Abstract
Objectives/Hypothesis
The purpose of this study was to evaluate the return of vibratory function and restoration of vibration amplitude and symmetry after vocal fold microflap surgery.
Study Design
Prospective in vivo animal model.
Methods
Microflap surgery was performed on 30 New Zealand white breeder rabbits. The left vocal fold received a 3-mm epithelial incision and mucosal elevation, while the contralateral vocal fold was left intact to serve as an internal control. Quantitative analysis of amplitude ratio and lateral phase difference were measured using high-speed laryngeal imaging at a frame rate of 10,000 frames per second from animals undergoing evoked phonation on postoperative days 0, 1, 3, 5, and 7.
Results
Quantitative measures revealed a significantly reduced amplitude ratio and lateral phase difference on day 0 after microflap. These impairments of vibratory function on day 0 were associated with separation of the vocal fold’s bodycover layer. Amplitude ratio increased significantly by day 3 after microflap, with further increases in vibration amplitude on days 5 and 7. While the amplitude ratio improved significantly on day 3, lateral phase difference decreased significantly on day 3, and returned to normal on days 5 and 7.
Conclusions
High-speed laryngeal imaging was used to investigate the natural time course of postmicroflap recovery of vibratory function. Results revealed the restoration of vibration amplitude and lateral phase difference by days 3 to 7 after microflap. The time period of improved vibratory function observed in this study coincides with the end of the well-documented inflammatory phase of vocal fold wound repair.
Keywords: Amplitude ratio, high-speed laryngeal imaging, lateral phase difference, phonation, vocal fold, microflap surgery
INTRODUCTION
One of the most important factors in the success of phonomicrosurgery for benign vocal fold lesions is the preservation of vocal fold mucosa. It is well-known that the human vocal fold has a layered structure that is comprised of epithelium, lamina propria, and muscle.1 The epithelium and superficial layer of the lamina propria are often referred to as the vocal fold “cover,” while the vocalis muscle is referred to as the “body.” The body and cover are connected by a transitional layer, which is comprised of the intermediate and deep layers of the lamina propria.2,3 Optimal vibration depends on the relationship between the loose and pliable mucosal cover and underlying layers of the vocal fold. A major goal of modern day phonomicrosurgery involves preserving as much of the epithelium and lamina propria as possible, a concept that is used during microflap procedures in the treatment of benign vocal fold disease.4 Clinical and experimental data have demonstrated the usefulness of the microflap approach in preservation of the vocal fold mucosa and improved postoperative voice outcomes.1,3,5,6
High-speed laryngeal imaging involves detailed visualization of the larynx under fast frame rates for careful inspection of the physiology of vocal fold vibration.7 Typical frame rates average 2,000 to 4,000 frames per second,8 which corresponds to 10 to 20 frames per cycle of vocal fold vibration at a fundamental frequency of 200 Hz (100 Hz–120 Hz for normal males; 200 Hz–220 Hz for normal females). Vibratory parameters that are commonly assessed during laryngeal imaging include amplitude of vibration, vibration symmetry, phase closure, mucosal wave, glottal configuration, and periodicity.9 The information acquired during high-speed laryngeal imaging provides useful information in the evaluation of vocal fold vibratory function. In the present study, measurements of the amplitude of vibration and vibration symmetry were used to provide insight into the return of normal vocal-fold vibratory function after microflap surgery.
Currently it is unknown when vibratory function returns to normal following vocal fold microflap procedures. These data regarding the return of vibratory function have important clinical implications. Specifically, there is a need for more information on the timing of tissue recovery and the return of normal vibratory function to help guide postoperative recommendations regarding voice use after phonomicrosurgery. High-speed videoendoscopy is a useful tool in this regard, with the potential to provide substantive information regarding tissue vibration and nonperiodic vocal fold behavior,10 particularly during the acute stages after vocal fold microflap. In the present study, we used high-speed laryngeal imaging to evaluate the natural time course of vibration recovery immediately after microflap and up to 7 days postmicroflap surgery using an in vivo rabbit phonation model.11–15
MATERIALS AND METHODS
Animal Welfare Assurance
This study was performed in accordance with the Public Health Service Policy on Humane Care and Use of Laboratory Animals, the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the Animal Welfare Act (7 U.S.C. et seq.); the animal use protocol was approved by the Institutional Animal Care and Use Committee of Vanderbilt University Medical Center.
Microflap Surgery Procedures
Anesthesia was induced through intramuscular administration of ketamine (35 mg/kg), xylazine (5 mg/kg), and acepromazine (0.75 mg/kg). The general well-being of the animals was monitored throughout the procedure by continually checking heart rate, temperature, and oxygen saturation levels. Subsequent intramuscular injections of ketamine (17.5 mg/kg) and acepromazine (0.375 mg/kg) were provided as necessary to maintain a surgical plane of anesthesia.
As described in detail elsewhere,16 transoral direct-suspension microlaryngoscopy was performed using an 11-cm Holinger-Tucker pediatric anterior commissure side-slotted laryngoscope (Karl Storz Endoscopy-America, Inc., El Segundo, CA). The surgical procedures were recorded using a 30-degree 2.7-mm rigid endoscope (Karl Storz Endoscopy-America, Inc.) coupled to a Telecam C-Mount camera (Karl Storz Endoscopy-America, Inc.). A 3-mm incision was made through the epithelium and mucosal cover of one vocal fold. Elevation was performed in an easily located surgical plane using a microlaryngeal fine-angled spatula. The contralateral vocal fold was left intact to serve as an internal control.
Phonation Procedure and High-Speed Laryngeal Imaging
Following induction of general anesthesia, the animals were shaved from the submentum down to the chest and prepped for surgery. Rabbits were placed in the supine position on an operating platform. Local anesthesia (0.2% lidocaine) was administered at the surgical site, and the larynx and trachea were exposed by a midline incision from the hyoid bone to the sternal notch. The trachea was transected just proximal to the sternum. To suspend the lower portion of the trachea to the sternal fascia, sutures were used to provide a stable airway via a tracheostomy. A 3.5-mm cuffed endotracheal tube (Mallinckrodt, Hennef, Germany) was then inserted into the upper portion of the bisected trachea and positioned to rest approximately 2 cm below the glottal aperture. The cuff of this endotracheal tube was inflated to seal off the trachea and deliver airflow through the glottis. Custom, stainless-steel hooked electrodes were prepared prior to each experiment. One electrode was inserted mediolaterally into the belly of each cricothyroid muscle in a direction perpendicular to the muscle fibers (approximately at a 45-degree angle off midline) to serve as cathodes for electrical stimulation. Two additional electrodes were then inserted into the cricothyroid membrane to serve as anodes for electrical stimulation. One electrode was placed into the membrane on each side at the intersection of a longitudinal line 1-mm lateral to midline and a transverse line 1-mm inferior to the thyroid cartilage. A Gilmont Instruments flowmeter (GF-8522-1700; Barrington, IL) coupled to a ConchaTherm Neptune humidifier (Hudson, RCI, Temecula, CA) was used to deliver compressed humidified air heated to 37°C to the glottis. A Grass S-88 stimulator (SA Instrumentation, Encinitas, CA) and constant current isolation unit (Grass Telefactor, model PSIU6; West Warwick, RI) were used to provide electrical stimulation and to produce evoked phonation, as described in detail in our previous studies.11–15
The larynx was suspended using an 11-cm Holinger-Tucker pediatric anterior commissure side-slotted laryngoscope (Karl Storz Endoscopy-America, Inc., El Segundo, CA). Vocal fold vibration was recorded using a 0-degree, 4.0-mm rigid endoscope coupled to a high-speed camera: FASTCAM MC 2.1 (KayPENTAX, Montvale, NJ). The images were acquired in black and white with 512 × 96 pixel resolution at a frame rate of 10,000 frames per second.
Animals and Group Assignment
Thirty New Zealand white breeder rabbits weighing 3.0 to 3.7 kg were used in this experiment. To collect baseline high-speed laryngeal recordings, six animals underwent the evoked phonation procedure described above. These same six animals then underwent the microflap procedure described above, followed by the evoked phonation procedure immediately after microflap (day 0). The remaining 24 animals were randomly assigned to four groups that underwent microflap surgery and the evoked phonation procedure on days 1, 3, 5, and 7.
Amplitude Ratio
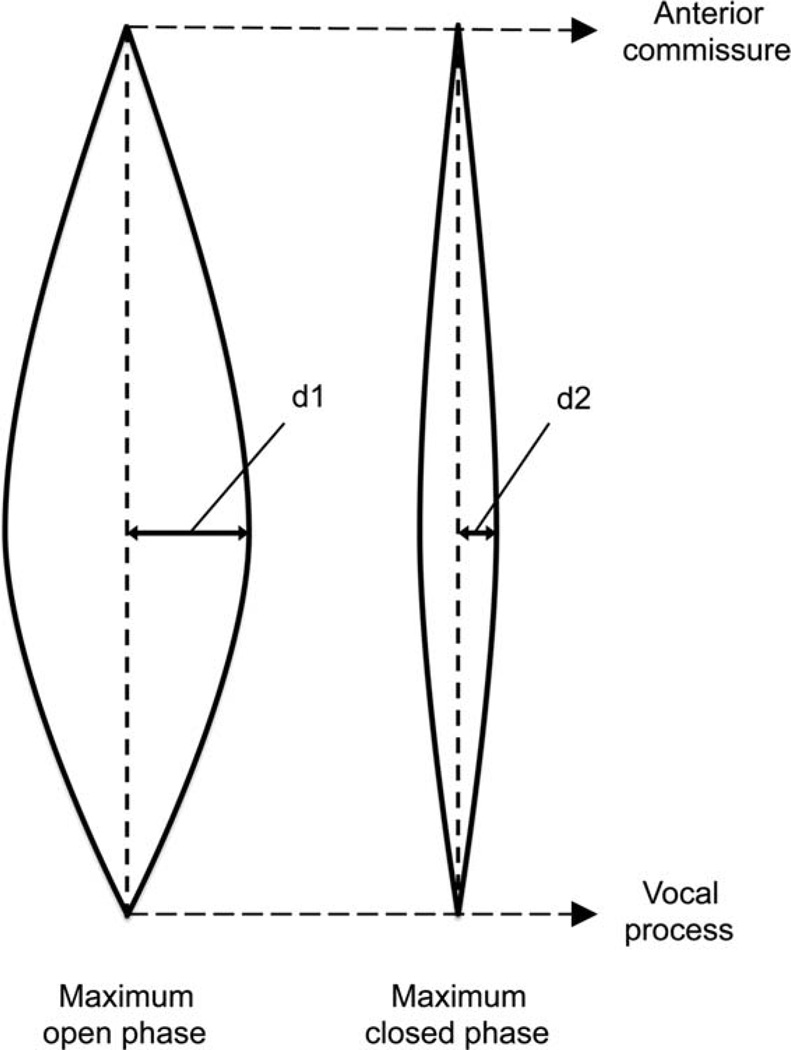
Quantitative analysis of high-speed laryngeal images was performed using the amplitude ratio between the injured and contralateral control vocal fold. In the maximum opening and closing phase, the distance from the midline to the furthest point was measured using ImageJ (NIH, Bethesda, MD)17 (Fig. 1). Amplitude was determined using the difference in amplitude between the maximum opening and closing phase of vibration. When vibration is symmetrical, the amplitude ratio is equal to 1.
Fig. 1.
Measurement of mucosal wave amplitude, defined as the difference between the maximum opening phase amplitude (d1) and maximum closed phase amplitude (d2) of vibration.
Lateral Phase Difference
Lateral phase difference is defined as the difference in phase between the vocal folds.18 In this study, digital kymography was performed from high-speed endoscopic recordings using ImageJ17 (Fig.2A and 2B). The mucosal wave motion in a kymograph was modeled using a sine wave.19 With this concept, a phase of a complete cycle is determined using 2 π radian. If the vocal folds vibrate symmetrically, the lateral phase difference is π radian. Maximum asymmetry occurs when the lateral phase difference is 0 radian. In the present study, we calculated the lateral phase difference using the difference of maximum opening phase between the vocal folds, which is multiplied by the difference cycle of maximum opening (&parderiv; = Fig.2C and 2D) divided by one cycle (T = Fig.2C and 2D) and 2 π radian together. The phase difference is determined by the absolute value, which is the difference of maximum opening phase (2 π × &parderiv;/ T) subtracted from π radian.
Fig. 2.
Lateral phase difference: (A) Digital kymography for normal vibration (control). The upper waves show the left vocal fold (intact) and the bottom waves show the right vocal fold (intact). (B) Digital kymography for day 0. The upper waves show the left vocal fold (microflap) and the bottom waves show the right vocal fold (intact). (C) Representation of symmetric phonation as a sine wave. Lateral phase difference is π radian. (∂ = difference of maximum opening cycle between the vocal folds; T = one cycle.) (D) Representation of asymmetric phonation as a sine wave. Lateral phase difference is 0.25 π radian. (∂ = Difference of maximum opening cycle between the vocal folds; T = one cycle.).
Statistical Analysis
To assess for an overall main effect of postmicroflap day on amplitude ratio and lateral phase difference, two separate Kruskal-Wallis tests were performed using an adjusted alpha level of 0.025 (0.05/2) to control for type I error. If the overall main effect was significant, posthoc testing was performed using Mann-Whitney U tests for two independent samples to investigate differences between postmicroflap days. To control for type I error during posthoc analyses, the alpha level was adjusted to 0.003 (Bonferroni correction: 0.05/15). All analyses were conducted using two-tailed P values. All data were analyzed using IBM SPSS 21.0 (International Business Machines Corp., Armonk, NY).
RESULTS
Amplitude Ratio
The amplitude ratio for rabbits that underwent phonation and no surgical intervention (normal condition) was approximately 1.00. Amplitude ratio revealed a significant main effect of postmicroflap day (P = 0.000). Immediately following microflap surgery, the amplitude ratio for the vocal folds undergoing microflap was significantly lower on day 0 compared to the control vocal fold (P = 0.002). Although postmicroflap day 1 revealed a slightly improved amplitude ratio, the ratio remained significantly lower than control (P = 0.002). However, the amplitude ratio on day 3 increased significantly to approximately 0.80 (P = 0.002), as compared to day 0, and continued to increase on days 5 and 7 postmicroflap (Fig. 3). See supplementary video file (Video I).
Fig. 3.
Vibration amplitude ratio as a function of postmicroflap day. The vertical axis represents the amplitude ratio and the horizontal axis represents the day after microflap. Data points represent the ratio for individual animals.
Significance (P = 0.002) denoted by*. N = normal (control) condition.
Lateral Phase Difference
In the control animals, the lateral phase difference was nearly π radian, indicating symmetrical vocal fold vibration. A significant main effect of postmicroflap day was found for lateral phase difference (P = 0.004). On day 0 postmicroflap, the lateral phase difference of the vocal fold receiving microflap decreased significantly to approximately 0.5 π radian (P = 0.002). Although the lateral phase difference improved on day 1 (0.75 π radian), the ratio decreased significantly on day 3 (0.5 π radian) compared to the control condition (P = 0.002), followed by a return to normal on days 5 and 7 postmicroflap (Fig. 4). See supplementary video file (Video I).
Fig. 4.
Lateral phase difference as a function of postmicroflap day. The vertical axis represents the lateral phase difference and the horizontal axis represents the day after microflap. Data points represent the lateral phase difference for individual animals.
Significance (P = 0.002) denoted by*. N = normal (control) condition.
DISCUSSION
This study examined the natural time course and restoration of vibratory function following microflap surgery in an in vivo rabbit phonation model. High-speed laryngeal imaging provides a useful tool for evaluating vocal fold vibration, allowing for the evaluation of highly complex voice signals. In high-speed imaging studies, frame rate is an important factor as it influences the brightness and resolution of the recording. The mean fundamental frequency evoked during rabbit phonation has been reported to be 672 Hz during modal phonation and 616 Hz during raised intensity phonation.13 Thus, high-speed laryngeal recordings were made at 10,000 frames per second in the present study to yield approximately 15 to 16 frames per vibratory cycle. Recordings were made in black and white using 512 × 96 pixel resolution, providing optimal resolution for the measurement of amplitude ratio and lateral phase difference.
Results of the present study revealed a significant reduction in both the amplitude ratio and lateral phase difference immediately after microflap (day 0). The surgical incision resulted in a separation of the vocal fold body and cover, resulting in vibration of primarily the cover layer. The resulting disruption in vibration at day 0 is likely related to differences in the physical property, weight, and tension of the epithelium and superficial layer of the lamina propria in comparison to the vocal fold body, which is comprised of the denser vocalis muscle. Regarding the general concern of when to initiate mobilization of tissue after microflap surgery, the results at day 0 of body-cover separation and a significant disruption of vibration amplitude and lateral phase difference appear to provide support for the general tenet of keeping the vocal folds immobilized immediately after surgery to allow for the microflap to redrape and heal by secondary intention. Although the amplitude ratio and lateral phase difference revealed general improvement on day 1 postmicroflap, vibration amplitude remained subnormal until day 3 after microflap. On day 3, different patterns emerged for vibration amplitude and lateral phase difference. While the amplitude of vibration improved significantly on day 3 postmicroflap, lateral phase difference decreased significantly on day 3, followed by a return to normal on days 5 and 7. The improvement in vibration amplitude on day 3 occurred shortly after adhesion of the microflap incision. Modern microflap procedures involve minimal disruption of the epithelium and surrounding tissues. Optimal preservation of the vocal fold mucosa minimizes the inflammatory response and results in a negligible influence on vibration amplitude after adhesion of the microflap incision.
Although amplitude of vibration improved on day 3 postmicroflap, lateral phase difference (vibration symmetry) did not return to normal until postmicroflap days 5 and 7. The improvement in vibration symmetry occurred after the peak of the proliferative phase and during the early remodeling phase of repair.20 The proliferative phase of repair is characterized by an increase in cell proliferation, reepithelialization, granulation tissue, and the formation of a provisional extracellular matrix. These early repair events may influence weight, tension, and stiffness of the vocal fold after microflap and result in a transient disruption in vibration symmetry. These impairments in vibration up to day 3 are consistent with known changes that take place during wound healing of the injured vocal fold, including increased deposition of fibronectin.21,22
Fibronectin acts as a chemoattractant for monocytes, fibroblasts, and endothelial cells, helping guide these cells to the site of injury and providing a substrate for the migration and adhesion of these cells within the extracellular matrix.23 Thus, the increased cellular infiltrate and activity associated with the first 3 days of tissue repair may provide an explanation for the impairments in vibration noted during this period. The formation of a provisional extracellular matrix to facilitate adhesion and repair of tissue likely results in alterations to tissue viscoelasticity and transient impairment of tissue vibratory properties. After postmicroflap day 3, both vibration amplitude and lateral phase difference continued to improve over the time period of observation with lateral phase difference reaching near normal values by postmicroflap day 7. The return of vibratory function observed during this period coincides with the end of the inflammatory phase of wound healing (0–3 days) and the beginning of the proliferative and remodeling phases of wound repair (3–7 days).20,22,24 These subsequent phases of the wound repair process (proliferation and remodeling) are characterized by neomatrix deposition and increases in the levels of collagen type I and collagen type III.
Because the intermediate and deep layers of the lamina propria, ligament, and muscle are typically not violated for microflap procedures involving simple dissection, it would appear reasonable to mobilize tissue and resume judicious voice use once inflammation has subsided and vocal fold vibratory function has returned to normal (3–7 days). However, for procedures and lesion excision involving more extensive dissection in the superficial lamina propria, the combined use of laryngeal imaging to assess the status of healing and vibratory function and sound clinical judgment at the first postoperative visit would be more prudent, with routine surveillance if necessary.
Despite the minimal disruption involved in microflap surgery, it remains quite common for otolaryngologists to recommend a period of tissue immobilization (e.g., voice rest) to allow the vocal folds to recover from surgery. While it is generally agreed that some period of vocal fold immobilization is necessary, there is no agreed upon duration, amount, or consensus on the type of voice restrictions provided. Behrman and Sulica25 found that the preferences and practices with regard to voice rest varied widely among practicing otolaryngologists. Because very few empirical studies have examined the effects of varying durations and amounts of tissue immobilization on recovery of the vocal folds from surgery, there remains a significant need for further systematic research in this area of laryngology. The use of animal models has emerged as an important and valuable approach in laryngology research. Basic science investigations are necessary to provide insight into underlying mechanisms contributing to enhanced postoperative outcomes. These investigations have the potential to inform and guide the design of clinical studies and future human trials with well-defined outcomes, which are ultimately needed to provide guidance regarding factors that constitute safe and unsafe voice use after phonomicrosurgery.
CONCLUSION
The data from the present study document the natural time course of postmicroflap healing and restoration of vibratory function following vocal fold microflap surgery in an in vivo rabbit phonation model. Vibration amplitude and lateral phase difference returned to normal within 3 to 7 days after vocal fold microflap. The return of vibratory function during this period coincides with the end of the inflammatory phase of wound healing (0–3 days), and the beginning of the proliferative and remodeling phases of vocal fold tissue repair (3–7 days).
Supplementary Material
Acknowledgements
The authors thank Carolyn Novaleski, MS, for assistance with manuscript preparation and data interpretation.
This work was supported by NIH grant R01 DC 011338-01 from the National Institute of Deafness and Other Communication Disorders (NIDCD).
Footnotes
This paper received first place in the 2013 American Laryngological Association Poster Competition at COSM in Orlando, Florida, U.S.A., April 10–11, 2013.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
BIBLIOGRAPHY
- 1.Courey MS, Shohet JA, Scott MA, Ossoff RH. Immunohistochemical characterization of benign laryngeal lesions. Ann Otol Rhinol Laryngol. 1996;105:525–531. doi: 10.1177/000348949610500706. [DOI] [PubMed] [Google Scholar]
- 2.Hirano M. Morphological structure of the vocal cord as a vibrator and its variations. Folia Phoniatr (Basel) 1974;26:89–94. doi: 10.1159/000263771. [DOI] [PubMed] [Google Scholar]
- 3.Garrett CG, Coleman JR, Reinisch L. Comparative histology and vibration of the vocal folds: implications for experimental studies in microlaryngeal surgery. Laryngoscope. 2000;110:814–824. doi: 10.1097/00005537-200005000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Hochman II, Zeitels SM. Phonomicrosurgical management of vocal fold polyps: the subepithelial microflap resection technique. J Voice. 2000;14:112–118. doi: 10.1016/s0892-1997(00)80101-0. [DOI] [PubMed] [Google Scholar]
- 5.Courey MS, Gardner GM, Stone RE, Ossoff RH. Endoscopic vocal fold microflap: a three-year experience. Ann Otol Rhinol Laryngol. 1995;104:267–273. doi: 10.1177/000348949510400402. [DOI] [PubMed] [Google Scholar]
- 6.Courey MS, Garrett CG, Ossoff RH. Medial microflap for excision of benign vocal fold lesions. Laryngoscope. 1997;107:340–344. doi: 10.1097/00005537-199703000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Bieging E, Tsui H, Jiang JJ. Efficient and effective extraction of vocal fold vibratory patterns from high-speed digital imaging. J Voice. 2010;24:21–29. doi: 10.1016/j.jvoice.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kendall KA. High-speed digital imaging of the larynx: recent advances. Curr Opin Otolaryngol Head Neck Surg. 2012;20:466–471. doi: 10.1097/MOO.0b013e328359840d. [DOI] [PubMed] [Google Scholar]
- 9.Kendall KA. High-speed laryngeal imaging compared with videostroboscopy in healthy subjects. Arch Otolaryngol Head Neck Surg. 2009;135:274–281. doi: 10.1001/archoto.2008.557. [DOI] [PubMed] [Google Scholar]
- 10.Ikuma T, Kunduk M, McWhorter AJ. Advanced waveform decomposition for high-speed videoendoscopy analysis. J Voice. 2013;27:369–375. doi: 10.1016/j.jvoice.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Rousseau B, Ge P, French LC, Zealear DL, Thibeault SL, Ossoff RH. Experimentally induced phonation increases matrix metalloproteinase-1 gene expression in normal rabbit vocal fold. Otolaryngol Head Neck Surg. 2008;138:62–68. doi: 10.1016/j.otohns.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ge PJ, French LC, Ohno T, Zealear DL, Rousseau B. Model of evoked rabbit phonation. Ann Otol Rhinol Laryngol. 2009;118:51–55. doi: 10.1177/000348940911800109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swanson ER, Abdollahian D, Ohno T, Ge P, Zealear DL, Rousseau B. Characterization of raised phonation in an evoked rabbit phonation model. Laryngoscope. 2009;119:1439–1443. doi: 10.1002/lary.20532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swanson ER, Ohno T, Abdollahian D, Garrett CG, Rousseau B. Effects of raised-intensity phonation on inflammatory mediator gene expression in normal rabbit vocal fold. Otolaryngol Head Neck Surg. 2010;143:567–572. doi: 10.1016/j.otohns.2010.04.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rousseau B, Suehiro A, Echemendia N, Sivasankar M. Raised intensity phonation compromises vocal fold epithelial barrier integrity. Laryngoscope. 2011;121:346–351. doi: 10.1002/lary.21364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suehiro A, Bock JM, Hall JE, Garrett CG, Rousseau B. Feasibility and acute healing of vocal fold microflap incisions in a rabbit model. Laryngoscope. 2012;122:600–605. doi: 10.1002/lary.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to Image J: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sulter AM, Schutte HK, Miller DG. Standardized laryngeal videostroboscopic rating: differences between untrained and trained male and female subjects, and effects of varying sound intensity, fundamental frequency, and age. J Voice. 1996;10:175–189. doi: 10.1016/s0892-1997(96)80045-2. [DOI] [PubMed] [Google Scholar]
- 19.Krausert CR, Ying D, Zhang Y, Jiang JJ. Quantitative study of vibrational symmetry of injured vocal folds via digital kymography in excised canine larynges. J Speech Lang Hear Res. 2011;54:1022–1038. doi: 10.1044/1092-4388(2010/10-0105). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Branski RC, Rosen CA, Verdolini K, Hebda PA. Acute vocal fold wound healing in a rabbit model. Ann Otol Rhinol Laryngol. 2005;114:19–24. doi: 10.1177/000348940511400105. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell JR, Kojima T, Wu H, Garrett CG, Rousseau B. Biochemical basis of vocal fold mobilization after microflap surgery in a rabbit model. Laryngoscope. 2013 Jun 17; doi: 10.1002/lary.24263. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tateya T, Tateya I, Sohn JH, Bless DM. Histological study of acute vocal fold injury in a rat model. Ann Otol Rhinol Laryngol. 2006;115:285–292. doi: 10.1177/000348940611500406. [DOI] [PubMed] [Google Scholar]
- 23.Hirschi SD, Gray SD, Thibeault SL. Fibronectin: an interesting vocal fold protein. J Voice. 2002;16:310–316. doi: 10.1016/s0892-1997(02)00102-9. [DOI] [PubMed] [Google Scholar]
- 24.Tateya T, Tateya I, Sohn JH, Bless DM. Histologic characterization of rat vocal fold scarring. Ann Otol Rhinol Laryngol. 2005;114:183–191. doi: 10.1177/000348940511400303. [DOI] [PubMed] [Google Scholar]
- 25.Behrman A, Sulica L. Voice rest after microlaryngoscopy: current opinion and practice. Laryngoscope. 2003;113:2182–2186. doi: 10.1097/00005537-200312000-00026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.