Abstract
Premature ovarian failure (POF) affects 1% of women in reproductive age, but its etiology remains uncertain. Whereas kisspeptins, the products of Kiss1 that act via Kiss1r (aka, Gpr54), are known to operate at the hypothalamus to control GnRH/gonadotropin secretion, additional actions at other reproductive organs, including the ovary, have been proposed. Yet, their physiological relevance is still unclear. We present here a series of studies in Kiss1r haplo-insufficient and null mice suggesting a direct role of kisspeptin signaling in the ovary, the defect of which precipitates a state of primary POF. Kiss1r hypomorph mice displayed a premature decline in ovulatory rate, followed by progressive loss of antral follicles, oocyte loss, and a reduction in all categories of preantral follicles. These alterations were accompanied by reduced fertility. Because of this precocious ovarian ageing, mice more than 48 weeks of age showed atrophic ovaries, lacking growing follicles and corpora lutea. This phenomenon was associated with a drop in ovarian Kiss1r mRNA expression, but took place in the absence of a decrease in circulating gonadotropins. In fact, FSH levels increased in aged hypomorph animals, reflecting loss of follicular function. In turn, Kiss1r-null mice, which do not spontaneously ovulate and have arrested follicular development, failed to show normal ovulatory responses to standard gonadotropin priming and required GnRH prestimulation during 1 week in order to display gonadotropin-induced ovulation. Yet, the magnitude of such ovulatory responses was approximately half of that seen in control immature wild-type animals. Altogether, our data are the first to demonstrate that Kiss1r haplo-insufficiency induces a state of POF, which is not attributable to defective gonadotropin secretion. We also show that the failure of follicular development and ovulation linked to the absence of Kiss1r cannot be fully rescued by (even extended) gonadotropin replacement. These findings suggest a direct ovarian role of kisspeptin signaling, the perturbation of which may contribute to the pathogenesis of POF.
Acquisition and maintenance of female reproductive capacity require the complex interplay of numerous regulatory signals that impinge at different levels of the so-called hypothalamic-pituitary-ovarian (HPO) axis (1–3). This is a complex neurohormonal system that is responsible for the regulated secretion of ovarian hormones and the episodic release of oocytes at ovulation (2, 4). Notably, successful ovulation critically depends on an adequate gonadotropin drive, which is responsible for the stimulation of late stages of follicular growth, mainly via FSH secretion, and for oocyte extrusion, caused by the preovulatory surge of LH (2, 4). In turn, patterned gonadotropin secretion is regulated by the hypothalamic decapeptide, GnRH, which is under the control of a myriad of central transmitters and peripheral signals (2, 4, 5). Among the neuropeptide factors involved in the regulation of pulsatile GnRH secretion, kisspeptins, the product of the Kiss1 gene that act via the surface receptor, Kiss1r (aka, Gpr54), have been recognized in recent years as indispensable upstream activators of GnRH neurons that are essential for the control of the pulsatile and surge release of gonadotropins (6–8).
In addition to the above neuroendocrine pathways, follicular assembly, maintenance, and local regulation are equally important to guarantee oocyte survival and release at ovulation (9, 10). In fact, major attention has been paid in recent years to the elucidation of the intraovarian regulatory signals responsible for the control of follicular integrity and dynamics during the reproductive lifespan (9). Better understanding of these mechanisms may help to define the basis of premature ovarian failure (POF), a condition that affects 1% of reproductive-age women (11, 12), and is linked to the premature loss of viable oocytes (13). Interest and concern on POF has risen considerably in recent years, given the major changes in human reproductive habits, with later age at first pregnancy, and the suspected decline in reproductive efficiency in human populations worldwide (14–16).
Recent progress in the area of ovarian physiology and pathophysiology has allowed not only the identification of ovarian-specific pathways responsible for the dynamic regulation of follicular development and oocyte survival (9) but has suggested also the involvement of signaling systems known to operate at other levels of the HPO axis, including the nervous system, which might play a role in the direct control of oocyte and follicular physiology. Among such central factors, fragmentary, as yet compelling evidence has pointed out the possibility that kisspeptins may be produced and/or operate locally in the ovary to modulate specific aspects of ovarian function (6, 17). Thus, initial studies demonstrated the expression of Kiss1 and Kiss1r genes in the rat ovary (18), and detailed analyses have more recently documented the presence of kisspeptin and/or Kiss1r immunoreactivity (IR) in ovarian tissue from the rat, hamster, and primates, including humans (19–22). Moreover, ovarian expression of Kiss1 and kisspeptin-IR is cycle dependent in the rat and hamster (19, 22), with peak Kiss1 mRNA levels in the rat ovary at the preovulatory stage (19), a phenomenon induced by the preovulatory surge of gonadotropins (19). Likewise, ovarian Kiss1 expression was up-regulated by β-adrenergic stimulation in vitro (23). Conversely, models of perturbed ovulation, eg, by the blockade of cyclooxygenase and prostaglandin synthesis, have been linked to defective ovarian expression of Kiss1 (20), whereas suppressed kisspeptin and Kiss1r IR has been detected in the ovaries of photo-inhibited Siberian hamsters (22).
Although the above observations do not question the predominant actions of kisspeptins at central (hypothalamic) levels to regulate ovarian function (6), they raise the intriguing possibility that local kisspeptin signaling may participate in the fine tuning of specific ovarian functions. Yet, such a role has been mainly inferred on the basis of expression data (19–22), and direct supporting evidence has not been provided. We report here the ovarian phenotypes of mice with Kiss1r haplo-insufficiency and characterize the ovulatory responses of mice with congenital absence of kisspeptin receptors following different protocols of gonadotropin priming. Taken together with the results of the accompanying paper (24), our data support a direct, local role of kisspeptins signaling in the control of follicular dynamics and ovulation and strongly suggest that defective ovarian actions of kisspeptins accelerates ovarian ageing, leading to a state of POF.
Materials and Methods
Mutant mice
All mice were maintained on a 12-hour light, 12-hour dark cycle (lights off at 7:00 pm) with food and water available ad libitum. The experimental protocols were approved by the Córdoba University Ethical Committee of animal experimentation and conducted in accordance with the European Union guidelines for use of experimental animals. Mice carrying one deleted Kiss1r allele on a C57BL/6J background were obtained by crossing Kiss1rloxP/loxP mice with transgenic animals expressing Cre under the control of the Protamine1 promoter (Prm1Cre+) (25), which is expressed in male germ cells (26). To generate mice lacking both Kiss1r alleles (Kiss1r−/−), we crossed F1 Kiss1r+/− males and female mice, as described elsewhere (25). Kiss1r heterozygous and knockout mice were genotyped as previously described (25), using the primer pair 5′-AGC GCA AGG CTC TGA AGC GGC-3′ and 5′-CAA TGT CGC CTC GGT GGC CAT-3′ to detect the wild-type (WT) allele, and the primer pair, 5′-AGC GCA AGG CTC TGA AGC GGC-3′ and 5′-AAC AAC CCG TCG GAT TCT CCG-3′ for the mutant allele.
Quantification of ovarian follicles, histologic analysis, and fertility assessment in Kiss1r+/− mice
Analyses of ovarian morphology were performed on heterozygous Kiss1r mice at 3, 16, 32, and more than 48 weeks of age. Upon euthanasia of the animals, the right ovaries (including the oviduct) were removed, fixed in Bouin's fluid for 24 to 48 hours, and processed for paraffin embedding. Serial sections (7 μm thick) were cut, stained with hematoxylin and eosin, and viewed under an Eclipse 400 microscope. The number of preantral and antral follicles was determined in WT and Kiss1r+/− mice. Preantral follicles were counted to include the following follicle classes: 1) primordial follicles (P0), composed of a single layer of flattened pregranulosa cells surrounding the oocyte; 2) primary follicles (P1), with one layer of cuboidal granulosa cells, including transitional follicles composed of a mixture of flattened and cuboidal granulosa cells; and 3) secondary follicles (Sc), with 2–5 granulosa cell layers and measuring up to 150 μm in diameter (for representative examples of the different classes of preantral follicles in mice, see Supplemental Figure 1). Every eighth section was examined under the microscope at ×400 magnification. The number of resting (P0 and P1) and Sc follicles were recorded when the oocyte nucleus or nucleolus were present in the section, for P0-P1 and Sc follicles, respectively. One of every eighth ovarian section was scored, and the total number of follicles per ovary was obtained by multiplying the sum of counted follicles by 8. Based on their morphologic features (ie, presence of antral coalescence and follicular size > 150 μm), the number of antral follicles was also counted, using the same protocol as described above.
In addition, changes in the ovarian follicle population were evaluated in all ovarian sections by assessing oocyte loss, as defined by signs of oocyte (or follicular) atresia at any stage of follicle development. Degenerating oocytes were recognized by the presence of morphologic alterations, such as shrinkage and/or fragmentation (27, 28), at early follicle stages, or by morphologic changes in the oocyte and/or somatic follicle cells at more advanced phases of follicular development, including also shrinkage/absence of the granulosa layer or presence of apoptotic granulosa cells. Note that in both WT and Kiss1r+/− mice, the number of oocytes showing morphologic alterations suggestive of cell death in primordial or primary follicles was rather low and, therefore, follicle loss could not be directly quantified. Instead, it was estimated from the changes in the number of primordial follicles, as a decrease in the number of resting follicles, without a concomitant increase in the number of growing follicles, constitutes an indirect evidence of increased resting follicle loss. On the other hand, in antral follicles, oocyte loss was estimated by the number of oocytes displaying morphologic signs of cell death and/or follicles showing signs of atresia, as described above.
Finally, the end point of follicle development was scored in Kiss1r+/− mice by assessing the number of ovulated oocytes, as estimated by counting the number of ova in the oviduct and/or the number of corpora lutea of the current cycle, using standard procedures running in our laboratory (29, 30). As complementary analysis, we implemented a retrospective assessment of fertility of Kiss1r+/− mice, estimated, using as proxy marker, the number of live offspring per litter (ie, litter size) from hypomorph breeding pairs. In detail, 2 sets of analyses were implemented: 1) a cross-sectional comparison of fertility between WT and Kiss1r+/− dams at a young age, namely between females of 16–24 weeks of age; and 2) a longitudinal comparison of the decline in fertility observed in Kiss1r+/− dams with increasing age (at the following age intervals: 16–24 weeks, 24–32 weeks, 32–40 weeks, and 40–46 weeks). For both analyses, only the age of the dam was taken into consideration; the Kiss1r+/− males were usually below 32 weeks of age. Note that, because of the retrospective nature of our analyses, applied to our breeding colony, we could only gather data from WT (Kiss1r+/+ male × Kiss1r+/+ female) and heterozygous (Kiss1r+/− male × Kiss1r+/− female) breeding pairs. For the same reason, WT breeding data are restricted to young females (16–32 weeks), because mating of older WT dams was not systematically conducted for breeding purposes.
Quantification of ovulatory responses in Kiss1r−/− mice following gonadotropin priming
Ovulatory responses were assayed in adult Kiss1r-null (Kiss1r−/−) mice subjected to 2 different ovulation-induction protocols. First, standard gonadotropin priming (protocol 1), consisting of 2 doses of pregnant mare serum gonadotropin (PMSG) (5 IU) 24-hours apart, followed by a single bolus of human chorionic gonadotropin (hCG) (10 IU), was applied; ovarian tissues were sampled 24 hours after the hCG injection. Second, an extended protocol (protocol 2) consisting of one daily bolus of 1 μg of GnRH for 5-days, followed by 2 doses PMSG + 1 ovulatory dose of hCG (as described for protocol 1), was implemented. For comparative purposes, similar PMSG + hCG priming protocols were applied to WT (Kiss1r+/+) mice, used as controls. Importantly, due to their state of hypogonadotropic hypogonadism, Kiss1r-null mice display severe ovarian immaturity, with secondary follicles and some occasional early antral follicles (with signs of atresia) as the most advanced stage (31). For this reason, WT controls were not paired by age but rather by the stage of ovarian development. In fact, preliminary studies involving gonadotropin priming (protocol 1) were applied to infantile (15-day) and young immature (21-day-old) female mice, as well as to adult animals; these analyses revealed lack of ovulatory responses in infantile mice and nonphysiological superovulatory responses in adult animals (data not shown). Hence, to allow for proper comparison, in further studies standard gonadotropin priming, consisting of PMSG + hCG administration (protocol 1), was applied to immature WT female mice on postnatal day 21. At this age, the ovaries of WT mice display a degree of folliculogenesis similar to that induced by protocol 2 in Kiss1r−/− mice (prior to the final ovulatory dose of hCG), thus assuring proper comparison of differences in ovulatory responses between the 2 genotypes as function of the presence/absence of kisspeptin signaling and not of differences in the prevailing degree of ovarian maturity.
After application of the above protocols of gonadotropin priming, WT and Kiss1r−/− mice were euthanized, and the right ovaries (including the oviduct) were removed, fixed in Bouin's fluid for 24–48 hours, and processed for paraffin embedding. Serial ovarian sections (7 μm-thick) were cut and stained with hematoxylin and eosin; assessment of ovulation was conducted by scoring the number of ova in the oviduct and/or the number of newly formed corpora lutea.
Quantification of Kiss1r mRNA expression in Kiss1r+/− mice
Relative expression levels of Kiss1r mRNA were evaluated in ovarian and hypothalamic samples from WT and Kiss1r+/− mice, at 48 weeks of age. Procedures for hypothalamic and ovarian dissection, as well as real-time quantitative PCR analysis of Kiss1r expression, were implemented as described in detail elsewhere (29).
Gonadotropin measurements in Kiss1r+/− mice
Hormonal measurements were conducted in WT and Kiss1r+/− mice in 3 age ranges: less than 24, 24–32, and more than 48 weeks of age. Serum LH and FSH levels were measured using RIA kits supplied by the National Institutes of Health (Dr. A.F. Parlow, National Hormone and Peptide Program, Torrance, CA). Rat LH-I-10 and FSH-I-9 were labeled with 125I using Iodo-gen tubes, following the instructions of the manufacturer (Pierce Chemical Co). Hormone concentrations were expressed using reference preparations LH-RP-3 and FSH-RP-2 as standards. Intra- and interassay coefficients of variation were < 8 and < 10%, respectively.
Presentation of data and statistics
Data are presented as the means ± SEM. Differences among several groups were analyzed (GraphPad Prism) by ANOVA followed by the Student-Neuman-Keuls multiple-comparison tests. Differences between 2 groups were analyzed using Student's t tests. Values were considered significantly different if P < .05.
Results
Kiss1r haplo-insufficiency induces a phenotype of POF
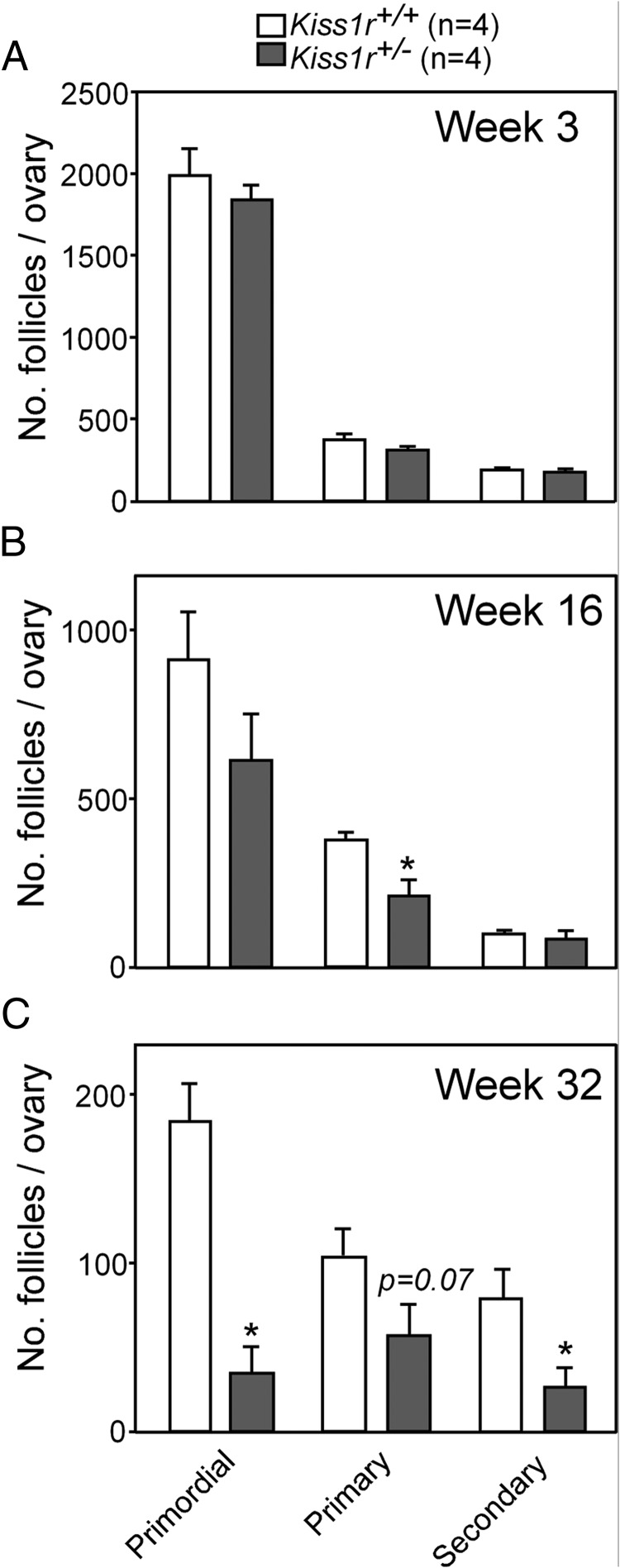
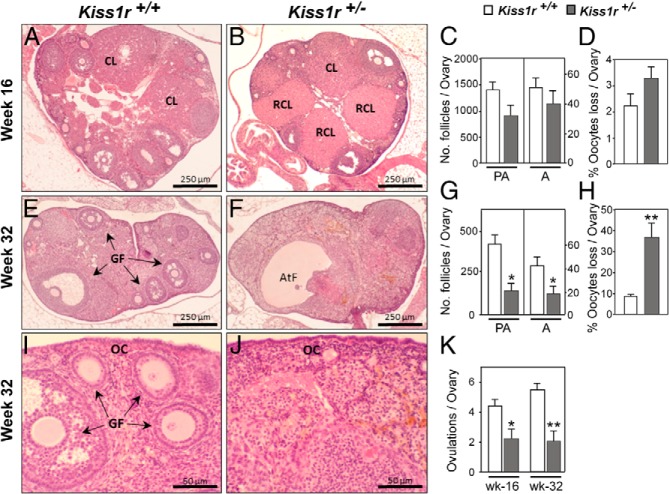
Kiss1 expression is negligible in prepubertal ovaries and increases abruptly at the time of the preovulatory surge of gonadotropins (19); yet, the functional role of local kisspeptin signaling remains unclear. To gain insights into the biological relevance of such local actions, we examined the ovarian phenotype of mice haplo-insufficient for Kiss1r (25). No significant differences in preantral follicle development, estimated by the total number and morphologic features, were observed between WT and Kiss1r+/− mice before puberty, ie, by 3 weeks of age (Figure 1A). Yet, progressive deterioration of ovarian architecture and follicular dynamics was detected in Kiss1r haplo-insufficient mice at later postnatal ages. Thus, by 16 weeks of age, histologic analyses of the ovaries of Kiss1r+/− mice revealed structures resembling persistent corpora lutea or luteinized foci of interstitial tissue (Figure 2, A and B). At this age, and despite a modest decrease in the number of primary follicles (Figure 1B), the total number of preantral and antral follicles was still statistically similar between WT and Kiss1r hypomorph mice (Figure 2C). Likewise, in 16-week-old Kiss1r+/− mice, the percentage of atretic follicles over the total number of antral follicles (40.7 ± 5.7 vs 34.4 ± 9 in WT), as well as the number (31.4 ± 4.2 vs 32.2 ± 6.5 in WT) and percentage (Figure 2D) of dying oocytes (scored as oocyte loss; see Materials and Methods), were not different from WT controls. Yet, the number of oocytes ovulated per ovary was reduced by 50% in mutant animals at this age (Figure 2K).
Figure 1.
Kiss1r haplo-insufficiency induces a progressive loss of ovarian follicles. Quantitative data from morphometric analyses of ovarian follicles, at early stages of folliculogenesis, in WT (Kiss1r+/+) and heterozygous (Kiss1r+/−) mice are shown, at 3 (A), 16 (B), and 32 weeks of age (C). Primordial, primary, and secondary preantral follicles were counted in serial 7-μm sections of ovaries from Kiss1r+/+ and Kiss1r+/− mice. No differences in the number of follicles at any stage of development were detected between genotypes at week 3, ie, before puberty. By 16 weeks of age, only the number of primary follicles was reduced significantly in Kiss1r+/− mice, but by 32 weeks of life there was a substantial loss of both primordial and secondary follicles, and a consistent trend for lower number of primary follicles in Kiss1r+/− mice (n = 4 mice/group). Bars represent means, and vertical bars are ± SEM. *, P < .05.
Figure 2.
Kiss1r haplo-insufficiency causes a precocious decline of ovarian follicular reserve and ovulatory defects. A and B, Representative photomicrographs of ovaries from 16-week-old WT (Kiss1r+/+) and heterozygous (Kiss1r+/−) mice. C, Number of preantral and antral follicles in each genotype. D, Percentage of dead oocytes within the ovary at 16 weeks compared with the total number of oocytes counted. E–J, Thirty-two week old ovaries from WT (E, higher magnification in I) and Kiss1r+/− mice (F, higher magnification in J). G, Number of preantral and antral follicles per ovary. H, Percentage of dead oocytes within the ovary at 32 weeks compared with the total number of oocytes counted. K, Mean number of ovulations at 16 and 32 weeks of age. For each genotype and age point, 6–8 independent ovaries per animals were used. *, P < .05; and **, P < .01 vs WT control. CL, corpus luteum; RCL, regressing corpus luteum; GF, growing follicle; AtF, atretic follicle; OC, ovarian cortex.
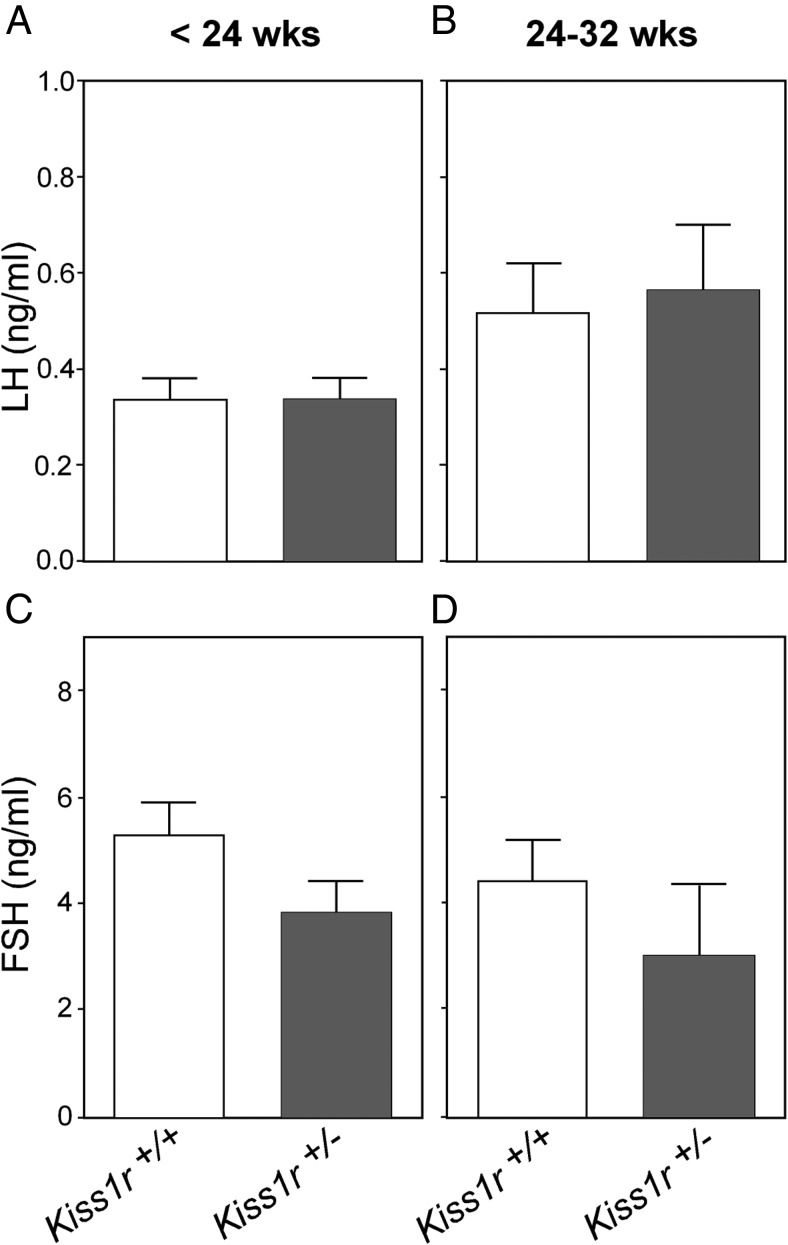
By 32 weeks of age, follicle loss became firmly established, with the ovaries of Kiss1r+/− mice showing a reduced number of both antral and preantral follicles (Figure 2, E–G). In fact, the incipient reduction in primordial, primary, and secondary preantral follicles first detected at 16 weeks of age, which was only significant for primary follicles at that age, became clearly evident at 32 weeks (Figure 1C). Note that notable reductions in the total numbers of all categories of preantral follicles were detected in 32-week-old Kiss1r+/− mice; yet, due to some variability, the drop in primary follicles, which was close to 45%, fell slightly below the limit of statistical significance (P = .07). In addition, at this age, the ovaries of some Kiss1r+/− mice displayed occasional cysts (Figure 2F), and an interstitial tissue of luteal-like appearance became the predominant feature of the ovarian parenchyma (Figure 2, F and J). This was accompanied by a substantial increase in the percentage of atretic follicles over the total number of antral follicles (68.5 ± 7.4 vs 39.5 ± 4.6 in WT), as well as in the number (53.6 ± 9 vs 35.6 ± 3.9 in WT) and percentage (Figure 2H) of dying oocytes (scored as oocyte loss), whereas there was a significant reduction in the number of ovulated oocytes per ovary at this age (Figure 2K). These deficiencies cannot be attributed to loss of gonadotropin support in haplo-insufficient mice, because neither serum FSH nor LH levels were different from WT at less than 24 or 32 weeks of age (Figure 3).
Figure 3.
Circulating gonadotropins levels in WT (Kiss1r+/+) and heterozygous (Kiss1r+/−) mice, at 2 different group ages: less than 24 weeks and 24–32 weeks of postnatal life. Hormonal values of at least 12 animals per group are presented. No significant differences between genotypes were detected, in striking contrast to the dramatic suppression of serum LH and FSH levels (>80% drop) detected in Kiss1r-null mice (data not shown) and the elevation of FSH levels observed at 48 weeks of age (see Figure 4).
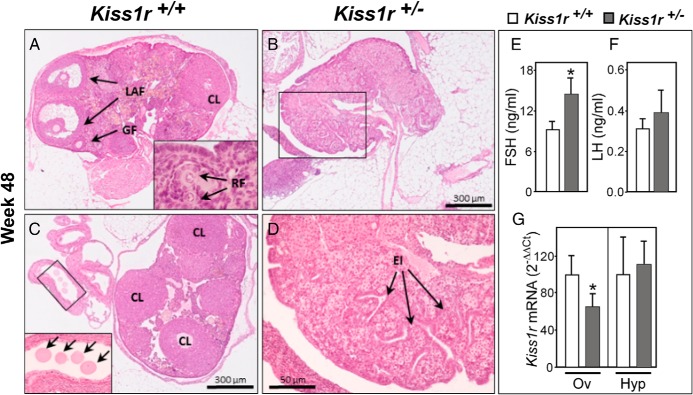
At more than 48 weeks of age, the loss of ovarian structures was definitive (see Figure 4). Thus, whereas WT ovaries continued to ovulate (Figure 4, A and C), the ovaries from Kiss1r+/− mice no longer did so (Figure 3, C and D). Moreover, whereas circulating LH remained unchanged, serum FSH levels were significantly greater in Kiss1r+/− mice than in WT at more than 48 weeks of age, thus suggesting a reduced gonadal negative feedback (Figure 4, E and F). In addition, we observed that Kiss1r mRNA abundance was reduced in the ovary, but not in the hypothalamus, of Kiss1r+/− mice at this age (Figure 4G).
Figure 4.
Kiss1r haplo-insufficiency causes premature ovarian failure. A, A representative ovary from a more than 48-week-old WT mouse, showing large antral follicles (long arrows) and growing preantral follicles (short arrows), is shown. Inset, resting follicles. B, Ovary from a pair-aged (>48-week-old) Kiss1r+/− mouse showing atrophy, absence of follicles, and deep surface epithelium invaginations. C, An ovary from a WT mouse of the same age showing numerous corpora lutea and ovulated oocytes in the fallopian tube (boxed area is magnified in inset). D, Magnification of boxed area in B. E and F, Serum FSH and LH levels in WT and Kiss1r+/− mice at more than 48 weeks of age. G, Kiss1r mRNA levels in the ovary and hypothalamus at the same age. For each genotype and age point, 6–8 independent ovaries/animals were used. *, P < .05 vs WT control. CL, corpus luteum; GF, growing follicle; OC, ovarian cortex; LAF, large antral follicle; EI, Epithelial invaginations. Released oocytes seen in the oviduct are denoted by arrows. Ov, ovary; Hyp, hypothalamus.
In good agreement with the ovarian phenotypic features described above, fertility analyses of Kiss1r+/− mice further supported that POF takes place in our hypomorph animals. Thus, as illustrated in Table 1, fertility of Kiss1r+/− breeding pairs, as estimated by the mean number of live offspring per litter, was significantly reduced vs that of WT animals already at a relatively young age (ie, between 16 and 24 weeks of age), in keeping with the ovulatory data (see Figure 2K). Moreover, a progressive decline in fertility was detected in Kiss1r+/− dams with early ageing, so that a significant (P = .01) 35% decrease of mean litter size was detected in 40- to 46-week-old Kiss1r+/− females vs heterozygous mice less than 32 weeks of age. Note that initial breeding attempts revealed that more than 48-week-old Kiss1r+/− dams are infertile and systematically failed to get pregnant after mating with Kiss1r+/− males of proven fertility (data not shown); because of these initial observations, crossings with more than 48-week-old Kiss1r+/− females were not routinely attempted and, for this reason, no data from this age-range are included in Table 1.
Table 1.
Assessment of Fertility, as Estimated by the Number of Live Offspring per Litter (Litter Size), Produced by Haplo-Insufficient Kiss1r+/− mice
| Fertility (Litter Size) | WT 16–24 Weeks | Kiss1r+/− 16–24 Weeks | Kiss1r+/− 24–32 Weeks | Kiss1r+/− 32–40 Weeks | Kiss1r+/− 40–46 Weeks |
|---|---|---|---|---|---|
| Mean | 7.3 | 4.87a | 4.91 | 3.78b | 3.17c |
| sem | 0.31 | 0.29 | 0.36 | 0.63 | 0.66 |
| N | 29 | 25 | 16 | 9 | 6 |
Both cross-sectional (ie, comparison of WT vs Kiss1r+/− dams of 16–24 weeks of age) and longitudinal (ie, comparison of Kiss1r+/− dams with increasing age) analyses are shown. N, Number of independent litters. Initial analyses revealed that >48-week-old Kiss1r+/− dams were infertile and systematically failed to get pregnant after mating with Kiss1r+/− males of proven fertility; thus, crossings with >48-week-old Kiss1r +/− females were not routinely attempted. Analysis of the offspring from Kiss1r+/− pairs revealed that the litters born to these animals had a roughly normal Mendelian ratio (Kiss1r−/−, 21.5%; Kiss1r+/−, 51.5%; and Kiss1r+/+, 27%). n = 40 litters.
P < .0001 vs WT mice (16–24 weeks old) (Student's t test).
P = .08 vs 16-to 24-week-old Kiss1r+/− dams (ANOVA followed by Student-Neuman-Keuls test).
P = 0.01 vs 16- to 24 week-old Kiss1r+/− dams (ANOVA followed by Student-Neuman-Keuls test).
Local Kiss1r signaling is required for complete ovulatory competence
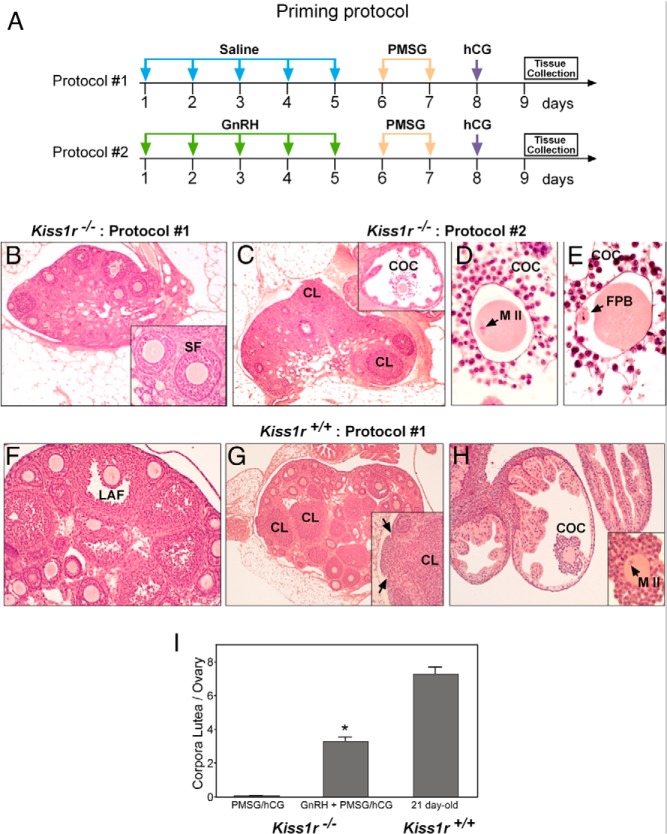
The importance of local Kiss1r signaling for ovulatory competence was further evidenced by experiments in which Kiss1r-null mice were treated with ovulatory doses of PMSG and hCG, without or with previous priming of the pituitary gland with GnRH (Figure 5A). Because of the lack of central kisspeptin effects, Kiss1r-deficient mice are hypogonadotropic and do not spontaneously ovulate (25, 31); yet, the predominance of such central actions of kisspeptins is likely to mask potential effects at other sites of the HPO axis, including the ovary (6). In fact, our histologic analyses confirmed that Kiss1r−/− female mice have small ovaries, with secondary follicles as the most advanced stage of follicular development, as described in detail elsewhere (32–34). Although some occasional early antral follicles were found in some animals, virtually all early-antral follicles showed signs of atresia, and no large antral follicles or corpora lutea were observed. However, Kiss1r-null mice can be forced to ovulate by gonadotropin priming (32), but the detailed characteristics of such ovulatory responses have not been reported to date.
Figure 5.
Follicular and ovulatory responses of adult Kiss1r−/− mice to gonadotropin stimulation. A, Schematic presentation of the two protocols used for hormone-induced ovulation: GnRH (1 μg/d) or saline for 5 days; PMSG (5 IU/mouse, ip), two boluses 24 hours apart; hCG (10 IU/mouse, ip), single injection. The ovaries were collected 24 hours after hCG. B, Kiss1r−/− mice do not ovulate in the absence of GnRH priming (protocol 1); in most cases, secondary follicles (inset) were the most advanced stage observed; large antral follicles and corpora lutea were always absent. C, Kiss1r−/− mice ovulate in response to gonadotropins (as assessed by the presence of corpora lutea) when pretreated with GnRH (protocol 2). Inset, Cumulus-oocyte complexes (COCs) in the oviduct. D and E, Normal appearance of oocytes in COCs retrieved from the oviduct of Kiss1r−/− mice. F–H, Immature (21-day-old) Kiss1r+/+ mice ovulate efficiently in response to Protocol 1, as evidenced by the presence of preovulatory follicles (F), corpora lutea (G, including the inset) and COCs in the oviduct (H); inset in H shows normal appearance of oocytes contained in these COCs. I, Quantification of the ovulatory response to hormonal treatment. *, P < .05 vs both Kiss1r−/− and WT mice not primed with GnRH (protocol 1). CL, corpus luteum; SF, secondary follicle; FPB, first polar body; MII, oocyte at metaphase-II.
Implementation of a standard protocol of gonadotropin priming, consisting of administration of PMSG + hCG to adult Kiss1r-deficient mice (without prior GnRH treatment) failed to induce complete follicular development and ovulation; thus, although early antral follicles were observed, these showed clear signs of atresia, and large antral follicles or corpora lutea were completely absent, which demonstrates the lack of ovulation (Figure 5B). In contrast, priming the pituitary with a daily bolus of GnRH for 5 days before PMSG administration enabled the ovary to respond to gonadotropins with ovulation (Figure 5, C–E). Newly formed corpora lutea were observed in the ovaries, and cumulus-oocyte complexes were found in the oviduct. Cumulus-oocyte complexes exhibited normal features, including progression to meiosis II (Figure 5D) and release of the first polar body (Figure 5E). In turn, immature 21-day-old WT mice ovulated efficiently in response to the standard protocol of gonadotropin priming (Figure 5, F–H), ie, in the absence of preceding GnRH priming; a procedure that was ineffective in Kiss1r-null animals. Although Kiss1r−/− mice did ovulate in response to the extended protocol of GnRH plus gonadotropin stimulation, null animals released significantly fewer oocytes than WT females exposed to standard gonadotropin priming, as assessed also by the number of corpora lutea detected in the ovaries (Figure 5I).
Discussion
The present study shows that Kiss1r haplo-insufficiency is associated with a discernible ovarian phenotype, characterized by a premature decline in the ovarian follicular reserve and early-onset anovulation, as revealed by detailed histologic analyses. This ovarian phenotype was closely mirrored by data from direct fertility assessments in our Kiss1r+/− mice, which display reduced litter size vs WT animals even at a relatively young age (16–24 weeks old), an index that further deteriorates with incipient ageing. In addition, even the combined replacement with GnRH and gonadotropins, as a means to bypass the central defect caused by the lack of kisspeptin signaling, was unable to fully rescue the ovulatory capacity of Kiss1r-null female mice. The latter model was selected as complementary to our ovarian analyses in Kiss1r haplo-insufficient mice, for proof-of-principle purpose: once POF was documented in Kiss1r+/− mice, despite preserved gonadotropin levels, studies in Kiss1r knockout mice were implemented to ascertain whether, in the absence of kisspeptin signaling, normal ovulatory responses could be achieved following the rescue of gonadotropin drive in Kiss1r-null animals.
Previous data had extensively documented the state of hypogonadotropic hypogonadism linked to the congenital absence of kisspeptin receptors (6, 32), a finding corroborated in our Kiss1r−/− mice. In line with a previous report (32), our Kiss1r-null mice could be forced to ovulate following gonadotropin stimulation. Yet, in this mouse line, the standard priming protocol, consisting of 2 boluses of PMSG followed by an ovulatory dose of hCG, was incapable of inducing ovulation. This seems to be in contrast to previous priming experiments, although the detailed features of the ovulatory responses in that previous study were not provided (32). In any event, application of a protocol of intensive ovarian stimulation by prepriming with daily doses of GnRH followed by administration of PMSG and an ovulatory dose of hCG did evoke ovulation. However, the magnitude of such responses was approximately half of that induced by gonadotropin priming alone (without prior GnRH stimulation) in immature WT mice. The fact that the ovulatory capacity of Kiss1r−/− mice is compromised even after restoration of the gonadotropin drive is fully compatible with a local role of kisspeptins in the direct control of folliculogenesis and/or ovulation. In line with such a local role, irregularities of estrous (ovarian) cyclicity, of as yet unknown basis, have been recently described in female rats after intraovarian treatment with a kisspeptin antagonist (23).
The mechanism underlying the direct ovarian role of kisspeptins and Kiss1r, as suggested by our phenotypic and hormonal analyses, is substantiated by the data of the accompanying study (24). This work suggests that local kisspeptins, produced by granulosa cells in response to gonadotropins, are required to activate NTRK2.FL receptor expression in the oocyte, thereby conveying the stimulatory effects of gonadotropins and driving a survival signal to the oocyte, which seems essential for maintenance of follicular integrity from puberty onward. Indeed, our previous studies had documented the ability of gonadotropins to increase ovarian Kiss1 expression, both during the pubertal transition and at the preovulatory stage (19). The fact that gonadotropin administration was unable to induce the ovarian expression of NTRK2.FL receptors specifically in Kiss1r−/− mice further demonstrates the obligatory role of ovarian kisspeptin signaling in mediating the stimulatory effect of gonadotropins on NTRK2.FL (24).
The similarities in the POF phenotype displayed by mice with selective elimination of NTRK in oocytes (the OoNtrk2−/− mouse) and our Kiss1r haplo-insufficient mice, which presumable display defective local kisspeptin signaling, reinforces the idea that both pathways converge at some point in the direct control of follicular dynamics. Yet, the time course for the instauration of the POF phenotype diverges between these 2 mouse lines, because ovarian function declines much more rapidly in OoNtrk2−/− than in Kiss1r+/− mice. This is likely due to the expected difference in the severity of the underlying deficiency, as in OoNtrk2−/− mice, NTRK2.FL-mediated signaling is totally disrupted and fails to become operational in oocytes from puberty onward, whereas in Kiss1r+/− mice, local kisspeptin signaling, which is seemingly required for gonadotropins to activate NTRK2 expression in oocytes at puberty, is only partially reduced. In any event, the fact that POF takes place in Kiss1r haplo-insufficient mice in the face of preserved Kiss1r mRNA expression at the hypothalamus and circulating levels of gonadotropins further suggests the pathophysiological relevance of decreased local kisspeptin signaling in this phenomenon. Indeed, more than 48-week-old Kiss1r+/− mice displayed not only exhaustion of the follicular reserve, infertility, and anovulation, but also a rise of serum levels of FSH; a feature that argues in favor of a primary ovarian failure and is a hallmark of human POF (12).
Recent studies have demonstrated that selective elimination of Kiss1r from GnRH cells is sufficient to recapitulate the major phenotypic features of hypogonadotropic hypogonadism seen in individuals with global nullification of the kisspeptin receptor (35, 36), thus supporting a major central site of action for the reproductive effects of kisspeptins. Our present findings, including those of the accompanying study (24), do not refute the major primary role of central kisspeptin signaling in the control of the HPO axis (6) but instead help to more precisely understand the spectrum of key effects of kisspeptins in the regulation of female reproduction. In fact, the ovarian actions of kisspeptins would be subordinate to the effects of gonadotropins, in part due to their ability to induce Kiss1 expression in the ovary (19, 24). It is notable that Kiss1r+/− mice displayed reduced expression levels of Kiss1r mRNA in the ovary, thus suggesting that the ovaries of Kiss1r+/− mice are less sensitive to the local actions of kisspeptins. Although this phenomenon needs further characterization (eg, at the cellular level), it is notable that such putatively defective ovarian Kiss1r expression takes place in the presence of preserved gonadotropin levels, and even against presumable compensatory mechanisms that might have occurred to cope with the congenital absence of one allele of Kiss1r. To our knowledge, isolated heterozygous mutations of KISS1R/Kiss1r had not been associated to an overt reproductive phenotype in humans or mice (37); yet, double Kiss1/Kiss1r heterozygous mouse mutants did show some (moderate) reproductive alterations, which were attributed to a presumable state of GnRH deficiency (37). The insidious ovarian phenotype of Kiss1r+/− mice revealed by our present analyses may help to explain why ovarian alterations were not noticed in heterozygous mice in previous studies, as deterioration of the ovarian reserve and defective ovulation become apparent only with incipient ageing, and the state of POF could remain unnoticed in the absence of detailed histologic analyses of the ovaries during earlier stages of postnatal life. Comparison of present results with the eventual ovarian phenotype of haplo-insufficient Kiss1 mice might supply valuable information, provided that mouse lines with strictly similar genetic background were available.
POF is a heterogeneous condition of unexplained etiology in most cases, but for which a strong genetic component is suspected (38). Candidate genes underlying POF include several members of the TGF-β family with key roles in ovarian physiology, such as growth differentiation factor-9 and bone morphogenetic protein-15 (39). Intriguingly, gene dosage appears to be a major determinant factor for the putative etiological role of growth differentiation factor-9 and bone morphogenetic protein-15 in POF, because data from sheep models have documented that only homozygous carriers of mutations in those genes have impaired fertility, whereas heterozygous display increased fertility (40). Our present results document, for the first time, a (presumably primary) ovarian phenotype compatible with POF in mice with Kiss1r haplo-insufficiency. In addition to their physiological interest, these data call for a more careful evaluation of the potential contribution of isolated heterozygous mutations of KISS1R to POF in humans, and/or the eventual usefulness of screening for such genetic variants in order to identify women at higher risk of developing POF later in life, especially when interacting with other genetic traits and/or environmental factors.
Acknowledgments
This work was supported by grant BFI2011–25021 from the Spanish Ministry of Economy & Science (cofunded with European Union funds from FEDER program; to M.T.-S.), grants P08-CVI-03788 and P12-FQM-01943 from Junta de Andalucía, Spain (to M.T.-S.), and a Marie Curie International Outgoing Fellowship of the Seventh European Community Framework Programme (FP7-PEOPLE-2010-IOF) (to J.M.C.).
Present address for M.D.D.: Division of Metabolism, Endocrinology, and Nutrition, Department of Medicine, University of Washington, Seattle, WA 98109.
Disclosure Summary: The authors declare that no conflict of interest exists.
For News & Views see page 2751
- hCG
- chorionic gonadotropin
- HPO
- hypothalamic-pituitary-ovarian
- IR
- immunoreactivity
- PMSG
- pregnant mare serum gonadotropin
- POF
- premature ovarian failure
- WT
- wild-type.
References
- 1. Fink G. Neuroendocrine regulation of pituitary function: general principles. In: Conn PM, Freeman ME, eds. Neuroendocrinology in Physiology and Medicine. Totowa, NJ: Humana Press; 2000:107–134. [Google Scholar]
- 2. Tena-Sempere M, Huhtaniemi I. Gonadotropins and gonadotropin receptors. In: Fauser BCJM, ed. Reproductive Medicine - Molecular, Cellular and Genetic Fundamentals. New York, NY: Parthenon Publishing; 2003:225–244. [Google Scholar]
- 3. Ojeda SR, Skinner MK. Puberty in the rat. In: Neill JD, ed. The Physiology of Reproduction. San Diego, CA: Academic Press/Elsevier; 2006:2061–2126. [Google Scholar]
- 4. Schwartz NB. Neuroendocrine regulation of reproductive cyclicity. In: Conn PM, Freeman ME, eds. Neuroendocrinology in Physiology and Medicine. Totowa, NJ: Humana Press; 2000:135–146. [Google Scholar]
- 5. Christian CA, Moenter SM. The neurobiology of preovulatory and estradiol-induced gonadotropin-releasing hormone surges. Endocr Rev. 2010;31:544–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pinilla L, Aguilar E, Dieguez C, Millar RP, Tena-Sempere M. Kisspeptins and reproduction: physiological roles and regulatory mechanisms. Physiol Rev. 2012;92:1235–1316. [DOI] [PubMed] [Google Scholar]
- 7. Uenoyama Y, Tsukamura H, Maeda KI. Kisspeptin/metastin: a key molecule controlling two modes of gonadotrophin-releasing hormone/luteinising hormone release in female rats. J Neuroendocrinol 2009;21:299–304. [DOI] [PubMed] [Google Scholar]
- 8. Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev. 2009;30:713–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Edson MA, Nagaraja AK, Matzuk MM. The mammalian ovary from genesis to revelation. Endocr Rev. 2009;30:624–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Richards JS. Perspective: the ovarian follicle–a perspective in 2001. Endocrinology 2001;142:2184–2193. [DOI] [PubMed] [Google Scholar]
- 11. Coulam CB, Adamson SC, Annegers JF. Incidence of premature ovarian failure. Obstet Gynecol. 1986;67:604–606. [PubMed] [Google Scholar]
- 12. Kokcu Premature ovarian failure from current perspective. Gynecol Endocrinol. 2010;26:555–562. [DOI] [PubMed] [Google Scholar]
- 13. Williams SA, Stanley P. Premature ovarian failure in mice with oocytes lacking core 1-derived O-glycans and complex N-glycans. Endocrinology. 2011;152:1057–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lassen TH, Sobotka T, Jensen TK, Jacobsen R, Erb K, Skakkebaek NE. Trends in rates of natural conceptions among Danish women born during 1960–1984. Hum Reprod. 2012;27:2815–2822. [DOI] [PubMed] [Google Scholar]
- 15. Andersson AM, Jørgensen N, Main KM, et al. Adverse trends in male reproductive health: we may have reached a crucial 'tipping point'. Int J Androl. 2008;31:74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Skakkebaek NE, Jørgensen N, Main KM, et al. Is human fecundity declining? Int J Androl. 2006;29:2–11. [DOI] [PubMed] [Google Scholar]
- 17. Roa J, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. New frontiers in kisspeptin/GPR54 physiology as fundamental gatekeepers of reproductive function. Front Neuroendocrinol. 2008;29:48–69. [DOI] [PubMed] [Google Scholar]
- 18. Terao Y, Kumano S, Takatsu Y, et al. Expression of KiSS-1, a metastasis suppressor gene, in trophoblast giant cells of the rat placenta. Biochim Biophys Acta 2004;1678:102–110. [DOI] [PubMed] [Google Scholar]
- 19. Castellano JM, Gaytan M, Roa J, et al. Expression of KiSS-1 in rat ovary: putative local regulator of ovulation? Endocrinology 2006;147:4852–4862. [DOI] [PubMed] [Google Scholar]
- 20. Gaytán F, Gaytán M, Castellano JM, et al. KiSS-1 in the mammalian ovary: distribution of kisspeptin in human and marmoset and alterations in KiSS-1 mRNA levels in a rat model of ovulatory dysfunction. Am J Physiol Endocrinol Metab 2009;296:E520–531. [DOI] [PubMed] [Google Scholar]
- 21. Cejudo Roman A, Pinto FM, Dorta I, et al. Analysis of the expression of neurokinin B, kisspeptin, and their cognate receptors NK3R and KISS1R in the human female genital tract. Fertil Steril. 2012;97:1213–1219. [DOI] [PubMed] [Google Scholar]
- 22. Shahed A, Young KA. Differential ovarian expression of KiSS-1 and GPR-54 during the estrous cycle and photoperiod induced recrudescence in Siberian hamsters (Phodopus sungorus). Mol Reprod Dev. 2009;76:444–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ricu MA, Ramirez VD, Paredes AH, Lara HE. Evidence for a celiac ganglion-ovarian kisspeptin neural network in the rat: intraovarian anti-kisspeptin delays vaginal opening and alters estrous cyclicity. Endocrinology. 2012;153:4966–4977. [DOI] [PubMed] [Google Scholar]
- 24. Dorfman MD, Garcia-Rudaz C, Alderman Z, et al. Loss of NTRK2/KISS1R signaling in oocytes causes premature ovarian failure Endocrinology 2014;155:3098–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. García-Galiano D, van Ingen Schenau D, Leon S, et al. Kisspeptin signaling is indispensable for neurokinin B, but not glutamate, stimulation of gonadotropin secretion in mice. Endocrinology 2012;153:316–328. [DOI] [PubMed] [Google Scholar]
- 26. O'Gorman S, Dagenais NA, Qian M, Marchuk Y. Protamine-Cre recombinase transgenes efficiently recombine target sequences in the male germ line of mice, but not in embryonic stem cells. Proc Natl Acad Sci U S A. 1997;94:14602–14607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hirshfield AN. Size-frequency analysis of atresia in cycling rats. Biol Reprod. 1988;38:1181–1188. [DOI] [PubMed] [Google Scholar]
- 28. Morita Y, Tilly JL. Oocyte apoptosis: like sand through an hourglass. Dev Biol 1999;213:1–17. [DOI] [PubMed] [Google Scholar]
- 29. Pinilla L, Castellano JM, Romero M, Tena-Sempere M, Gaytán F, Aguilar E. Delayed puberty in spontaneously hypertensive rats involves a primary ovarian failure independent of the hypothalamic KiSS-1/GPR54/GnRH system. Endocrinology. 2009;150:2889–2897. [DOI] [PubMed] [Google Scholar]
- 30. Roa J, Garcia-Galiano D, Varela L, et al. The mammalian target of rapamycin as novel central regulator of puberty onset via modulation of hypothalamic Kiss1 system. Endocrinology. 2009;150:5016–5026. [DOI] [PubMed] [Google Scholar]
- 31. Colledge WH. Transgenic mouse models to study Gpr54/kisspeptin physiology. Peptides. 2009;30:34–41. [DOI] [PubMed] [Google Scholar]
- 32. Seminara SB, Messager S, Chatzidaki EE, et al. The GPR54 gene as a regulator of puberty. N Engl J Med 2003;349:1614–1627. [DOI] [PubMed] [Google Scholar]
- 33. Lapatto R, Pallais JC, Zhang D, et al. Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology 2007;148:4927–4936. [DOI] [PubMed] [Google Scholar]
- 34. Funes S, Hedrick JA, Vassileva G, et al. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun 2003;312:1357–1363. [DOI] [PubMed] [Google Scholar]
- 35. Kirilov M, Clarkson J, Liu X, et al. Dependence of fertility on kisspeptin-Gpr54 signaling at the GnRH neuron. Nat Commun. 2013;4:2492. [DOI] [PubMed] [Google Scholar]
- 36. Novaira HJ, Sonko ML, Hoffman G, et al. Disrupted kisspeptin signaling in GnRH neurons leads to hypogonadotrophic hypogonadism. Mol Endocrinol. 2014;28:225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chan YM, Broder-Fingert S, Paraschos S, et al. GnRH-deficient phenotypes in humans and mice with heterozygous variants in KISS1/Kiss1. J Clin Endocrinol Metab 2011;96:E1771–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Persani L, Rossetti R, Cacciatore C, Fabre S. Genetic defects of ovarian TGF-β-like factors and premature ovarian failure. J Endocrinol Invest. 2011;34:244–251. [DOI] [PubMed] [Google Scholar]
- 39. Otsuka F, McTavish KJ, Shimasaki S. Integral role of GDF-9 and BMP-15 in ovarian function. Mol Reprod Dev. 2011;78:9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liao WX, Moore RK, Shimasaki S. Functional and molecular characterization of naturally occurring mutations in the oocyte-secreted factors bone morphogenetic protein-15 and growth and differentiation factor-9. J Biol Chem. 2004;279:17391–17396. [DOI] [PubMed] [Google Scholar]