Abstract
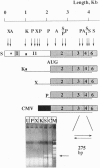
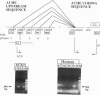
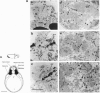
We have employed Xenopus embryos to express human acetylcholinesterase (AcChoEase; EC 3.1.1.7) in developing synapses. Transcription of AcChoEase mRNA was driven by a 2.2-kb sequence upstream from the initiator AUG in the ACHE gene encoding AcChoEase, with multiple potential sites for binding universal and tissue-specific transcription factors. These included clustered MyoD elements, E-box, SP1, EGR1, AP-2, and the development-related GAGA motif. A DNA construct composed of this sequence linked to a 2.1-kb sequence encoding human AcChoEase was designated human AcChoEase promoter-reporter (HpACHE). HpACHE but none of its several 5'-truncated derivatives was transcriptionally active in developing Xenopus embryos. Furthermore, PCR analysis using chimeric PCR primers revealed usage of the same 1.5-kb intron and 74-bp exon within the HpACHE sequence in microinjected embryos and various human tissues. Cytochemical staining revealed conspicuous accumulation of overexpressed AcChoEase in neuromuscular junctions and within muscle fibers of apparently normal 2-day Xenopus embryos injected with HpACHE. The same reporter driven by the cytomegalovirus promoter was similarly efficient in directing the heterologous human enzyme toward neuromuscular junctions, attributing the evolutionary conservation of AcChoEase targeting to the coding sequence. Our findings demonstrate that a short DNA sequence is sufficient to promote the exogenous transcription and faithful splicing of human AcChoEase mRNA in developing Xenopus embryos and foreshadow their use for integrative studies of cholinergic signaling and synapse formation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benvenisty N., Shoshani T., Farkash Y., Soreq H., Reshef L. trans activation of rat phosphoenolpyruvate carboxykinase (GTP) gene expression by micro-coinjection of rat liver mRNA in Xenopus laevis oocytes. Mol Cell Biol. 1989 Nov;9(11):5244–5247. doi: 10.1128/mcb.9.11.5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J. P. Compartmentalized transcription of acetylcholine receptor genes during motor endplate epigenesis. New Biol. 1991 May;3(5):413–429. [PubMed] [Google Scholar]
- Cohen M. W. Development of an amphibian neuromuscular junction in vivo and in culture. J Exp Biol. 1980 Dec;89:43–56. doi: 10.1242/jeb.89.1.43. [DOI] [PubMed] [Google Scholar]
- Doctor B. P., Chapman T. C., Christner C. E., Deal C. D., De La Hoz D. M., Gentry M. K., Ogert R. A., Rush R. S., Smyth K. K., Wolfe A. D. Complete amino acid sequence of fetal bovine serum acetylcholinesterase and its comparison in various regions with other cholinesterases. FEBS Lett. 1990 Jun 18;266(1-2):123–127. doi: 10.1016/0014-5793(90)81522-p. [DOI] [PubMed] [Google Scholar]
- Ehrlich G., Viegas-Pequignot E., Ginzberg D., Sindel L., Soreq H., Zakut H. Mapping the human acetylcholinesterase gene to chromosome 7q22 by fluorescent in situ hybridization coupled with selective PCR amplification from a somatic hybrid cell panel and chromosome-sorted DNA libraries. Genomics. 1992 Aug;13(4):1192–1197. doi: 10.1016/0888-7543(92)90037-s. [DOI] [PubMed] [Google Scholar]
- Faisst S., Meyer S. Compilation of vertebrate-encoded transcription factors. Nucleic Acids Res. 1992 Jan 11;20(1):3–26. doi: 10.1093/nar/20.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getman D. K., Eubanks J. H., Camp S., Evans G. A., Taylor P. The human gene encoding acetylcholinesterase is located on the long arm of chromosome 7. Am J Hum Genet. 1992 Jul;51(1):170–177. [PMC free article] [PubMed] [Google Scholar]
- KARNOVSKY M. J., ROOTS L. A "DIRECT-COLORING" THIOCHOLINE METHOD FOR CHOLINESTERASES. J Histochem Cytochem. 1964 Mar;12:219–221. doi: 10.1177/12.3.219. [DOI] [PubMed] [Google Scholar]
- Krejci E., Coussen F., Duval N., Chatel J. M., Legay C., Puype M., Vandekerckhove J., Cartaud J., Bon S., Massoulié J. Primary structure of a collagenic tail peptide of Torpedo acetylcholinesterase: co-expression with catalytic subunit induces the production of collagen-tailed forms in transfected cells. EMBO J. 1991 May;10(5):1285–1293. doi: 10.1002/j.1460-2075.1991.tb08070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullberg R. W., Lentz T. L., Cohen M. W. Development of the myotomal neuromuscular junction in Xenopus laevis: an electrophysiological and fine-structural study. Dev Biol. 1977 Oct 1;60(1):101–129. doi: 10.1016/0012-1606(77)90113-0. [DOI] [PubMed] [Google Scholar]
- Lapidot-Lifson Y., Patinkin D., Prody C. A., Ehrlich G., Seidman S., Ben-Aziz R., Benseler F., Eckstein F., Zakut H., Soreq H. Cloning and antisense oligodeoxynucleotide inhibition of a human homolog of cdc2 required in hematopoiesis. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):579–583. doi: 10.1073/pnas.89.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapidot-Lifson Y., Prody C. A., Ginzberg D., Meytes D., Zakut H., Soreq H. Coamplification of human acetylcholinesterase and butyrylcholinesterase genes in blood cells: correlation with various leukemias and abnormal megakaryocytopoiesis. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4715–4719. doi: 10.1073/pnas.86.12.4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Camp S., Rachinsky T. L., Getman D., Taylor P. Gene structure of mammalian acetylcholinesterase. Alternative exons dictate tissue-specific expression. J Biol Chem. 1991 Dec 5;266(34):23083–23090. [PubMed] [Google Scholar]
- Lu B., Greengard P., Poo M. M. Exogenous synapsin I promotes functional maturation of developing neuromuscular synapses. Neuron. 1992 Mar;8(3):521–529. doi: 10.1016/0896-6273(92)90280-q. [DOI] [PubMed] [Google Scholar]
- McMahan U. J. The agrin hypothesis. Cold Spring Harb Symp Quant Biol. 1990;55:407–418. doi: 10.1101/sqb.1990.055.01.041. [DOI] [PubMed] [Google Scholar]
- Neville L. F., Gnatt A., Loewenstein Y., Seidman S., Ehrlich G., Soreq H. Intramolecular relationships in cholinesterases revealed by oocyte expression of site-directed and natural variants of human BCHE. EMBO J. 1992 Apr;11(4):1641–1649. doi: 10.1002/j.1460-2075.1992.tb05210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotundo R. L. Nucleus-specific translation and assembly of acetylcholinesterase in multinucleated muscle cells. J Cell Biol. 1990 Mar;110(3):715–719. doi: 10.1083/jcb.110.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz i Altaba A., Melton D. A. Involvement of the Xenopus homeobox gene Xhox3 in pattern formation along the anterior-posterior axis. Cell. 1989 Apr 21;57(2):317–326. doi: 10.1016/0092-8674(89)90969-0. [DOI] [PubMed] [Google Scholar]
- Rychlik W., Rhoads R. E. A computer program for choosing optimal oligonucleotides for filter hybridization, sequencing and in vitro amplification of DNA. Nucleic Acids Res. 1989 Nov 11;17(21):8543–8551. doi: 10.1093/nar/17.21.8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soreq H., Ben-Aziz R., Prody C. A., Seidman S., Gnatt A., Neville L., Lieman-Hurwitz J., Lev-Lehman E., Ginzberg D., Lipidot-Lifson Y. Molecular cloning and construction of the coding region for human acetylcholinesterase reveals a G + C-rich attenuating structure. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9688–9692. doi: 10.1073/pnas.87.24.9688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soreq H., Gnatt A., Loewenstein Y., Neville L. F. Excavations into the active-site gorge of cholinesterases. Trends Biochem Sci. 1992 Sep;17(9):353–358. doi: 10.1016/0968-0004(92)90314-y. [DOI] [PubMed] [Google Scholar]
- Soreq H., Seidman S. Xenopus oocyte microinjection: from gene to protein. Methods Enzymol. 1992;207:225–265. doi: 10.1016/0076-6879(92)07016-h. [DOI] [PubMed] [Google Scholar]
- Taylor P. The cholinesterases. J Biol Chem. 1991 Mar 5;266(7):4025–4028. [PubMed] [Google Scholar]
- Velan B., Kronman C., Grosfeld H., Leitner M., Gozes Y., Flashner Y., Sery T., Cohen S., Ben-Aziz R., Seidman S. Recombinant human acetylcholinesterase is secreted from transiently transfected 293 cells as a soluble globular enzyme. Cell Mol Neurobiol. 1991 Feb;11(1):143–156. doi: 10.1007/BF00712806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vize P. D., Melton D. A., Hemmati-Brivanlou A., Harland R. M. Assays for gene function in developing Xenopus embryos. Methods Cell Biol. 1991;36:367–387. doi: 10.1016/s0091-679x(08)60288-5. [DOI] [PubMed] [Google Scholar]
- Zakut H., Ehrlich G., Ayalon A., Prody C. A., Malinger G., Seidman S., Ginzberg D., Kehlenbach R., Soreq H. Acetylcholinesterase and butyrylcholinesterase genes coamplify in primary ovarian carcinomas. J Clin Invest. 1990 Sep;86(3):900–908. doi: 10.1172/JCI114791. [DOI] [PMC free article] [PubMed] [Google Scholar]