Abstract
Background
During the past decade, in the United States, an increasing number of hepatitis B outbreaks have been reported in assisted living facilities (ALFs) as a result of breaches in infection control practices. We evaluated the seroprotection rates conferred by hepatitis B vaccine among older adults during a response to an outbreak that occurred in multiple ALFs and assessed the influence of demographic and clinical factors on vaccine response.
Methods
Residents were screened for hepatitis B and C infection prior to vaccination and susceptible residents were vaccinated against hepatitis B with one dose of 20 μg Engerix-B™ (GSK) given at 0, 1, and 4 months. Blood samples were collected 80–90 days after the third vaccine dose to test for anti-HBs levels.
Results
Of the 48 residents who had post-vaccination blood specimens collected after the third vaccine dose, 16 (33.3%) achieved anti-HBs concentration ≥10 mIU/mL. Age was a significant determinant of seroprotection with rates decreasing from 88% among persons aged ≤60 years to 12% among persons aged ≤90 years (p = 0.001). Geometric mean concentrations were higher among non-diabetic than diabetic residents, however, the difference was not statistically significant (5.1 vs. 3.8 mIU/mL, p = 0.7).
Conclusions
These findings highlight that hepatitis B vaccination is of limited effectiveness when administered to older adults. Improvements in infection control and vaccination at earlier ages might be necessary to prevent spread of infection in ALFs.
Keywords: Hepatitis B vaccine, Seroprotection, Older adults, Immunity, Outbreaks
1. Introduction
Hepatitis B virus (HBV) infection is a bloodborne and sexually transmitted infection that affects almost 730,000 persons in the United States with highest prevalence rates reported among persons aged 50 years or older compared to younger age groups [1]. The main consequences of HBV infection include cirrhosis, liver cancer, and death. Hepatitis B vaccination is the most effective measure to prevent HBV infection and its consequences. The Centers for Disease Control and Prevention (CDC) and the Advisory Committee for Immunization Practices (ACIP) recommend universal vaccination for all children and adolescents, as well as adults who are at high risk for HBV infection [2].
While the achievement of seroprotection (anti-HBs ≥ 10 mIU/mL) after receipt of hepatitis B vaccine has been established among persons aged less than 40 years, limited data are available regarding anti-HBs response among those aged 60 years or older. Outbreaks of HBV infection, with many acute infections resulting in death, are increasingly being recognized in long-term care (LTC) facilities as a result of improper cleaning and sharing of blood glucose monitoring devices [3–6]. Projections indicate that the number of persons in the United States 65 years of age or older is expected to double to more than 70 million by 2030 with a concomitant increase in the number of residents in LTC facilities [7]. To prevent HBV infections among residents of LTC facilities, assiduous adherence to infection control guidelines is essential; however, adherence has proven challenging in such settings in the absence of federal oversight and variable state regulations regarding infection control practices [7].
The extent to which hepatitis B vaccination might be useful for susceptible persons living in LTC facilities is not clear, particularly in the acute context of prevention of HBV transmission during an outbreak. We evaluated the immunogenicity of monovalent hepatitis B vaccine administered to older adults during an outbreak that occurred in multiple assisted living facilities (ALFs) in one city and assessed the influence of demographic and clinical factors on vaccine response.
2. Materials and methods
2.1. Study population
In June 2010, an outbreak of acute hepatitis B was identified in four ALFs housing a total of 289 older adults in the city of Houston, TX. Investigation of the outbreak suggested transmission through sharing of improperly cleaned blood glucose monitoring devices. In August 2010, the Houston Department of Health and Human Services with assistance from CDC implemented hepatitis B screening and vaccination of residents in the four facilities to prevent further transmission of hepatitis B.
2.2. Data collection and vaccine administration
Informed consent for hepatitis screening and vaccination was obtained from residents and/or their guardians. Those who consented were screened for hepatitis B and C infection and those who were found to be susceptible for hepatitis B infection (antibodies to hepatitis B surface antigen (anti-HBs) = 0 mIU/mL, negative hepatitis B surface antigen (HBsAg), and negative hepatitis B core antibody (anti-HBc)) were vaccinated using a 23-gauge one inch needle in the right or left deltoid muscles with one dose of 20 μg Engerix-B™ (GlaxoSmithKline Biologicals, lot no.: 11,AHBVB798AA) on a 0, 1, and 4 month schedule. Vaccines and serological testing were provided by the Texas Department of Health and Human Services and CDC, respectively, as part of the outbreak response.
Information was abstracted from medical records of the residents at the four facilities to obtain basic demographic (age, sex, race, and ethnicity) and clinical characteristics which might affect vaccine response such as smoking, body mass index (BMI), diabetes status, alcohol use, renal disease (ranging from moderate renal disease to renal failure and hemodialysis), liver disease (any liver disease including hepatitis C infection, liver failure, cirrhosis), cancer (previous or current), HIV status, use of immunosuppressive drugs or chemotherapy, and the presence of comorbid conditions. Body mass index was classified according to guidelines as underweight (BMI < 18.5), normal (18.5 ≤ BMI < 25 kg/m2), overweight (25 ≤ BMI < 30 kg/m2), and obese (BMI ≥ 30 kg/m2) [8].
2.3. Serology testing
Blood samples were collected for serological testing at pre-vaccination (day 0) and 80–90 days after the third vaccine dose. The level of total antibodies to hepatitis B surface antigen (quantitative anti-HBs), and qualitative determination of hepatitis B core antibody (total anti-HBc and anti-HBc IgM), hepatitis B surface antigen (HBsAg), and immunoglobulin G antibody to hepatitis C virus (IgG anti-HCV) were determined using the VITROS ECi Immunodiagnostic System (Ortho-Clinical Diagnostics, Inc., Rochester, NY). Anti-HBs concentrations ≥ 10 mIU/mL were considered indicators of seroprotection.
2.4. Statistical analysis
Response to hepatitis B vaccine (anti-HBs concentrations ≥ 10 mIU/mL) was assessed by demographic and clinical characteristics. Quantitative anti-HBs levels were calculated as geometric mean concentrations (GMCs) with 95% confidence intervals (95%CI). Anti-HBs levels were log transformed and anti-HBs values below 1 mIU/mL were assigned a value of 1 mIU/mL before log transformation. Fisher’s exact test was used to test associations between demographic and clinical characteristics with seroprotection and t-tests were used to compare anti-HBs GMC between groups. SAS v9.2 was used for statistical analysis and a p-value <0.05 was considered statistically significant. Houston Department of Health and Human Services and CDC IRB approval were not required as a licensed vaccine was administered to adults at risk for HBV infection within the context of an acute outbreak.
3. Results
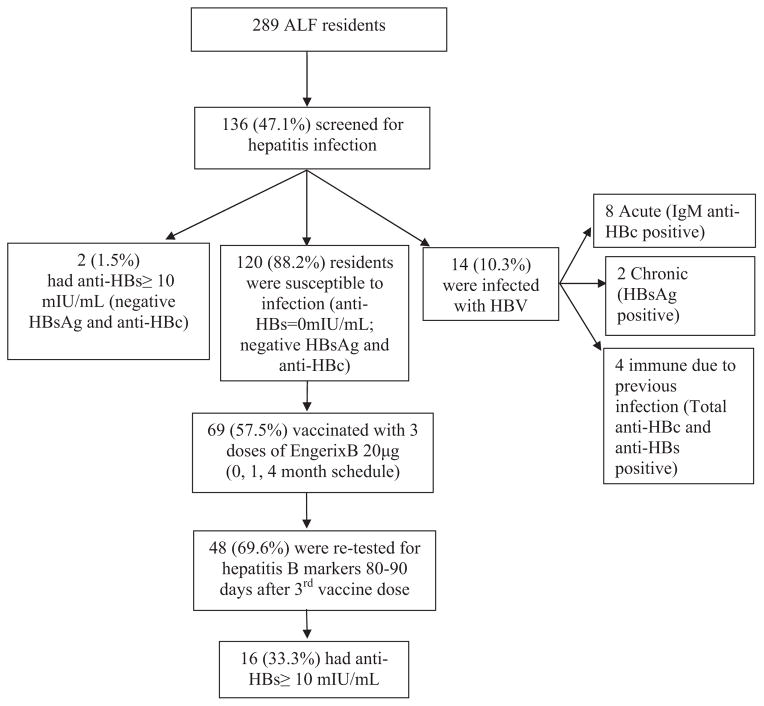
Of the 289 ALF residents, 136 (47.1%) consented to be screened for hepatitis B and C infection; of these, 120 (88.2%) were found to be susceptible to HBV infection, 2 had anti-HBs ≥ 10 mIU/mL and were negative for HBsAg and anti-HBc, and 14 (10.3%) were infected with hepatitis B of which 8 were acute infections (Fig. 1). Of the 69 residents who consented to receive 3 doses of hepatitis B vaccine, 48 (69.6%) residents had post-vaccination blood specimens collected 80–90 days after the third vaccine dose; of these, 16 (33.3%) achieved anti-HBs concentration ≥ 10 mIU/mL. Among the 32 residents with anti-HBs concentration <10 mIU/mL, 8 (25%) had detectable anti-HBs concentration (between 1 and 9.9 mIU/mL) with 2 residents having borderline anti-HBs concentrations of 9.3 mIU/mL. The anti-HBs GMC among the 48 residents was 4.8 mIU/mL. The basic characteristics of residents who did not complete vaccination or did not have serological testing were similar to those who completed vaccination and serological testing.
Fig 1.
Participant flow chart for hepatitis B screening and vaccination—Houston, 2010. ALF: assisted living facility; HBV: hepatitis B virus; anti-HBs: antibody to hepatitis B surface antigen; anti-HBc: hepatitis B core antibody; HBsAg: hepatitis B surface antigen.
The mean age of vaccinated residents was 82.2 ± 14.2 years; the median age was 86 years, range (45–102 years). Demographic and clinical characteristics are shown in Table 1. The residents included more women than men and all residents with available race and ethnicity information were non-Hispanic white. Although there were more than 30 residents with diabetes living in the four ALFs, only five agreed to be tested and vaccinated. Residents had a mean BMI of 25.4 ± 4.6 (range: 18.9–36.8) and almost all were non-smokers. A total of 6.3% had a previous history of cancer but none were on chemotherapy, and 12.5% had moderate renal disease. None of the residents who agreed to get vaccinated had liver disease, HIV infection, were on hemodialysis, or on immunosuppressive drugs. Information on medication was available for 27 residents; most were on anti-hypertensive, cardiac and thyroid medications.
Table 1.
Level of antibody to hepatitis B surface antigen (anti-HBs) after 3 doses of hepatitis B vaccine among older adults by demographic and clinical characteristics—Houston, 2010.
| Characteristic | Total sample N (%) | Anti-HBs < 10 mIU/ml N (%) | Anti-HBs ≥ 10 mIU/ml N (%) | p-Value | Anti-HB4s GMC
|
|
|---|---|---|---|---|---|---|
| GMC | 95%CI | |||||
| Total | 48 | 32 (66.7%) | 16 (33.3%) | – | 4.8 | 2.58–8.91 |
| Age (years) | ||||||
| ≤60 | 8 (16.7%) | 1 (12.5%) | 7 (87.5%) | 0.001 | 72.7 | 11.35–466.00 |
| 61–69 | 0 (0.0%) | – | – | – | – | |
| 70–79 | 3 (6.2%) | 2 (66.7%) | 1 (33.3%) | 9.8 | 0.0–124012.6 | |
| 80–89 | 20 (41.7%) | 14 (70.0%) | 6 (30.0%) | 3.7 | 1.67–8.28 | |
| ≥90 | 17 (35.4%) | 15 (88.2%) | 2 (11.7%) | 1.6 | 1.02–2.46 | |
| Sex | ||||||
| Male | 7 (14.6%) | 6 (85.7%) | 1 (14.3%) | 1.0 | 3.0 | 0.30–29.83 |
| Female | 25 (52.1%) | 19 (76.0%) | 6 (24.0%) | 3.0 | 1.60–5.83 | |
| Missing | 16 (33.3%) | 7 (43.7%) | 9 (56.3%) | |||
| Race | ||||||
| White | 32 (66.7%) | 25 (78.1%) | 7 (21.9%) | N/A | 3.0 | 1.61–5.73 |
| Missing | 16 (33.3%) | 7 (43.7%) | 9 (56.3%) | |||
| Ethnicity | ||||||
| Non-Hispanic | 23 (47.9%) | 18 (78.3%) | 5 (21.7%) | N/A | 3.6 | 1.57–8.10 |
| Missing | 25 (52.1%) | 14 (56.0%) | 11 (44.0%) | |||
| Diabetes | ||||||
| No | 42 (87.5%) | 27 (64.3%) | 15 (35.7%) | 0.6 | 5.1 | 2.69–9.74 |
| Yes N (%) | 5 (10.4%) | 4 (80.0%) | 1 (20.0%) | 3.8 | 0.09–154.51 | |
| Missing | 1 (2.1%) | 1 (100.0%) | 0 (0.0%) | |||
| BMI | ||||||
| Normal (18.5 to <25) | 11 (22.9%) | 10 (90.9%) | 1 (9.1%) | 0.5 | 2.1 | 0.78–5.64 |
| Overweight (25–29) | 13 (27.1%) | 9 (69.2%) | 4 (30.8%) | 3.1 | 1.18–7.95 | |
| Obese (≥30) | 5 (10.4%) | 4 (80.0%) | 1 (20.0%) | 6.5 | 0.19–219.76 | |
| Missing | 19 (39.6%) | 9 (47.4%) | 10 (52.6%) | |||
| Smoking | ||||||
| Non-smoker | 29 (60.4%) | 23 (79.3%) | 6 (20.7%) | 0.4 | 2.6 | 1.48–4.63 |
| Smoker (ex and current) | 2 (4.2%) | 1 (50.0%) | 1 (50.0%) | 28.1 | 0.00–7.27 × 1019 | |
| Missing | 17 (35.4%) | 8 (47.1%) | 9 (52.9%) | |||
| Alcohol | ||||||
| No | 9 (18.7%) | 7 (77.8%) | 2 (22.2%) | 1.0 | 5.4 | 0.84–34.57 |
| Yes | 12 (25.0%) | 9 (75.0%) | 3 (25.0%) | 2.7 | 0.98–7.31 | |
| Missing | 27 (56.3%) | 16 (59.2%) | 11 (40.7%) | |||
| History of cancer | ||||||
| No | 30 (62.5%) | 24 (80.0%) | 6 (20.0%) | 0.5 | 2.9 | 1.52–5.65 |
| Yes | 3 (6.3%) | 2 (66.7%) | 1 (33.3%) | 3.0 | 0.03–352.27 | |
| Missing | 15 (31.2%) | 6 (40.0%) | 9 (60.0%) | |||
| Moderate renal disease | ||||||
| No | 27 (56.3%) | 21 (77.8%) | 6 (22.2%) | 1.0 | 3.0 | 1.52–6.07 |
| Yes | 6 (12.5%) | 5 (83.3%) | 1 (16.7%) | 2.5 | 0.37–17.68 | |
| Missing | 15 (31.2%) | 6 (40.0%) | 9 (60.0%) | |||
Age was the only significant determinant of seroprotection. GMCs were significantly lower among residents aged 70 years or older compared to those who were aged 60 years or younger (mean age: 53; age range: 45–60) (p < 0.001) (Table 1). No statistically significant difference was found between diabetic and non-diabetic residents who developed anti-HBs concentration ≥ 10 mIU/mL even after controlling for age; however, GMC were higher among non-diabetic compared to diabetic residents (5.1 vs. 3.8 mIU/mL, p = 0.7). Of the 5 diabetic residents, only one responded to the vaccine and was relatively younger in age compared to those who did not respond (77 years old vs. 80, 84, and 93 (n = 2) years old). In addition, residents with higher BMI had higher anti-HBs GMCs but the difference was not statistically significant. We could not determine any association between seroprotection or anti-HBs GMCs and smoking, alcohol use, cancer, and renal disease among persons who had complete information on those variables (Table 1).
4. Discussion
This paper describes the use of hepatitis B vaccine among older adults in several ALFs during an outbreak of hepatitis B. Seroprotection (anti-HBs concentration ≥ 10 mIU/mL) was achieved by one third of vaccinated respondents with available anti-HBs levels after receipt of the third vaccine dose. Very few studies have assessed the response to hepatitis B vaccine among older adults (aged 60 years or older); available data suggest variable seroprotection rates, ranging between 30% and 80%, depending on the characteristics of the study population, the type of vaccine, and vaccination schedule [9–14]. Using a 0, 1, 6 month vaccination schedule might have achieved a better booster effect with the third dose given at 6 months and residents with anti-HBs concentrations between 1 and 10 mIU/mL might have achieved seroprotection if the third dose was given at a later time. However, we have used the 0, 1, 4 month vaccination schedule given the urgency to respond to the outbreak, and the need to achieve seroprotection as early as possible using an approved hepatitis B vaccination schedule for adults [2]. Moreover, using the shortest approved schedule was operationally easier to implement in this situation and ensured the ability to complete all three doses and cover most residents before their discharge.
Although a small proportion of residents achieved seroprotection, we did not detect additional new infections after re-testing for hepatitis B and C markers post-vaccination which might be a result of multiple factors. While anti-HBs concentration continues to be the standard used to evaluate vaccine-induced immunity, recent studies have shown that other components of the immune system, such as cell-mediated immunity, may play a role in long-term protection [15]. In addition, the timing of anti-HBs testing influences the seroprotection rates [2,9]. Testing for anti-HBs concentration is recommended 30–60 days after the third vaccine dose; however, because we tested for anti-HBs concentration 80–90 days after the last vaccine dose, we could have missed some who initially responded to the vaccine. Therefore, the eight residents with anti-HBs levels between one and 10 mIU/mL may have had anti-HBs levels ≥ 10 mIU/mL during the 30–60 day period but their antibody levels declined thereafter. In addition, improvements in infection control practices as a result of the outbreak might have contributed to the prevention of new infections.
Age was the only significant predictor of seroprotection in our study population. Residents aged 60 years or younger achieved a high seroprotection rate of 88%; however, rates reached 30% and 12% among those aged 80–89 and ≥90 years, respectively. In addition, as age increased there was a significant decrease in anti-HBs GMCs. These findings are consistent with previously published reports [2,14,16] and are presumably the result of the phenomenon of immunosenescence [15,17]. In this older population, higher BMI may have been a surrogate marker for better nutritional status, which in turn contributes to a stronger immune system, leading to higher seroprotection rates among those who are overweight or obese compared to those having normal BMI (27.8% vs. 9.1%). Only five diabetic residents had post-vaccine testing results which probably hindered our ability to detect any association between diabetes status and vaccine response.
This study had several limitations, including the small sample size, which precluded the determination of statistically significant associations between risk factors and seroprotection. Moreover, our population consisted of older adults living in ALFs who might not be representative of older adults living in the community. In addition, almost one third of the study population had missing information in their medical records on co-morbid conditions and a reliable verbal history was difficult to obtain given the high prevalence of dementia in this population. Similarly, information on receipt of prior hepatitis B vaccination was obtained strictly through review of medical records available at ALFs. Some persons with undetectable anti-HBs could have been vaccinated previously. In such cases, a response to vaccination would truly have represented a response to revaccination, thereby falsely elevating our estimate of primary vaccine response.
Despite these limitations, the findings in this study contribute valuable observational data regarding response to hepatitis B vaccination of older adults in LTC settings. Only one third of the vaccinated older adults developed seroprotection. From an outbreak control standpoint, the combination of poor vaccine response and time interval required to complete the vaccine series are likely to limit the usefulness of such an intervention among elderly persons. However, recent trends suggest that hepatitis B outbreaks in LTC facilities resulting from improper infection control practices are likely to continue [3–6]. Although implementation of proper infection control practices is essential in LTC facilities, studies have shown the difficulty in sustaining these practices even after an outbreak has occurred in these facilities [7]. Until more immunogenic hepatitis B vaccines are available, populations deemed presently at risk – or those likely to incur risk in the future – are more likely to benefit from vaccination at earlier ages when immune response is more favorable. If hepatitis B vaccination of an older population is necessary, an alternative approach such as a double dose or extra doses of either single- or combined-antigen hepatitis B vaccine may be useful, as has been demonstrated among other immunosuppressed populations at risk for HBV infection [2].
Acknowledgments
We would like to thank the following persons for their generous assistance with this project: William Bryant, Decrecia Robinson, MaryJane Lowery, and Howard Tuner from the Houston Department of Health and Human Services; Yenlik Zheteyeva, MD, MPH, Pritish Tosh, MD, Saleem Kamili, PhD, Yury Khudyakov, PhD, Ngoc-Thao Le, BS, and Natasha Khudyakov, MS, from the Centers for Disease Control and Prevention.
Funding: None.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention. This paper was presented in part as a poster during the 5th Vaccine and ISV Annual Global Congress, October 2–4, 2011, Seattle, USA.
Conflict of interest statement: None declared.
References
- 1.Wasley A, Kruszon-Moran D, Kuhnert W, Simard E, Finelli L, McQuillan G, Bell B. The prevalence of hepatitis B virus infection in the United States in the era of vaccination. J Infect Dis. 2010;202:192–201. doi: 10.1086/653622. [DOI] [PubMed] [Google Scholar]
- 2.CDC. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the advisory committee on immunization practices (ACIP). Part II: immunization of adults. MMWR. 2006;55(RR-16):1–33. [PubMed] [Google Scholar]
- 3.Counard CA, Perz JF, Linchangco PC, Christiansen D, Ganova-Raeva L, Xia G, et al. Acute hepatitis B outbreaks related to fingerstick blood glucose monitoring in two assisted living facilities. J Am Geriatr Soc. 2010;58:306–11. doi: 10.1111/j.1532-5415.2009.02669.x. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Nosocomial hepatitis B virus infection associated with reusable fingerstick blood sampling devices—Ohio and New York City, 1996. MMWR Morb Mortal Wkly Rep. 1997;46:217–21. [PubMed] [Google Scholar]
- 5.Centers for Disease Control Prevention. Transmission of hepatitis B virus among persons undergoing blood-glucose monitoring in long-term care facilities—Mississippi, North Carolina, and Los Angeles County, California, 2003–2004. MMWR Morb Mortal Wkly Rep. 2005;54:220–3. [PubMed] [Google Scholar]
- 6.Thompson ND, Perz JF, Moorman AC, Holmberg SD. Nonhospital health care-associated hepatitis B and C virus transmission: United States, 1998–2008. Ann Intern Med. 2009;150:33–9. doi: 10.7326/0003-4819-150-1-200901060-00007. [DOI] [PubMed] [Google Scholar]
- 7.Patel AS, White-Comstock MB, Woolard CD, Perz JF. Infection Control practices in assisted living facilities: a response to hepatitis B virus infection outbreaks. Infect Control Hosp Epidemiol. 2009;30:209–14. doi: 10.1086/595693. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. [accessed 21.06.11]; http://www.cdc.gov/obesity/defining.html.
- 9.Wolters B, Junge U, Dziuba S, Roggendorf M. Immunogenicity of combined hepatitis A and B vaccine in elderly persons. Vaccine. 2003;21:3623–8. doi: 10.1016/s0264-410x(03)00399-2. [DOI] [PubMed] [Google Scholar]
- 10.Van der Wielen M, Van Damme P, Chlibek R, Smetana J, Von Sonnenburg F. Hepatitis A/B vaccination of adults over 40 years old: comparison of three vaccine regimens and effect of influencing factors. Vaccine. 2006;24:5509–15. doi: 10.1016/j.vaccine.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 11.Rendi-Wagner P, Kundi M, Stemberger H, Wiedermann G, Holzmann H, Hofer M, et al. Antibody-response to three recombinant hepatitis B vaccines: comparative evaluation of multicenter travel-clinic based experience. Vaccine. 2001;19:2055–60. doi: 10.1016/s0264-410x(00)00410-2. [DOI] [PubMed] [Google Scholar]
- 12.Stoffel M, Lievens M, Dieussaert I, Martin I, Andre F. Immunogenicity of Twinrix in older adults: a critical analysis. Expert Rev Vaccines. 2003;2(1):9–14. doi: 10.1586/14760584.2.1.9. [DOI] [PubMed] [Google Scholar]
- 13.Averhoff F, Mahoney F, Coleman P, Schatz G, Hurwitz E, Margolis H. Immuno-genicity of hepatitis B vaccines. Implications for persons at occupational risk of hepatitis B virus infection. Am J Prev Med. 1998;15:1–8. doi: 10.1016/s0749-3797(98)00003-8. [DOI] [PubMed] [Google Scholar]
- 14.Denis F, Mounier M, Hessel L, Michel JP, Gualde N, Dubois F, et al. Hepatitis-B vaccination in the elderly. J Infect Dis. 1984;149(6):1019. doi: 10.1093/infdis/149.6.1019. [DOI] [PubMed] [Google Scholar]
- 15.Höhler T, Groeger-Bicanic G, Hoet B, Stoffel M. Antibody persistence and immune memory elicited by combined hepatitis A and B vaccination in older adults. Vaccine. 2007;25:1503–8. doi: 10.1016/j.vaccine.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 16.Fisman DN, Agrawal D, Leder K. The effect of age on immunologic response to recombinant hepatitis B vaccine: a meta-analysis. Clin Infect Dis. 2002;35:1368–75. doi: 10.1086/344271. [DOI] [PubMed] [Google Scholar]
- 17.Effros RB. Role of T lymphocyte replicative senescence in vaccine efficacy. Vaccine. 2007;25:599–604. doi: 10.1016/j.vaccine.2006.08.032. [DOI] [PubMed] [Google Scholar]