notably though, by day 1 of life, the vast majority of Vγ6+ γδT-cells in wild type mice were CD27−CD44+ (77%, range 74-81%), indicating that Vγ6+ γδT-cells acquire this phenotype during the late fetal stages. PLZF-deficiency led to a decrease in both the absolute numbers of Vγ6+ γδT-cells as well as in the percentage of Vγ6+ cells with a CD27−CD44+ phenotype (50%, range 34-64%)(Figure 2f). This change in phenotype resulted in reduced numbers of CD27−CD44+ Vγ6+ γδT-cells in PLZF-deficient mice and similar numbers of CD27+CD44− Vγ6+ γδT-cells in wild type and PLZF-deficient mice (Figure 2g). These results suggest that PLZF specifically regulates the generation of the CD27−CD44+ subset from immature precursors.
PLZF is required for the IL-17 effector differentiation of Vγ6+ γδT-cells
Our results indicated that intrathymic Vγ6+ γδT-cells can be subdivided into different subpopulations based on the expression of CD27 and CD44. They also showed that the predominant Vγ6+ γδT-cell subset in the fetal E16 thymus was CD27+CD44− while a CD27−CD44+ phenotype was predominant in the neonatal thymus. We therefore assessed whether these phenotypes corresponded to differences in the ability to secrete IL-17, and furthermore, whether PLZF was required for the acquisition of this effector function. Approximately 40% of wild type Vγ6+ γδT-cells from the fetal thymus had the capacity to secrete IL-17 and this proportion increased to approximately 80% in the neonatal thymus. Interestingly, PLZF-deficiency led to a reduced proportion and numbers of IL-17+ Vγ6+ γδT-cells in both the fetal and neonatal thymus (Figure 2h and 2i), indicating that in the absence of PLZF, the effector maturation of Vγ6+ γδT-cells was affected. The impaired acquisition of IL-17 secretion in PLZF-deficient Vγ6+γδT-cells did not correlate with increased IFN-γ secretion as shown by intracellular staining (Supplementary Figure 3), indicating that PLZF does not prevent an alternative differentiation fate into IFN-γ-secreting cells. These results show that PLZF is required for the acquisition of IL-17 effector functions in Vγ6+ γδT-cells during the fetal period. Impaired effector-maturation of PLZF-deficient Vγ6+ γδT-cells was also evident as cells failed to downregulate CD62L levels, a common characteristic of maturation in innate-like T-cells (Figure 2j).
In Sum, these data indicate that PLZF expression is required for the acquisition of IL-17-secretion in Vγ6+ γδT-cells, as well as their maturation into a CD62L−CD27−CD44+ phenotype.
PLZF is required for immature CD27+CD44− Vγ6+ γδT-cells to develop into mature CD27−CD44+ IL-17 secreting cells
To specifically determine whether CD27+CD44− Vγ6+ γδT-cells found in the E16 fetal thymus were the precursors of CD27−CD44+ cells, we sorted CD27+CD44− Vγ6+ γδT-cell subsets from wild type and PLZF-deficient E16 thymus and assessed their differentiation potential in vitro in co-cultures with the stroma cell line OP9-DL1 for 3 days. Wild type CD27+CD44− Vγ6+ γδT-cells gave rise to a majority of CD27−CD44+ cells after culture, indicating that CD27+CD44− cells are the precursors of CD27−CD44+ cells. Importantly, CD27+CD44− Vγ6+ γδT-cells were unable to generate the CD27−CD44+ subset in the absence of PLZF, revealing a requirement for PLZF at this maturation step (Figure 3a). Thus, PLZF is required for the maturation of CD27+CD44− Vγ6+γδT-cells to a CD27−CD44+ phenotype.
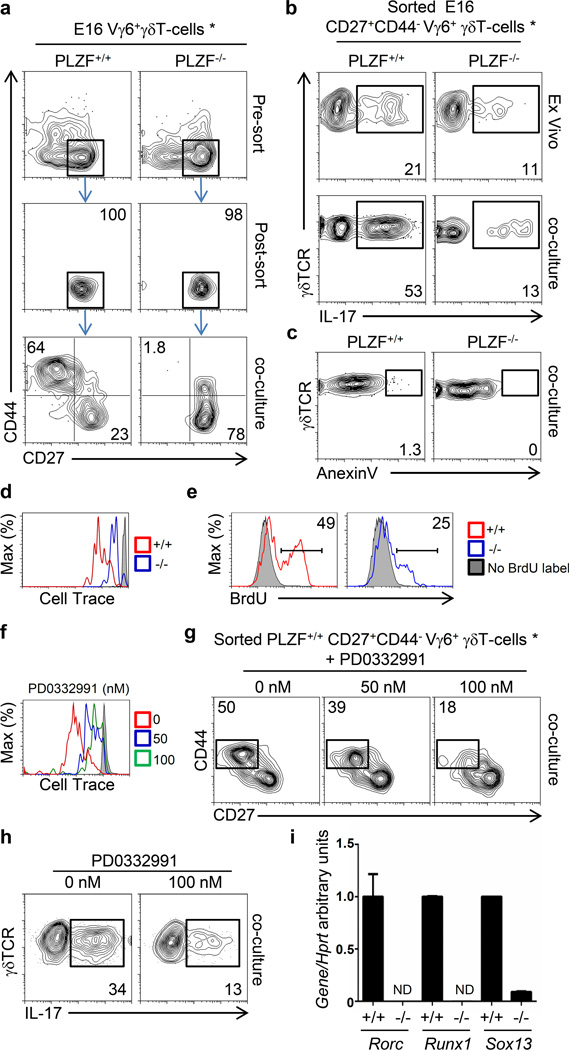
Figure 3. PLZF is required for the effector maturation of Vγ6+ γδT-cells in vitro.
a) Gating strategy indicating the subpopulations of Vγ6+ γδT-cells that were sorted and placed for 3 days in OP9-DL1 co-cultures in the presence of IL-7 to evaluate their ability to generate different subpopulations. FACs analysis showing the phenotypic profile of sorted cells post-sort and after co-culture. The numbers indicate the proportion of cells within the quadrants. b) FACs analysis for intracellular IL-17 staining after PMA plus Ionomycin stimulation of sorted CD27+CD44−Vγ6+γδT-cells from fetal (E16) thymus after sort (Ex vivo) or after a 3 day differentiation in OP9-DL1 co-cultures as in panel (a). The percentage of IL-17+ cells is indicated. c) FACs analysis of Anexin V positive cells after 3 day OP9-DL1 co-culture. The percentage of anexinV positive cells is indicated. d) Histogram showing the dilution of cell trace violet on sorted wild type (+/+) and PLZF-deficient (−/−) CD27+CD44− Vγ6+ γδT-cells after 3 day co-culture as in (a). The gray histogram represent non-proliferating T-cells. e) histograms showing the incorporation of BrdU in fetal E16 Vγ6+ γδT-cells 24 hours after a single BrdU injection to the mother. f) Histogram showing the dilution of cell trace violet on sorted wild type CD27+CD44− Vγ6+ γδT-cells after co-culture in the presence of the CDK4/6 inhibitor (PD0332991). g) FACs analysis showing the phenotypic profile of sorted cells after co-culture. h) FACs analysis showing intracellular IL-17 staining in sorted cells after co-culture. The numbers indicate the proportion of cells within the quadrants. i) RT-PCR analysis of sorted immature E16 Vγ6+CD27+CD44− γδT-cells for the indicated genes. Data correspond to two independent experiments. * Vγ6+ cells are identified as GL3+2C11+Vγ5−17D1+ cells.
We next assessed whether PLZF-deficient immature CD27+CD44− Vγ6+ γδT-cells from fetal E16 thymus could acquire IL-17 secretion after in vitro culture. Approximately 20% of sorted immature CD27+CD44− E16 Vγ6+ wild type γδT-cells were capable of secreting IL-17 and this proportion increased to 50% after a 3-day in vitro co-culture on OP9-DL1 stroma, indicating that maturation into a CD27−CD44+ phenotype correlates with the acquisition of IL-17 secretion. Immature PLZF-deficient CD27+CD44− Vγ6+ γδT-cells already presented a reduced IL-17-secretion profile and consistent with their lack of maturation in the OP9-DL1 system, the proportion of IL-17+ cells did not increase (Figure 3b). During these in vitro differentiation co-cultures we did not detect a significant increase in apoptosis in either wild type or PLZF-deficient Vγ6+ γδT-cells (Figure 3c), suggesting that PLZF is not providing a pro-survival role.
Interestingly, analysis of proliferation by dilution of cell trace label identified that PLZF-deficient CD27+CD44− Vγ6+ γδT-cells proliferated at a lower rate than wild type cells during the OP9-DL1 co-cultures (Figure 3d). We also observed that PLZF-deficient E16 Vγ6+γδT-cells incorporated lower levels of BrdU twenty four hours after BrdU injection into the mother (Figure 3e). These results indicate that PLZF is required for the proliferation of immature Vγ6+ γδT-cells, opening the possibility that lack of proliferation may be a causal mechanism impairing the maturation of PLZF-deficient cells
To test if impaired proliferation was sufficient to prevent the maturation of Vγ6+ γδT-cells, we sorted wild type CD27+CD44− Vγ6+ γδT-cells and co-cultured them with OP9-DL1 stroma in the presence of different concentrations of a cyclin kinase 4/6 inhibitor (PD0332991) to inhibit their proliferation. We observed that 100nM treatment led to stronger inhibition of proliferation than 50nM treatment, as observed by dilution of cell trace violet (Figure 3f). Interestingly, 50nM and 100nM PD0332991 treatment led to approximately 20% and 65% inhibition in the generation of mature CD27−CD44+ Vγ6+ γδT-cells from immature CD27+CD44− progenitors respectively (Figure 3g), strongly suggesting that the inability of PLZF-deficient CD27+CD44− Vγ6+ γδT-cells to proliferate is causative of their maturation defect. In correlation with the requirement of proliferation for cells to acquire a mature CD27−CD44+ phenotype, inhibition of proliferation during the in vitro cultures also led to a reduction of IL-17 secretion in wild type Vγ6+ γδT-cells (Figure 3h). Impaired IL-17 effector maturation of PLZF-deficient Vγ6+ γδT-cells correlated with impaired expression of Sox13, Rorc and Runx1, which control the IL-17 program in γδT-cells and Th17 cells (Figure 3i).
Altogether, these results show that PLZF-deficient CD27+CD44− Vγ6+ γδT-cells fail to proliferate and acquire a mature CD27−CD44+ phenotype with increased IL-17 secretion.
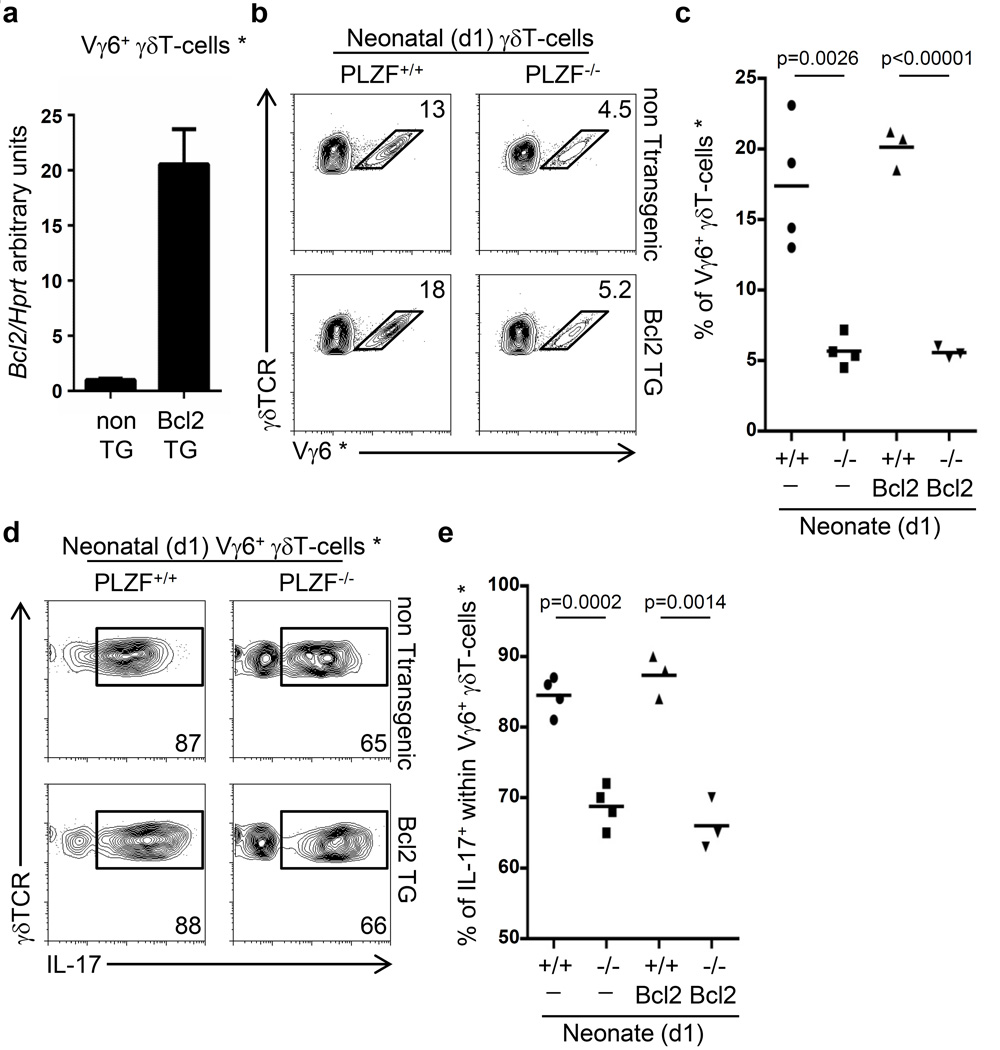
Transgenic Bcl2 expression does not rescue Vγ6+ γδT-cell development in PLZF-deficient mice
Although we didn’t observe increased apoptosis of PLZF-deficient Vγ6+ γδT-cells in vitro, reduced survival of PLZF-deficient Vγ6+ cells in vivo could still contribute to their impaired development. To evaluate this hypothesis, we generated compound PLZF-deficient x lck-Bcl-2 transgenic mice because transgenic expression of Bcl2 has been shown to prevent the apoptosis of lymphocytes to different stimuli (21). While Bcl2 expression in sorted Vγ6+ γδT-cells from transgenic neonatal thymus was increased by twenty-fold (Figure 4a), there was no rescue of Vγ6+ γδT-cells in the Bcl2-transgenic PLZF-deficient neonatal thymus (Figure 4b and 4c). The reduced proportion of IL-17+Vγ6+ γδT-cell in PLZF-deficient neonates was also not rescued by transgenic Bcl2 expression (Figure 4d and 4e). These results suggest that increased apoptosis is not the underlying mechanism responsible for the developmental impairment of PLZF-deficient Vγ6+γδT-cells.
Figure 4. Transgenic Bcl2 expression fails to rescue the effector maturation of PLZF-deficient Vγ6+γδT-cells.
a) RT-PCR from wild type and Bcl2-TG neonatal Vγ6+γδT-cells. b) FACs analysis on γδT-cells from PLZF-wild type or PLZF-deficient neonatal thymus that are also transgenic for Bcl2 (Bcl2 TG). The numbers indicate the percentage of cells within the gate. c) Gram plot indicating the percentage of Vγ6+ γδT-cells represented in panel (b). d) FACs analysis for intracellular IL-17 staining after PMA plus Ionomycin stimulation of Vγ6+γδT-cells indicated in panel (b). Numbers represent the percentage of events within the gate. e) Gram plot of the percentage of IL-17+ Vγ6+γδT-cells as represented in panel (d). Data is representative of two independent experiments. Each dot in the gram plots represents data from a neonate and horizontal lines represent the mean value. p values corresponding to the significance using T-test are shown. * Vγ6+ cells are identified as GL3+2C11+Vγ5−17D1+ cells.
PLZF-deficiency impairs the IL-17 effector maturation of Skint1-mutant Vγ5+ γδT-cells
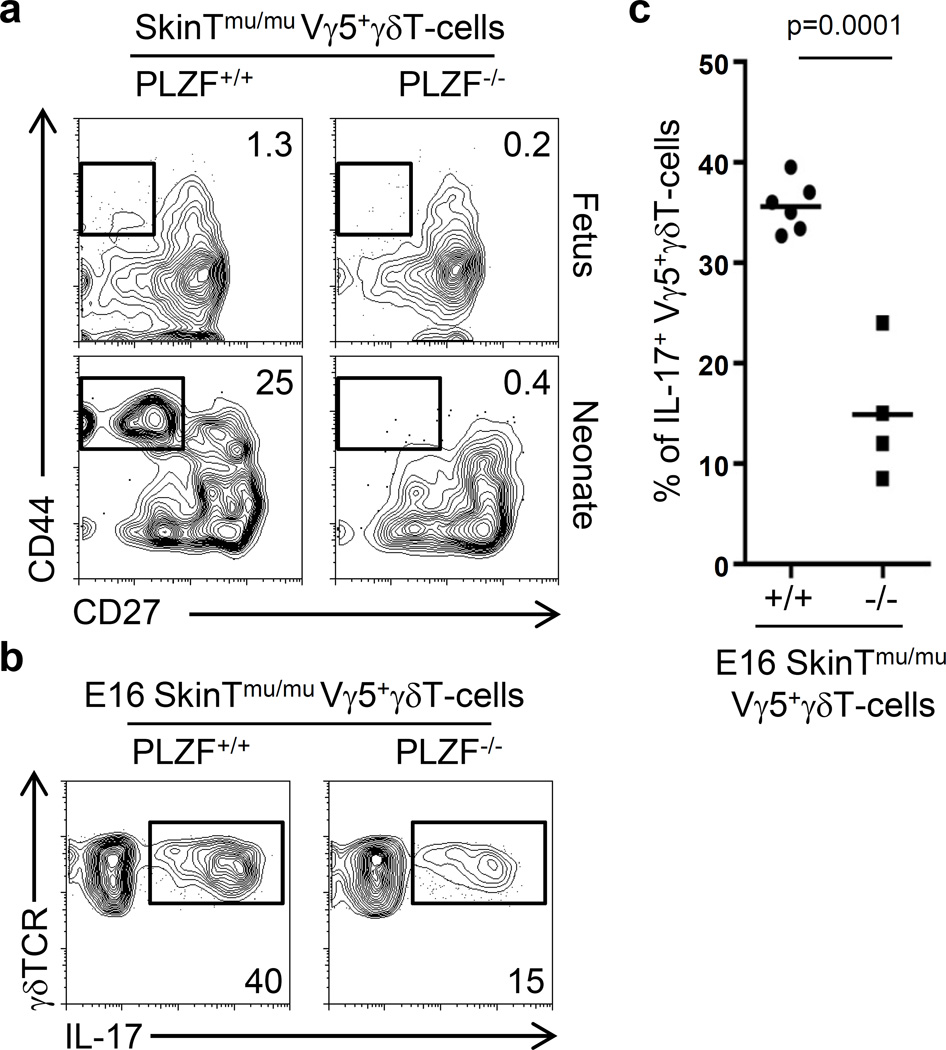
Skint-1 is an immunoglobulin family gene expressed in Thymic epithelial cells (TECs) and keratinocytes. Interestingly, in Skint-1 mutant mice, Vγ5+γδT-cells do not acquire the ability to secrete IFN-γ and instead acquire a IL-17-secreting fate and migrate to the epithelia of the uterus instead of the skin, similar to Vγ6+ γδT-cells (22). Based on this, it was postulated that the IL-17 program in γδT-cells is a default pathway that occurs under weak or absent TCR signals. In order to interrogate if the requirement of PLZF expression for the effector maturation of Vγ6+ γδT-cells was related to the specificity of the TCR or to the IL-17 program, we assessed whether PLZF may also contribute to the IL-17 effector maturation of Vγ5+ γδT-cells in Skint1-deficient mice. To evaluate this, we generated compound Skint1-mutant x PLZF-deficient mice and analyzed the phenotype of Vγ5+ γδT-cells in the fetal E16 and neonatal d1 thymus. Fetal E16 wild type and PLZF-deficient Skint1-mutant Vγ5+ γδT-cells remained CD27+CD44−, phenotypically resembling Vγ6+ γδT-cells. Interestingly, while some Vγ5+ γδT-cells acquired a CD27−CD44+ phenotype in Skint1-mutant neonates, this differentiation was completely abrogated in compound Skint1-mutant x PLZF-deficient neonates (Figure 5a). Thus, under conditions where Vγ5+ γδT-cells are programmed to differentiate into IL-17 secreting cells, PLZF is required for the acquisition of a CD27−CD44+ phenotype. To directly assess the role of PLZF in the IL-17 effector differentiation of Skint1-mutant Vγ5+ γδT-cells, we analyzed the proportion of IL-17+ γδT-cells within the Vγ5+ γδT-cells by intracellular staining. Skint1-mutant x PLZF-deficient Vγ5+ γδT-cells had a significantly reduced proportion of IL-17+ cells as compared to Skint1-mutant x PLZF-wild type Vγ5+ γδT-cells (35 ± 0.5 % vs 15 ± 2.2 %; p<0.0001) (Figure 5b and 5c).
Figure 5. Impaired IL-17 effector maturation of PLZF-deficient SkinT-mutant Vγ5+ γδT-cells.
a) FACs analysis of gated Vγ5+ γδT-cells from fetal (E16) and neonatal (d1) thymus in the indicated strain. The numbers indicate the percentage of cells within the gate. b) FACs analysis for intracellular IL-17 staining after PMA plus Ionomycin stimulation of Vγ5+ γδT-cells in Skint1-mutant (E16) thymus. c) Gram plot of the percentage of IL-17+ Vγ5+ γδT-cells as represented in panel (b). Data is representative of two independent experiments. Each dot in the gram plots represents data from a fetus and horizontal lines represent the mean value. p values corresponding to the significance using T-test are shown.
Altogether, these results indicate that the requirement of PLZF expression for the development and effector maturation of Vγ6+ γδT-cells is associated with the IL-17 program and it is independent of the Vγ6 TCR, as a similar phenotype is observed in Vγ5+ γδT-cells that are forced to acquire an IL-17 phenotype in Skint1-mutant mice.
DISCUSSION
The mechanisms controlling the intrathymic effector differentiation of innate-like IL-17+γδT-cells are different to those required for the differentiation of Th17 cells in the periphery (23–27).
We have identified a novel requirement of PLZF for the development of IL-17+Vγ6+ γδT-cells. We show that PLZF expression precedes the selection of innate-like IL-17+γδT-cells, suggesting that PLZF expression is not induced downstream of TCR signals in these cells as it has been proposed for γδNKT cells (5).
Although PLZF is not expressed at the earliest stages of T-cell development in the adult thymus, we have found that ETPs in the fetal thymus are PLZF-expressing cells. A unique characteristic of fetal HSCs is the expression of Lin28b, which blocks the production of mature Let-7 microRNAs (28). As PLZF was recently shown to be regulated by Let-7 microRNAs (29), it is reasonable to speculate that the increase of PLZF levels during fetal hematopoiesis might be due to increased Lin28b and reduced Let-7 levels in fetal progenitors. We have also observed that PLZF levels are further increased in DN2 thymocytes in the fetal thymus. Interestingly, Zbtb16 (PLZF) expression has been previously identified in DN2 thymocytes of adult mice (30), indicating that PLZF may be induced as cells develop into DN2a thymocytes. As IL-7 plays a critical role for the generation of DN2a thymocytes as well as in the maintenance of IL-17+ γδT-cells, it is possible that PLZF is positively regulated or maintained by IL-7 signals. Although, PLZF expression already occurs before γδT-cell selection in the fetal thymus, a role of TCR signals in regulating PLZF expression cannot be ruled out. It was originally postulated that the IL-17 program in fetal Vγ6+ γδT-cells occurs in the absence of TCR engagement (22), however, later work identified that Vγ6+ γδT-cells have received TCR signals during development (31), therefore, it is possible that TCR signals maintain PLZF expression in Vγ6+ γδT-cells.
We also show that PLZF specifically allows the differentiation of fetal-derived IL-17+γδT-cells, but not IFN-γ+γδT-cells, despite sharing a common PLZF+ precursor. Therefore, PLZF-driven innate-like differentiation is not exclusive to NKT cells or IL-4+T-cell subsets (5, 32), but it also controls the differentiation of innate-like IL-17+γδT-cells.
We have observed that PLZF is required for both the development and acquisition of IL-17-secretion in Vγ6+γδT-cells as well as in Vγ5+γδT-cells when redirected into a IL-17-secreting phenotype in Skint-1 mutant mice, indicating that the requirement of PLZF expression for the development of IL-17-secreting cells is independent of the specificity of the TCR. Interestingly, development of Vγ5+γδT-cells was not affected by the absence of PLZF in Skint-1 wild type mice, when acquiring their IFN-γ program, suggesting that TCR signals during differentiation may compensate the requirement of PLZF expression. In fact, the ability of transgenic PLZF expression to confer effector functions to the conventional naïve CD4 T-cell population in the absence of TCR stimulation suggests that PLZF and TCR signals may play redundant roles in providing the effector maturation to T-cells (8, 33).
Lack of surface CD27 identifies IL-17+γδT-cells in peripheral tissues (12, 13). However, in the fetal thymus, some Vγ6+ γδT-cells acquired IL-17 secretion but have not yet downregulated CD27 levels, indicating that downregulation of CD27 is not required for their effector maturation.
We have characterized the different developmental stages of Vγ6+γδT-cells and have shown that CD27+CD44− Vγ6+γδT-cells are the precursors of CD27−CD44+ Vγ6+γδT-cells, which are the major subset present in the neonatal day 1 thymus and secrete IL-17. PLZF was required for this developmental step as the number of mature CD27−CD44+ Vγ6+γδT-cells was highly reduced in the neonatal thymus and PLZF-deficient immature CD27+CD44− Vγ6+γδT-cells did not proliferate and generate mature CD27−CD44+ Vγ6+γδT-cells in vitro. PLZF-mediated proliferation of immature CD27+CD44− Vγ6+γδT-cells is required for the effector maturation into a CD27−CD44+IL-17+ phenotype as block of proliferation in wild type immature CD27+CD44− Vγ6+γδT-cells also prevented their maturation. Impaired maturation of PLZF-deficient Vγ6+γδT-cells was also evident as inefficient CD62L downregulation, a phenotype associated with the maturation of “innate-like” T-cells that is disrupted in PLZF-deficient iNKT and γδNKT cells (4, 6). It is not clear how a defect in proliferation leads to a maturation block in Vγ6+ γδT-cells. However, previous reports identified a requirement of proliferation for developmental progression in T-cells. The effector maturation of helper T-cells into IFN-γ, IL-4 and IL-10 secreting cells was shown to be dependent on proliferation (34, 35), and more recently, β-selection-induced proliferation was shown required for the generation of DP thymocytes (36). Presumably, epigenetic changes during cell cycle may be required for developmental progression (37).
It is also not clear how PLZF controls the proliferation of immature Vγ6+γδT-cells. Although overexpression of PLZF leads to a block of cell cycle progression in a myelomonocytic cell line (U937T) (38), it was recently described that PLZF expression may stimulate proliferation in NIH3T3 cells by repressing expression of Cdkn1a (p21), a negative regulator of cell cycle progression (39), however, we did not find increased Cdkn1a levels in PLZF-deficient Vγ6+γδT-cells (data not shown), indicating that PLZF is required for other mechanisms of cell cycle control in Vγ6+ γδT-cells.
Despite the deficient development of Vγ6+γδT-cells in the absence of PLZF, Vγ6+γδT-cells were present in the lungs and uterus of adult mice, indicating that PLZF-deficient Vγ6+γδT-cells are able to self-renew in the periphery.
Although transgenic PLZF expression protects from super-antigen mediated negative selection in conventional T-cells (33), a pro-survival function does not seem to be the main role of PLZF in controlling the effector differentiation of Vγ6+ γδT-cells. We did not detect increased apoptosis of PLZF-deficient Vγ6+ γδT-cells, also transgenic Bcl2 expression, which protects against several apoptotic stimuli in thymocytes, didn’t rescue the development of PLZF-deficient Vγ6+ γδT-cells.
Interestingly, a recent report identified that PLZF is expressed in human Th17 cells, binds to the CCR6 promoter and regulates the expression of IL-17-associated genes (40), suggesting that PLZF may control IL-17 effector maturation in other cell types.
In Sum, we have identified that PLZF not only controls the effector maturation of NKT and IL-4+γδT-cells but also that of “innate-like” IL-17+γδT-cells.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. Naomi Taylor and Dr. Avinash Bhandoola for critically reviewing the manuscript. Dr. Derek Sant’Angelo for providing the PLZF-CRE x Rosa-Tdtomato strain. Dr. Robert Tigelaar for the 17D1 antibody and Dr. Zuniga-Pflucker for the OP9-DL1 cell line.
Footnotes
This work was funded by NIH intramural support, NCI investigator-initiated intramural Research Projects (ZIA) # 1ZIABC011429
REFERENCES
- 1.Vantourout P, Hayday A. Six-of-the-best: unique contributions of gammadelta T cells to immunology. Nature reviews. Immunology. 2013;13:88–100. doi: 10.1038/nri3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Constantinides MG, Bendelac A. Transcriptional regulation of the NKT cell lineage. Current opinion in immunology. 2013;25:161–167. doi: 10.1016/j.coi.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kovalovsky D, Uche OU, Eladad S, Hobbs RM, Yi W, Alonzo E, Chua K, Eidson M, Kim HJ, Im JS, Pandolfi PP, Sant'Angelo DB. The BTB-zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nature immunology. 2008;9:1055–1064. doi: 10.1038/ni.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Savage AK, Constantinides MG, Han J, Picard D, Martin E, Li B, Lantz O, Bendelac A. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity. 2008;29:391–403. doi: 10.1016/j.immuni.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kreslavsky T, Savage AK, Hobbs R, Gounari F, Bronson R, Pereira P, Pandolfi PP, Bendelac A, von Boehmer H. TCR-inducible PLZF transcription factor required for innate phenotype of a subset of gammadelta T cells with restricted TCR diversity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:12453–12458. doi: 10.1073/pnas.0903895106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alonzo ES, Gottschalk RA, Das J, Egawa T, Hobbs RM, Pandolfi PP, Pereira P, Nichols KE, Koretzky GA, Jordan MS, Sant'Angelo DB. Development of promyelocytic zinc finger and ThPOK-expressing innate gamma delta T cells is controlled by strength of TCR signaling and Id3. J Immunol. 2010;184:1268–1279. doi: 10.4049/jimmunol.0903218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerart S, Siberil S, Martin E, Lenoir C, Aguilar C, Picard C, Lantz O, Fischer A, Latour S. Human iNKT and MAIT cells exhibit a PLZF-dependent proapoptotic propensity that is counterbalanced by XIAP. Blood. 2013;121:614–623. doi: 10.1182/blood-2012-09-456095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kovalovsky D, Alonzo ES, Uche OU, Eidson M, Nichols KE, Sant'Angelo DB. PLZF induces the spontaneous acquisition of memory/effector functions in T cells independently of NKT cell-related signals. J Immunol. 2010;184:6746–6755. doi: 10.4049/jimmunol.1000776. [DOI] [PubMed] [Google Scholar]
- 9.Thomas SY, Scanlon ST, Griewank KG, Constantinides MG, Savage AK, Barr KA, Meng F, Luster AD, Bendelac A. PLZF induces an intravascular surveillance program mediated by long-lived LFA-1-ICAM-1 interactions. The Journal of experimental medicine. 2011;208:1179–1188. doi: 10.1084/jem.20102630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girardi M, Lewis J, Glusac E, Filler RB, Geng L, Hayday AC, Tigelaar RE. Resident skin-specific gammadelta T cells provide local, nonredundant regulation of cutaneous inflammation. The Journal of experimental medicine. 2002;195:855–867. doi: 10.1084/jem.20012000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jameson J, Ugarte K, Chen N, Yachi P, Fuchs E, Boismenu R, Havran WL. A role for skin gammadelta T cells in wound repair. Science. 2002;296:747–749. doi: 10.1126/science.1069639. [DOI] [PubMed] [Google Scholar]
- 12.Ribot JC, deBarros A, Pang DJ, Neves JF, Peperzak V, Roberts SJ, Girardi M, Borst J, Hayday AC, Pennington DJ, Silva-Santos B. CD27 is a thymic determinant of the balance between interferon-gamma- and interleukin 17-producing gammadelta T cell subsets. Nature immunology. 2009;10:427–436. doi: 10.1038/ni.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haas JD, Ravens S, Duber S, Sandrock I, Oberdorfer L, Kashani E, Chennupati V, Fohse L, Naumann R, Weiss S, Krueger A, Forster R, Prinz I. Development of interleukin-17-producing gammadelta T cells is restricted to a functional embryonic wave. Immunity. 2012;37:48–59. doi: 10.1016/j.immuni.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Michel ML, Pang DJ, Haque SF, Potocnik AJ, Pennington DJ, Hayday AC. Interleukin 7 (IL-7) selectively promotes mouse and human IL-17-producing gammadelta cells. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:17549–17554. doi: 10.1073/pnas.1204327109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuzaki G, Umemura M. Interleukin-17 as an effector molecule of innate and acquired immunity against infections. Microbiology and immunology. 2007;51:1139–1147. doi: 10.1111/j.1348-0421.2007.tb04008.x. [DOI] [PubMed] [Google Scholar]
- 16.O'Brien RL, Born WK. gammadelta T cell subsets: a link between TCR and function? Seminars in immunology. 2010;22:193–198. doi: 10.1016/j.smim.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Linette GP, Li Y, Roth K, Korsmeyer SJ. Cross talk between cell death and cell cycle progression: BCL-2 regulates NFAT-mediated activation. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:9545–9552. doi: 10.1073/pnas.93.18.9545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roark CL, Aydintug MK, Lewis J, Yin X, Lahn M, Hahn YS, Born WK, Tigelaar RE, O'Brien RL. Subset-specific, uniform activation among V gamma 6/V delta 1+ gamma delta T cells elicited by inflammation. Journal of leukocyte biology. 2004;75:68–75. doi: 10.1189/jlb.0703326. [DOI] [PubMed] [Google Scholar]
- 20.Lynch L, Michelet X, Zhang S, Brennan PJ, Moseman A, Lester C, Besra G, Vomhof-Dekrey EE, Tighe M, Koay HF, Godfrey DI, Leadbetter EA, Sant'Angelo DB, von Andrian U, Brenner MB. Regulatory iNKT cells lack expression of the transcription factor PLZF and control the homeostasis of T(reg) cells and macrophages in adipose tissue. Nature immunology. 2015;16:85–95. doi: 10.1038/ni.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chao DT, Linette GP, Boise LH, White LS, Thompson CB, Korsmeyer SJ. Bcl-XL and Bcl-2 repress a common pathway of cell death. The Journal of experimental medicine. 1995;182:821–828. doi: 10.1084/jem.182.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turchinovich G, Hayday AC. Skint-1 identifies a common molecular mechanism for the development of interferon-gamma-secreting versus interleukin-17-secreting gammadelta T cells. Immunity. 2011;35:59–68. doi: 10.1016/j.immuni.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 23.Rachitskaya AV, Hansen AM, Horai R, Li Z, Villasmil R, Luger D, Nussenblatt RB, Caspi RR. Cutting edge: NKT cells constitutively express IL-23 receptor and RORgammat and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion. J Immunol. 2008;180:5167–5171. doi: 10.4049/jimmunol.180.8.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lochner M, Peduto L, Cherrier M, Sawa S, Langa F, Varona R, Riethmacher D, Si-Tahar M, Di Santo JP, Eberl G. In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ RORgamma t+ T cells. The Journal of experimental medicine. 2008;205:1381–1393. doi: 10.1084/jem.20080034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shibata K, Yamada H, Sato T, Dejima T, Nakamura M, Ikawa T, Hara H, Yamasaki S, Kageyama R, Iwakura Y, Kawamoto H, Toh H, Yoshikai Y. Notch-Hes1 pathway is required for the development of IL-17-producing gammadelta T cells. Blood. 2011;118:586–593. doi: 10.1182/blood-2011-02-334995. [DOI] [PubMed] [Google Scholar]
- 26.Malhotra N, Narayan K, Cho OH, Sylvia KE, Yin C, Melichar H, Rashighi M, Lefebvre V, Harris JE, Berg LJ, Kang J. A network of high-mobility group box transcription factors programs innate interleukin-17 production. Immunity. 2013;38:681–693. doi: 10.1016/j.immuni.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Serre K, Silva-Santos B. Molecular Mechanisms of Differentiation of Murine Pro-Inflammatory gammadelta T Cell Subsets. Frontiers in immunology. 2013;4:431. doi: 10.3389/fimmu.2013.00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuan J, Nguyen CK, Liu X, Kanellopoulou C, Muljo SA. Lin28b reprograms adult bone marrow hematopoietic progenitors to mediate fetal-like lymphopoiesis. Science. 2012;335:1195–1200. doi: 10.1126/science.1216557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pobezinsky LA, Etzensperger R, Jeurling S, Alag A, Kadakia T, McCaughtry TM, Kimura MY, Sharrow SO, Guinter TI, Feigenbaum L, Singer A. Let-7 microRNAs target the lineage-specific transcription factor PLZF to regulate terminal NKT cell differentiation and effector function. Nature immunology. 2015;16:517–524. doi: 10.1038/ni.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li L, Leid M, Rothenberg EV. An early T cell lineage commitment checkpoint dependent on the transcription factor Bcl11b. Science. 2010;329:89–93. doi: 10.1126/science.1188989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wencker M, Turchinovich G, Di Marco Barros R, Deban L, Jandke A, Cope A, Hayday AC. Innate-like T cells straddle innate and adaptive immunity by altering antigen-receptor responsiveness. Nature immunology. 2014;15:80–87. doi: 10.1038/ni.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee YJ, Holzapfel KL, Zhu J, Jameson SC, Hogquist KA. Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nature immunology. 2013;14:1146–1154. doi: 10.1038/ni.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Savage AK, Constantinides MG, Bendelac A. Promyelocytic leukemia zinc finger turns on the effector T cell program without requirement for agonist TCR signaling. J Immunol. 2011;186:5801–5806. doi: 10.4049/jimmunol.1100119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bird JJ, Brown DR, Mullen AC, Moskowitz NH, Mahowald MA, Sider JR, Gajewski TF, Wang CR, Reiner SL. Helper T cell differentiation is controlled by the cell cycle. Immunity. 1998;9:229–237. doi: 10.1016/s1074-7613(00)80605-6. [DOI] [PubMed] [Google Scholar]
- 35.Richter A, Lohning M, Radbruch A. Instruction for cytokine expression in T helper lymphocytes in relation to proliferation and cell cycle progression. The Journal of experimental medicine. 1999;190:1439–1450. doi: 10.1084/jem.190.10.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kreslavsky T, Gleimer M, Miyazaki M, Choi Y, Gagnon E, Murre C, Sicinski P, von Boehmer H. beta-Selection-induced proliferation is required for alphabeta T cell differentiation. Immunity. 2012;37:840–853. doi: 10.1016/j.immuni.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mullen AC, Hutchins AS, Villarino AV, Lee HW, High FA, Cereb N, Yang SY, Hua X, Reiner SL. Cell cycle controlling the silencing and functioning of mammalian activators. Current biology : CB. 2001;11:1695–1699. doi: 10.1016/s0960-9822(01)00533-4. [DOI] [PubMed] [Google Scholar]
- 38.Konrad TA, Karger A, Hackl H, Schwarzinger I, Herbacek I, Wieser R. Inducible expression of EVI1 in human myeloid cells causes phenotypes consistent with its role in myelodysplastic syndromes. Journal of leukocyte biology. 2009;86:813–822. doi: 10.1189/jlb.0109042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi WI, Kim MY, Jeon BN, Koh DI, Yun CO, Li Y, Lee CE, Oh J, Kim K, Hur MW. Role of promyelocytic leukemia zinc finger (PLZF) in cell proliferation and cyclin-dependent kinase inhibitor 1A (p21WAF/CDKN1A) gene repression. The Journal of biological chemistry. 2014;289:18625–18640. doi: 10.1074/jbc.M113.538751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh SP, Zhang HH, Tsang H, Gardina PJ, Myers TG, Nagarajan V, Lee CH, Farber JM. PLZF regulates CCR6 and is critical for the acquisition and maintenance of the Th17 phenotype in human cells. J Immunol. 2015;194:4350–4361. doi: 10.4049/jimmunol.1401093. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.