ABSTRACT
Strains of emm89 Streptococcus pyogenes have become one of the major causes of invasive infections worldwide in the last 10 years. We recently sequenced the genome of 1,125 emm89 strains and identified three major phylogenetic groups, designated clade 1, clade 2, and the epidemic clade 3. Epidemic clade 3 strains, which now cause the great majority of infections, have two distinct genetic features compared to clade 1 and clade 2 strains. First, all clade 3 organisms have a variant 3 nga promoter region pattern, which is associated with increased production of secreted cytolytic toxins SPN (S. pyogenes NADase) and SLO (streptolysin O). Second, all clade 3 strains lack the hasABC locus mediating hyaluronic acid capsule synthesis, whereas this locus is intact in clade 1 and clade 2 strains. We constructed isogenic mutant strains that produce different levels of SPN and SLO toxins and capsule (none, low, or high). Here we report that emm89 strains with elevated toxin production are significantly more virulent than low-toxin producers. Importantly, we also show that capsule production is dispensable for virulence in strains that already produce high levels of SPN and SLO. Our results provide new understanding about the molecular mechanisms contributing to the rapid emergence and molecular pathogenesis of epidemic clade 3 emm89 S. pyogenes.
IMPORTANCE
S. pyogenes (group A streptococcus [GAS]) causes pharyngitis (“strep throat”), necrotizing fasciitis, and other human infections. Serious infections caused by emm89 S. pyogenes strains have recently increased in frequency in many countries. Based on whole-genome sequence analysis of 1,125 strains recovered from patients on two continents, we discovered that a new emm89 clone, termed clade 3, has two distinct genetic features compared to its predecessors: (i) absence of the genes encoding antiphagocytic hyaluronic acid capsule virulence factor and (ii) increased production of the secreted cytolytic toxins SPN and SLO. emm89 S. pyogenes strains with the clade 3 phenotype (absence of capsule and high expression of SPN and SLO) are highly virulent in mice. These findings provide new understanding of how new virulent clones emerge and cause severe infections worldwide. This newfound knowledge of S. pyogenes virulence can be used to help understand future epidemics and conduct new translational research.
INTRODUCTION
Understanding the evolutionary genetic changes underpinning emergence of new pathogenic strains is of practical importance because it provides useful information for development of effective strategies to control infectious diseases in humans, domesticated animals, and crops. The combination of population genomics, epidemiology, evolutionary biology, molecular genetics, and microbial pathogenesis makes it possible to precisely delineate these genetic changes at the nucleotide level.
Streptococcus pyogenes (group A streptococcus [GAS]) is a human pathogen that causes many diseases ranging in severity from minor skin and throat infections to fatal invasive episodes (1). Based on variation in the emm gene encoding the antiphagocytic M protein, S. pyogenes can be classified into ~200 emm types (2). For reasons that are not well understood, in the last 10 years emm89 strains have rapidly become one of the major emm types causing severe invasive S. pyogenes infections in several geographic regions (3–18). We recently sequenced the genomes of 1,125 emm89 strains isolated on two continents from 1995 to 2013 and discovered the existence of three major phylogenetic groups of emm89 strains, designated clade 1, clade 2, and epidemic clade 3 (19). We discovered that clade 3 strains emerged, disseminated extensively, and rapidly displaced clade 1 and clade 2 strains in an epidemic wave of emm89 disease.
Population genomic analysis revealed two major features of epidemic clade 3 emm89 strains. First, all clade 3 emm89 strains have the variant 3 nga promoter region sequence, which is identical to the nga promoter region present in pandemic emm1 strains. The variant 3 promoter region is associated with elevated production of SPN (S. pyogenes NADase) and SLO (streptolysin O), two potent secreted cytolytic toxins that contribute to virulence (19). Second, all clade 3 emm89 strains studied lack the hasABC gene region containing hyaluronic acid (HA) capsule synthesis genes.
We hypothesized that the genetic changes in the nga promoter region and capsule synthesis genes have altered the virulence phenotype of emm89 strains. To test this hypothesis, we constructed a panel of isogenic mutant strains that vary in level of production of secreted SPN and SLO cytolytic toxins and HA capsule. These mutant strains recapitulate the level of SPN and SLO toxin and capsule production made by genomically representative members of each of the three emm89 clades. The isogenic mutant strains were tested for virulence in a mouse model of necrotizing fasciitis.
RESULTS
Variant 3 nga promoter sequence is associated with increased production of secreted SPN and SLO toxins.
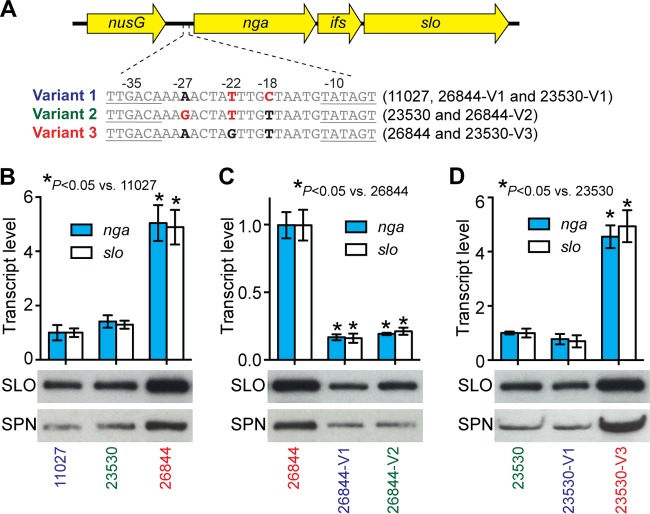
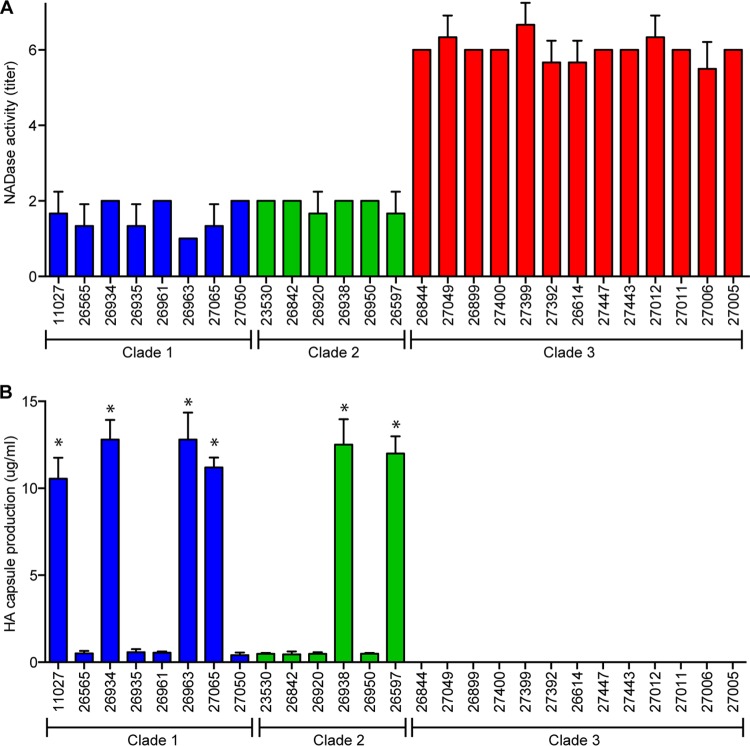
Based on our analysis of 1,125 genome sequences, emm89 strains cluster into three major phylogenetic groups referred to as clades. Strains in each clade have a distinct nga promoter sequence (2). That is, clade 1 strains all have the variant 1 pattern, clade 2 strains have the variant 2 pattern, and epidemic clade 3 strains have the variant 3 pattern. Three reference strains, MGAS11027 (clade 1), MGAS23530 (clade 2), and MGAS26844 (clade 3), whose genomes were sequenced to closure at high fold coverage, were chosen for subsequent analyses. The nga, slo, and intervening ifs genes are organized as an operon and expressed as a single transcript (20–22) (see Fig. 2A). nga and slo encode SPN and SLO, respectively (22). In principle, variation in the promoter region sequence could alter the level of nga and slo transcripts and thereby SPN and SLO production. To test this idea, we first compared the secreted SPN (NADase) activities of 27 emm89 isolates belonging to clade 1, clade 2, and clade 3 (All 27 strains are wild type in transcriptional regulators known to control SPN, SLO production, and capsule production, i.e., covR/S and rocA [23–26]). Compared to clade 1 and clade 2 isolates, clade 3 isolates produced increased NADase activity (Fig. 1). We next examined the transcript levels of nga and slo and SPN and SLO production in the three reference strains representing the three clades. Consistent with the hypothesis, strain MGAS26844 (variant 3 pattern) had a significantly higher nga and slo transcript levels and production of SPN and SLO compared to strain MGAS23530 (variant 2 pattern) and strain MGAS11027 (variant 1 pattern) (Fig. 2B).
FIG 2 .
In vitro characteristics of emm89 reference strains and isogenic mutant strains with different nga promoter region sequences. (A) Schematic showing the nga promoter region sequences of the strains assayed. (B to D) qRT-PCR analysis of nga and slo transcript levels and Western immunoblot analysis of secreted SLO and SPN in the culture supernatant. Transcript analysis was done in triplicate on three separate occasions.
FIG 1 .
In vitro characteristics of 27 emm89 isolates. (A) NADase activities of emm89 isolates in the culture supernatant. (B) Production of hyaluronic acid capsule by emm89 isolates. Asterisks indicate that strains 11027, 26934, 26963, 27065, 26938, and 26957, which produce higher levels of HA capsule, also have a 38-bp deletion in the hasA upstream region (Fig. 3). NADase assays and capsule assays were performed in triplicate on three separate occasions. Replicate data are expressed as the mean ± SD.
To further test the hypothesis that variation in the promoter region alters gene transcript levels, we generated isogenic mutant strains by replacing the nga promoter of the variant-3 parental strain 26844. The results (Fig. 2C) show that converting the nga promoter region of strain MGAS26844 to either variant 1 sequence or variant 2 sequence significantly reduced both nga and slo transcript levels and production of SPN and SLO cytotoxins. We next generated isogenic mutant strains by converting the nga promoter region of parental strain MGAS23530 (variant 2) to variant 1 sequence and variant 3 sequence. Consistent with the hypothesis, the isogenic mutant strain with the variant 3 sequence expressed significantly more nga and slo transcript and SPN and SLO proteins (Fig. 2D). In contrast, conversion to the variant 1 nga promoter sequence did not significantly alter the level of transcript or SLO and SPN production. Collectively, these results show that sequence variation in the nga promoter region alters transcription of nga and slo and production of SPN and SLO. Epidemic clade 3 emm89 strains with the variant 3 nga promoter pattern have the highest nga-slo transcript level and produce the largest amount of secreted SPN and SLO.
A 38-bp deletion in the upstream region of hasA is associated with increased production of HA capsule.
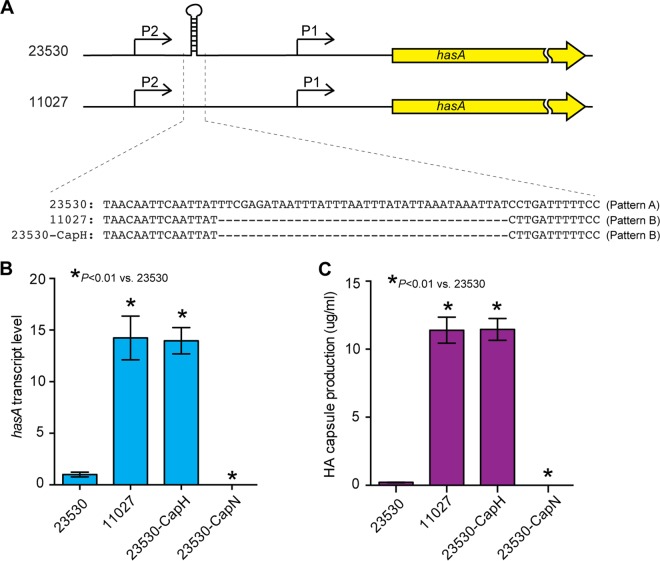
Epidemic clade 3 strains lack the hasABC genes for capsule synthesis, whereas clade 1 and clade 2 strains have these genes. However, the abundance of capsule production varies from strain to strain among clade 1 and clade 2 strains (Fig. 1B). For example, representative strain MGAS23530 (clade 2) produces a moderate amount of capsule (~0.3 µg/ml), and HA capsule production by representative strain MGAS11027 (clade 1) is ~40 times higher (~12 µg/ml) (Fig. 3C). Analysis of the genome sequence data found two distinct hasA promoter region patterns present in clade 1 and clade 2 strains. We designate these two variants as pattern A and pattern B. Patterns A and B are present in both clade 1 and clade 2 strains. Compared to pattern A, pattern B has a 38-bp deletion that may remove a transcriptional terminator (Fig. 3A). Consistent with this idea, we found that regardless of clade assignment, strains with the 38-bp deletion produce a higher level of capsule (Fig. 1B and 3A). Falaleeva et al. showed that deletion of this transcriptional terminator region resulted in increased capsule production (27). To directly study if the 38-bp deletion results in increased production of capsule, we generated an isogenic mutant derivative of parental reference strain MGAS23530 (23530-CapH) by replacing the hasA promoter with the promoter sequence present in pattern B strains. Deletion of the 38-bp region resulted in an ~15-fold increase in hasA expression and ~40-fold increase in HA capsule production, levels similar to those obtained for the naturally occurring pattern B strain MGAS11027 (Fig. 3B and C). These results demonstrate that removal of the 38-bp region upstream of the hasA promoter region results in increased hasA transcript and HA capsule synthesis.
FIG 3 .
In vitro characteristics of emm89 reference strains and isogenic mutant strains with different hasA upstream promoter region sequences. (A) Schematic showing the hasA upstream promoter region sequences of the assayed strains. The relative position of the “region of deletion” relative to P1 and P2 was graphed according to Falaleeva et al. (27). (B and C) hasA transcript level and hyaluronic acid capsule production of assayed strains. Transcript analysis and capsule assays were done in triplicate on three separate occasions.
An isogenic acapsular emm89 strain with the variant 3 nga promoter region is highly virulent in a mouse model of necrotizing fasciitis.
Two key genetic features of the epidemic emm89 clone are absence of HA capsule synthesis genes and presence of the variant 3 nga promoter region that results in increased production of the secreted SPN and SLO toxin virulence factors. We hypothesized that the absence of capsule together with increased SPN/SLO production (phenotypes that occur naturally in clade 3 strains) significantly increases strain virulence of emm89 S. pyogenes. We have previously shown that reduction of SPN/SLO production in the genetically representative clade 3 strain MGAS26844 significantly reduced its virulence in a mouse infection model (19). These data confirmed our hypothesis that increased toxin production is associated with increased virulence. However, strain MGAS26844 is acapsular, as are the isogenic mutant derivatives, which means that the effect of capsule loss on virulence in these strains was not determined.
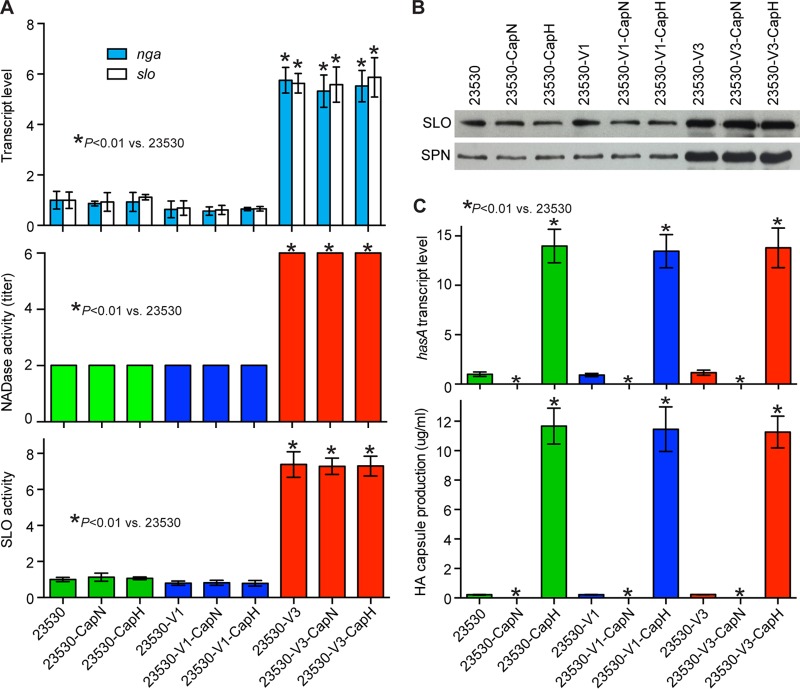
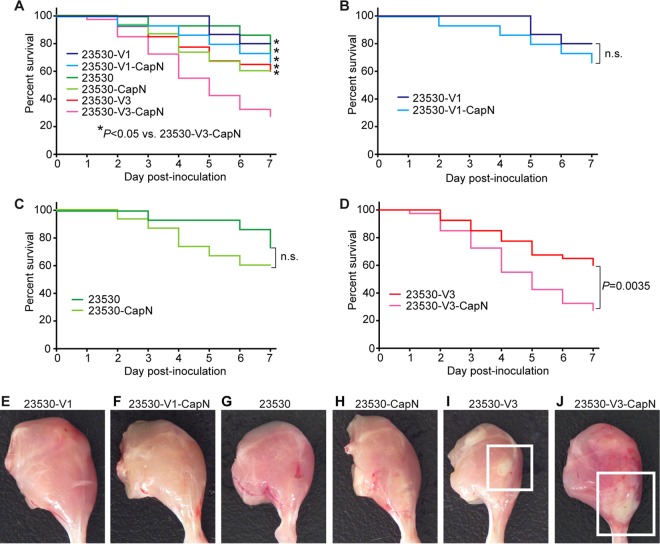
We next generated a panel of isogenic mutant strains of clade 2 strain MGAS23530 (weakly encapsulated, low SPN/SLO production) by altering the nga promoter region sequence and deleting the hasABC locus (Fig. 4; Table 1). The virulence of these strains was compared using a mouse model of necrotizing fasciitis (Fig. 5). Consistent with our hypothesis, the acapsular variant 3 strain (23530-V3-CapN) caused significantly greater near mortality than the acapsular or weakly encapsulated strains that express comparatively smaller amounts of SPN and SLO toxin (Fig. 5A, 23530-V1-CapN, 23530-V1, 23530-V2-CapN, and 23530-V2). Similarly, gross and microscopic examination of infected limbs found that the acapsular variant 3 isogenic mutant strain (23530-V3-CapN) caused significantly larger lesions with more tissue destruction (Fig. 5E to J). Interestingly, loss of HA capsule did not result in decreased virulence (Fig. 5). To the contrary, loss of capsule caused a substantial increase of virulence in an emm89 strain with the variant 3 nga promoter (Fig. 5D and 6). This result suggests production of a low level of capsule is not sufficient to protect emm89 organisms from host innate immunity. Taken together, these data strongly support our hypothesis that the epidemic clade 3 genotype (absence of capsule combined with strong nga promoter expression) confers an increased virulence phenotype to the epidemic emm89 clade 3 organisms.
FIG 4 .
In vitro characteristics of nine emm89 strains with three nga promoter patterns that produce different levels of hyaluronic acid capsule. (A) Relative transcript levels of nga and slo and enzymatic activities of SPN (NADase) and SLO in the culture supernatant. (B) Western immunoblot analysis of SLO and SPN in the culture supernatant. (C) Transcript level of hasA and hyaluronic acid capsule production of strains analyzed.
TABLE 1 .
Characteristics of the strains used in this study
| Strain (MGAS no.)a | nga promoter | Production of: |
Clade | |
|---|---|---|---|---|
| SPN or SLO | Capsule | |||
| 11027 | Variant 1 | Low | High | 1 |
| 26844 | Variant 3 | High | None | 3 |
| 26844-V1 | Variant 1 | Low | None | |
| 26844-V2 | Variant 2 | Low | None | |
| 23530 | Variant 2 | Low | Low | 2 |
| 23530-V1 | Variant 1 | Low | Low | |
| 23530-V3 | Variant 3 | High | Low | |
| 23530-CapH | Variant 2 | Low | High | |
| 23530-V1-CapH | Variant 1 | Low | High | |
| 23530-V3-CapH | Variant 3 | High | High | |
| 23530-CapN | Variant 2 | Low | None | |
| 23530-V1-CapN | Variant 1 | Low | None | |
| 23530-V3-CapN | Variant 3 | High | None | |
MGAS no., Musser group A Streptococcus number.
FIG 5 .
Acapsular emm89 strain with a variant 3 nga promoter is highly virulent in a mouse model of necrotizing fasciitis. (A) Kaplan-Meier near-survival curves of mice infected with strain MGAS23530 (low toxin, low capsule), 23530-V1 (low toxin, low capsule), 23530-V3 (high toxin, low capsule), 23530-CapN (low toxin, no capsule), 23530-V1-CapN (low toxin, no capsule), and 23530-V3-CapN (high toxin, no capsule). (B to D) Subsections of panel A that show comparisons between capsule-positive strains and capsule-negative strains. (E to J) Gross pathology analysis of strain virulence in a mouse model of necrotizing fasciitis. The boxed region in panels I and J marks the comparatively larger lesion caused by variant 3 strains expressing large amounts of SPN and SLO toxins.
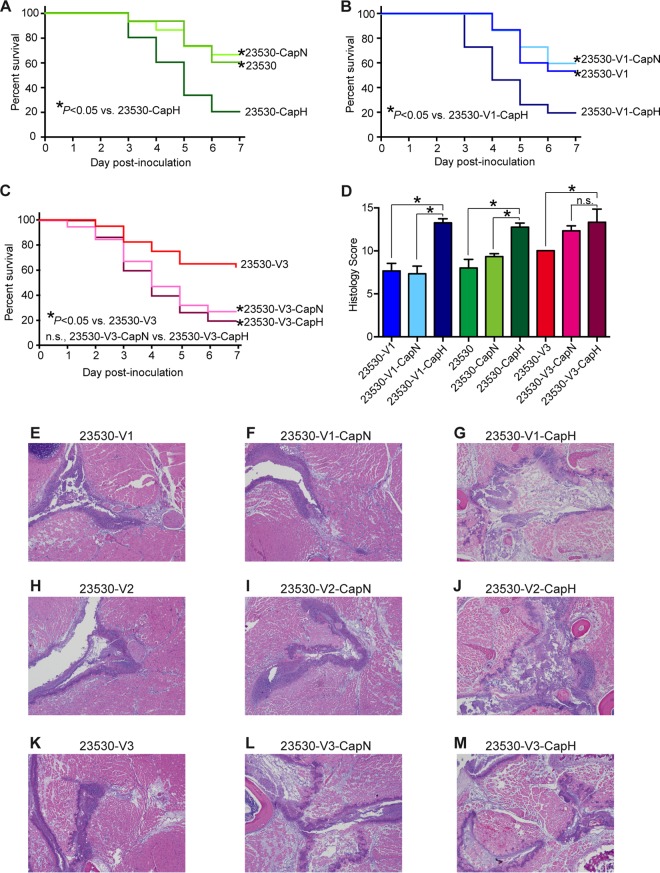
FIG 6 .
Capsule production is dispensable for emm89 virulence among strains that produce high levels of SPN and SLO. (A to C) Kaplan-Meier near-survival curves of mice infected with strains that produce different levels of capsule and toxins. (D) Histopathology scores of mouse limb tissue infected with each strain. Data are expressed as means ± SEM. (E to M) Histopathologic analysis of the virulence of isogenic mutant strains in a mouse model of necrotizing fasciitis. Original magnification, 4×.
HA capsule production is dispensable for full virulence in emm89 strains that produce high levels of SPN and SLO toxins.
The data show that transition from low HA capsule production to complete absence of capsule did not result in decreased virulence (Fig. 5). To further study if HA capsule production plays a role in the virulence in emm89 strains, we compared the virulence levels of heavily encapsulated emm89 strains (generated by deleting a 38-bp region upstream of hasA [Fig. 4; Table 1]) and acapsular organisms. Our virulence study results show that for emm89 strains that produce low levels of toxins (strains with a variant 1 or variant 2 nga promoter region), high capsule production rendered the organisms more virulent (Fig. 6A and B). However, for emm89 strains that produce high levels of SLO and SPN cytolytic toxins (strains with a variant 3 nga promoter region), the acapsular isogenic mutant strain is equally virulent to the heavily encapsulated strain (Fig. 6C and D). In other words, production of a high level of HA capsule did not increase virulence in strains producing large amounts of SLO and SPN. Again, intriguingly, the poorly encapsulated strain was less virulent (23530-V3) (Fig. 6C and D). Taken together, capsule production (regardless of whether high or low) is dispensable for virulence in emm89 strains that produce high levels of the secreted cytolytic toxins SLO and SPN.
DISCUSSION
One distinctive feature of the epidemic emm89 population is the lack of the hasABC locus responsible for production of HA capsule. Capsule has been studied for more than 100 years and has long been considered an important virulence factor for S. pyogenes strains, in part because of its role in resisting phagocytosis and killing by human polymorphonuclear leukocytes (PMNs) (28–30). Many lines of evidence, including virulence studies using isogenic mutant strains, have strongly supported the concept that HA capsule production is a crucial factor in the complex interaction between pathogen and host (28–30). These extensive studies led to the generally accepted idea that HA capsule is required for invasive infections and colonization. However, Flores et al. (31) recently reported that human disease isolates of emm4 and emm22 S. pyogenes lack the hasABC genes, a discovery effectively ruling out the idea that production of HA capsule is obligatory for virulence. In addition, the investigators showed that emm4 and emm22 strains proliferated ex vivo in human blood. Our work here adds emm89 strains to the list of S. pyogenes organisms that do not require HA capsule to cause human infections or, unexpectedly, epidemic disease.
A second distinctive feature of epidemic clade 3 emm89 strains is that they all have the variant 3 nga promoter region pattern. Compared to the variant 1 and variant 2 nga promoter region sequences present in clade 1 and clade 2 strains, respectively, the variant 3 promoter region sequence is associated with increased expression of nga and slo and increased production of SPN and SLO cytotoxins. SPN and SLO have been reported to protect S. pyogenes from phagocytic killing and increase intracellular survival (32, 33). In the absence of HA capsule, a high level of SPN and SLO production could provide a critical defense against host immunity. In this regard, we note that for emm89 strains that are high producers of SPN and SLO, HA capsule production is dispensable for virulence in a mouse infection model. The acapsular strain 23530-V3-CapN is as virulent as heavily encapsulated strain 23530-V3-CapH, as assessed by ability to cause near mortality and tissue destruction. In contrast, for strains that produce relatively smaller amounts of SPN and SLO, a high level of HA capsule production is important for virulence.
Population genomic analysis showed that epidemic clade 3 emm89 evolved from a clade 2 strain (19). The precise nature and order of the molecular events that generated epidemic clade 3 is not known. In particular, we do not know if loss of the hasABC capsule synthesis genes preceded acquisition of the variant 3 nga-slo region or vice versa. Our inability to differentiate between these two possibilities is due in part to a lack of strains that have one of these two genotypic characteristics in the absence of the other. That is, our analysis did not identify strains with the hasABC capsule genes together with the variant 3 nga-slo region or which have the variant 2 nga-slo region but lack the HA capsule synthesis genes. The mouse infection data suggest that increased production of SPN and SLO is a more important contributor to virulence than HA capsule production. This leads us to speculate that the key event immediately preceding the successful emergence of epidemic clade 3 organisms was gain of the variant 3 nga-slo region by horizontal gene transfer and homologous recombination in an emm89 strain that lacked the hasABC genes. It is possible that analysis of additional emm89 strains may identify strains that will permit us to differentiate between the possibilities. We note that it is a formal possibility that the evolutionary genetic events occurred simultaneously, although this seems unlikely.
One intriguing finding of our study is that low HA capsule production is not sufficient to provide adequate protection against host defenses for emm89 S. pyogenes. For strains that produce low levels of SPN and SLO, poorly encapsulated strains are as attenuated as acapsular strains (Fig. 5 and 6). Interestingly, for high-toxin producers, poorly encapsulated strain 23530-V3 is significantly less virulent than acapsular strain 23530-V3-CapN (Fig. 5 and 6). One potential explanation for this unusual phenomenon is that acapsular emm89 organisms that produce high levels of SPN and SLO are more cytotoxic for host cells. Under this idea, compared to capsule-positive strains (even poorly encapsulated strains), acapsular organisms attach to the host cell better and are more likely to be internalized and intoxicate the host cell. For high-toxin producers, more internalization may result in more abundant cytotoxicity, tissue destruction, and host pathology.
In summary, by analyzing isogenic mutant strains that recapitulate key genetic aspects of emm89 strains from different phylogenetic clades, we discovered that an acapsular emm89 strain producing high level of SPN and SLO (the two main features of epidemic emm89 strains) is highly virulent in a mouse model of necrotizing fasciitis. In addition, our data show that HA capsule production is dispensable for virulence in emm89 strains that produce high levels of SPN and SLO cytotoxins. The sum of the data provide additional evidence to support the idea we recently put forward that upregulation of SPN and SLO production is a key trigger for epidemic disease caused by S. pyogenes.
MATERIALS AND METHODS
Construction of isogenic mutant strains.
Strain MGAS23530 was used as the parental organism for construction of isogenic mutant derivatives. Isogenic mutant strain 23530-V1 was generated by converting the nga promoter to the variant 1 pattern. Briefly, using MGAS23530 genomic DNA as the template, overlap extension PCR was performed with primer sets ngapro-1/V1A and ngapro-V1B/4 to generate a 1,097-bp fragment flanking the nga promoter region. SNPs G-27A and T-22G were introduced into the amplicon with primers ngapro-V1A and ngapro-V1B. The double-SNP-containing amplicon was digested with BamHI and ligated into the BamHI site of plasmid pBBL740 (34). The ligation product was column purified and transformed into strain MGAS23530. Allelic exchange was performed as previously described (34). The resulting derivative strain, 23530-V1, was sequenced (sequencing primer, ngapro-seq) (Table 2) to ensure that the native MGAS23530 sequence GACTAT was replaced with the derived two-single-nucleotide polymorphism (SNP) sequence AACTAG located in the −10 and −35 spacer region of the nga promoter. (The changed residues are underlined.) Analogous to the procedure used to generate 23530-V1, strain 23530-V3 was generated by converting the nga promoter to the variant 3 pattern. SNPs G-27A and T-18C were introduced into the nga promoter region with primers ngapro-V3A and ngapro-V3B. Capsule-negative derivative strain 23530-V3-CapN was generated by deleting the has operon (hasA, hasB, and hasC) genes. Briefly, primer sets hasABCdel-1/2 and hasABCdel-3/4 (Table 2) were used for overlap extension PCR to amplify and join the two DNA fragments upstream and downstream of the hasABC locus. PCR product was cloned into the BamHI site of suicide plasmid pBBL740 and then transformed into the designated S. pyogenes strains. Allelic exchange was performed as previously described. PCR (primer set hasABCdel-1/4) (Table 2) was used to screen for hasABC-negative strains. Capsule-negative derivative strains 23530-CapN and 23530-V1-CapN were made acapsular by disrupting the hasA gene with suicide plasmid pBBL740. Briefly, primers hasA-1 and hasA-2 (Table 2) were used to amplify an ~350-bp internal region of hasA. The PCR product was cloned into the BamHI site of pBBL740. Recombinant plasmid was used to transform S. pyogenes strains and disrupt hasA.
TABLE 2 .
Primers used for construction of isoallelic mutant strains
| Primer | Sequence | Sequence underlined |
|---|---|---|
| ngapro-1 | CGCGTGGATCCCCGTGCTATCTTGTTGTCTATGGGT | BamHI |
| ngapro-V1A | TAAGTAAACTATACATTAGCAAATAGTTTTTGTCAATGCGA | SNPs to introduce |
| ngapro-V1B | TCGCATTGACAAAAACTATTTGCTAATGTATAGTTTACTTA | SNPs to introduce |
| ngapro-4 | CGCGTGGATCCTTTATCAATCTCAATGTGATGCGGT | BamHI site |
| ngapro-V3A | TAAGTAAACTATACATTAACAACTAGTTTTTGTCAATGCGA | SNPs to introduce |
| ngapro-V3B | TCGCATTGACAAAAACTAGTTGTTAATGTATAGTTTACTTA | SNPs to introduce |
| ngapro-seq | CGTGTTTACAAACCAATGGATGACT | |
| hasABCdel-1 | CGCGTGGATCCCTTTAGGTTGATACTGGGCGATGGA | BamHI site |
| hasABCdel-2 | TGGTCCTCGATAGAGCGTTTAGCTTTATTTTAGCAAACTTTTCAAATAGT | |
| hasABCdel-3 | ACTATTTGAAAAGTTTGCTAAAATAAAGCTAAACGCTCTATCGAGGACCA | |
| hasABCdel-4 | CGCGTGGATCCAATCCAAATTTATCCCCAACATCGT | BamHI site |
| 11027has-1 | CGCGTGGATCCCTTTAGGTTGATACTGGGCGATGGA | BamHI site |
| 11027has-2 | CGCGTGGATCCTCTACGGTTAAAAAAACGTCAGCGT | BamHI site |
| hasA−1 | CCGCGTGGATCCAATAAAGGAAAACGCCATGCTCAAG | BamHI site |
| hasA−2 | CCGCGTGGATCCATCATCCCCAATGCTAACAGGTAAA | BamHI site |
Isogenic mutant strain 23530-V3-CapH was constructed by converting the hasA promoter region of strain 23530-V3 to the sequence present in strain MGAS11027. Briefly, using genomic DNA of strain MGAS11027 as the template, primers 11027has-1 and 11027has-2 were used to amplify a 1.4-kb fragment that spans the hasA promoter region. The amplicon was ligated into the BamHI site of plasmid pBBL740 and transformed into strain MGAS23530. Allelic exchange was performed as previously described (34). Isogenic mutant strains 23530-V1-CapH and 23530-CapH were generated by the same method.
The genome of all mutant strains was sequenced, and no spurious mutations were identified.
qRT-PCR analysis of nga, slo, and hasA transcripts in emm89 S. pyogenes strains grown in THY broth.
RNA prepared from S. pyogenes cultures grown to an optical density at 600 nm (OD600) of 0.5 was extracted with an RNeasy minikit (Qiagen) and converted into cDNA using a high-capacity cDNA reverse transcription (RT) kit (Applied Biosystems). Quantitative RT-PCR (qRT-PCR) was performed with TaqMan Fast Universal PCR master mix (Applied Biosystems) and an ABI 7500 Fast System (Life Technologies) instrument. The sequences of the TaqMan primers and probes used are listed in Table 3. Each experiment was performed in triplicate with the mean values (± standard deviation [SD]) shown. Statistical differences between strains were determined using Student’s t test.
TABLE 3 .
Primers used for TaqMan qRT-PCR analysis
| Primer name | Sequencea |
|---|---|
| rpsl-forward | CGTGTTGGAACAATGACACCTAA |
| rpsl-reverse | CTTCGATAAGGTTGCTCAAACGT |
| rpsl-probe | 6FAM-CCTAACTCAGCCCTTCGTAAATTCGCTCGT-TAMRA |
| M89hasA-forward | ACCGTTCCCTTGTCAATAAAGG |
| M89hasA-reverse | CGTCAGCGTCAGATCTTTCAAA |
| M89hasA-probe | 6FAM-CGCCATGCTCAAGCGTGGGC-TAMRA |
| M89nga-forward | GAATTAGGCGACACCTACACTAA |
| M89nga-reverse | GTGACCTCTGACAAGGCTAAA |
| M89nga-probe | 6FAM-TGAGGTAACAGAGGTCCATCAGGGA-TAMRA |
| M89slo-forward | TGGGACAACAACTGGTATAGTAAG |
| M89slo-reverse | GTGCACTCTCTAGCCATGATAC |
| M89slo-probe | 6FAM-AGCACAGTTATCCCACTAGGAGCT-TAMRA |
6FAM, 6-carboxyfluorescein; TAMRA, 6-carboxytetramethylrhodamine.
Western immunoblot analysis of SLO and SPN in the culture supernatant.
Western immunoblot analysis was done as described previously (19). Briefly, cell-free supernatants of S. pyogenes cultures grown to OD600 of 0.5 were collected by centrifugation at 4,000 rpm for 10 min and filtered through a 0.2-µm filter. Proteins in the supernatant were concentrated with 2-ml centrifugal filters (Amicon) and assayed for presence of SLO and SPN with anti-SLO antibody (American Research Product) and anti-NADase antibody (Abcam), respectively.
Measurement of SLO and NADase activities in the culture supernatant.
SLO activity and NADase activity in the culture supernatant was assayed as described previously (35).
Capsule assay.
Hyaluronic acid capsule production was assayed as described previously (31).
Mouse virulence experiments.
Six-week-old female CD1 mice (Harlan Laboratories) were used for the necrotizing fasciitis studies as described previously (36). This study was approved by the Institutional Animal Care and Use Committee of the Houston Methodist Research Institute (AUP-0615-0041). Mice were randomly assigned to treatment groups and inoculated in the right hindlimb to a uniform depth with 2.5 × 108 CFU of emm89 strains in 100 µl phosphate-buffered saline (PBS). Stocks of each strain were prepared at known CFU and stored at −80°C. Inocula were prepared immediately before infection by diluting frozen stocks in PBS to the desired number of CFU. For survival experiments (n = 15 mice/strain for variant 1 and 2 strains and n = 40 mice/strain for variant 3 strains), near mortality was determined by observation using predefined internationally recognized criteria for sacrificing mice, including loss of >10% of body weight, failure to eat or drink for 24 h, reduction of body condition score by <2, becoming unable to ambulate, rupture of the abscess at the inoculation site, or rupture of an abscess at a site of dissemination (37). Data are shown as a Kaplan-Meier survival curve, with statistical differences between strain groups determined with the log-rank test. The larger number of mice used for the variant 3 strains was determined using a power calculation after conducting a pilot experiment. For histology evaluation (n = 3 mice/strain), lesions were excised, visually inspected, and photographed. The tissue was fixed in 10% phosphate-buffered formalin, decalcified, and embedded in paraffin using standard automated instruments. Histology was scored by a pathologist blinded to the strain treatment groups as described previously (38). Data are shown as means ± standard errors of the means (SEM), with statistical differences between strain groups determined using the Wilcoxon rank sum test.
Footnotes
Citation Zhu L, Olsen RJ, Nasser W, de la Riva Morales I, Musser JM. 2015. Trading capsule for increased cytotoxin production: contribution to virulence of a newly emerged clade of emm89 Streptococcus pyogenes. mBio 6(5):e01378-15. doi:10.1128/mBio.01378-15.
REFERENCES
- 1.Cunningham MW. 2000. Pathogenesis of group A streptococcal infections. Clin Microbiol Rev 13:470–511. doi: 10.1128/CMR.13.3.470-511.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steer AC, Law I, Matatolu L, Beall BW, Carapetis JR. 2009. Global emm type distribution of group A streptococci: systematic review and implications for vaccine development. Lancet Infect Dis 9:611–616. doi: 10.1016/S1473-3099(09)70178-1. [DOI] [PubMed] [Google Scholar]
- 3.Friães A, Lopes JP, Melo-Cristino J, Ramirez M, Portuguese Group for the Study of Streptococcal Infections . 2013. Changes in Streptococcus pyogenes causing invasive disease in Portugal: evidence for superantigen gene loss and acquisition. Int J Med Microbiol 303:505–513. doi: 10.1016/j.ijmm.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Syrogiannopoulos GA, Grivea IN, Al-Lahham A, Panagiotou M, Tsantouli AG, Ralf M, Rene Reinert AN, van der Linden M. 2013. Seven-year surveillance of emm types of pediatric group A streptococcal pharyngitis isolates in western Greece. PLoS One 8:e71558. doi: 10.1371/journal.pone.0071558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tamayo E, Montes M, García-Arenzana JM, Pérez-Trallero E. 2014. Streptococcus pyogenes emm-types in northern Spain; population dynamics over a 7-year period. J Infect 68:50–57. doi: 10.1016/j.jinf.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 6.Karaky NM, Araj GF, Tokajian ST. 2014. Molecular characterization of Streptococcus pyogenes group A isolates from a tertiary hospital in Lebanon. J Med Microbiol 63:1197–1204. doi: 10.1099/jmm.0.063412-0. [DOI] [PubMed] [Google Scholar]
- 7.Ikebe T, Tominaga K, Shima T, Okuno R, Kubota H, Ogata K, Chiba K, Katsukawa C, Ohya H, Tada Y, Okabe N, Watanabe H, Ogawa M, Ohnishi M, Working Group for Beta-Haemolytic Streptococci in Japan . 2015. Increased prevalence of group A streptococcus isolates in streptococcal toxic shock syndrome cases in Japan from 2010 to 2012. Epidemiol Infect 143:864–872. doi: 10.1017/S0950268814001265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turner CE, Abbott J, Lamagni T, Holden MT, David S, Jones MD, Turner CE, Abbott J, Lamagni T, Holden MT, David S, Jones MD, Game L, Efstratiou A, Sriskandan S. 2015. Emergence of a new highly successful acapsular group A Streptococcus clade of genotype emm89 in the United Kingdom. mBio 6:e00622-15. doi: 10.1128/mBio.00622-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shea PR, Ewbank AL, Gonzalez-Lugo JH, Martagon-Rosado AJ, Martinez-Gutierrez JC, Rehman HA, Serrano-Gonzalez M, Fittipaldi N, Beres SB, Flores AR, Low DE, Willey BM, Musser JM. 2011. Group A Streptococcus emm gene types in pharyngeal isolates, Ontario, Canada, 2002–2010. Emerg Infect Dis 17:2010–2017. doi: 10.3201/eid1711.110159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darenberg J, Luca-Harari B, Jasir A, Sandgren A, Pettersson H, Schalén C, Norgren M, Romanus V, Norrby-Teglund A, Normark BH. 2007. Molecular and clinical characteristics of invasive group A streptococcal infection in Sweden. Clin Infect Dis 45:450–458. doi: 10.1086/519936. [DOI] [PubMed] [Google Scholar]
- 11.Friães A, Pinto FR, Silva-Costa C, Ramirez M, Melo-Cristino J, Portuguese Group for the Study of Streptococcal Infections . 2012. Group A streptococci clones associated with invasive infections and pharyngitis in Portugal present differences in emm types, superantigen gene content and antimicrobial resistance. BMC Microbiol 12:280. doi: 10.1186/1471-2180-12-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imöhl M, Reinert RR, Ocklenburg C, van der Linden M. 2010. Epidemiology of invasive Streptococcus pyogenes disease in Germany during 2003–2007. FEMS Immunol Med Microbiol 58:389–396. doi: 10.1111/j.1574-695X.2010.00652.x. [DOI] [PubMed] [Google Scholar]
- 13.Lepoutre A, Doloy A, Bidet P, Leblond A, Perrocheau A, Bingen E, Trieu-Cuot P, Bouvet A, Poyart C, Lévy-Bruhl D, Microbiologists of the Epibac Network . 2011. Epidemiology of invasive Streptococcus pyogenes infections in France in 2007. J Clin Microbiol 49:4094–4100. doi: 10.1128/JCM.00070-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luca-Harari B, Darenberg J, Neal S, Siljander T, Strakova L, Tanna A, Creti R, Ekelund K, Koliou M, Tassios PT, van der Linden M, Straut M, Vuopio-Varkila J, Bouvet A, Efstratiou A, Schalén C, Henriques-Normark B, Strep-EURO Study Group, Jasir A. 2009. Clinical and microbiological characteristics of severe Streptococcus pyogenes disease in Europe. J Clin Microbiol 47:1155–1165. doi: 10.1128/JCM.02155-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montes M, Ardanuy C, Tamayo E, Domènech A, Liñares J, Pérez-Trallero E. 2011. Epidemiological and molecular analysis of Streptococcus pyogenes isolates causing invasive disease in Spain (1998–2009): comparison with non-invasive isolates. Eur J Clin Microbiol Infect Dis 30:1295–1302. doi: 10.1007/s10096-011-1226-x. [DOI] [PubMed] [Google Scholar]
- 16.O’Loughlin RE, Roberson A, Cieslak PR, Lynfield R, Gershman K, Craig A, Albanese BA, Farley MM, Barrett NL, Spina NL, Beall B, Harrison LH, Reingold A, Van Beneden C, Active Bacterial Core Surveillance Team . 2007. The epidemiology of invasive group A streptococcal infection and potential vaccine implications: United States, 2000–2004. Clin Infect Dis 45:853–862. [DOI] [PubMed] [Google Scholar]
- 17.Plainvert C, Doloy A, Loubinoux J, Lepoutre A, Collobert G, Touak G, Trieu-Cuot P, Bouvet A, Poyart C, CNR-Strep Network . 2012. Invasive group A streptococcal infections in adults, France (2006–2010). Clin Microbiol Infect 18:702–710. doi: 10.1111/j.1469-0691.2011.03624.x. [DOI] [PubMed] [Google Scholar]
- 18.Safar A, Lennon D, Stewart J, Trenholme A, Drinkovic D, Peat B, Taylor S, Read K, Roberts S, Voss L. 2011. Invasive group A streptococcal infection and vaccine implications, Auckland, New Zealand. Emerg Infect Dis 17:983–989. doi: 10.3201/eid/1706.100804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu L, Olsen RJ, Nasser W, Beres SB, Vuopio J, Kristinsson KG, Gottfredsson M, Porter AR, DeLeo FR, Musser JM. 2015. A molecular trigger for intercontinental epidemics of group A streptococcus. J Clin Invest 125:3545–3559. doi: 10.1172/JCI82478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meehl MA, Pinkner JS, Anderson PJ, Hultgren SJ, Caparon MG. 2005. A novel endogenous inhibitor of the secreted streptococcal NAD-glycohydrolase. PLoS Pathog 1:e35. doi: 10.1371/journal.ppat.0010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimoto H, Fujii Y, Hirano S, Yokota Y, Taketo A. 2006. Genetic and biochemical properties of streptococcal NAD-glycohydrolase inhibitor. J Biol Chem 281:9181–9189. doi: 10.1074/jbc.M506879200. [DOI] [PubMed] [Google Scholar]
- 22.Kimoto H, Fujii Y, Yokota Y, Taketo A. 2005. Molecular characterization of NADase-streptolysin O operon of hemolytic streptococci. Biochim Biophys Acta 1681:134–149. doi: 10.1016/j.bbaexp.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 23.Lynskey NN, Goulding D, Gierula M, Turner CE, Dougan G, Edwards RJ, Sriskandan S. 2013. RocA truncation underpins hyper-encapsulation, carriage longevity and transmissibility of serotype M18 group A streptococci. PLoS Pathog 9:e1003842. doi: 10.1371/journal.ppat.1003842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shelburne SA, Olsen RJ, Suber B, Sahasrabhojane P, Sumby P, Brennan RG, Musser JM. 2010. A combination of independent transcriptional regulators shapes bacterial virulence gene expression during infection. PLoS Pathog 6:e1000817. doi: 10.1371/journal.ppat.1000817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernish B, van de Rijn I. 1999. Characterization of a two-component system in Streptococcus pyogenes which is involved in regulation of hyaluronic acid production. J Biol Chem 274:4786–4793. doi: 10.1074/jbc.274.8.4786. [DOI] [PubMed] [Google Scholar]
- 26.Sumby P, Whitney AR, Graviss EA, DeLeo FR, Musser JM. 2006. Genome-wide analysis of group A streptococci reveals a mutation that modulates global phenotype and disease specificity. PLoS Pathog 2:e5. doi: 10.1371/journal.ppat.0020005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Falaleeva M, Zurek OW, Watkins RL, Reed RW, Ali H, Sumby P, Voyich JM, Korotkova N. 2014. Transcription of the Streptococcus pyogenes hyaluronic acid capsule biosynthesis operon is regulated by previously unknown upstream elements. Infect Immun 82:5293–5307. doi: 10.1128/IAI.02035-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wessels MR, Moses AE, Goldberg JB, DiCesare TJ. 1991. Hyaluronic acid capsule is a virulence factor for mucoid group A streptococci. Proc Natl Acad Sci U S A 88:8317–8321. doi: 10.1073/pnas.88.19.8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moses AE, Wessels MR, Zalcman K, Albertí S, Natanson-Yaron S, Menes T, Hanski E. 1997. Relative contributions of hyaluronic acid capsule and M protein to virulence in a mucoid strain of the group A streptococcus. Infect Immun 65:64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dale JB, Washburn RG, Marques MB, Wessels MR. 1996. Hyaluronate capsule and surface M protein in resistance to opsonization of group A streptococci. Infect Immun 64:1495–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flores AR, Jewell BE, Fittipaldi N, Beres SB, Musser JM. 2012. Human disease isolates of serotype m4 and m22 group A streptococcus lack genes required for hyaluronic acid capsule biosynthesis. mBio 3:e00413-12. doi: 10.1128/mBio.00413-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Seaghdha M, Wessels MR. 2013. Streptolysin O and its co-toxin NAD-glycohydrolase protect group A Streptococcus from xenophagic killing. PLoS Pathog 9:e1003394. doi: 10.1371/journal.ppat.1003394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bastiat-Sempe B, Love JF, Lomayesva N, Wessels MR. 2014. Streptolysin O and NAD-glycohydrolase prevent phagolysosome acidification and promote group A Streptococcus survival in macrophages. mBio 5:e01690-01614. doi: 10.1128/mBio.01690-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramirez-Peña E, Treviño J, Liu Z, Perez N, Sumby P. 2010. The group A Streptococcus small regulatory RNA FasX enhances streptokinase activity by increasing the stability of the ska mRNA transcript. Mol Microbiol 78:1332–1347. doi: 10.1111/j.1365-2958.2010.07427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sumby P, Porcella SF, Madrigal AG, Barbian KD, Virtaneva K, Ricklefs SM, Sturdevant DE, Graham MR, Vuopio-Varkila J, Hoe NP, Musser JM. 2005. Evolutionary origin and emergence of a highly successful clone of serotype M1 group A Streptococcus involved multiple horizontal gene transfer events. J Infect Dis 192:771–782. doi: 10.1086/432514. [DOI] [PubMed] [Google Scholar]
- 36.Olsen RJ, Sitkiewicz I, Ayeras AA, Gonulal VE, Cantu C, Beres SB, Green NM, Lei B, Humbird T, Greaver J, Chang E, Ragasa WP, Montgomery CA, Cartwright J Jr, McGeer A, Low DE, Whitney AR, Cagle PT, Blasdel TL, DeLeo FR, Musser JM. 2010. Decreased necrotizing fasciitis capacity caused by a single nucleotide mutation that alters a multiple gene virulence axis. Proc Natl Acad Sci U S A 107:888–893. doi: 10.1073/pnas.0911811107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals 2011. Guide for the care and use of laboratory animals, 8th ed. National Academies Press, Washington, DC. [Google Scholar]
- 38.Nasser W, Beres SB, Olsen RJ, Dean MA, Rice KA, Long SW, Kristinsson KG, Gottfredsson M, Vuopio J, Raisanen K, Caugant DA, Steinbakk M, Low DE, McGeer A, Darenberg J, Henriques-Normark B, Van Beneden CA, Hoffmann S, Musser JM. 2014. Evolutionary pathway to increased virulence and epidemic group A Streptococcus disease derived from 3,615 genome sequences. Proc Natl Acad Sci U S A 111:E1768–E1776. doi: 10.1073/pnas.1403138111. [DOI] [PMC free article] [PubMed] [Google Scholar]