Abstract
Damage to peripheral nerves or the spinal cord is often accompanied by neuropathic pain, which is a complex, chronic pain state. Increasing evidence indicates that alterations in the expression and activity of gap junction channels in the spinal cord are involved in the development of neuropathic pain. Thus, this review briefly summarizes evidence that regulation of the expression, coupling, and activity of spinal gap junction channels modulates pain signals in neuropathic pain states induced by peripheral nerve or spinal cord injury. We particularly focus on connexin 43 and pannexin 1 because their regulation vastly attenuates symptoms of neuropathic pain. We hope that the study of gap junction channels eventually leads to the development of a suitable treatment tool for patients with neuropathic pain.
Keywords: Connexin, Gap junction channel, Glial cell, Neuropathic pain, Pannexin 1, Spinal cord
INTRODUCTION
Neuropathic pain often occurs after damage to the peripheral nervous system (PNS) or central nervous system (CNS), and its symptoms include spontaneous pain, augmented pain from noxious stimulation (hyperalgesia), and pain induced by normally non-noxious stimulation (allodynia). Since multiple mechanisms are involved in the early and late pathophysiological processes of neuropathic pain [1], the treatment of neuropathic pain is complicated and the need is yet unmet. Many of the suggested mechanisms of neuropathic pain are related to the neuronal system in the PNS and CNS. However, findings continuously demonstrate critical involvement of immune cells and glia and gap junction channels expressed on these non-neuronal cells in the pathophysiological changes following damage to the PNS or CNS [2]. It is hoped that these findings will offer an avenue toward the development of a new armamentarium for the treatment of neuropathic pain.
Gap junctions are specialized transmembrane channels that allow the rapid passage of electrical signals and direct cytoplasmic communication between opposing cells such as astrocytes, oligodendrocytes, and ependymal cells [2]. In this review, we will briefly summarize recent findings that address the significant role of spinal gap junction channels, such as connexin 43 and pannexin 1 in the development of neuropathic pain.
GLIAL CELLS AFTER INJURY OF PERIPHERAL NERVES AND SPINAL CORD
In the early period of the pathophysiology of neuropathic pain development, resident macrophages rush to the lesion site and secrete matrix metalloproteinases, leading to an interruption of the blood-nerve barrier [3]. Within two days of injury, the lesion site is further infiltrated by many neutrophils, monocytes, T lymphocytes, and mast cells from peripheral blood; the infiltration is regulated by chemokines, e.g., C-C motif ligands (CCLs) 2 and 3. Following infiltration, the immune cells, together with Schwann cells, release prostaglandins and proinflammatory cytokines [interleukins (ILs)-1β, 6, 12, and 18, interferon (IFN)-γ, tumor necrosis factor (TNF)-α, and leukemia inhibitory factor (LIF)] [2]. These immune and inflammatory substances contribute to the peripheral mechanism of neuropathic pain.
In addition to the activity at the peripheral lesion site, peripheral nerve injury causes population and morphological changes in immune and glial cells in the dorsal horn (DH) of the spinal cord, an important region that transmits and integrates sensory and pain signals from the periphery. Typically, recruitment and activation of microglia in the spinal DH reaches its peak within a week after peripheral nerve injury, followed by a slow decline of microglia but a further invasion of T lymphocytes over several weeks [2]. In contrast, proliferation and activation of astrocytes begins relatively late and progresses slowly, but is sustained for a longer period. It has been known that microglia and astrocytes are activated by various molecules that are released from inflammatory cells and injured cells and nerves or leaked from injured vessels, e.g., glutamate, ATP, K+, Ca2+, reactive oxygen species (ROS), nitric oxide (NO), proinflammatory cytokines (TNF-α, IL-1β, IFN-γ, etc.), and neurotrophic factors. Although glial cells in the CNS play an important role in maintaining neural homeostasis by scavenging excess K+ or debris, the activated glial cells release various substances that may be neuroprotective or neurotoxic. In the early period of injury, the substances may protect neural tissues or reduce the size of the injured area [4,5,6]. However, the sustained activity of glial cells causes further release of neurotoxic substances such as ATP and glutamate. In peripheral nerve injury-induced neuropathic pain, ATP signaling is associated with pain transduction within the spinal cord via activation of microglia through P2X4 receptors, a type of ionotropic ATP-gated receptor [7]. On the other hand, the activation of microglia and astrocytes is also prominent in the spinal DH after traumatic, compressive, or ischemic spinal cord injury (SCI) [8]. The sustained activity of glial cells after SCI produces high levels of ATP at the site of injury in the spinal cord [9,10], leading to abnormal activity of neurons and glial cells [4,11]. This ATP-mediated vicious cycle induces the release of proinflammatory cytokines (e.g., TNF-α, IL-1β, IL-6, and IFN-γ) [10,12], which results in the central sensitization of the neuropathic pain mechanism [13] and the broadening of the injured spinal area following SCI [14].
FUNCTION OF GAP JUNCTION CHANNELS IN GLIAL CELLS OF INJURED NEURAL TISSUES
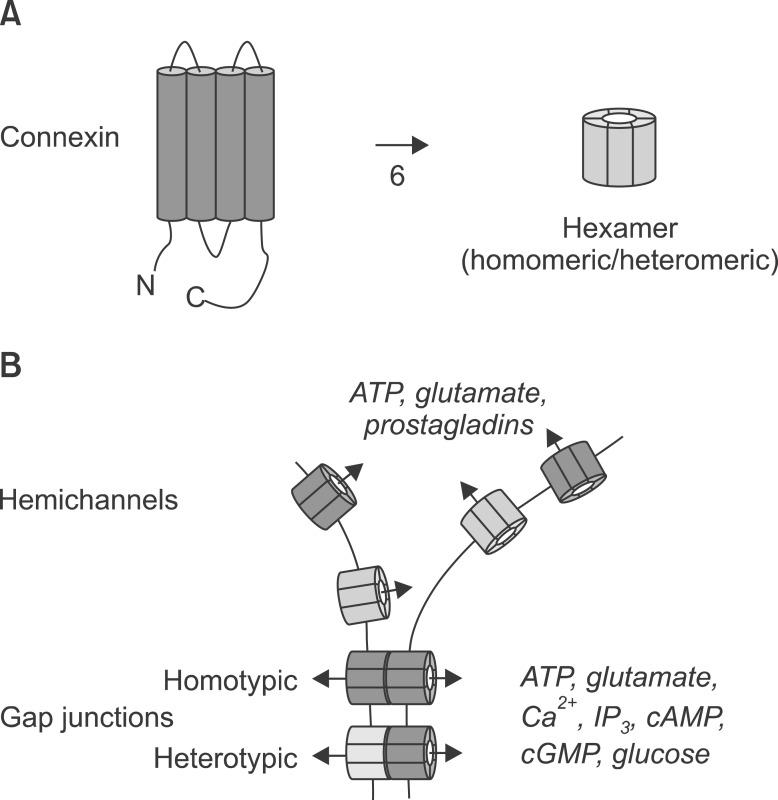
On the other hand, recent studies indicate that the activated glial cells use gap junction channels to release various neurotoxic substances into the surrounding neural tissue. Gap junction channels are homomeric or heteromeric hexamers that are composed of connexin (Cx) proteins (Fig. 1A); 21 mammalian genes produce Cx proteins. Each Cx has four transmembrane domains, one intracellular and two extracellular loops, and intracellular N- and C-terminals. Homotypic or heterotypic couplings of gap junction channels form cell-to-cell connections [15]. Gap junction channels become open after depolarization, extracellular alkalization, metabolic inhibition, mechanical stimulation, or in the presence of low concentrations of extracellular Ca2+ [15]. Although various types of Cxs exist in glia, Cx32, Cx36, and Cx43 are predominantly expressed in microglia, and Cx30 and Cx43 are expressed in astrocytes [16]. Generally, gap junction channels play a role in passing molecules less than 1 kD (e.g., Ca2+, IP3, cAMP, cGMP, glutamate, glucose, ATP, and ADP) between cells (Fig. 1B) [17]. However, some gap junction channels in glia are not opposed by existing gap junctions in other neighboring glial cells; these unopposed channels are called "hemichannels" (Fig. 1B). It has been shown that glia release cytotoxic substances (e.g., ATP, glutamate, and prostaglandins) through the unopposed hemichannels to the extracellular space, affecting neighboring glia and neurons [16,18,19,20,21]. In practice, the Cx hemichannel activator quinine evokes ATP release, and the mechanical stimulation-induced release of ATP is inhibited by the Cx channel inhibitor flufenamic acid [22], indicating the mediation of unopposed hemichannels in ATP release. In the case of glutamate, its extracellular release through the Cx hemichannels is induced by lowering the extracellular Ca2+ concentration because of the extracellular location of the Ca2+ binding site and the release is not related to glutamate transporters or P2X7 receptors [23], indicating that the extracellular release of glutamate is specific to the opening of Cx hemichannels. Recently, it has been shown that TNF-α stimulates the release of the chemokine CXCL1, and this release is blocked by Cx43-specific peptide inhibitors, indicating that the CXCL1 release is mediated by Cx43-hemichannels on astrocytes [24]. This result is somewhat unusual because the molecular weight of CXCL1 (8 kD) is beyond the classical molecular weight limit (~1 kD) for the passage of gap junction channels [17]. Therefore, it would be interesting to investigate whether certain activity of gap junction channels changes the size of channel pores [24]. Altogether, these results suggest that the unopposed hemichannels release various substances, toxic or nontoxic, to the extracellular space, potentially aggravating pathophysiological conditions after injury of neural tissues. In addition, this kind of mechanism may contribute to the development of neuropathic pain [25].
Fig. 1. Diagram showing the topological structure of connexin and roles of gap junction channels and hemichannels. (A) A connexin (Cx) protein has one intracellular and two extracellular loops and intracellular N- and C-terminals, and six homomeric or heteromeric Cx proteins compose a functional gap junction channel. (B) Homotypic or heterotypic gap junction channels coupled between cells pass molecules less than 1 kD, such as Ca2+, IP3, cAMP, cGMP, glutamate, glucose, ATP, and ADP, but unopposed hemichannels, upon their activation, release those molecules, particularly, ATP, glutamate, and prostaglandins.
ROLE OF SPINAL GAP JUNCTIONS IN NEUROPATHIC PAIN DEVELOPMENT: CONNEXIN 43 AND PANNEXIN 1
In the spinal cord, there is increasing evidence that the expression of gap junction channels is increased under various pathological conditions and may contribute to chronic pain states including neuropathic pain. Under pathological conditions, abnormal release of neurotransmitters, growth factors, peptides, and cytokines may increase connexin expression and the permeability of gap junction channels [25]. Although the augmented activity of gap junction channels may be neuroprotective through the removal of harmful substances, it may also cause extensive Ca2+ waves through coupled cells, facilitating the release of proinflammatory cytokines and pain-enhancing molecules such as ATP, glutamate, and prostaglandins. These changes may occur in the spinal cord after injury to the spinal cord and peripheral nerves, contributing to central sensitization [1,2]. Following this notion, recent studies have reported on the role of spinal gap junction channels in neuropathic pain modulation. Intrathecal delivery of carbenoxolone, a non-specific and reversible gap junction decoupler [26], has been shown to ameliorate neuropathic pain induced by inflammation or chronic constriction injury (CCI) of the sciatic nerve in a dose-dependent manner [27]. In addition, intrathecal administration of carbenoxolone reversed SCI-induced neuropathic pain by inhibiting astrocyte activation via gap junction decoupling [28].
Regarding specific types of Cxs related to neuropathic pain, an early report has shown that expression of Cx43 increases rapidly after facial nerve axotomy in the facial nucleus [29]. In astrocytes of the spinal DH that densely express Cx43 [30], the Cx43 protein is upregulated following SCI [31,32,33,34], involving the release of ATP by forming hemichannels and then activation of neighboring astrocytes [14]. This consequence may lead to the expansion of the injured area of the spinal cord [32,35]. Thus, a peptide (so-called "peptide 5"), mimicking the extracellular loop of Cx43, is able to recover the loss of motor function after SCI [34]. Interestingly, thermal hyperalgesia and mechanical allodynia induced by SCI is prevented in transgenic mice with Cx43/Cx30 deletions [33]. In addition, Cx43 is persistently upregulated in the spinal astrocytes in models of neuropathic pain with peripheral nerve injury such as spinal nerve ligation (SNL) [36] and CCI [24]. Notably, suppression of Cx43 with siRNA decreases mechanical hypersensitivity in the SNL pain model [36], and the Cx43-specific peptide inhibitors Gap26 and Gap27 alleviate mechanical allodynia established by CCI [24], suggesting that Cx43 is a potential target for neuropathic pain treatment.
In addition to Cx proteins, pannexins also form gap junction channels and are widely expressed in mammalian tissues. Pannexins have a similar structure to that of Cxs, comprising four transmembrane domains, one intracellular and two extracellular loops, and N- and C-terminals. Pannexin 1 is the most ubiquitously expressed pannexin, while pannexin 2 is predominantly expressed in the CNS, and pannexin 3 is expressed in skin and bone [37]. Pannexin 1 is mechanosensitive, has large conductance, and is permeant for ATP by forming hemichannels [38]. It has recently been suggested that pannexin 1 plays a role in the mechanisms underlying central sensitization in a neuropathic pain model with sural nerve transection [39]. Although western blot analysis shows similar levels of pannexin 1 expression in the DH of the lumbar spinal cord from sham-operated and neuropathic rats, pannexin 1 blockers suppress spinal C-reflex wind-up activity and mechanical hypersensitivity in the neuropathic rats [39]. In contrast to Cx43, it is of interest that pannexin 1 channels can be opened by ATP and glutamate, which mediate acute and chronic pain signaling in the spinal DH. Therefore, pannexin 1 may not only release pain-related substances through its hemichannels but also may enhance the release by responding to ATP and glutamate, exacerbating neuropathic pain.
CONCLUSIONS
In the present review, we briefly address the importance of gap junction channels in the development of neuropathic pain that is tightly related to immunological reactions in the spinal DH after various types of injury in the spinal cord and peripheral nerves. The gap junction channels may be activated, or upregulated, by proinflammatory cytokines following injury of the spinal cord or peripheral nerves, for example, the increased activity of Cx43 hemichannels by TNF-α after CCI [24]. Alternatively, immunological and inflammatory molecules released through gap junction hemichannels further reinforce adverse immunological reactions, eventually causing exacerbation of the pathological conditions in the spinal cord. These processes are important in the development of neuropathic pain. Therefore, to prevent and inhibit the mechanisms underlying neuropathic pain after damage to the spinal cord or peripheral nerves, it would be essential to control the activity and expression levels of gap junction channels, particularly Cx43 and pannexin 1, in the spinal DH.
References
- 1.Scholz J, Woolf CJ. Can we conquer pain? Nat Neurosci. 2002;5(Suppl):1062–1067. doi: 10.1038/nn942. [DOI] [PubMed] [Google Scholar]
- 2.Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;10:1361–1368. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- 3.Shubayev VI, Angert M, Dolkas J, Campana WM, Palenscar K, Myers RR. TNFalpha-induced MMP-9 promotes macrophage recruitment into injured peripheral nerve. Mol Cell Neurosci. 2006;31:407–415. doi: 10.1016/j.mcn.2005.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bethea JR. Spinal cord injury-induced inflammation: a dual-edged sword. Prog Brain Res. 2000;128:33–42. doi: 10.1016/S0079-6123(00)28005-9. [DOI] [PubMed] [Google Scholar]
- 5.Farahani R, Pina-Benabou MH, Kyrozis A, Siddiq A, Barradas PC, Chiu FC, et al. Alterations in metabolism and gap junction expression may determine the role of astrocytes as "good samaritans" or executioners. Glia. 2005;50:351–361. doi: 10.1002/glia.20213. [DOI] [PubMed] [Google Scholar]
- 6.Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004;24:2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, et al. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- 8.Gwak YS, Kang J, Unabia GC, Hulsebosch CE. Spatial and temporal activation of spinal glial cells: role of gliopathy in central neuropathic pain following spinal cord injury in rats. Exp Neurol. 2012;234:362–372. doi: 10.1016/j.expneurol.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, Arcuino G, Takano T, Lin J, Peng WG, Wan P, et al. P2X7 receptor inhibition improves recovery after spinal cord injury. Nat Med. 2004;10:821–827. doi: 10.1038/nm1082. [DOI] [PubMed] [Google Scholar]
- 10.Peng W, Cotrina ML, Han X, Yu H, Bekar L, Blum L, et al. Systemic administration of an antagonist of the ATP-sensitive receptor P2X7 improves recovery after spinal cord injury. Proc Natl Acad Sci U S A. 2009;106:12489–12493. doi: 10.1073/pnas.0902531106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Springer JE, Azbill RD, Knapp PE. Activation of the caspase-3 apoptotic cascade in traumatic spinal cord injury. Nat Med. 1999;5:943–946. doi: 10.1038/11387. [DOI] [PubMed] [Google Scholar]
- 12.Collo G, Neidhart S, Kawashima E, Kosco-Vilbois M, North RA, Buell G. Tissue distribution of the P2X7 receptor. Neuropharmacology. 1997;36:1277–1283. doi: 10.1016/s0028-3908(97)00140-8. [DOI] [PubMed] [Google Scholar]
- 13.Kawasaki Y, Zhang L, Cheng JK, Ji RR. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci. 2008;28:5189–5194. doi: 10.1523/JNEUROSCI.3338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cotrina ML, Nedergaard M. Physiological and pathological functions of P2X7 receptor in the spinal cord. Purinergic Signal. 2009;5:223–232. doi: 10.1007/s11302-009-9138-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reyes EP, Cerpa V, Corvalán L, Retamal MA. Cxs and Panx-hemichannels in peripheral and central chemosensing in mammals. Front Cell Neurosci. 2014;8:123. doi: 10.3389/fncel.2014.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takeuchi H, Suzumura A. Gap junctions and hemichannels composed of connexins: potential therapeutic targets for neurodegenerative diseases. Front Cell Neurosci. 2014;8:189. doi: 10.3389/fncel.2014.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldberg GS, Lampe PD, Nicholson BJ. Selective transfer of endogenous metabolites through gap junctions composed of different connexins. Nat Cell Biol. 1999;1:457–459. doi: 10.1038/15693. [DOI] [PubMed] [Google Scholar]
- 18.De Vuyst E, Decrock E, De Bock M, Yamasaki H, Naus CC, Evans WH, et al. Connexin hemichannels and gap junction channels are differentially influenced by lipopolysaccharide and basic fibroblast growth factor. Mol Biol Cell. 2007;18:34–46. doi: 10.1091/mbc.E06-03-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Retamal MA, Froger N, Palacios-Prado N, Ezan P, Sáez PJ, Sáez JC, et al. Cx43 hemichannels and gap junction channels in astrocytes are regulated oppositely by proinflammatory cytokines released from activated microglia. J Neurosci. 2007;27:13781–13792. doi: 10.1523/JNEUROSCI.2042-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laird DW. The gap junction proteome and its relationship to disease. Trends Cell Biol. 2010;20:92–101. doi: 10.1016/j.tcb.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Giaume C, Leybaert L, Naus CC, Sáez JC. Connexin and pannexin hemichannels in brain glial cells: properties, pharmacology, and roles. Front Pharmacol. 2013;4:88. doi: 10.3389/fphar.2013.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stout CE, Costantin JL, Naus CC, Charles AC. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J Biol Chem. 2002;277:10482–10488. doi: 10.1074/jbc.M109902200. [DOI] [PubMed] [Google Scholar]
- 23.Ye ZC, Wyeth MS, Baltan-Tekkok S, Ransom BR. Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J Neurosci. 2003;23:3588–3596. doi: 10.1523/JNEUROSCI.23-09-03588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen G, Park CK, Xie RG, Berta T, Nedergaard M, Ji RR. Connexin-43 induces chemokine release from spinal cord astrocytes to maintain late-phase neuropathic pain in mice. Brain. 2014;137:2193–2209. doi: 10.1093/brain/awu140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu A, Green CR, Rupenthal ID, Moalem-Taylor G. Role of gap junctions in chronic pain. J Neurosci Res. 2012;90:337–345. doi: 10.1002/jnr.22764. [DOI] [PubMed] [Google Scholar]
- 26.Rozental R, Giaume C, Spray DC. Gap junctions in the nervous system. Brain Res Brain Res Rev. 2000;32:11–15. doi: 10.1016/s0165-0173(99)00095-8. [DOI] [PubMed] [Google Scholar]
- 27.Spataro LE, Sloane EM, Milligan ED, Wieseler-Frank J, Schoeniger D, Jekich BM, et al. Spinal gap junctions: potential involvement in pain facilitation. J Pain. 2004;5:392–405. doi: 10.1016/j.jpain.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Roh DH, Yoon SY, Seo HS, Kang SY, Han HJ, Beitz AJ, et al. Intrathecal injection of carbenoxolone, a gap junction decoupler, attenuates the induction of below-level neuropathic pain after spinal cord injury in rats. Exp Neurol. 2010;224:123–132. doi: 10.1016/j.expneurol.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Rohlmann A, Laskawi R, Hofer A, Dermietzel R, Wolff JR. Astrocytes as rapid sensors of peripheral axotomy in the facial nucleus of rats. Neuroreport. 1994;5:409–412. doi: 10.1097/00001756-199401120-00009. [DOI] [PubMed] [Google Scholar]
- 30.Ochalski PA, Frankenstein UN, Hertzberg EL, Nagy JI. Connexin-43 in rat spinal cord: localization in astrocytes and identification of heterotypic astro-oligodendrocytic gap junctions. Neuroscience. 1997;76:931–945. doi: 10.1016/s0306-4522(96)00394-6. [DOI] [PubMed] [Google Scholar]
- 31.Theriault E, Frankenstein UN, Hertzberg EL, Nagy JI. Connexin43 and astrocytic gap junctions in the rat spinal cord after acute compression injury. J Comp Neurol. 1997;382:199–214. [PubMed] [Google Scholar]
- 32.Cronin M, Anderson PN, Cook JE, Green CR, Becker DL. Blocking connexin43 expression reduces inflammation and improves functional recovery after spinal cord injury. Mol Cell Neurosci. 2008;39:152–160. doi: 10.1016/j.mcn.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Chen MJ, Kress B, Han X, Moll K, Peng W, Ji RR, et al. Astrocytic CX43 hemichannels and gap junctions play a crucial role in development of chronic neuropathic pain following spinal cord injury. Glia. 2012;60:1660–1670. doi: 10.1002/glia.22384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Carroll SJ, Alkadhi M, Nicholson LF, Green CR. Connexin 43 mimetic peptides reduce swelling, astrogliosis, and neuronal cell death after spinal cord injury. Cell Commun Adhes. 2008;15:27–42. doi: 10.1080/15419060802014164. [DOI] [PubMed] [Google Scholar]
- 35.Huang C, Han X, Li X, Lam E, Peng W, Lou N, et al. Critical role of connexin 43 in secondary expansion of traumatic spinal cord injury. J Neurosci. 2012;32:3333–3338. doi: 10.1523/JNEUROSCI.1216-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu Q, Cheong YK, He SQ, Tiwari V, Liu J, Wang Y, et al. Suppression of spinal connexin 43 expression attenuates mechanical hypersensitivity in rats after an L5 spinal nerve injury. Neurosci Lett. 2014;566:194–199. doi: 10.1016/j.neulet.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sandilos JK, Bayliss DA. Physiological mechanisms for the modulation of pannexin 1 channel activity. J Physiol. 2012;590:6257–6266. doi: 10.1113/jphysiol.2012.240911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 2004;572:65–68. doi: 10.1016/j.febslet.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 39.Bravo D, Ibarra P, Retamal J, Pelissier T, Laurido C, Hernandez A, et al. Pannexin 1: a novel participant in neuropathic pain signaling in the rat spinal cord. Pain. 2014;155:2108–2115. doi: 10.1016/j.pain.2014.07.024. [DOI] [PubMed] [Google Scholar]