Abstract
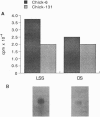
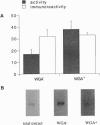
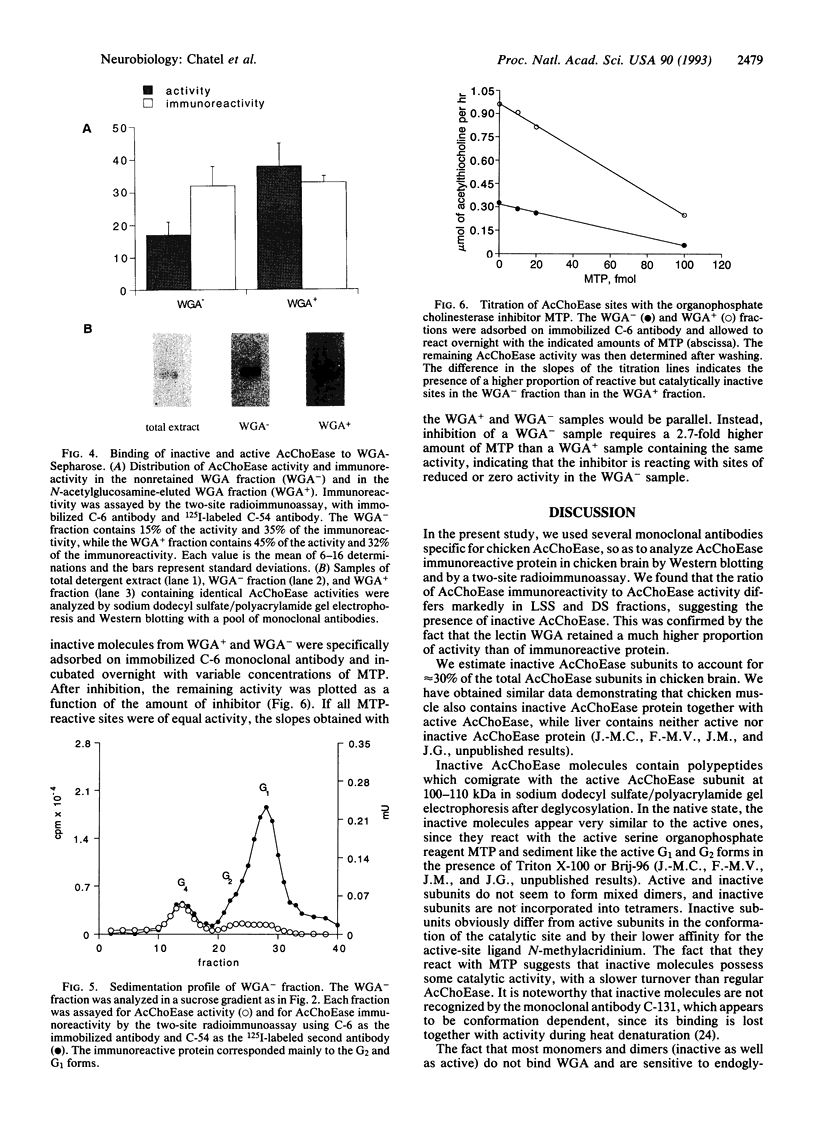
We analyzed acetylcholinesterase (AcChoEase; EC 3.1.1.7) activity and AcChoEase immunoreactive protein in chicken brain by using five monoclonal antibodies raised against chicken AcChoEase. Four of them specifically recognized AcChoEase catalytic subunits in Western blots and one, C-131, recognized only enzymatically active AcChoEase. We observed considerable differences in the ratio of immunoreactive protein to catalytic activity in various fractions, indicating the existence of inactive AcChoEase protein. This inactive AcChoEase component was more abundant in a low-salt-soluble extract than in a subsequent detergent-soluble extract. On the basis of the ratio between activity and immunoreactivity, we calculated that the inactive component represents about 30% of the total AcChoEase subunits in chicken brain. The immunoreactive AcChoEase protein sedimented in sucrose gradients like the active molecular forms; the G1 and G2 peaks contained inactive molecules, whereas the G4 peak appeared to contain only active AcChoEase. The bulk of inactive AcChoEase reacted with the organophosphate cholinesterase inhibitor O-ethyl S-[2-(diisopropylamino)ethyl]methylphosphonothioate (MTP) but was found to bind the active site affinity ligand N-methylacridinium poorly and was not recognized by the active-form-specific monoclonal antibody, C-131. In addition, most of this fraction is sensitive to endoglycosidase H and binds the lectin wheat germ agglutinin poorly, suggesting that it was not processed in the Golgi apparatus. From these observations, we propose that the active and inactive AcChoEase components are differently folded.
Full text
PDF
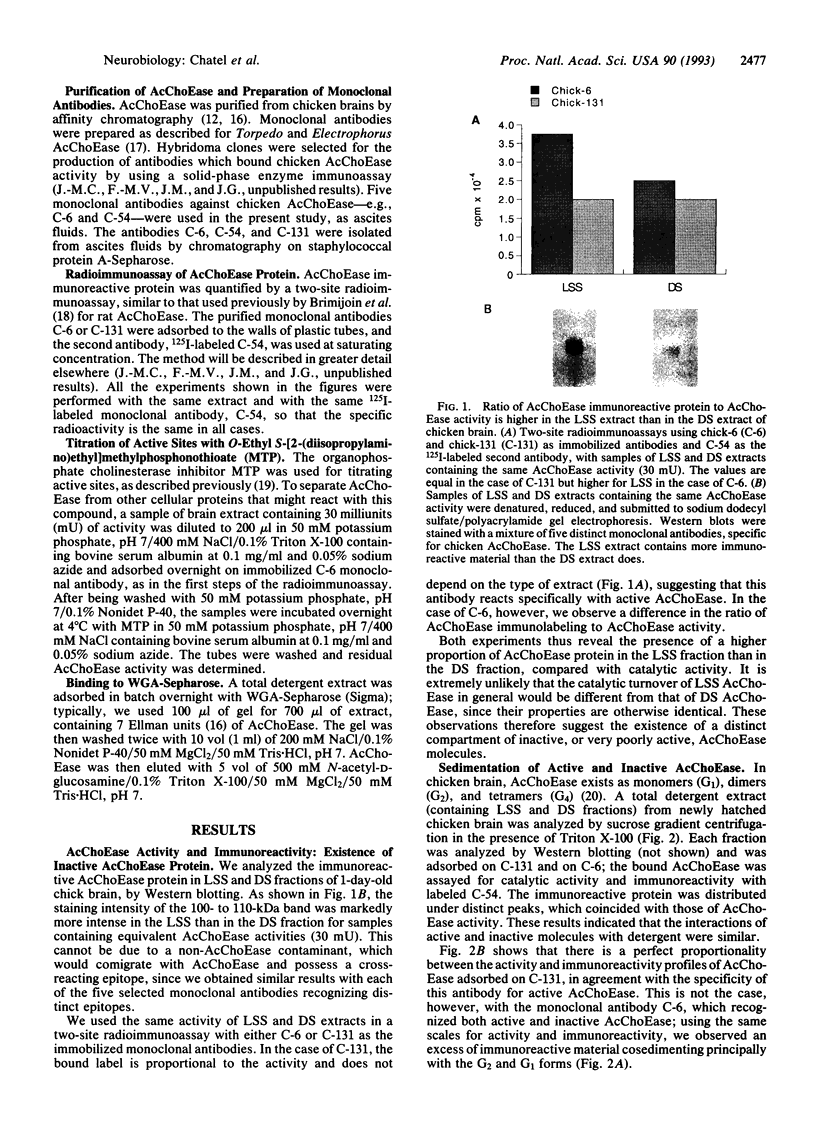
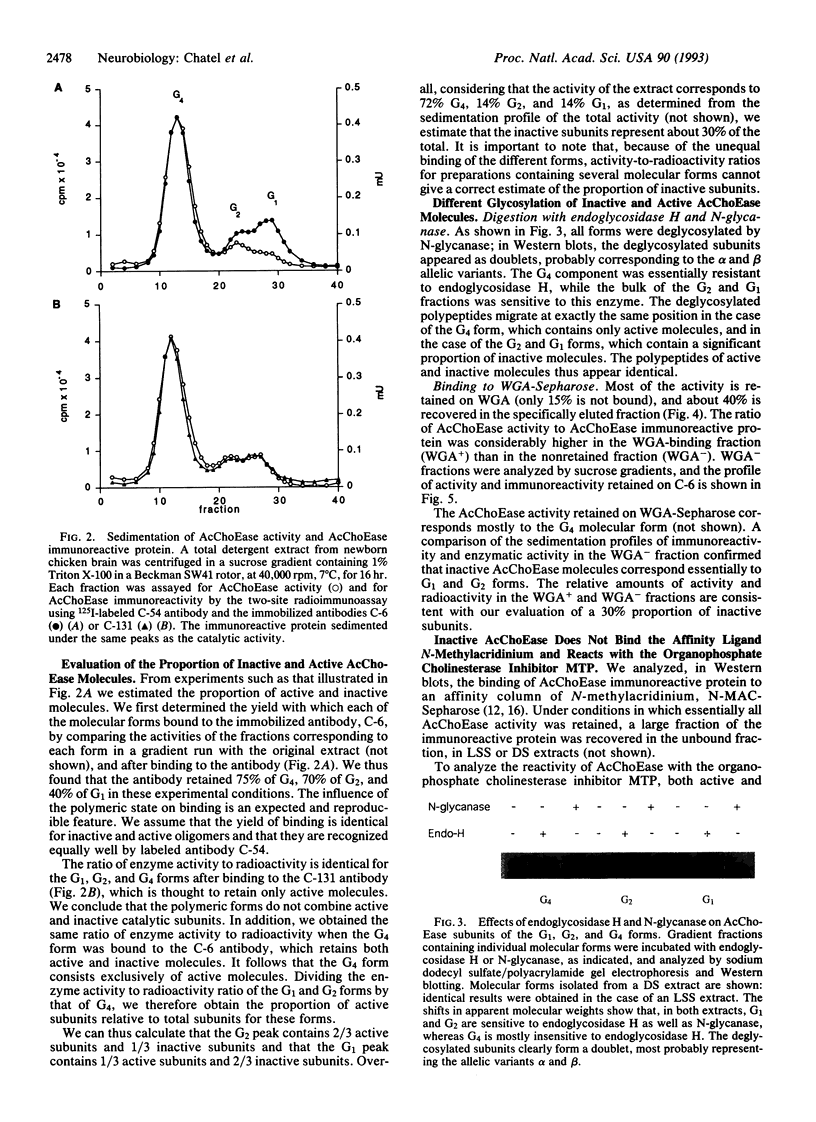

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allemand P., Bon S., Massoulié J., Vigny M. The quaternary structure of chicken acetylcholinesterase and butyrylcholinesterase; effect of collagenase and trypsin. J Neurochem. 1981 Mar;36(3):860–867. doi: 10.1111/j.1471-4159.1981.tb01673.x. [DOI] [PubMed] [Google Scholar]
- Appleyard M. E. Secreted acetylcholinesterase: non-classical aspects of a classical enzyme. Trends Neurosci. 1992 Dec;15(12):485–490. doi: 10.1016/0166-2236(92)90100-m. [DOI] [PubMed] [Google Scholar]
- Bon S., Vigny M., Massoulié J. Asymmetric and globular forms of acetylcholinesterase in mammals and birds. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2546–2550. doi: 10.1073/pnas.76.6.2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimijoin S., Hammond P., Rakonczay Z. Two-site immunoassay for acetylcholinesterase in brain, nerve, and muscle. J Neurochem. 1987 Aug;49(2):555–562. doi: 10.1111/j.1471-4159.1987.tb02900.x. [DOI] [PubMed] [Google Scholar]
- Dudai Y., Silman I. Affinity chromatography of acetylcholinesterase. Methods Enzymol. 1974;34:571–580. doi: 10.1016/s0076-6879(74)34076-1. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L., COURTNEY K. D., ANDRES V., Jr, FEATHER-STONE R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961 Jul;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Hammond P., Brimijoin S. Acetylcholinesterase in Huntington's and Alzheimer's diseases: simultaneous enzyme assay and immunoassay of multiple brain regions. J Neurochem. 1988 Apr;50(4):1111–1116. doi: 10.1111/j.1471-4159.1988.tb10580.x. [DOI] [PubMed] [Google Scholar]
- Jedrzejczyk J., Silman I., Lyles J. M., Barnard E. A. Molecular forms of the cholinesterases inside and outside muscle endplates. Biosci Rep. 1981 Jan;1(1):45–51. doi: 10.1007/BF01115148. [DOI] [PubMed] [Google Scholar]
- Krejci E., Duval N., Chatonnet A., Vincens P., Massoulié J. Cholinesterase-like domains in enzymes and structural proteins: functional and evolutionary relationships and identification of a catalytically essential aspartic acid. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6647–6651. doi: 10.1073/pnas.88.15.6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layer P. G. Cholinesterases during development of the avian nervous system. Cell Mol Neurobiol. 1991 Feb;11(1):7–33. doi: 10.1007/BF00712798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar M., Salmeron E., Vigny M., Massoulié J. Heavy isotope-labeling study of the metabolism of monomeric and tetrameric acetylcholinesterase forms in the murine neuronal-like T 28 hybrid cell line. J Biol Chem. 1984 Mar 25;259(6):3703–3713. [PubMed] [Google Scholar]
- Marsh D., Grassi J., Vigny M., Massoulié J. An immunological study of rat acetylcholinesterase: comparison with acetylcholinesterases from other vertebrates. J Neurochem. 1984 Jul;43(1):204–213. doi: 10.1111/j.1471-4159.1984.tb06698.x. [DOI] [PubMed] [Google Scholar]
- Musset F., Frobert Y., Grassi J., Vigny M., Boulla G., Bon S., Massoulié J. Monoclonal antibodies against acetylcholinesterase from electric organs of Electrophorus and Torpedo. Biochimie. 1987 Feb;69(2):147–156. doi: 10.1016/0300-9084(87)90247-1. [DOI] [PubMed] [Google Scholar]
- Rogers A. W., Darzynkiewicz Z., Salpeter M. M., Ostrowski K., Barnard E. A. Quantitative studies on enzymes in structures in striated muscles by labeled inhibitor methods. I. The number of acetylcholinesterase molecules and of other DFP-reactive sites at motor endplates, measured by radioautography. J Cell Biol. 1969 Jun;41(3):665–685. doi: 10.1083/jcb.41.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotundo R. L. Asymmetric acetylcholinesterase is assembled in the Golgi apparatus. Proc Natl Acad Sci U S A. 1984 Jan;81(2):479–483. doi: 10.1073/pnas.81.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotundo R. L., Gomez A. M., Fernandez-Valle C., Randall W. R. Allelic variants of acetylcholinesterase: genetic evidence that all acetylcholinesterase forms in avian nerves and muscles are encoded by a single gene. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7805–7809. doi: 10.1073/pnas.85.20.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotundo R. L., Thomas K., Porter-Jordan K., Benson R. J., Fernandez-Valle C., Fine R. E. Intracellular transport, sorting, and turnover of acetylcholinesterase. Evidence for an endoglycosidase H-sensitive form in Golgi apparatus, sarcoplasmic reticulum, and clathrin-coated vesicles and its rapid degradation by a non-lysosomal mechanism. J Biol Chem. 1989 Feb 25;264(6):3146–3152. [PubMed] [Google Scholar]
- Stieger S., Brodbeck U., Witzemann V. Inactive monomeric acetylcholinesterase in the low-salt-soluble extract of the electric organ from Torpedo marmorata. J Neurochem. 1987 Aug;49(2):460–467. doi: 10.1111/j.1471-4159.1987.tb02887.x. [DOI] [PubMed] [Google Scholar]
- Vallette F. M., Marsh D. J., Muller F., Massoulié J., Marçot B., Viel C. Comparative affinity chromatography of acetylcholinesterases from five vertebrate species. J Chromatogr. 1983 Mar 4;257(2):285–296. doi: 10.1016/s0021-9673(01)88184-x. [DOI] [PubMed] [Google Scholar]
- Vallette F. M., Massoulié J. Regulation of the expression of acetylcholinesterase by muscular activity in avian primary cultures. J Neurochem. 1991 May;56(5):1518–1525. doi: 10.1111/j.1471-4159.1991.tb02046.x. [DOI] [PubMed] [Google Scholar]
- Vigny M., Bon S., Massoulié J., Leterrier F. Active-site catalytic efficiency of acetylcholinesterase molecular forms in Electrophorus, torpedo, rat and chicken. Eur J Biochem. 1978 Apr 17;85(2):317–323. doi: 10.1111/j.1432-1033.1978.tb12241.x. [DOI] [PubMed] [Google Scholar]
- Walker C. R., Wilson B. W. Regulation of acetylcholinesterase in chick muscle cultures after treatment with diisopropylphosphorofluoridate: ribonucleic acid and protein synthesis. Neuroscience. 1976 Dec;1(6):509–513. doi: 10.1016/0306-4522(76)90103-2. [DOI] [PubMed] [Google Scholar]