ABSTRACT
Cerebral malaria (CM) is a major contributor to malaria deaths, but its pathophysiology is not well understood. While sequestration of parasitized erythrocytes is thought to be critical, the roles of inflammation and coagulation are controversial. In a large series of Malawian children hospitalized with CM, HIV coinfection was more prevalent than in pediatric population estimates (15% versus 2%, P < 0.0001, chi-square test), with higher mortality than that seen in HIV-uninfected children (23% versus 17%, P = 0.0178, chi-square test). HIV-infected (HIV+) children with autopsy-confirmed CM were older than HIV-uninfected children (median age, 99 months versus 32 months, P = 0.0007, Mann-Whitney U test) and appeared to lack severe immunosuppression. Because HIV infection is associated with dysregulated inflammation and platelet activation, we performed immunohistochemistry analysis for monocytes, platelets, and neutrophils in brain tissue from HIV+ and HIV-uninfected children with fatal CM. Children with autopsy-confirmed CM had significantly (>9 times) more accumulations of intravascular monocytes and platelets, but not neutrophils, than did children with nonmalarial causes of coma. The monocyte and platelet accumulations were significantly (>2-fold) greater in HIV+ children than in HIV-uninfected children with autopsy-confirmed CM. Our findings indicate that HIV is a risk factor for CM and for death from CM, independent of traditional measures of HIV disease severity. Brain histopathology supports the hypotheses that inflammation and coagulation contribute to the pathogenesis of pediatric CM and that immune dysregulation in HIV+ children exacerbates the pathological features associated with CM.
Importance There are nearly 1 million malaria deaths yearly, primarily in sub-Saharan African children. Cerebral malaria (CM), marked by coma and sequestered malaria parasites in brain blood vessels, causes half of these deaths, although the mechanisms causing coma and death are uncertain. Sub-Saharan Africa has a high HIV prevalence, with 3 million HIV-infected (HIV+) children, but the effects of HIV on CM pathogenesis and mortality are unknown. In a study of pediatric CM in Malawi, HIV prevalence was high and CM-attributed mortality was higher in HIV+ than in HIV-uninfected children. Brain pathology in children with fatal CM was notable not only for sequestered malaria parasites but also for intravascular accumulations of monocytes and platelets that were more severe in HIV+ children. Our findings raise the possibility that HIV+ children at risk for malaria may benefit from targeted malaria prophylaxis and that adjunctive treatments targeting inflammation and/or coagulation may improve CM outcomes.
Importance
There are nearly 1 million malaria deaths yearly, primarily in sub-Saharan African children. Cerebral malaria (CM), marked by coma and sequestered malaria parasites in brain blood vessels, causes half of these deaths, although the mechanisms causing coma and death are uncertain. Sub-Saharan Africa has a high HIV prevalence, with 3 million HIV-infected (HIV+) children, but the effects of HIV on CM pathogenesis and mortality are unknown. In a study of pediatric CM in Malawi, HIV prevalence was high and CM-attributed mortality was higher in HIV+ than in HIV-uninfected children. Brain pathology in children with fatal CM was notable not only for sequestered malaria parasites but also for intravascular accumulations of monocytes and platelets that were more severe in HIV+ children. Our findings raise the possibility that HIV+ children at risk for malaria may benefit from targeted malaria prophylaxis and that adjunctive treatments targeting inflammation and/or coagulation may improve CM outcomes.
INTRODUCTION
There are 1.2 billion people at high risk for malaria infection worldwide. Plasmodium falciparum is the most virulent of the malaria species that infect humans, and it is responsible for the majority of morbidity and mortality related to malaria infection. While malaria incidence and malaria-associated mortality have decreased significantly over the past several years, the disease burden is still high, with an estimated 198 million malaria infections and 584,000 malaria-associated deaths in 2013 (1). The majority of these deaths are due to cerebral malaria (CM) and severe malarial anemia (SMA) in sub-Saharan African children, in whom 1 in 7 deaths are due to malaria (1–3).
Human immunodeficiency virus type 1 (HIV) also disproportionally affects sub-Saharan Africa. There are 35.3 million people living with HIV worldwide, with 25 million in sub-Saharan Africa, including 3 million children (4). Despite the geographic overlap of areas heavily affected by malaria and HIV and the presumed likelihood of widespread HIV-malaria coinfection, the effect of coinfection on disease pathogenesis and outcome has only recently received attention, and most studies have not examined the effects of HIV coinfection in children with severe malaria.
Initial observational studies of HIV and P. falciparum coinfection from the late 1980s and early 1990s found no significant differences in prevalence or severity of malaria disease (5–8). By the late 1990s, HIV infection was found to negatively affect a pregnant woman’s ability to control P. falciparum parasitemia and placental infection (9–12). More-recent studies on children and nonpregnant adults in both endemic malaria transmission and sporadic malaria transmission zones have shown that HIV infection is associated with more frequent and more severe P. falciparum infection (13–18), and this increased incidence of symptomatic and severe malaria is inversely related to CD4+ T lymphocyte counts (19–21). The mechanisms behind these associations are not fully understood, and the impact of HIV infection on CM has been largely unexplored.
The World Health Organization (WHO) defines CM as unarousable coma accompanied by asexual P. falciparum parasitemia with no other identifiable cause for coma (22). While CM is a relatively rare complication of malaria, it accounts for roughly half of all malaria deaths (23). CM predominantly affects children younger than 5 years living in endemic malaria transmission zones and, less commonly, people of all ages living in meso-endemic transmission zones (24). Death from CM is rapid, and the case fatality rate is 15 to 20% even among patients receiving appropriate treatment (25, 26). Intravenous (i.v.) artesunate treatment may improve survival (27).
The pathophysiology of CM is controversial and may differ between children and adults, but sequestration of parasites in the brain microvasculature is a characteristic feature (28, 29). There is scant evidence of recruitment of inflammatory cells to intravascular or extravascular sites in adult CM (30), but monocytes and platelets have been implicated in the pathogenesis of pediatric and murine experimental CM and in placental malaria (28, 31–33). Chronic HIV infection is associated with expansion of monocyte subsets and platelet activation, leading to monocyte activation and formation of circulating monocyte-platelet complexes (34–36). This dysregulation of platelets and monocytes persists even with effective antiretroviral therapy (ART) and is linked with a variety of conditions, including HIV-associated neurocognitive disorders (HAND) (34, 35, 37).
In an ongoing study of the clinicopathological correlates of pediatric CM based in Blantyre, Malawi, we enrolled >3,000 subjects and completed 103 autopsies of children with clinically defined fatal CM and children dying with nonmalarial causes of coma. We found 3 patterns of brain pathology in children meeting WHO clinical criteria for CM prior to death: intravascular sequestered parasites with no parenchymal changes (termed CM1); intravascular sequestered parasites and parenchymal changes, including ring hemorrhages (termed CM2); and no pathology suggestive of CM, with a nonmalarial anatomic cause of death identified from autopsy (termed CM3) (28, 38). CM is a clinical diagnosis that may be incorrect in a quarter of children meeting existing clinical criteria. Adding retinopathy to the criteria improves specificity for identifying autopsy-confirmed CM (CM1 and CM2) (39).
Malawi has a population of 15.9 million, half of whom are <15 years old, and the entire population is at risk for malaria. The average age of children presenting with CM is 3.5 years, consistent with Malawi’s hyperendemic malaria transmission (3, 40). The HIV prevalence is 10% in the adult population and is estimated to be ≤2.5% in children (4). Before ART was widely available in Malawi, the majority of children with perinatal HIV infection died before age 3 (41).
Because of the important role that monocytes and platelets play in HIV-associated inflammation, and the high rate of HIV infection in children previously enrolled, we hypothesized that HIV coinfection exacerbates host factors that contribute to the pathogenesis of CM. We retrospectively compared autopsy-derived samples and clinical data from HIV-seropositive (HIV+) and HIV-seronegative (HIV-uninfected) children with fatal CM to identify differences or similarities in presentation and pathology.
RESULTS
HIV prevalence was higher than expected and conferred higher mortality in CM patients.
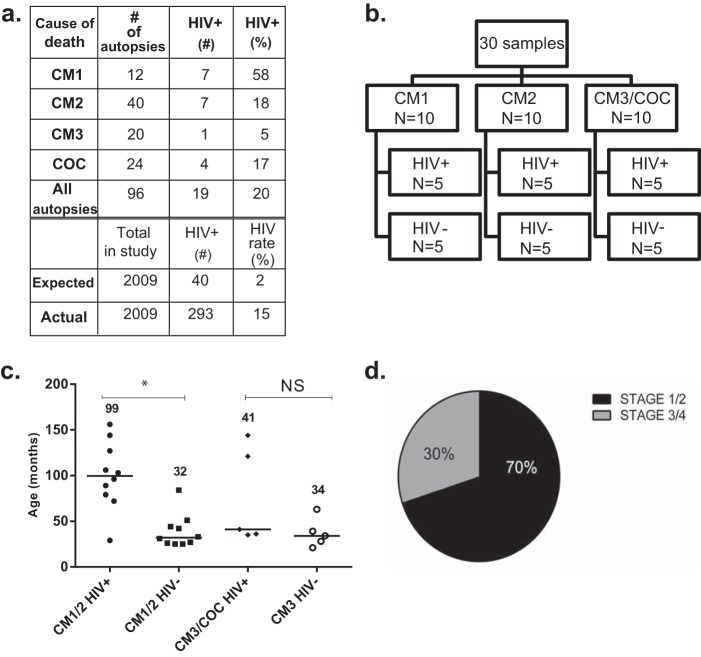
During the period of the autopsy study (1996 to 2010), 2,464 children were admitted to the Paediatric Research Ward. Of these, 2,009 (81%) had HIV antibody testing (Fig. 1a; see also Fig. S1 in the supplemental material). HIV prevalence was 14.5% in children enrolled in the cohort and 2% in Malawi pediatric population estimates (odds ratio [OR] = 8.338; 95% confidence interval [CI], 7.364 to 9.441; P < 0.0001). Mortality was 23.3% in HIV+ children and 17.5% in HIV-uninfected children (OR = 1.433; 95% CI, 1.063 to 1.932; P = 0.0178). Twenty percent of autopsy cases were HIV+ (19/96 children with definitive HIV test results). HIV serostatus was associated with CM brain pathology classification: 58% of children with the CM1 pathology pattern were HIV+ compared with 18% of the CM2 pathology group (OR = 6.6; 95% CI, 1.614 to 26.99; P = 0.0095). Forty out of 52 (77%) autopsy-confirmed CM cases had the CM2 pathology pattern. HIV+ children were split equally between the CM1 and CM2 pathology patterns (50%, or 7, had the CM1 pattern), whereas 33 of 38 (87%) HIV-uninfected children had the CM2 pattern.
FIG 1 .
Features of HIV+ and HIV-uninfected autopsy subjects. (a) Classification of pathology pattern and HIV status among autopsy subjects. Twenty percent of autopsy subjects were HIV+, compared to 15% in the larger study group. CM cases were defined as in the work of Taylor et al. (38): CM1, autopsy-confirmed cerebral malaria cases with sequestered parasites and no perivascular changes in brain pathology; CM2, autopsy-confirmed cerebral malaria cases with sequestered parasites and perivascular hemorrhages in brain pathology; CM3, clinically defined CM cases with no pathological evidence to suggest CM; and COC, cases with a nonmalarial cause of coma. Fifty-eight percent (7/12) of cases with the CM1 pathology pattern were HIV+. One HIV+ CM1 patient aged 6 months, whose HIV-1 status could not be confirmed, was censored (including this patient, 8 of 13 CM1 patients were HIV+). HIV prevalence is estimated to be 2% in Malawian children. (b) Diagram of HIV status and brain pathology patterns of patients evaluated by immunohistochemistry. Thirty patients from the autopsy series were studied in a blinded immunohistochemistry study. The groups comprised 10 CM1 (5 HIV+ and 5 HIV-uninfected), 10 CM2 (5 HIV+ and 5 HIV-uninfected), and 10 CM3/COC (5 HIV+ and 5 HIV-uninfected) patients. Because there was only 1 HIV+ patient with a CM3 diagnosis, the HIV+ group included both CM3 and COC, whereas the HIV-uninfected group members all had a histopathological CM3 classification. (c) Age differences in children who underwent autopsy. Horizontal bars and numerical values are medians. Among children with autopsy-confirmed CM (CM1 and CM2), HIV+ children were significantly older than HIV-uninfected children. *, P = 0.007 by Mann-Whitney U test; NS, not significant. The age distributions of CM1 and CM2 groups are shown in Fig. S3 in the supplemental material. (d) HIV clinical staging of children with autopsy-confirmed CM. Chart review identified 11 out of 14 HIV+ children with WHO stage 1 or 2 HIV, indicating mild disease. The 3 children with severe disease met criteria for WHO stage 3 or 4 based on weight-for-height z score.
HIV-infected children with CM were older than HIV-uninfected children and were not severely immunocompromised.
To define pathological features that might distinguish HIV-associated CM, we performed a blinded histopathological study on a subset of 30 subjects from the autopsy series (Fig. 1b; see also Fig. S2 in the supplemental material). Clinical characteristics of the 96 autopsy subjects with definitive HIV testing (72 with clinically defined CM and 24 with nonmalarial cause of coma or indeterminate cause of death; Table 1) and clinical characteristics of the 30-patient subset (see Table S1) were similar.
TABLE 1 .
Clinical features of the entire autopsy cohorta
| Clinical feature | Value for CM type and HIV status (n): |
P value by ANOVA or chi-square testm | |||||
|---|---|---|---|---|---|---|---|
| CM1 |
CM2 |
CM3; HIV+ (n = 1), HIV− (n = 19) | Other or indeterminate cause of death; HIV+ (n = 4), HIV−/unknown (n = 26) | ||||
| HIV+ (n = 7) | HIV− (n = 5) | HIV+ (n = 7) | HIV− (n = 33) | ||||
| Age (mo) | 79 (41, 103) | 44 (26, 67.5) | 96 (37, 144) | 26 (18, 40.5) | 28 (18.75, 47.75) | 34.5 (19, 60.75) | 0.006 |
| Sex (% female) | 71.4 | 80 | 42.9 | 42.4 | 25 | 60 | NS |
| Admission coma scoreb | 1 | 1 | 1 | 1 | 1 | 1 | NS |
| Retinopathy (% positive)c | 100 | 100 | 100 | 100 | 13.3 | 22.7 | <0.0001 |
| Body mass index (kg/m2)d | 15.5 (14.6, 16.4) | 13.6 (12.5, 15.7) | 15.1 (14, 15.4) | 14.4 (13.4, 15.3) | 15.2 (13.75, 17.8) | 14.5 (12.9, 16.3) | NS |
| Total WBC (103/µl)e | 14.5 (12.1, 17.1) | 11.2 (7.3, 15.7) | 13.2 (10.9, 19.3) | 12.4 (9.1, 21.5) | 13.4 (10.5, 23.95) | 16.1 (10.7, 23.1) | NS |
| Lymphocyte count (103/µl)f | 2.4 (1.5, 5.1) | 2.3 (2, 5.5) | 2.7 (1.5, 5.3) | 5.3 (2.2, 7.8) | 2.9 (2.5, 11.9) | 3.8 (2.4, 5.2) | NS |
| Monocyte count (103/µl)g | 0.7 (0.1, 1.7) | 1 (0.8, 2.5) | 1.1 (0.5, 2.4) | 1.7 (0.6, 2) | 0.6 (0.3, 1.3) | 1.2 (0.8, 3.3) | NS |
| Parasitemia (103/µl)h | 98.3 (48.2, 324.8) | 49.2 (5.1, 717.6) | 56.4 (28.8, 308.7) | 45.3 (7.5, 433.3) | 13.6 (0.9, 147.6) | (0, 0.3) | NS (excluding last column) |
| Hematocrit (%)i | 22 (17, 35) | 29 (22.5, 32) | 21 (17, 26) | 20 (12.3, 22.7) | 31 (20.7, 36) | 24.8 (7.8, 30.3) | 0.02 |
| Platelet count (103/µl)j | 74.4 (36, 144) | 49.4 (31.5, 87.6) | 117.4 (42.7, 351.5) | 45.2 (26, 73) | 155.8 (123, 276) | 169.2 (70, 370) | <0.0001 (excluding last column) |
| Coma duration (h)k | 19 (10.3, 33) | 25 (13, 34) | 28 (24, 43) | 26 (14, 37) | 17 (8, 30) | 13.5 (8.3, 65.7) | NS |
| Duration of illness (h)l | 39.5 (24.7, 59) | 35 (30, 158.5) | 48 (36, 56) | 79 (55.5, 106) | 32 (19, 54) | 71 (29.5, 144) | 0.01 |
Clinical characteristics of children who underwent autopsy for clinically defined CM or coma of other cause. Continuous variables are shown as medians with 25th and 75th percentiles in parentheses, except for parasitemia and platelet counts, values for which are geometric means with 25th and 75th percentiles.
Admission coma score, n = 100 (n = 28 for other/indeterminate cause of death).
Retinopathy, n = 78 (n = 6 for CM1 HIV+, n = 6 for CM2 HIV+, n = 4 for CM1 HIV−, n = 25 for CM2 HIV−, n = 15 for CM3, n = 22 for other/indeterminate cause of death).
Body mass index, n = 100 (n = 32 for CM2 HIV−, n = 29 for other/indeterminate case of death).
Total white blood cells (WBC), n = 85 (n = 6 for CM2 HIV+, n = 29 for CM2 HIV−, n = 13 for CM3, n = 25 for other/indeterminate cause of death).
Lymphocyte count, n = 48 (n = 5 for CM1 HIV+, n = 4 for CM1 HIV−, n = 4 for CM2 HIV+, n = 14 for CM2 HIV−, n = 10 for CM3, n = 11 for other/indeterminate cause of death).
Monocyte count, n = 39 (n = 4 for CM1 HIV+ and CM2 HIV+, n = 3 for CM1 HIV−, n = 11 for CM2 HIV−, n = 8 for CM3, n = 7 for other/indeterminate cause of death).
Parasitemia, n = 100 (n = 28 for other/indeterminate cause of death).
Hematocrit, n = 101 (n = 19 for CM3).
Platelet count, n = 81 (n = 6 for CM2 HIV+, n = 25 for CM2 HIV−, n = 19 for CM3, n = 19 for other/indeterminate cause of death).
Coma duration, n = 82 (n = 6 for CM1 HIV+, n = 5 for CM2 HIV+, n = 31 for CM2 HIV−, n = 17 for CM3, n = 18 for other/indeterminate cause of death).
Duration of illness, n = 98 (n = 6 for CM1 HIV+, n = 18 for CM3, n = 29 for other/indeterminate cause of death). Duration of illness was determined by combining the duration of fever with duration of coma. Note that when data from HIV+/HIV− patients are merged and CM1 is compared with CM2, CM2 has a significantly longer duration of illness.
Differences between groups were analyzed by 1-way analysis of variance (ANOVA) (normal data) or Kruskal-Wallis test (nonnormal data) for continuous variables and by chi-square test for dichotomous variables. NS, not significant.
Among children who had a dilated retinal exam, all 41 (100%) children with autopsy-confirmed CM (CM1 and CM2) had evidence of malarial retinopathy, whereas 2 of the 15 (13.3%) children with CM3 and 5 of the 22 (22.7%) children with nonmalarial causes of coma had signs of retinopathy (P < 0.0001, chi-square test). The same relationship between retinopathy and autopsy-confirmed CM was true for the subset of 30 patients (P < 0.0001, chi-square test). In both the entire autopsy series and the subset of 30 patients with immunohistochemistry analysis, HIV+ children with autopsy-confirmed CM were significantly older than HIV-uninfected children (CM1 and CM2 groups combined; median age, 84 months versus 26.5 months [P = 0.0002] and 99.5 months versus 32 months, respectively [P = 0.0007; Mann-Whitney U test for both]) (Fig. 1c and Table 1; see also Fig. S3 in the supplemental material). Ten of the 14 HIV+ children with autopsy-confirmed CM were ≥5 years old (71%), whereas 5 of the 38 HIV-uninfected children with autopsy-confirmed CM were ≥5 years old (13%). Higher peripheral platelet counts were noted in HIV+ than in HIV-uninfected children with autopsy-confirmed CM in the entire cohort (P = 0.01) and the subset of 30 patients (P = 0.04, Mann-Whitney U test for both) (Table 1; see also Table S1 and Fig. S3). There were no differences in gender, body mass index, coma duration, fever duration, or admission values for peripheral parasitemia, white blood cell count, or coma score in children with clinically defined CM (Table 1; see also Table S1 and Fig. S3). CM1 and CM2 subjects had similar hematocrit values, regardless of HIV status (Table 1; see also Table S1).
No child was known to be HIV+ prior to study enrollment, and none was receiving ART. CD4+ T lymphocyte quantification was not performed at study enrollment because, during the early years of the study, ART was not available in Malawi. Total white blood cell count and total lymphocyte count did not differ between HIV+ and HIV-uninfected children in the entire autopsy series or in the immunohistochemistry subset of 30 patients (Table 1; see also Fig. S3 and Table S1 in the supplemental material). Clinical staging using WHO criteria for HIV severity from chart review, including lymphocyte count, autopsy findings, and weight-for-height z score, identified three out of 14 HIV+ children with autopsy-confirmed CM with stage 3 or 4 HIV, considered advanced disease (Fig. 1d; see also Fig. S3). The children with stage 3/4 HIV were classified as such because of low weight-for-height z scores, which were not significantly different between HIV+ and HIV-uninfected children. Four out of five HIV+ children with nonmalarial causes of death had stage 3/4 HIV, including disseminated tuberculosis, meningitis, and complicated pneumonia. Analysis of archived frozen plasma by real-time PCR of HIV+ children (n = 14 in total, 12 of whom were included in the subset of 30) confirmed HIV infection in all samples, with a geometric mean of 158,691 or 5.172 log10 copies/ml and an interquartile range (IQR) of 69,285 to 488,520 or 4.825 to 5.673 log10 copies/ml. There was no correlation between HIV load and white blood cell count or lymphocyte count.
Pathology from complete autopsy for HIV+ children with autopsy-confirmed CM did not identify AIDS-defining conditions (e.g., Pneumocystis pneumonia, Kaposi sarcoma, or central nervous system [CNS] lymphoma). Eleven of 96 autopsy cases with lung pathology analysis had lymphoid interstitial pneumonitis (LIP), eight of whom were HIV+. All eight had autopsy-confirmed CM, whereas only one of the three HIV-uninfected children with LIP had autopsy-confirmed CM. Other diagnoses included subdural hematoma and pneumonia with pulmonary edema and diffuse alveolar damage. Clinically symptomatic LIP with radiographic findings of reticulonodular infiltrates constitutes a WHO stage 3 HIV diagnosis. None of the HIV+ children had preceding respiratory complaints, and none with available oxygen saturation data were hypoxic at initial hospitalization.
Fatal CM was associated with intravascular monocyte sequestration that was greater in HIV-infected subjects.
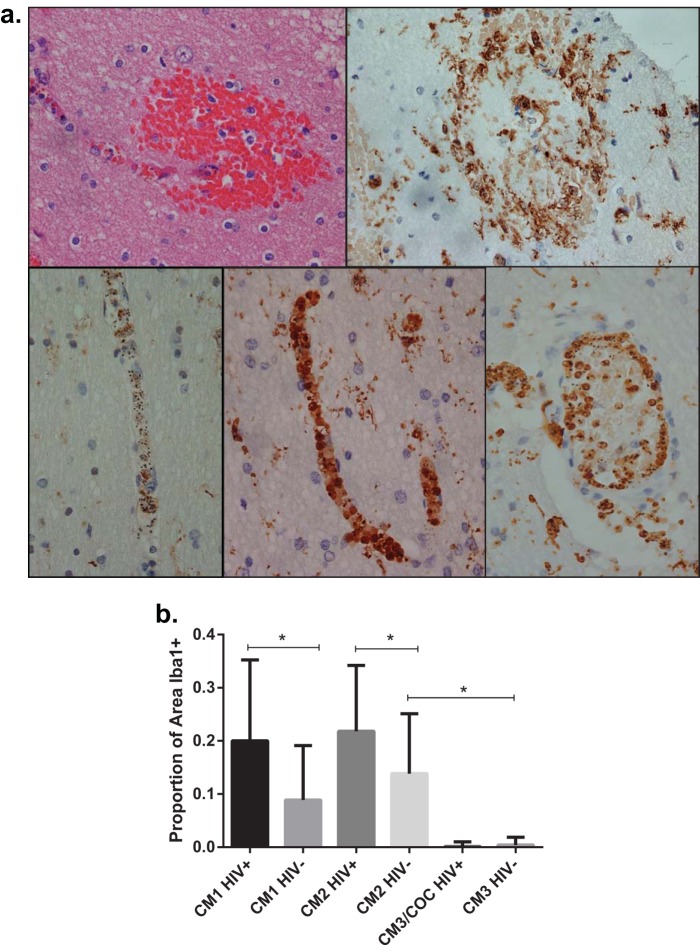
Examination of hematoxylin-and-eosin (H&E)-stained brain sections suggested that cells other than parasitized red blood cells contributed to intravascular pathology (see Fig. S4 in the supplemental material), so we performed immunohistochemistry staining to characterize these cells. Ionized calcium binding adapter molecule 1 (Iba1) is specific for the monocyte lineage and is commonly used as a marker for monocytes, macrophages, and microglia in immunohistochemical studies of inflammation in the brain (42). Iba1 staining revealed activated microglia surrounding microhemorrhages in CM2 sections (Fig. 2a, top right). In several CM1 and CM2 (but not CM3) sections, blood vessels appeared packed with Iba1+ cells containing hemozoin, indicating monocytes that had phagocytosed late-stage parasites. The degree to which these monocytes were present did not always correspond to the amount of sequestered parasites in the brain, as some subjects had blood vessels filled with parasites but with few monocytes (Fig. 2a, bottom left), while others had both parasites and many monocytes (Fig. 2a, bottom middle). Monocytes lined the inner surface of large-caliber vessels, appearing to adhere to the endothelium (Fig. 2a, bottom right).
FIG 2 .
Immunohistochemistry of brain tissue for microglia and monocytes. (a) Micrographs (×400) of brain sections stained with H&E (top left) and immunohistochemically labeled for Iba1 (top right and 3 bottom panels). Brown color indicates the presence of Iba1. (Top left) Ring hemorrhage in close proximity to a blood vessel; (top right) microglia surrounding a ring hemorrhage; (bottom left) blood vessel packed with parasites (denoted by black pigment of hemozoin) with scant monocytes; (bottom middle) blood vessel packed with monocytes containing hemozoin; (bottom right) large vessel with monocytes containing hemozoin lining the endovascular surface. (b) Comparison of the proportions of blood vessel surface areas with Iba1 staining. Values are shown as medians with interquartile ranges. COC, children with an obvious nonmalarial cause of coma. *, P < 0.0001 by Mann-Whitney U test. Autopsy-confirmed CM cases (CM1 and CM2) have more intravascular monocytes than do cases with coma of other cause (CM3 and COC). Among cases of autopsy-confirmed CM, HIV+ children have more intravascular monocytes than do children not infected with HIV.
To further evaluate the association between monocytes and CM, we performed blinded quantification of intravascular monocytes by measuring the areas of 50 blood vessels in cross section as well as the areas of Iba1+ cells in each vessel, calculating the proportions of Iba1+ blood vessel surface areas (ImageJ software). Autopsy-confirmed CM cases had significantly more (greater than 600 times more) brain intravascular monocytes than did children with other causes of death (CM3 and nonmalarial causes of coma) (Fig. 2b). Among autopsy-confirmed CM cases, HIV+ children had 1.9 times more intravascular monocytes than did HIV-uninfected subjects. Intravascular monocytes did not correlate with coma or illness duration, peripheral monocyte counts, or the monocyte-lymphocyte ratio, a potential marker for clinical malaria (43).
Fatal CM was associated with intravascular platelet accumulation that was greater in HIV-infected subjects but was not associated with neutrophil accumulation.
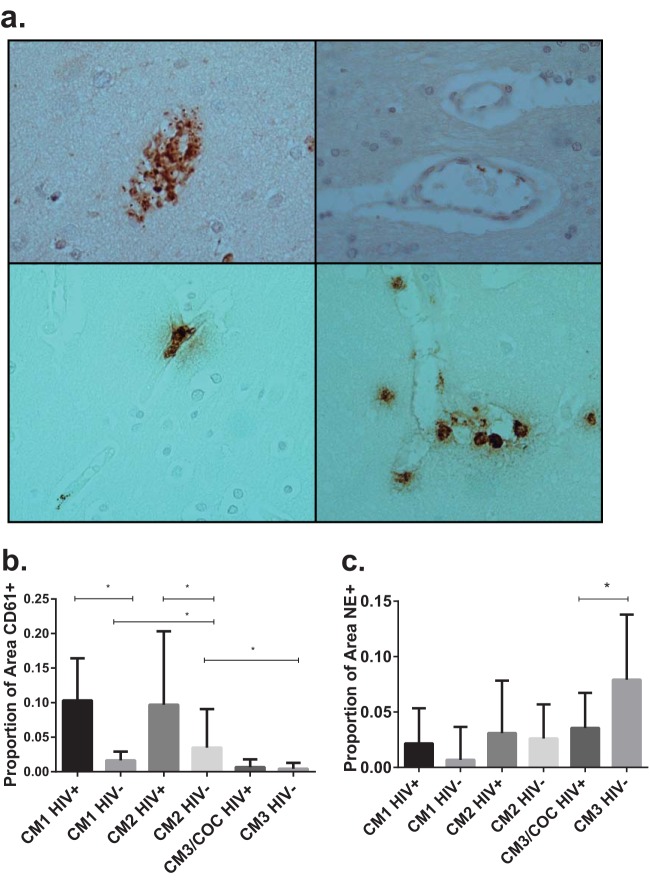
Evaluation of tissue sections labeled for the platelet marker CD61 identified a range of intravascular platelet findings; some subjects had scant intravascular platelets, and others had significant intravascular staining, suggesting the presence of thrombus (Fig. 3a, top left and right, respectively). Quantification of intravascular platelets using ImageJ software identified an 8.8-times-greater proportion of CD61+ blood vessel surface area in children with autopsy-confirmed CM than in children with other causes of death (CM3 and nonmalarial cause of coma) (Fig. 3b). Among autopsy-confirmed CM cases, HIV+ children had 4.5 times more intravascular platelets than did HIV-uninfected children. The proportion of intravascular platelet staining did not correlate with peripheral platelet counts or with duration of illness.
FIG 3 .
Immunohistochemistry of brain tissue for platelets and neutrophils. (a) Micrographs (×400) of brain sections labeled for CD61 (top left and right) and neutrophil elastase (bottom left and right). Brown color indicates the presence of CD61 or neutrophil elastase. COC, cases with a nonmalarial cause of coma. (Top left) Blood vessel filled with platelets and parasites (denoted by black pigment of hemozoin); (top right) blood vessel with scant platelets; (bottom left) blood vessel with one intravascular neutrophil at top and intravascular hemozoin at bottom, from a child with autopsy-confirmed CM; (bottom right) multiple intra- and perivascular neutrophils, from a child with bacterial meningitis. (b) Comparison of the proportions of blood vessel surface areas with CD61 staining. Values are shown as medians with interquartile ranges. *, P < 0.0001 by Mann-Whitney U test. Autopsy-confirmed CM cases (CM1 and CM2) have more intravascular platelets than do cases with coma of other causes (CM3 and COC). Among cases of autopsy-confirmed CM, HIV+ children have more intravascular platelets than do children not infected with HIV. (c) Comparison of the proportions of blood vessel surface areas with neutrophil elastase staining. Values are shown as medians with interquartile ranges. *, P < 0.0001 by Mann-Whitney U test. Autopsy-confirmed CM cases (CM1 and CM2) have low levels of intravascular neutrophils, with no difference based on HIV status. HIV-uninfected CM3 cases have more intravascular neutrophils than do other groups, possibly because three patients had bacterial infection with meningeal involvement or meningoencephalitis diagnosed at autopsy.
Because malaria affects neutrophil function (44), we looked for evidence of neutrophil recruitment to the vasculature during CM. Immunohistochemistry analysis for neutrophil elastase (NE; a serine protease secreted by neutrophils during inflammation) identified scattered intravascular neutrophils and areas of perivascular degranulation (Fig. 3a, bottom). There was no evidence of small-caliber vessels filled with neutrophils or of neutrophils adherent to the walls of larger vessels, as was seen with monocytes. Quantification of intravascular NE staining using ImageJ software found no differences in intravascular neutrophils based on HIV serostatus or pathology pattern in children with autopsy-confirmed CM (Fig. 3c). HIV-uninfected children with the CM3 pattern had significantly more intravascular neutrophils than did any other group. Three of these children had pathological evidence of meningitis or meningoencephalitis, processes that lead to recruitment and accumulation of neutrophils in the brain.
We detected no HIV-1 p24 with immunohistochemistry analysis of brain sections in any of the 30 autopsy subjects, regardless of HIV serostatus (data not shown), consistent with earlier brain pathology studies of HIV+ children (45). HIV-1 p24 was detected in positive controls (tonsil and brain tissue from U.S. adults with HIV encephalitis and fixed HIV-infected microglia from in vitro culture).
Fatal CM was associated with high parasite burdens that were greater in HIV-infected subjects.
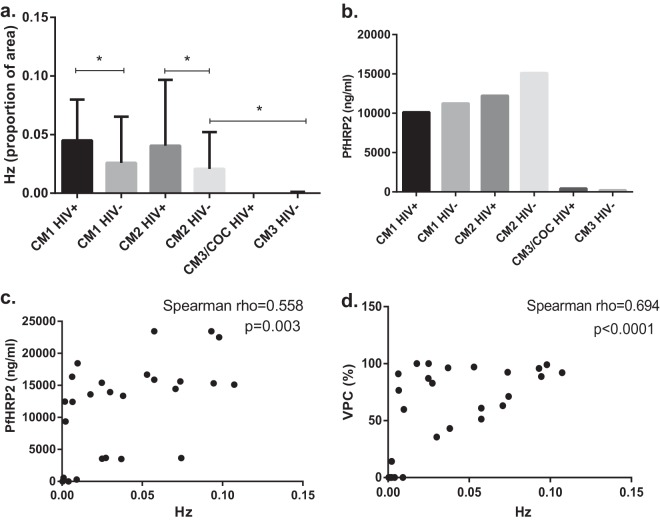
Peripheral parasitemia was not predictive of CM (Table 1), but differences in brain parasite burden were seen in the proportion of blood vessel surface area covered by hemozoin (Fig. 4a). Autopsy-confirmed CM cases had significantly more brain intravascular hemozoin than did cases with another cause of death (CM3 and obvious nonmalarial cause of coma), and among autopsy-confirmed CM cases, HIV+ children had 1.8 times more intravascular hemozoin than did HIV-uninfected children. Intravascular monocytes and intravascular platelets correlated with hemozoin measurements (Spearman rho = 0.8274, P < 0.0001, for monocytes; Spearman rho = 0.6793, P < 0.0001, for platelets).
FIG 4 .
Comparison of parasite burden using intravascular hemozoin area, plasma PfHRP2, and percentage of parasitized vessels. (a) Comparison of the proportions of intravascular hemozoin surface areas. Values are shown as medians with interquartile ranges. COC, cases with an obvious nonmalarial cause of coma. *, P < 0.0001 by Mann-Whitney U test. Autopsy-confirmed CM cases (CM1 and CM2) have more intravascular hemozoin than do cases with coma of other causes. Among cases of autopsy-confirmed CM, HIV+ children have more intravascular hemozoin than do children not infected with HIV. (b) Comparison of plasma PfHRP2 levels. Plasma PfHRP2 levels were measured by enzyme-linked immunosorbent assay from archived plasma samples. Values are shown as geometric means. Autopsy-confirmed CM cases (CM1 and CM2) had higher PfHRP2 levels in plasma than did parasitemic cases with coma of other causes (CM3 and COC; P < 0.02, 1-way analysis of variance). No difference was detected based on HIV serostatus. Twenty-six children had PfHRP2 levels measured. Only one of the HIV+ CM3/COC children had parasitemia and PfHRP2 testing, and so this group was excluded from statistical analysis. (c) Spearman correlation of plasma PfHRP2 with hemozoin surface area measurements. PfHRP2 plasma levels (n = 26) positively correlate with hemozoin measurements from brain pathology (Spearman rho = 0.558, P = 0.003). (d) Spearman correlation of brain blood vessels parasitized (VPC) with hemozoin surface area measurements. Percent counts of parasitized vessels positively correlate with hemozoin measurements from brain pathology (Spearman rho = 0.694, P < 0.0001).
P. falciparum histidine-rich protein 2 (PfHRP2) is used as an antigen in malaria rapid diagnostic tests. More PfHRP2 is released as the parasite matures within the erythrocyte, with the largest amount being released at schizont rupture, when parasites are sequestered in deep tissues such as the brain (46). Quantification of PfHRP2 can help to distinguish uncomplicated malaria from severe malaria (47) and may act as a biomarker for sequestered parasite burden. We compared plasma PfHRP2 levels in the autopsy study patients with immunohistochemistry results analyzed here, excluding the HIV+ CM3/nonmalarial-cause-of-coma group because only 1 out of 5 children had parasitemia and PfHRP2 testing. The HIV-uninfected CM3 group had significantly less PfHRP2 than did children with autopsy-confirmed CM (Fig. 4b). Similar differences were noted between children with and without malarial retinopathy in the larger cohort (48). There was no difference in PfHRP2 levels between CM1 and CM2 groups regardless of HIV status. Brain intravascular hemozoin measurements using ImageJ correlated with plasma PfHRP2 levels (Fig. 4c) and with the percentage of blood vessels containing parasites (Fig. 4d). Neither PfHRP2, hemozoin, nor the percentage of vessels parasitized correlated with fever or coma duration.
DISCUSSION
We have identified a unique and pervasive pathology pattern in pediatric CM, marked by intravascular monocytes and platelets, which is more severe in HIV+ children. This has not been previously described in adult CM studies (49) but has been noted in the rodent experimental CM (ECM) model (50). We previously showed that children with fatal CM have more brain intravascular platelets than do children with fatal SMA or nonmalarial cause of coma (51). Intravascular monocytes have also been noted in studies of CM (28, 52), but they have never been quantified or correlated with intravascular platelets. ECM is not universally accepted as a model for human CM (53), in part because sequestered parasitized erythrocytes are not as prominent as in human CM, but there is growing evidence that infected erythrocytes do become sequestered and are necessary for ECM to develop (54–57).
Understanding how HIV exacerbates CM may provide insight into the pathological processes underlying CM in all children. HIV infection is associated with platelet activation and the formation of monocyte-platelet complexes, and these complexes are more adherent to the vascular endothelium than are monocytes alone (36). In our histologic analysis, monocytes appear to actively migrate to sites of malaria sequestration and accumulate in brain microvasculature with infected erythrocytes. HIV infection may further enhance this phenomenon, through production of chemoattractants such as CCL2 in the brain, increased responsiveness to chemoattractants in HIV-infected monocytes, and enhanced binding of monocytes to the endothelial surface (34, 35).
Monocytes and platelets contribute to other severe malaria syndromes, including placental malaria (33, 58), a syndrome more common in HIV+ mothers (12, 33). Circulating hemozoin-containing monocytes are associated with malarial anemia in HIV+ children (59). Platelets can act as a bridge between parasitized red blood cells and brain endothelial cells in vitro by upregulating endothelial CD36 (60), a receptor that is also involved in the in vitro clumping of platelets and parasitized red blood cells from adults and children with severe malaria (61, 62).
Severe malaria, particularly CM, is associated with higher parasite burdens measured by plasma PfHRP2 than are found in uncomplicated malaria (63). We found higher parasite burdens, measured by surface area of intravascular hemozoin, in autopsy-confirmed CM cases than in parasitemic children with nonmalarial cause of coma, and also in HIV+ than in HIV-uninfected children with fatal CM. Similar differences were noted using plasma PfHRP2 measurements in a study of HIV and severe malaria in Mozambique (64). The correlation between brain intravascular hemozoin, circulating PfHRP2, and percentage of vessels parasitized reinforces the utility of PfHRP2 quantification as a biomarker for total parasite burden.
Based on population estimates of pediatric HIV prevalence in Malawi, HIV+ Malawian children are at increased risk for developing CM. Risk of symptomatic malaria has been linked to low CD4+ T lymphocyte counts (65), but in our study, the increased risk of CM was not due to severe immunosuppression as seen in advanced AIDS. HIV infection may impair clearance of malaria parasites by monocytes and macrophages independent of T cells, resulting in an increased parasite burden and therefore a greater likelihood of developing CM.
Within the autopsy series, HIV+ children were significantly older than HIV-uninfected children. This age disparity was previously noted in the larger clinicopathological study (16) and specifically in children with malarial retinopathy (E. Mbale, C. A. Moxon, M. E. Molyneux, and T. E. Taylor, unpublished data), as well as in children with severe malaria in Mozambique (64) and Kenya (66). Untreated perinatally acquired HIV confers high mortality during infancy (41), and thus, these older HIV+ children presenting with CM are drawn from a survivor minority. Although clinical parameters did not indicate severe immune suppression, some had pathological evidence of immune dysregulation; eight of 14 (57%) HIV+ children with CM had lung pathology with LIP, a lymphoproliferation associated with slower progression of HIV disease in perinatally infected children (67). Children with clinically symptomatic LIP have greater immune dysregulation than do age-matched HIV+ children without LIP (68).
Older age at presentation with severe malaria among HIV+ children may be due to loss of acquired malarial immunity as HIV progressively weakens the immune system; alternatively, effective antibodies to variant antigens may not develop because of B cell or memory cell dysfunction. These children may represent a pediatric population similar to adults with slowly progressive HIV disease, who often have evidence of ongoing chronic inflammation (37, 69). Future studies evaluating malaria antibody response in HIV+ adults and perinatally infected or exposed children may identify high-risk groups who would benefit from targeted malaria chemoprophylaxis.
While ART likely improves B cell immunity, lingering immune dysfunction associated with monocyte and endothelial activation persists in HIV+ individuals even with viral suppression. Therefore, the optimal management of HIV+ children at risk for malaria remains unknown, particularly since widespread P. falciparum resistance may limit the antimalarial efficacy of trimethoprim-sulfamethoxazole, recommended by the WHO for prophylaxis in all HIV+ adults and children (70). Although further study is needed, in recent malaria seasons, increasing numbers of HIV+ children with CM receiving ART have been admitted to the Paediatric Research Ward while the HIV prevalence of hospitalized children with CM has not changed, suggesting that ART does not reduce the risk of severe malaria (K. B. Seydel and T. E. Taylor, unpublished data).
Previous studies have demonstrated that the loss of the endothelial protein C receptor (EPCR) is associated with foci of localized microvascular coagulation in CM (71), and EPCR has now been identified as the major endothelial receptor for Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) subtypes linked to severe malaria, including CM (72). Taken together, we can postulate a model in which infected erythrocytes displaying virulent PfEMP1 subtypes adhere to endothelial cells, resulting in activation of endothelium with conversion of the endothelium from an anticoagulant to procoagulant state. In many pathological states, including HIV infection, endothelial dysfunction is associated with platelet activation, adhesion to endothelium, clumping, and complex formation with monocytes (56). Both endothelial activation and platelet activation upregulate expression of endothelial adhesion molecules and release of inflammatory cytokines and chemokines (56). We have reported that brain edema is associated with death in CM (73), but the mechanism for these clinical findings is not yet understood. HIV infection likely exacerbates endothelial dysfunction in response to parasitized erythrocytes.
An inherent limitation of any autopsy study and most human clinical studies is that while they can identify correlations suggestive of causality, they cannot be used to deduce causal relationships. We describe a significant association of intravascular monocytes and platelets (and sequestered parasites) with pediatric CM. It is notable that our human histopathology findings support some observations reported in ECM, a rodent model whose relevance to human pathophysiology is uncertain (53). If ECM studies are combined with human blood-brain barrier in vitro studies utilizing parasitized erythrocytes, peripheral blood mononuclear cells, and platelets (68), it may be possible to identify mechanisms by which monocytes and platelets contribute to CM pathogenesis.
We now show that fatal pediatric CM is associated with intravascular accumulation of infected erythrocytes, monocytes, and platelets that is qualitatively similar, but more pronounced, in those children with CM who are HIV+. Further investigation of how HIV infection affects the microvasculature of children with CM may illuminate how brain microvascular coagulation and inflammation contribute to brain edema and pediatric CM pathogenesis.
MATERIALS AND METHODS
Clinicopathological study and consent.
The institutional review boards (IRBs) of the University of Malawi College of Medicine, the Albert Einstein College of Medicine, Michigan State University, and the Brigham and Women’s Hospital approved all aspects of this study, including informed written consent from parents/guardians.
Children aged 6 months to 12 years presenting to Queen Elizabeth Central Hospital (QECH) in Blantyre, Malawi, who met the CM clinical case definition (peripheral P. falciparum parasitemia, a Blantyre coma score of ≤2 [unrousable coma], and no other identifiable cause for coma) were treated in the Paediatric Research Ward and enrolled in an ongoing observational study of malaria pathogenesis run by the Blantyre Malaria Project (BMP) and the Malawi-Liverpool-Wellcome Trust Clinical Research Programme (MLW) previously described (16, 38, 74). Blood cultures and cerebrospinal fluid cultures were routinely obtained unless contraindicated. From 1996 to 2010, a study of the clinicopathological correlates of fatal CM was pursued, and during that time, 2,464 children were evaluated and 420 died (17% mortality). One hundred three autopsies were performed, constituting the largest controlled autopsy series of CM to date. In the event of death, permission was requested from family members for autopsy, where brain and other organs were sectioned and fixed in 10% buffered formalin. We define “autopsy-confirmed CM” as fatal clinically defined CM with sequestered parasitized erythrocytes in >20% of intracerebral microvessels and no other identifiable cause of death (38).
Voluntary counseling and testing for HIV were incorporated into the study in 2001. Plasma from autopsy cases not tested prior to death and archived specimens from 1996 to 2000 were tested retrospectively with IRB approval. All patients with clinically defined CM prior to autopsy had HIV antibody testing. Six children with another cause or an indeterminate cause of death prior to autopsy were not tested for HIV. One child with autopsy-confirmed CM had detectable HIV antibody but was 6 months old with no sample available for confirmatory testing with HIV PCR and was censored. Twenty of 96 autopsy cases with definitive testing were HIV+. All 14 HIV+ cases with archived plasma had quantifiable HIV loads (Abbott m2000 system). The tissues analyzed in our study represent a subset of the autopsy series, including 12 of the 14 HIV+ children with archived plasma.
We analyzed temporal lobe brain tissue from 30 autopsy subjects: 10 patients with the CM1 pattern (P. falciparum sequestration with no parenchymal changes), 10 patients with the CM2 pattern (P. falciparum sequestration with parenchymal changes, including hemorrhage), and 10 patients with either the CM3 pattern (those who met the clinical case definition of CM but for whom a nonmalarial cause of death was determined from autopsy) or a nonmalarial cause of coma prior to death. Five from each group were HIV+, determined by antibody-based test. These cases were selected by D.A.M., who was not involved in immunohistochemistry analysis, and matched by histopathology pattern and degree of parasite sequestration (defined by percentage of parasitized vessels and described in detail previously) (75). There were only five HIV-uninfected CM1 cases and five HIV+ patients with a nonmalarial cause of death in the entire autopsy series. This HIV+ group comprised one HIV+ CM3 patient plus four HIV+ patients with a nonmalarial cause of death. The HIV-uninfected subjects with nonmalarial causes of death were all CM3. These patients were chosen so that peripheral parasitemia was comparable to that in HIV-uninfected CM groups. The investigators who performed immunohistochemistry and microscopic analysis (S.E.H., T.F.M., N.C., and S.L.) were blinded to all patient-related information, including histopathology pattern, HIV antibody status, and the criteria used to pick cases.
A retrospective chart review was performed to identify clinical characteristics and history used as criteria for WHO Clinical Staging of HIV/AIDS in Infants and Children guidelines. Children were classified as stage 1/2 (mild disease) or stage 3/4 (if they met criteria for severe disease).
Pathological analysis of lung tissue.
Lungs from 101 of 103 autopsies were previously examined (76). Lung histology was graded on a scale of 0 to 3 by a single pathologist (R.O.W.) who was blinded to final anatomic diagnosis and HIV status.
Immunohistochemistry analysis of temporal lobe tissue.
Five-micrometer sections of fixed, paraffin-embedded temporal lobe tissue from 30 subjects who underwent autopsy were evaluated. Tissue slides were labeled for HIV-1 p24, a capsid protein expressed during HIV replication; ionized calcium binding adapter molecule 1 (Iba1), a marker specific for the monocyte lineage that is present in microglia, macrophages, and monocytes; CD61, a platelet glycoprotein; and neutrophil elastase (NE), a serine protease in neutrophil granules that is released during neutrophil activation.
Sections were deparaffinized and rehydrated in successive xylene and alcohol solutions, boiled for antigen retrieval at 95°C for 20 min in sodium citrate buffer (Dako), treated with 3% H2O2 to block endogenous peroxidase activity, and incubated with 10% normal goat or horse serum to block nonspecific antibody binding. No antigen retrieval was performed for NE.
Immunohistochemistry labeling.
Primary antibodies were (i) mouse anti-HIV-1 p24 (IgG1; Dako), 1:10 concentration, 4-h incubation; (ii) rabbit anti-Iba1 (polyclonal; Wako), 1:250 concentration, 1-h incubation; (iii) mouse anti-CD61 IOPath (IgG1; clone SZ21; Beckman Coulter) undiluted, 2-h incubation; and (iv) mouse anti-neutrophil elastase (NE; IgG1; clone NP57; Dako), 1:100 dilution, 2-h incubation. p24 and Iba1 antibody labeling was followed by incubation with an avidin-biotin complex method kit (ABC Vectastain kit; Vector Labs) according to the manufacturer’s instructions. CD61 and NE primary antibody labeling was followed by incubation with anti-mouse micropolymer-linked secondary antibody (ImmPRESS kit; Vector Labs) according to the manufacturer’s instructions. Color was developed with diaminobenzidine (DAB), with hematoxylin counterstaining. Positive controls for p24 and Iba1 included paraffin-embedded brain tissue with a neuropathologic diagnosis of HIV encephalitis, paraffin-embedded tonsil from HIV+ donors, and fixed HIV-infected microglia from in vitro culture. Sections incubated with an irrelevant mouse IgG1 antibody were used as negative controls for p24, CD61, and NE. Sections incubated with an irrelevant polyclonal rabbit antibody were used as negative controls for Iba1.
Quantification of intravascular monocytes, platelets, and neutrophils.
Fifty to 100 high-power fields (hpf; ×400) from each slide were digitally photographed. ImageJ software was used to outline and measure the total blood vessel area, the area of intravascular Iba1 staining, and the area of intravascular hemozoin in 50 vessels from 50 hpf by setting threshold values for DAB and hemozoin intensity. No distinction was made between free hemozoin, intraerythrocytic hemozoin, and hemozoin within monocytes. Measurements were conducted in duplicate by two independent, blinded observers (S.E.H. and T.F.M.). Statistical analysis for each observer was performed for each series with similar results, and there was significant statistical correlation between individual patient values for each observer (see below). Results from one observer are shown in the figures.
PfHRP2 measurement.
Enzyme-linked immunosorbent assay was performed on archived frozen plasma, using plates precoated with anti-PfHRP2 antibody (Cellabs, Brookvale, Australia). Plasma was thawed, diluted 1:500, and plated in duplicate along with a stock solution of recombinant PfHRP2. The manufacturer’s protocol was followed with the exception of incubation steps, which were carried out at 37°C in a humidified chamber. After sample incubation, secondary conjugated antibody incubation, and addition of substrate, plates were analyzed at an optical density at 450 nm (OD450). A standard curve was generated from wells containing recombinant PfHRP2, and readings from diluted plasma samples were compared to this curve to calculate PfHRP2 levels. Twenty-six out of 30 children in this autopsy series had parasitemia and PfHRP2 testing.
Statistical analyses.
Clinical data were analyzed using the Kruskal-Wallis one-way analysis of variance and Mann-Whitney U test or unpaired t test with Welch’s correction for continuous variables and the chi-square test or Fisher’s exact test for dichotomous variables. Quantification of intravascular hemozoin, monocytes, neutrophils, and platelets was analyzed by Kruskal-Wallis one-way analysis of variance and by Mann-Whitney U test. All applicable P values are two-tailed. Correlation of ImageJ measurements between observers was analyzed using Lin’s concordance correlation coefficient (77). There was significant correlation of Iba1 measurements (rho = 0.727, P < 0.001), CD61 measurements (rho = 0.859, P < 0.001), and NE measurements (rho = 0.652, P < 0.001) between observers. Analysis was performed using GraphPad Prism version 6 and Stata version 12.0.
SUPPLEMENTAL MATERIAL
Flow chart of children enrolled in the BMP cohort study. HIV+ mortality rate was 23.3% (68/292), compared to 17.5% (300/1716) in HIV-uninfected children (P < 0.02, chi-square test). One child was HIV seropositive but under the age of 1 year and died before HIV infection could be confirmed by DNA PCR. Download
Flow chart of all children undergoing autopsy as part of the clinicopathological study. The subset of 30 patients characterized in this paper was drawn from this autopsy cohort. HIV testing was performed for all participants with clinically defined CM but not for 6 children with nonmalarial coma or indeterminate cause of death. One child with the CM1 pathology pattern was HIV seropositive but under the age of 1 year and died before HIV infection could be confirmed by DNA PCR. Data from this child were censored. Download
Dot plot graphs demonstrating values for age, peripheral platelet count, and lymphocyte count for the 30 children with brain tissue characterized in this paper. Horizontal bars denote mean values. COC, cases with nonmalarial cause of coma. (A) Age distribution. Among children with autopsy-confirmed CM, HIV+ children were older than HIV-uninfected children (unpaired t test for CM1 comparison and unpaired t test with Welch’s correction for CM2 comparison). (B) Distribution of peripheral platelet counts. While thrombocytopenia is common in autopsy-confirmed CM groups, HIV+ children had greater peripheral platelet counts than did HIV-uninfected children with autopsy-confirmed CM (unpaired t test with Welch’s correction). (C) Distribution of peripheral lymphocyte counts. Peripheral lymphocyte levels, a surrogate for immune function under older WHO HIV staging criteria, were not significantly different among groups. CD4+ T lymphocytes were not quantified. Download
Images of blood vessels from hematoxylin-and-eosin-stained temporal lobe sections from children with fatal cerebral malaria. (A) A vessel in cross section from an HIV-uninfected child with the CM1 pathology pattern. Infected red blood cells (iRBCs), monocytes, and the parasite pigment hemozoin (both within monocytes and within iRBCs) are identified. (B) A vessel in cross section from an HIV+ child with the CM1 pathology pattern. iRBCs, a monocyte containing hemozoin, and intraerythrocytic hemozoin are demonstrated. Download
Clinical features of the 30 children with brain tissue analyzed in this paper. Continuous variables are shown as medians, with interquartile ranges in parentheses, except for parasitemia and platelet counts, values for which are geometric means with 25th and 75th percentiles. Differences between groups were analyzed by 1-way analysis of variance or Kruskal-Wallis test for continuous variables and by chi-square test for dichotomous variables. Duration of illness was determined by combining the duration of fever with duration of coma. All analyses included data from 30 patients except where indicated below. COC, cases with an obvious nonmalarial cause of coma. *, excludes HIV+ CM3/COC; 4 of these patients had no malaria parasitemia; **, P = nonsignificant (NS) when CM3/COC data are excluded. Retinopathy status determined: n = 24 (n = 4 for CM1 HIV+, CM1 HIV−, CM2 HIV+, CM2 HIV−, and CM3/COC HIV+; n = 3 for CM3 HIV−). White blood cell count: n = 27 (n = 4 for CM2 HIV−, n = 3 for CM3/COC HIV+). Lymphocyte count: n = 21 (n = 4 for CM1 HIV+, CM1 HIV−, CM3/COC HIV+, and CM3 HIV−; n = 3 for CM2 HIV+; n = 2 for CM2 HIV−). Monocyte count: n = 17 (n = 4 for CM3 HIV−; n = 3 for CM1 HIV+, CM1 HIV−, and CM2 HIV+; n = 2 for CM2 HIV− and CM3/COC HIV+). Platelet count: n = 27 (n = 4 for CM2 HIV−; n = 3 for CM3/COC HIV+). Duration of coma: n = 26 (n = 4 for CM1 HIV+ and CM2 HIV+; n = 3 for CM3 HIV−). Duration of illness: n = 28 (n = 4 for CM1 HIV+ and CM3 HIV−).
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants K08MH089848 (S.E.H.), T32 AI 070117 awarded to the Albert Einstein College of Medicine (S.E.H.), and 2 R01 AI034969 (T.E.T. and K.B.S.); the Wellcome Trust United Kingdom (M.E.M.); a Burke Global Health Scholars award (D.A.M.); pilot awards from the Einstein-Montefiore Center for AIDS Research (K.K. and S.E.H.), which is funded by the National Institutes of Health (NIH AI051519); and microgrants from the Einstein Global Health Center (K.K. and T.F.M.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We extend special thanks to the patients and families of the Paediatric Research Ward who made this research possible. We thank Moonseong Heo, an Einstein-Montefiore Center for AIDS Research biostatistician, for review of the statistical analysis, and Anne Kessler for assistance with statistical tests. We thank Joan Berman for ongoing scientific discussion and David Sullivan for the gift of recombinant PfHRP2.
Footnotes
Citation Hochman SE, Madaline TF, Wassmer SC, Mbale E, Choi N, Seydel KB, Whitten RO, Varughese J, Grau GER, Kamiza S, Molyneux ME, Taylor TE, Lee S, Milner DA, Jr, Kim K. 2015. Fatal pediatric cerebral malaria is associated with intravascular monocytes and platelets that are increased with HIV coinfection. mBio 6(5):e01390-15. doi:10.1128/mBio.01390-15.
REFERENCES
- 1.WHO 2014. World malaria report 2014. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.UNIGME 2013. Levels and trends in child mortality. United Nations Children’s Fund, New York, NY. [Google Scholar]
- 3.WHO 2013. World malaria report 2013. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 4.UNAIDS 2013. Global report: UNAIDS report on the global AIDS epidemic 2013. UNAIDS, Geneva, Switzerland. [Google Scholar]
- 5.Taha TE, Canner JK, Dallabetta GA, Chiphangwi JD, Liomba G, Wangel AM, Saah AJ, Miotti PG. 1994. Childhood malaria parasitaemia and human immunodeficiency virus infection in Malawi. Trans R Soc Trop Med Hyg 88:164–165. doi: 10.1016/0035-9203(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg AE, Nsa W, Ryder RW, Medi M, Nzeza M, Kitadi N, Baangi M, Malanda N, Davachi F, Hassig SE. 1991. Plasmodium falciparum malaria and perinatally acquired human immunodeficiency virus type 1 infection in Kinshasa, Zaire. A prospective, longitudinal cohort study of 587 children. N Engl J Med 325:105–109. doi: 10.1056/NEJM199107113250206. [DOI] [PubMed] [Google Scholar]
- 7.Colebunders R, Bahwe Y, Nekwei W, Ryder R, Perriens J, Nsimba K, Turner A, Francis H, Lebughe I, Van der Stuyft P, Piot P. 1990. Incidence of malaria and efficacy of oral quinine in patients recently infected with human immunodeficiency virus in Kinshasa, Zaire. J Infect 21:167–173. doi: 10.1016/0163-4453(90)91701-E. [DOI] [PubMed] [Google Scholar]
- 8.Leaver RJ, Haile Z, Watters DA. 1990. HIV and cerebral malaria. Trans R Soc Trop Med Hyg 84:201. doi: 10.1016/0035-9203(90)90253-B. [DOI] [PubMed] [Google Scholar]
- 9.Steketee RW, Wirima JJ, Bloland PB, Chilima B, Mermin JH, Chitsulo L, Breman JG. 1996. Impairment of a pregnant woman’s acquired ability to limit Plasmodium falciparum by infection with human immunodeficiency virus type-1. Am J Trop Med Hyg 55:42–49. [DOI] [PubMed] [Google Scholar]
- 10.Verhoeff FH, Brabin BJ, Hart CA, Chimsuku L, Kazembe P, Broadhead RL. 1999. Increased prevalence of malaria in HIV-infected pregnant women and its implications for malaria control. Trop Med Int Health 4:5–12. doi: 10.1046/j.1365-3156.1999.00349.x. [DOI] [PubMed] [Google Scholar]
- 11.Van Eijk AM, Ayisi JG, ter Kuile FO, Misore AO, Otieno JA, Rosen DH, Kager PA, Steketee RW, Nahlen BL. 2003. HIV increases the risk of malaria in women of all gravidities in Kisumu, Kenya. AIDS 17:595–603. doi: 10.1097/01.aids.0000042975.95433.a5. [DOI] [PubMed] [Google Scholar]
- 12.Perrault SD, Hajek J, Zhong K, Owino SO, Sichangi M, Smith G, Shi YP, Moore JM, Kain KC. 2009. Human immunodeficiency virus co-infection increases placental parasite density and transplacental malaria transmission in Western Kenya. Am J Trop Med Hyg 80:119–125. [PMC free article] [PubMed] [Google Scholar]
- 13.Chalwe V, Van Geertruyden JP, Mukwamataba D, Menten J, Kamalamba J, Mulenga M, D’Alessandro U. 2009. Increased risk for severe malaria in HIV-1-infected adults, Zambia. Emerg Infect Dis 15:749. doi: 10.3201/eid1505.081009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grimwade K, French N, Mbatha DD, Zungu DD, Dedicoat M, Gilks CF. 2004. HIV infection as a cofactor for severe falciparum malaria in adults living in a region of unstable malaria transmission in South Africa. AIDS 18:547–554. doi: 10.1097/00002030-200402200-00023. [DOI] [PubMed] [Google Scholar]
- 15.Kamya MR, Gasasira AF, Yeka A, Bakyaita N, Nsobya SL, Francis D, Rosenthal PJ, Dorsey G, Havlir D. 2006. Effect of HIV-1 infection on antimalarial treatment outcomes in Uganda: a population-based study. J Infect Dis 193:9–15. doi: 10.1086/498577. [DOI] [PubMed] [Google Scholar]
- 16.Bronzan RN, Taylor TE, Mwenechanya J, Tembo M, Kayira K, Bwanaisa L, Njobvu A, Kondowe W, Chalira C, Walsh AL, Phiri A, Wilson LK, Molyneux ME, Graham SM. 2007. Bacteremia in Malawian children with severe malaria: prevalence, etiology, HIV coinfection, and outcome. J Infect Dis 195:895–904. doi: 10.1086/511437. [DOI] [PubMed] [Google Scholar]
- 17.Grimwade K, French N, Mbatha DD, Zungu DD, Dedicoat M, Gilks CF. 2003. Childhood malaria in a region of unstable transmission and high human immunodeficiency virus prevalence. Pediatr Infect Dis J 22:1057–1063. doi: 10.1097/01.inf.0000101188.95433.60. [DOI] [PubMed] [Google Scholar]
- 18.Moxon CA, Chisala NV, Wassmer SC, Taylor TE, Seydel KB, Molyneux ME, Faragher B, Kennedy N, Toh CH, Craig AG, Heyderman RS. 2014. Persistent endothelial activation and inflammation after Plasmodium falciparum infection in Malawian children. J Infect Dis 209:610–615. doi: 10.1093/infdis/jit419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.French N, Nakiyingi J, Lugada E, Watera C, Whitworth JA, Gilks CF. 2001. Increasing rates of malarial fever with deteriorating immune status in HIV-1-infected Ugandan adults. AIDS 15:899–906. doi: 10.1097/00002030-200105040-00010. [DOI] [PubMed] [Google Scholar]
- 20.Cohen C, Karstaedt A, Frean J, Thomas J, Govender N, Prentice E, Dini L, Galpin J, Crewe-Brown H. 2005. Increased prevalence of severe malaria in HIV-infected adults in South Africa. Clin Infect Dis 41:1631–1637. doi: 10.1086/498023. [DOI] [PubMed] [Google Scholar]
- 21.Van Geertruyden JP, Mulenga M, Kasongo W, Polman K, Colebunders R, Kestens L, D’Alessandro U. 2006. CD4 T-cell count and HIV-1 infection in adults with uncomplicated malaria. J Acquir Immune Defic Syndr 43:363–367. doi: 10.1097/01.qai.0000243125.98024.da. [DOI] [PubMed] [Google Scholar]
- 22.WHO 2013. Management of severe malaria. A practical handbook, 3rd ed. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 23.World Health Organization 2000. Severe falciparum malaria. Trans R Soc Trop Med Hyg 94:1–90. [PubMed] [Google Scholar]
- 24.Trape JF, Rogier C. 1996. Combating malaria morbidity and mortality by reducing transmission. Parasitol Today 12:236–240. doi: 10.1016/0169-4758(96)10015-6. [DOI] [PubMed] [Google Scholar]
- 25.Molyneux ME, Taylor TE, Wirima JJ, Borgstein A. 1989. Clinical features and prognostic indicators in paediatric cerebral malaria: a study of 131 comatose Malawian children. Q J Med 71:441–459. [PubMed] [Google Scholar]
- 26.Beare NA, Taylor TE, Harding SP, Lewallen S, Molyneux ME. 2006. Malarial retinopathy: a newly established diagnostic sign in severe malaria. Am J Trop Med Hyg 75:790–797. [PMC free article] [PubMed] [Google Scholar]
- 27.Dondorp AM, Fanello CI, Hendriksen IC, Gomes E, Seni A, Chhaganlal KD, Bojang K, Olaosebikan R, Anunobi N, Maitland K, Kivaya E, Agbenyega T, Nguah SB, Evans J, Gesase S, Kahabuka C, Mtove G, Nadjm B, Deen J, Mwanga-Amumpaire J, Nansumba M, Karema C, Umulisa N, Uwimana A, Mokuolu OA, Adedoyin OT, Johnson WB, Tshefu AK, Onyamboko MA, Sakulthaew T, Ngum WP, Silamut K, Stepniewska K, Woodrow CJ, Bethell D, Wills B, Oneko M, Peto TE, von Seidlein L, Day NP, White NJ, AQUAMAT Group . 2010. Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an open-label, randomised trial. Lancet 376:1647–1657. doi: 10.1016/S0140-6736(10)61924-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dorovini-Zis K, Schmidt K, Huynh H, Fu W, Whitten RO, Milner D, Kamiza S, Molyneux M, Taylor TE. 2011. The neuropathology of fatal cerebral malaria in Malawian children. Am J Pathol 178:2146–2158. doi: 10.1016/j.ajpath.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spitz S. 1946. The pathology of acute falciparum malaria. Mil Surg 99:555–572. [PubMed] [Google Scholar]
- 30.Ponsford MJ, Medana IM, Prapansilp P, Hien TT, Lee SJ, Dondorp AM, Esiri MM, Day NP, White NJ, Turner GD. 2012. Sequestration and microvascular congestion are associated with coma in human cerebral malaria. J Infect Dis 205:663–671. doi: 10.1093/infdis/jir812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Combes V, Taylor TE, Juhan-Vague I, Mège JL, Mwenechanya J, Tembo M, Grau GE, Molyneux ME. 2004. Circulating endothelial microparticles in Malawian children with severe falciparum malaria complicated with coma. JAMA 291:2542–2544. doi: 10.1001/jama.291.21.2542-b. [DOI] [PubMed] [Google Scholar]
- 32.Srivastava K, Field DJ, Aggrey A, Yamakuchi M, Morrell CN. 2010. Platelet factor 4 regulation of monocyte KLF4 in experimental cerebral malaria. PLoS One 5:e10413. doi: 10.1371/journal.pone.0010413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rogerson SJ, Pollina E, Getachew A, Tadesse E, Lema VM, Molyneux ME. 2003. Placental monocyte infiltrates in response to Plasmodium falciparum malaria infection and their association with adverse pregnancy outcomes. Am J Trop Med Hyg 68:115–119. [PubMed] [Google Scholar]
- 34.Williams DW, Calderon TM, Lopez L, Carvallo-Torres L, Gaskill PJ, Eugenin EA, Morgello S, Berman JW. 2013. Mechanisms of HIV entry into the CNS: increased sensitivity of HIV infected CD14+CD16+ monocytes to CCL2 and key roles of CCR2, JAM-A, and ALCAM in diapedesis. PLoS One 8:e69270. doi: 10.1371/journal.pone.0069270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams DW, Eugenin EA, Calderon TM, Berman JW. 2012. Monocyte maturation, HIV susceptibility, and transmigration across the blood brain barrier are critical in HIV neuropathogenesis. J Leukoc Biol 91:401–415. doi: 10.1189/jlb.0811394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh MV, Davidson DC, Kiebala M, Maggirwar SB. 2012. Detection of circulating platelet-monocyte complexes in persons infected with human immunodeficiency virus type-1. J Virol Methods 181:170–176. doi: 10.1016/j.jviromet.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson EM, Sereti I. 2013. Immune restoration after antiretroviral therapy: the pitfalls of hasty or incomplete repairs. Immunol Rev 254:343–354. doi: 10.1111/imr.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor TE, Fu WJ, Carr RA, Whitten RO, Mueller JS, Fosiko NG, Lewallen S, Liomba NG, Molyneux ME, Mueller JG. 2004. Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat Med 10:143–145. doi: 10.1038/nm986. [DOI] [PubMed] [Google Scholar]
- 39.Maccormick IJ, Beare NA, Taylor TE, Barrera V, White VA, Hiscott P, Molyneux ME, Dhillon B, Harding SP. 2014. Cerebral malaria in children: using the retina to study the brain. Brain 137:2119–2142. doi: 10.1093/brain/awu001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reyburn H, Mbatia R, Drakeley C, Bruce J, Carneiro I, Olomi R, Cox J, Nkya WM, Lemnge M, Greenwood BM, Riley EM. 2005. Association of transmission intensity and age with clinical manifestations and case fatality of severe Plasmodium falciparum malaria. JAMA 293:1461–1470. doi: 10.1001/jama.293.12.1461. [DOI] [PubMed] [Google Scholar]
- 41.Taha TE, Graham SM, Kumwenda NI, Broadhead RL, Hoover DR, Markakis D, van der Hoeven L, Liomba GN, Chiphangwi JD, Miotti PG. 2000. Morbidity among human immunodeficiency virus-1-infected and -uninfected African children. Pediatrics 106:E77. doi: 10.1542/peds.106.6.e77. [DOI] [PubMed] [Google Scholar]
- 42.Ahmed Z, Shaw G, Sharma VP, Yang C, McGowan E, Dickson DW. 2007. Actin-binding proteins coronin-1a and IBA-1 are effective microglial markers for immunohistochemistry. J Histochem Cytochem 55:687–700. doi: 10.1369/jhc.6A7156.2007. [DOI] [PubMed] [Google Scholar]
- 43.Warimwe GM, Murungi LM, Kamuyu G, Nyangweso GM, Wambua J, Naranbhai V, Fletcher HA, Hill AV, Bejon P, Osier FH, Marsh K. 2013. The ratio of monocytes to lymphocytes in peripheral blood correlates with increased susceptibility to clinical malaria in Kenyan children. PLoS One 8:e57320. doi: 10.1371/journal.pone.0057320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cunnington AJ, Njie M, Correa S, Takem EN, Riley EM, Walther M. 2012. Prolonged neutrophil dysfunction after Plasmodium falciparum malaria is related to hemolysis and heme oxygenase-1 induction. J Immunol 189:5336–5346. doi: 10.4049/jimmunol.1201028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kure K, Llena JF, Lyman WD, Soeiro R, Weidenheim KM, Hirano A, Dickson DW. 1991. Human immunodeficiency virus-1 infection of the nervous system: an autopsy study of 268 adult, pediatric, and fetal brains. Hum Pathol 22:700–710. doi: 10.1016/0046-8177(91)90293-X. [DOI] [PubMed] [Google Scholar]
- 46.Desakorn V, Silamut K, Angus B, Sahassananda D, Chotivanich K, Suntharasamai P, Simpson J, White NJ. 1997. Semi-quantitative measurement of Plasmodium falciparum antigen PfHRP2 in blood and plasma. Trans R Soc Trop Med Hyg 91:479–483. doi: 10.1016/S0035-9203(97)90292-3. [DOI] [PubMed] [Google Scholar]
- 47.Fox LL, Taylor TE, Pensulo P, Liomba A, Mpakiza A, Varela A, Glover SJ, Reeves MJ, Seydel KB. 2013. Histidine-rich protein 2 plasma levels predict progression to cerebral malaria in Malawian children with Plasmodium falciparum infection. J Infect Dis 208:500–503. doi: 10.1093/infdis/jit176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seydel KB, Fox LL, Glover SJ, Reeves MJ, Pensulo P, Muiruri A, Mpakiza A, Molyneux ME, Taylor TE. 2012. Plasma concentrations of parasite histidine-rich protein 2 distinguish between retinopathy-positive and retinopathy-negative cerebral malaria in Malawian children. J Infect Dis 206:309–318. doi: 10.1093/infdis/jis371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pongponratn E, Turner GD, Day NP, Phu NH, Simpson JA, Stepniewska K, Mai NT, Viriyavejakul P, Looareesuwan S, Hien TT, Ferguson DJ, White NJ. 2003. An ultrastructural study of the brain in fatal Plasmodium falciparum malaria. Am J Trop Med Hyg 69:345–359. [PubMed] [Google Scholar]
- 50.Piguet PF, Da Laperrousaz C, Vesin C, Tacchini-Cottier F, Senaldi G, Grau GE. 2000. Delayed mortality and attenuated thrombocytopenia associated with severe malaria in urokinase- and urokinase receptor-deficient mice. Infect Immun 68:3822–3829. doi: 10.1128/IAI.68.7.3822-3829.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grau GE, Mackenzie CD, Carr RA, Redard M, Pizzolato G, Allasia C, Cataldo C, Taylor TE, Molyneux ME. 2003. Platelet accumulation in brain microvessels in fatal pediatric cerebral malaria. J Infect Dis 187:461–466. doi: 10.1086/367960. [DOI] [PubMed] [Google Scholar]
- 52.Patnaik JK, Das BS, Mishra SK, Mohanty S, Satpathy SK, Mohanty D. 1994. Vascular clogging, mononuclear cell margination, and enhanced vascular permeability in the pathogenesis of human cerebral malaria. Am J Trop Med Hyg 51:642–647. [PubMed] [Google Scholar]
- 53.White NJ, Turner GD, Medana IM, Dondorp AM, Day NP. 2010. The murine cerebral malaria phenomenon. Trends Parasitol 26:11–15. doi: 10.1016/j.pt.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baptista FG, Pamplona A, Pena AC, Mota MM, Pied S, Vigário AM. 2010. Accumulation of Plasmodium berghei-infected red blood cells in the brain is crucial for the development of cerebral malaria in mice. Infect Immun 78:4033–4039. doi: 10.1128/IAI.00079-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Amante FH, Haque A, Stanley AC, Rivera FDL, Randall LM, Wilson YA, Yeo G, Pieper C, Crabb BS, de Koning-Ward TF, Lundie RJ, Good MF, Pinzon-Charry A, Pearson MS, Duke MG, McManus DP, Loukas A, Hill GR, Engwerda CR. 2010. Immune-mediated mechanisms of parasite tissue sequestration during experimental cerebral malaria. J Immunol 185:3632–3642. doi: 10.4049/jimmunol.1000944. [DOI] [PubMed] [Google Scholar]
- 56.Morrell CN, Aggrey AA, Chapman LM, Modjeski KL. 2014. Emerging roles for platelets as immune and inflammatory cells. Blood 123:2759–2767. doi: 10.1182/blood-2013-11-462432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wassmer SC, Combes V, Candal FJ, Juhan-Vague I, Grau GE. 2006. Platelets potentiate brain endothelial alterations induced by Plasmodium falciparum. Infect Immun 74:645–653. doi: 10.1128/IAI.74.1.645-653.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jaworowski A, Kamwendo DD, Ellery P, Sonza S, Mwapasa V, Tadesse E, Molyneux ME, Rogerson SJ, Meshnick SR, Crowe SM. 2007. CD16+ monocyte subset preferentially harbors HIV-1 and is expanded in pregnant Malawian women with Plasmodium falciparum malaria and HIV-1 infection. J Infect Dis 196:38–42. doi: 10.1086/518443. [DOI] [PubMed] [Google Scholar]
- 59.Davenport GC, Ouma C, Hittner JB, Were T, Ouma Y, Ong’echa JM, Perkins DJ. 2010. Hematological predictors of increased severe anemia in Kenyan children coinfected with Plasmodium falciparum and HIV-1. Am J Hematol 85:227–233. doi: 10.1002/ajh.21653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wassmer SC, Lépolard C, Traoré B, Pouvelle B, Gysin J, Grau GE. 2004. Platelets reorient Plasmodium falciparum-infected erythrocyte cytoadhesion to activated endothelial cells. J Infect Dis 189:180–189. doi: 10.1086/380761. [DOI] [PubMed] [Google Scholar]
- 61.Chotivanich K, Sritabal J, Udomsangpetch R, Newton P, Stepniewska KA, Ruangveerayuth R, Looareesuwan S, Roberts DJ, White NJ. 2004. Platelet-induced autoagglutination of Plasmodium falciparum-infected red blood cells and disease severity in Thailand. J Infect Dis 189:1052–1055. doi: 10.1086/381900. [DOI] [PubMed] [Google Scholar]
- 62.Pain A, Ferguson DJ, Kai O, Urban BC, Lowe B, Marsh K, Roberts DJ. 2001. Platelet-mediated clumping of Plasmodium falciparum-infected erythrocytes is a common adhesive phenotype and is associated with severe malaria. Proc Natl Acad Sci U S A 98:1805–1810. doi: 10.1073/pnas.98.4.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dondorp AM, Desakorn V, Pongtavornpinyo W, Sahassananda D, Silamut K, Chotivanich K, Newton PN, Pitisuttithum P, Smithyman AM, White NJ, Day NP. 2005. Estimation of the total parasite biomass in acute falciparum malaria from plasma PfHRP2. PLoS Med 2:e204. doi: 10.1371/journal.pmed.0020204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hendriksen IC, Ferro J, Montoya P, Chhaganlal KD, Seni A, Gomes E, Silamut K, Lee SJ, Lucas M, Chotivanich K, Fanello CI, Day NP, White NJ, von Seidlein L, Dondorp AM. 2012. Diagnosis, clinical presentation, and in-hospital mortality of severe malaria in HIV-coinfected children and adults in Mozambique. Clin Infect Dis 55:1144–1153. doi: 10.1093/cid/cis590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Patnaik P, Jere CS, Miller WC, Hoffman IF, Wirima J, Pendame R, Meshnick SR, Taylor TE, Molyneux ME, Kublin JG. 2005. Effects of HIV-1 serostatus, HIV-1 RNA concentration, and CD4 cell count on the incidence of malaria infection in a cohort of adults in rural Malawi. J Infect Dis 192:984–991. doi: 10.1086/432730. [DOI] [PubMed] [Google Scholar]
- 66.Berkley JA, Bejon P, Mwangi T, Gwer S, Maitland K, Williams TN, Mohammed S, Osier F, Kinyanjui S, Fegan G, Lowe BS, English M, Peshu N, Marsh K, Newton CR. 2009. HIV infection, malnutrition, and invasive bacterial infection among children with severe malaria. Clin Infect Dis 49:336–343. doi: 10.1086/600299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Scott GB, Hutto C, Makuch RW, Mastrucci MT, O’Connor T, Mitchell CD, Trapido EJ, Parks WP. 1989. Survival in children with perinatally acquired human immunodeficiency virus type 1 infection. N Engl J Med 321:1791–1796. doi: 10.1056/NEJM198912283212604. [DOI] [PubMed] [Google Scholar]
- 68.Tripathi AK, Sullivan DJ, Stins MF. 2007. Plasmodium falciparum-infected erythrocytes decrease the integrity of human blood-brain barrier endothelial cell monolayers. J Infect Dis 195:942–950. doi: 10.1086/512083. [DOI] [PubMed] [Google Scholar]
- 69.Krishnan S, Wilson EM, Sheikh V, Rupert A, Mendoza D, Yang J, Lempicki R, Migueles SA, Sereti I. 2014. Evidence for innate immune system activation in HIV type 1-infected elite controllers. J Infect Dis 209:931–939. doi: 10.1093/infdis/jit581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.WHO 2006. Guidelines on co-trimoxazole prophylaxis for HIV-related infections among children, adolescents and adults: recommendations for a public health approach. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 71.Moxon CA, Wassmer SC, Milner DA Jr, Chisala NV, Taylor TE, Seydel KB, Molyneux ME, Faragher B, Esmon CT, Downey C, Toh CH, Craig AG, Heyderman RS. 2013. Loss of endothelial protein C receptors links coagulation and inflammation to parasite sequestration in cerebral malaria in African children. Blood 122:842–851. doi: 10.1182/blood-2013-03-490219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Turner L, Lavstsen T, Berger SS, Wang CW, Petersen JEV, Avril M, Brazier AJ, Freeth J, Jespersen JS, Nielsen MA, Magistrado P, Lusingu J, Smith JD, Higgins MK, Theander TG. 2013. Severe malaria is associated with parasite binding to endothelial protein C receptor. Nature 498:502–505. doi: 10.1038/nature12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Seydel KB, Kampondeni SD, Valim C, Potchen MJ, Milner DA, Muwalo FW, Birbeck GL, Bradley WG, Fox LL, Glover SJ, Hammond CA, Heyderman RS, Chilingulo CA, Molyneux ME, Taylor TE. 2015. Brain swelling and death in children with cerebral malaria. N Engl J Med 372:1126–1137. doi: 10.1056/NEJMoa1400116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Milner DA Jr, Lee JJ, Frantzreb C, Whitten RO, Kamiza S, Carr RA, Pradham A, Factor RE, Playforth K, Liomba G, Dzamalala C, Seydel KB, Molyneux ME, Taylor TE. 2015. Quantitative assessment of multiorgan sequestration of parasites in fatal pediatric cerebral malaria. J Infect Dis. doi: 10.1093/infdis/jiv205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Milner DA Jr, Valim C, Carr RA, Chandak PB, Fosiko NG, Whitten R, Playforth KB, Seydel KB, Kamiza S, Molyneux ME, Taylor TE. 2013. A histological method for quantifying Plasmodium falciparum in the brain in fatal paediatric cerebral malaria. Malar J 12:191. doi: 10.1186/1475-2875-12-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Milner D Jr, Factor R, Whitten R, Carr RA, Kamiza S, Pinkus G, Molyneux M, Taylor T. 2013. Pulmonary pathology in pediatric cerebral malaria. Hum Pathol 44:2719–2726. doi: 10.1016/j.humpath.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lin LI. 1989. A concordance correlation coefficient to evaluate reproducibility. Biometrics 45:255–268. doi: 10.2307/2532051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flow chart of children enrolled in the BMP cohort study. HIV+ mortality rate was 23.3% (68/292), compared to 17.5% (300/1716) in HIV-uninfected children (P < 0.02, chi-square test). One child was HIV seropositive but under the age of 1 year and died before HIV infection could be confirmed by DNA PCR. Download
Flow chart of all children undergoing autopsy as part of the clinicopathological study. The subset of 30 patients characterized in this paper was drawn from this autopsy cohort. HIV testing was performed for all participants with clinically defined CM but not for 6 children with nonmalarial coma or indeterminate cause of death. One child with the CM1 pathology pattern was HIV seropositive but under the age of 1 year and died before HIV infection could be confirmed by DNA PCR. Data from this child were censored. Download
Dot plot graphs demonstrating values for age, peripheral platelet count, and lymphocyte count for the 30 children with brain tissue characterized in this paper. Horizontal bars denote mean values. COC, cases with nonmalarial cause of coma. (A) Age distribution. Among children with autopsy-confirmed CM, HIV+ children were older than HIV-uninfected children (unpaired t test for CM1 comparison and unpaired t test with Welch’s correction for CM2 comparison). (B) Distribution of peripheral platelet counts. While thrombocytopenia is common in autopsy-confirmed CM groups, HIV+ children had greater peripheral platelet counts than did HIV-uninfected children with autopsy-confirmed CM (unpaired t test with Welch’s correction). (C) Distribution of peripheral lymphocyte counts. Peripheral lymphocyte levels, a surrogate for immune function under older WHO HIV staging criteria, were not significantly different among groups. CD4+ T lymphocytes were not quantified. Download
Images of blood vessels from hematoxylin-and-eosin-stained temporal lobe sections from children with fatal cerebral malaria. (A) A vessel in cross section from an HIV-uninfected child with the CM1 pathology pattern. Infected red blood cells (iRBCs), monocytes, and the parasite pigment hemozoin (both within monocytes and within iRBCs) are identified. (B) A vessel in cross section from an HIV+ child with the CM1 pathology pattern. iRBCs, a monocyte containing hemozoin, and intraerythrocytic hemozoin are demonstrated. Download
Clinical features of the 30 children with brain tissue analyzed in this paper. Continuous variables are shown as medians, with interquartile ranges in parentheses, except for parasitemia and platelet counts, values for which are geometric means with 25th and 75th percentiles. Differences between groups were analyzed by 1-way analysis of variance or Kruskal-Wallis test for continuous variables and by chi-square test for dichotomous variables. Duration of illness was determined by combining the duration of fever with duration of coma. All analyses included data from 30 patients except where indicated below. COC, cases with an obvious nonmalarial cause of coma. *, excludes HIV+ CM3/COC; 4 of these patients had no malaria parasitemia; **, P = nonsignificant (NS) when CM3/COC data are excluded. Retinopathy status determined: n = 24 (n = 4 for CM1 HIV+, CM1 HIV−, CM2 HIV+, CM2 HIV−, and CM3/COC HIV+; n = 3 for CM3 HIV−). White blood cell count: n = 27 (n = 4 for CM2 HIV−, n = 3 for CM3/COC HIV+). Lymphocyte count: n = 21 (n = 4 for CM1 HIV+, CM1 HIV−, CM3/COC HIV+, and CM3 HIV−; n = 3 for CM2 HIV+; n = 2 for CM2 HIV−). Monocyte count: n = 17 (n = 4 for CM3 HIV−; n = 3 for CM1 HIV+, CM1 HIV−, and CM2 HIV+; n = 2 for CM2 HIV− and CM3/COC HIV+). Platelet count: n = 27 (n = 4 for CM2 HIV−; n = 3 for CM3/COC HIV+). Duration of coma: n = 26 (n = 4 for CM1 HIV+ and CM2 HIV+; n = 3 for CM3 HIV−). Duration of illness: n = 28 (n = 4 for CM1 HIV+ and CM3 HIV−).