Abstract
The experiment investigated the effect of maternal dietary supplementation of seaweed-derived polysaccharides (SDP) (–SDP v. +SDP, n 20) from day 83 of gestation until weaning (day 28) on selected sow faeces and piglet digesta microbiota populations, piglet small-intestinal morphology, and intestinal nutrient transporter and inflammatory cytokine gene expression at birth, 48 h after birth and weaning. The effect of maternal dietary treatment on the piglet gene expression profile of inflammatory cytokines in the colon following a lipopolysaccharide (LPS) challenge was also investigated. Dietary SDP reduced sow faecal Enterobacteriaceae gene numbers at parturition. Small-intestinal morphology, nutrient transporter and cytokine gene expression in newborn piglets did not differ between maternal dietary treatments (P > 0·10). At 48 h after birth, sodium–glucose-linked transporter 1 gene expression was down-regulated in the ileum of piglets suckling the SDP-supplemented sows compared with those suckling the basal sows (P = 0·050). There was a SDP × LPS challenge interaction on IL-1 and IL-6 gene expression in the colon of piglets (P < 0·05). The gene expression of IL-1 and IL-6 was down-regulated in the LPS-challenged colon of piglets suckling the SDP sows compared with those suckling the basal sows (P < 0·05). However, there was no difference in IL-1 and IL-6 gene expression in the unchallenged colon between treatment groups. At weaning, piglets suckling the SDP-supplemented sows had increased villus height in the jejunum and ileum compared with those suckling the basal-fed sows (P < 0·05). In conclusion, maternal dietary SDP supplementation enhanced the immune response of suckling piglets and improved gut morphology, making them more immune competent to deal with post-weaning adversities.
Key words: Cytokines, Intestinal morphology, Microbiota, Piglets, Seaweed-derived polysaccharides
Abbreviations: BW, body weight; CD, crypt depth; cDNA, complementary DNA; Ct, cycle threshold; FABP2, fatty acid binding protein 2; FOXP3, forkhead box P3; GCN, gene copy number; GIT, gastrointestinal tract; GLUT1, glucose transporter 1; HMBS, hydroxymethyl-bilane synthase; IFN-γ, interferon γ; LPS, lipopolysaccharide; PEPT1, peptide transporter 1; PPIA, peptidylprolylisomerase A; RT-qPCR, real-time PCR; SDP, seaweed-derived polysaccharide; SGLT1, sodium–glucose-linked transporter 1; TGF-β1, transforming growth factor β1; VH, villus height
Piglets are born in an agammaglobulinaemic state(1), due to the six-layered placenta of the dam(2). The gastrointestinal tract (GIT) of the piglet encounters numerous challenges at birth and weaning(3). Immediately after birth, the neonatal GIT is rapidly colonised by bacteria originating from the mother or the environment(4). It has become clear that this primary colonisation is important for the right development and programming of the animal's local and systemic immune system(5). Furthermore, the GIT takes complete responsibility for the provision of nutrients, and shifts from processing amniotic fluid swallowed during and after birth to a nutrient-enriched colostrum ingested after birth(3). At weaning, pigs are exposed to a large number of stressors, such as separation from the dam, transition from milk to a diet based on plant polysaccharides(6), and withdrawal of maternal IgA from milk that acts locally in the intestine of suckling piglets(7). These factors combined can disturb the intestinal immune system and microbiota equilibrium(8), which contribute toward a decrease in daily gain immediately post-weaning. In order to adapt to the changes encountered at birth and weaning, the GIT undergoes accelerated tissue growth (birth) and functional maturation (birth and weaning)(9), characterised by increases in intestinal weight and length, villus height (VH) and crypt depth (CD), cell migration rate, RNA and DNA contents, and the adaptation of intestinal enzymic activity(10). The intestinal epithelium is not only a physical barrier and a nutritional site; it also acts as a crucial regulator of intestinal immune homeostasis(11). In response to bacteria colonisation at birth and weaning, the enterocytes can act as a component of the immune system by secreting cytokines(12,13).
Recent research has indicated that supplementing sows with seaweed-derived polysaccharides (SDP), containing laminarin and fucoidan, bioactives containing antimicrobial, prebiotic and immunomodulatory properties, from day 107 of gestation influenced piglet immune status at weaning, and had beneficial effects on weaning-associated intestinal dysfunction and growth depression immediately after weaning(14–18). However there is no information about the effect of maternal dietary treatment of SDP on intestinal dysfunction immediately after birth and after ingestion of colostrum. Furthermore, Leonard et al.(17) found no effect of maternal SDP supplementation on piglet body weight (BW) at birth. The starting date of the supplementation (day 107 of gestation)(17) may have been too late to promote an effect on piglet birth weight. Studies have been shown that the nutrient needs for fetal growth increase from day 69 of gestation(19), and the transfer of nutrients to the fetus increases between days 90 and 100 of gestation(20).
Thus, the objective of the present study was to investigate the effect of maternal dietary supplementation of SDP from day 83 of gestation until weaning (day 28) on piglet BW at birth, and small-intestinal morphology, nutrient transporter and inflammatory cytokine gene expression, and colonic microbiota population of piglets at birth (0 h), 48 h after birth and weaning. The effect of maternal dietary treatment on the piglets’ gene expression profile of inflammatory cytokines in the colon following a lipopolysaccharide (LPS) challenge was also investigated. The hypothesis of this study is that maternal SDP supplementation from day 83 of gestation would improve piglet BW at birth and modulate selected intestinal microbial populations, the inflammatory response and aspects of intestinal health of piglets at birth and during the suckling period, making them more immune competent to deal with post-weaning adversities.
Materials and methods
All procedures described in the present experiment were conducted under experimental license from the Irish Department of Health in accordance with the Cruelty to Animals Act 1876 and the European Communities (Amendments of the Cruelty to Animals Act, 1876) Regulations.
Experimental design and dam diets – gestation and lactation period
A total of twenty crossbred pregnant gilts (Large White × Landrace genetic lines; Hermitage) were randomly assigned to one of the two dietary treatments (ten gilts/treatment): (T1) basal gestation/lactation diet (control) and (T2) basal gestation/lactation diet supplemented with 10·0 g SDP/d from day 83 of gestation until weaning (day 28). The quantity of SDP (Bioatlantis Ltd) used was based on previous work by Leonard et al.(17). The SDP supplement (10·0 g/d) contained laminarin (1·0 g), fucoidan (0·8 g) and ash (8·2 g), and was extracted from a Laminaria spp. according to the procedure described by Lynch et al.(21). The gestation diet contained 140 g/kg of crude protein (CP), 13·5 MJ/kg of digestible energy (DE) and 4·4 g/kg of standardised ileal digestible (SID) lysine. The lactation diet contained 190 g/kg of CP, 14·5 MJ/kg of DE and 8·5 g/kg of SID lysine. All amino acid requirements were met relative to lysine(22). The ingredient composition and chemical analysis of the diets are given in Table 1.
Table 1.
Ingredients and chemical composition of the experimental diets (g/kg, unless otherwise indicated)
| Gestation diet* | Lactation diet* | |
|---|---|---|
| Composition | ||
| Wheat | 303·8 | 352·5 |
| Barley | 300·0 | 300·0 |
| Soyabean meal | 67·0 | 182·0 |
| Dried maize distillers grains | 60·0 | 100·0 |
| Soya hulls | 70·0 | |
| Beet pulp | 100·0 | |
| Soya oil | 70·0 | 30·0 |
| Vitamins and minerals† | 3·0 | 2·5 |
| Salt | 3·0 | 5·0 |
| Dicalcium phosphate | 11·2 | 12·0 |
| Limestone | 12·0 | 12·0 |
| Lysine HCl | 2·0 | |
| dl-Methionine | 1·0 | |
| l-Threonine | 1·0 | |
| Analysis of chemical composition | ||
| DM | 870·9 | 873·2 |
| Crude protein (N × 6·25) | 140·0 | 190·0 |
| Gross energy (MJ/kg) | 16·9 | 17·1 |
| Ash | 55·2 | 57·1 |
| Digestible energy (MJ/kg)‡ | 13·5 | 14·5 |
| Lysine‡ | 5·5 | 10·0 |
| Methionine and cysteine‡ | 3·3 | 6·0 |
| Threonine‡ | 3·85 | 7·0 |
| Tryptophan‡ | 1·0 | 1·8 |
| Ca‡ | 8·7 | 9·3 |
| P‡ | 5·0 | 5·2 |
T1 = basal diet; T2 = basal diet supplemented with 10·0 g seaweed-derived polysaccharides containing laminarin and fucoidan/d.
Gilt/sow diet provided (per kg diet): 250 mg choline chloride; 140 mg Fe; 120 mg Zn as ZnO; 67 mg α-tocopherol; 47 mg Mn as MnO; 25 mg Cu as CuSO4; 12 mg nicotinic, acid; 10 mg pantothenic, acid; 4 mg phytylmenaquinone; 2 mg riboflavin; 2 mg thiamin; 1·8 mg retinol; 0·6 mg iodine as calcium iodate on a calcium sulphate/calcium carbonate carrier; 0·3 mg Se as sodium selenite; 0·025 mg cholecalciferol; 0·015 mg pyridoxine; 0·01 mg cyanocobalamin.
Calculated from amino acid and digestible energy values of ingredients(67).
Prior to day 83 of gestation, the gilts were housed in groups of ten. From day 83 until day 106 of gestation, they were housed individually in crates (2·0 × 0·6 m) in the gestation house. In the farrowing house, the gilts were housed individually in farrowing pens (2·2 × 2·4 m). The gestation and farrowing houses were maintained at 20°C throughout the experiment.
The experimental supplement (SDP) was top-dressed on the gestation diet and added to the trough prior to feeding the lactation diet each morning (09·00 hours) to ensure consumption. The dams received specific amounts of feed in the following quantities: 2·5 kg/d of gestation diet from day 83 until day 106 of gestation. They were fed 2·0 kg/d of lactation diet from day 107 of gestation until the day of farrowing and then the feed supply was increased by 1·0 kg/d until day 3 post-farrowing and by 0·5 kg/d until day 6 post-farrowing. Afterwards, the sows were allowed semi-ad libitum consumption of the diet, which was adjusted for each sow depending on daily intake. The sows were fed in three equal meals provided at 09·00, 13·00 and 17·00 hours. They had ad libitum access to drinking water throughout the experimental period.
On the expected farrowing date, fresh sow faecal samples (approximately 10 (sd 1·0) g) were collected from the ground into sterile containers (Sarstedt) and stored at −20°C for quantification (microbial genomic DNA analysis) of Lactobacillus spp. and Enterobacteriaceae.
Management of piglets and collection of colostrum samples
All farrowings were supervised. At parturition, piglets (Meat line boars × Large White × Landrace genetic lines gilts) were individually weighed and ear-tagged. Between 6 and 12 h after the birth of the last piglet, litter size was adjusted by cross-fostering piglets within sow dietary treatments to ensure that sows nursed a similar number of piglets (n 12 piglets/sow), and this was maintained throughout the suckling period. Cross-fostering performed on average at 20 h after birth has no adverse effects on growth performance(23) and IgG serum concentration(2) of both adopted and biological piglets. The individual piglet BW was recorded at birth and weaning and the average daily gain calculated from these data. The piglets received an intramuscular injection of Fe-dextran (Ferdex 100; Medion Farma Jaya) on day 7 after birth. No creep feed was offered to the piglets throughout the lactation period, and piglets did not have access to the sows’ feed.
During parturition, approximately 30 ml of colostrum sample was collected from each sow after hand-milking the first pair of mammary glands. To facilitate colostrum sampling, piglets were removed from the sow, and milk ejection was induced after administration of 1 ml of oxytocin (Pitocina, Watson Laboratories Inc.) into the marginal ear vein of the sow. The colostrum samples were immediately frozen at −20°C before analysis for IgG concentration.
A diarrhoea score was recorded daily on litter basis from birth to weaning using a scale from 0 to 3: (0) no diarrhoea; (1) slight; (2) middle; (3) acute(24). Diarrhoea score was performed by one trained person with no prior knowledge of the dietary treatment of the sows.
A total of sixteen piglets (n 8 piglets/treatment group, one piglet/sow) were selected at birth, with an average BW of 1·3 (sd 0·3) kg. The selection was based on the parturition of the third piglet. One trained operator was positioned at the back of the sow from the initiation of parturition, in order to make sure that the third piglet would not touch anything other than the sow vaginal tract and the gloved hand of the operator.
A total of sixteen piglets were selected at 48 h after birth (n 8 piglets/treatment group, one piglet/sow), with an average BW of 1·8 (sd 0·1) kg.
A total of sixteen piglets were selected at weaning (n 8 piglets/treatment group, one piglet/sow), with an average BW of 7·4 (sd 0·9) kg. The selection of piglets at 48 h after birth and weaning was based on the litter average BW.
The piglets were humanely killed on each day (immediately after birth (0 h), at 48 h after birth and at weaning) by lethal injection with pentobarbital sodium (Euthatal Solution, 200 mg/ml; Merial Animal Health Ltd) at a rate of 0·71 ml/kg BW to the cranial vena cava, and the entire intestinal tract was immediately removed.
Chemical analysis
The feed samples were milled through a hammer mill provided with a 1-mm screen (Christy and Norris hammer mill; Christy Turner Ltd). The gestation and lactation feed samples were analysed for N, DM, ash and gross energy as described by Heim et al.(25). The feed and dried faeces samples were milled through a hammer mill provided with a 1-mm screen (Christy and Norris). The DM of dried faeces and feed was determined after drying overnight at 103°C. Ash was determined after ignition of a known weight of concentrates or faeces in a muffle furnace (Nabertherm) at 500°C. The N content of both feed and faeces was determined using the LECO FP 528 instrument (Leco Instruments, U.K Ltd). Neutral-detergent fibre was determined using a Fibertec extraction unit (Tecator). The gross energy of the feed and faeces was determined using a Parr 1201 oxygen bomb calorimeter (Parr). The total laminarin content of the SDP supplement was determined using a Megazyme kit (Megazyme International Ireland Limited). Fucoidan levels were determined using the method of Usov et al.(26).
Collection of piglet tissue and digesta samples at birth (0 h), 48 h after birth and weaning
Immediately after slaughter, the entire intestinal tract was removed by blunt dissection and sections of the duodenum (10 cm from the stomach), jejunum (60 cm from stomach) and ileum (15 cm from caecum) were excised and fixed in 10 % phosphate-buffered formalin for VH and CD measurements. Ileum and colon tissues (second loop of the proximal colon) were excised, emptied by dissecting them along the mesentery and rinsed using sterile PBS (Oxoid). Two tissue sections of 1 cm2, which had been stripped of the overlying smooth muscle, were cut from each tissue. Then, one section from each tissue was placed in 1 ml of Dulbecco's modified Eagle's medium (Gibco, Life Technologies) in the presence or absence of bacterial LPS (source: Escherichia coli strain B4; Sigma Aldrich Ireland Limited) at a concentration of 10 mg/ml. Both LPS-challenged and unchallenged tissues were incubated at 37°C for 120 min before being removed, blotted dry and weighed. Approximately 1–2 g of the ileum and colon tissues were cut into small pieces and stored in 15 ml RNAlater® solution (Applied Biosystems) overnight at 4°C. RNAlater® was then removed before storing the samples at −80°C.
Colon digesta samples (approximately 2 g) were collected into sterile containers (Sarstedt) and stored at −20°C for quantification (microbial genomic DNA analysis) of Lactobacillus spp. and Enterobacteriaceae.
Colostrum IgG quantification
An assay for colostrum concentration of IgG was performed using a specific pig-ELISA IgG quantification kit (Bethyl Laboratories Inc.). For colostrum sample preparation, 10 ml of colostrum were centrifuged (15 min at 2500 g; Rotanta 460 R; Hettich Lab Technology) and 10 µl of the defatted fraction containing the IgG were added into 990 µl of 1× Dilution Buffer B to give a 1:100 dilution. The serial dilutions were repeated until the dilution recommended for colostrum: 1:1 000 000. The IgG was quantified according to the manufacturer's instructions.
Microbiology – DNA extraction and quantitative real-time PCR
Microbial genomic DNA was extracted from sow faeces and piglet colon digesta samples using a QIAamp DNA stool kit (Qiagen) in accordance with the manufacturer's instructions. Quantity and quality of DNA were assessed using a NanoDrop Spectrophotometer (ND1000, Thermo Scientific). Standard curves were prepared as described by O'Shea et al.(27). Briefly, genomic DNA from all samples was pooled and amplified through routine quantitative real-time PCR (RT-qPCR) using Lactobacillus spp. and Enterobacteriaceae primers. The primer sequences were as follows: Lactobacillus spp. – forward 5′–AGCAGTAGGGAATCTTCCA–3′ and reverse 5′–CACCGCTACACATGGAG–3′, 58°C; 341 bp and Enterobacteriaceae – forward 5′–CATTGACGTTACCCGCAGAAGAAGC–3′ and reverse 5′–CTCTACGAGACTCAAGCTTGC–3′, 58°C; 190 bp(28). All primers were designed using Primer Express™ Software (Applied Biosystems) and synthesised by MWG Biotech. Serial dilutions of these amplicons served to generate standard curves using RT-qPCR (ABI 7500 Real-Time PCR System; Applied Biosystems Ltd) permitting estimations of absolute quantification based on gene copy number (GCN)(29). The RT-qPCR were performed in a final reaction volume of 20 µl containing 2 µl template DNA, 1 µl of forward (100 pm) and 1 µl of reverse primers (100 pm), 10 µl SYBR Green PCR Master Mix (Applied Biosystems) and 6 µl nuclease-free water. The thermal cycling conditions involved an initial denaturation step at 95°C for 10 min followed by forty cycles of 95°C for 15 s and 65°C for 1 min. Dissociation analyses of the RT-qPCR product were performed to confirm the specificity of the resulting RT-qPCR products. All samples were prepared in duplicate. The mean cycle threshold (Ct) values of duplicates of each sample were used for calculations.
Small-intestinal morphology
The preserved segments (duodenum, jejunum and ileum) were prepared using standard paraffin-embedding techniques. The samples were sectioned at 5 µm thickness and stained with haemotoxylin and eosin(30). Measurements of fifteen well-orientated and intact villi and crypts were taken for each segment. The VH and the CD were measured as described by Heim et al.(25). The results are expressed as mean VH or CD in μm. The VH:CD ratio was calculated.
Nutrient transporter and inflammatory cytokine gene expression – RNA extraction, complementary DNA synthesis and quantitative real-time PCR
RNA was extracted from approximately 50 mg of ileum and colon tissue samples using the GenElute Mammalian Total RNA Miniprep Kit (Sigma Aldrich Corporation) according to the manufacturer's instructions. The total RNA was quantified using 1·5 µl of total RNA on a NanoDrop Spectrophotometer (ND1000; Thermo Scientific) and samples with a 260:280 ratio ≥2·0 were considered suitable for complementary DNA (cDNA) synthesis. Total RNA integrity (i.e. quality and quantity) was assessed by analysing 1 µl of total RNA using the Agilent 2100 Bioanalyser version A.02.12 (Agilent Technologies, Inc.) using RNA Nano LabChips® (Caliper Technologies Corporation). The cDNA synthesis was performed using 1 µg of total RNA and oligo(dT)20 primers in a final reaction volume of 20 µl using a Superscript™ III First-Strand synthesis kit (Invitrogen, Life Technologies) following the manufacturer's instructions. The final reaction volume of 20 µl was then adjusted to 250 µl using nuclease-free water. All primers for the selected nutrient transporter (peptide transporter 1 (PEPT1/SLC15A1), sodium–glucose-linked transporter 1 (SGLT1/SLC5A1), glucose transporter 1 (GLUT1/SLC2A1), GLUT2/SLC2A and fatty acid binding proteins 2 (FABP2/I-FABP)) and inflammatory cytokine (IL-1, IL-6, IL-8, IL-10, IL-12A (p35), IL-17R, TNF-α, interferon γ (IFN-γ), transforming growth factor β1 (TGF-β1) and forkhead box P3 (FOXP3)) genes were designed using Primer Express™ (Applied Biosystems) and synthesised by MWG Biotech. Primer sequences are presented in Table 2. The nutrient transporter and inflammatory cytokine genes chosen were selected based on previous work by Heim et al.(18,25). The efficiency of all primer sets was established using a semi-log curve of quantity v. control of 2-fold serial dilutions of cDNA as reported previously by Smith et al.(31). The following porcine reference genes were used as described previously by Ryan et al.(32): hydroxymethyl-bilane synthase (HMBS) and peptidylprolylisomerase A (PPIA) used for 0 h (immediately after birth) and weaning samples, and HMBS and tyrosine 3-mono-oxygenase/tryptophan 5-mono-oxygenase activation protein, zeta polypeptide (YWHAZ) used for 48 h after birth samples. The RT-qPCR was then carried out on cDNA using the ABI PRISM 7500 Fast sequence detection system for ninety-six-well plates (Applied Biosystems). All samples were prepared in duplicate using the SYBR Green Fast PCR Master Mix (Applied Biosystems), cDNA as the template and specific primers for the genes selected. For each reaction, 5 µl cDNA, 1·2 µl forward and reverse primer mix (300 nm), 3·8 µl nuclease-free water and 10 µl Fast SYBR Green PCR Master Mix were added and made up to a final volume of 20 µl. The two-step PCR programme was as follows: 95°C for 10 min for one cycle, followed by 95°C for 15 s and 60°C for 1 min for forty cycles.
Table 2.
Porcine oligonucleotide primers used for real-time PCR
| Gene | Accession no. | Primer (5′ 3′) 3′) |
Product length (bp) |
|---|---|---|---|
| IL-1 | NM_214029.1 | F: CAGCCAACGGGAAGATTCTG | 77 |
| R: AATGGCTTCCAGGTCGTCAT | |||
| IL-6 | NM_214399.1 | F:GACAAAGCCACCACCCCTAA | 69 |
| R:CTCGTTCTGTGACTGCAGCTTATC | |||
| IL-8 | NM_213867.1 | F:TGCACTTACTCTTGCCAGAACTG | 82 |
| R:CAAACTGGCTGTTGCCTTCTT | |||
| IL-10 | NM_214041.1 | F:GCCTTCGGCCCAGTGAA | 71 |
| R:AGAGACCCGGTCAGCAACAA | |||
| IL-12A (p35) | NM_213993.1 | F:CGTGCCTCGGGCAATTATA | 68 |
| R:CGCAGGTGAGGTCGCTAGTT | |||
| IL-17R | NM_001005729.1 | F:CAAGCGGTGGCGTTTTGCCT | 57 |
| R:GTCTCCGTCGGGGATGGGCT | |||
| IFN-γ | NM_213948.1 | F:TCTAACCTAAGAAAGCGGAAGAGAA | 81 |
| R:TTGCAGGCAGGATGACAATTA | |||
| TGF-β1 | NM_214015.1 | F:AGGGCTACCATGCCAATTTCT | 101 |
| R:CGGGTTGTGCTGGTTGTACA | |||
| TNF-α | NM_214022.1 | F:TGGCCCCTTGAGCATCA | 68 |
| R:CGGGCTTATCTGAGGTTTGAGA | |||
| FOXP3 | NM_001128438.1 | F:GTGGTGCAGTCTCTGGAACAAC | 68 |
| R:AGGTGGGCCTGCATAGCA | |||
| PPIA | NM_214353.1 | F: CGGGTCCTGGCATCTTGT | 75 |
| R: TGGCAGTGCAAATGAAAAACTG | |||
| HMBS | NM_001097412.1 | F: CTGAACAAAGGTGCCAAGAACA | 74 |
| R: GCCCCGCAGACCAGTTAGT | |||
| YWHAZ | XM_001927228.1 | F: GGACATCGGATACCCAAGGA | 71 |
| R: AAGTTGGAAGGCCGGTTAATTT |
F, forward; R, reverse; IFN-γ, interferon γ; TGF-β1, transforming growth factor β1; FOXP3, forkhead box P3; PPIA, peptidylprolylisomerase A; HMBS, hydroxymethyl-bilane synthase; YWHAZ, tyrosine 3-mono-oxygenase/tryptophan 5-mono-oxygenase activation protein, zeta polypeptide.
The raw Ct values for the reference genes were converted to relative quantities using the formula Q = EΔCt where E is the PCR efficiency of the assay and ΔCt is the value calculated for the difference between the lowest Ct value and the Ct value of the sample in question for each gene. The relative quantities of the endogenous controls were then analysed for stability in geNorm(33). The stability ‘M’ value generated by the geNorm application for the selected endogenous controls (β-actin (ACTB), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and PPIA) which was less than 1·5 indicated their suitability as endogenous controls for these intestinal samples. The geometric mean of the relative quantities for HMBS, YWHAZ and PPIA (normalisation factor) was then calculated using geNorm. The relative quantities were divided by the normalisation factor (obtained in geNorm) for that sample to give the final normalised relative expression for each target gene.
Statistical analysis
All data were analysed by using the General Linear Model procedure of SAS(34). All data were initially checked for normality using the Univariate procedure in SAS(34). Inflammatory cytokine and nutrient transporter gene expression in ileum tissue, small-intestinal morphology and Lactobacillus spp. and Enterobacteriaceae quantification data were analysed as a complete randomised design, with the piglet (sow pen) as the experimental unit. GCN of Lactobacillus spp. and Enterobacteriaceae were log-transformed before statistical analysis. Inflammatory cytokine gene expression in colon tissue data was analysed as a 2 × 2 factorial arrangement, with the piglet (sow pen) as the experimental unit. The statistical model used included the effects of maternal dietary treatment and challenge (LPS or PBS), and their associated two-way interactions. The sow lactation data were analysed as a complete randomised design, with the sow pen as the experimental unit. For piglet diarrhoea score, data were analysed by repeated-measures analysis using the PROC MIXED procedure of SAS. The model included the effects of maternal dietary treatment and time (days after birth) and the associated two-way interactions. The pdiff function of SAS was used to separate means. Simple regression models were used to determine the relationships between BW and small-intestinal morphology, between small-intestinal morphology and nutrient transporter gene expression, and between inflammatory cytokine and nutrient transporter gene expression. Animal (piglet) was modelled as a random effect. All data presented in the tables are expressed as least-square means with their standard errors. The probability level that denotes significance is P < 0·05, a numerical trend is P > 0·05 and P < 0·10, and not significant P > 0·10.
Results
Sow reproductive performance, colostrum IgG concentration and suckling piglet growth performance
Gilts offered diet supplemented with SDP had a longer gestation period compared with the basal-fed gilts (114·5 v. 113·5 (se 0·27) d; P < 0·05). Colostrum IgG concentration did not differ between maternal dietary treatments; however, it did tend towards significance (57·0 v. 92·4 (se 16·9) mg/ml, for basal-fed and SDP-supplemented sows, respectively; P = 0·07). Litter size (14·4 (se 0·72) piglets) and the number of live born piglets (12·9 (se 0·62) piglets) were not influenced by maternal dietary treatments (P > 0·10). Piglet BW at birth (1·76 (se 0·66) kg) and weaning (8·33 (se 0·22) kg), and average daily gain from birth to weaning (0·24 (se 0·07) kg/d) (P > 0·10) did not differ between sow dietary treatments.
Diarrhoea score
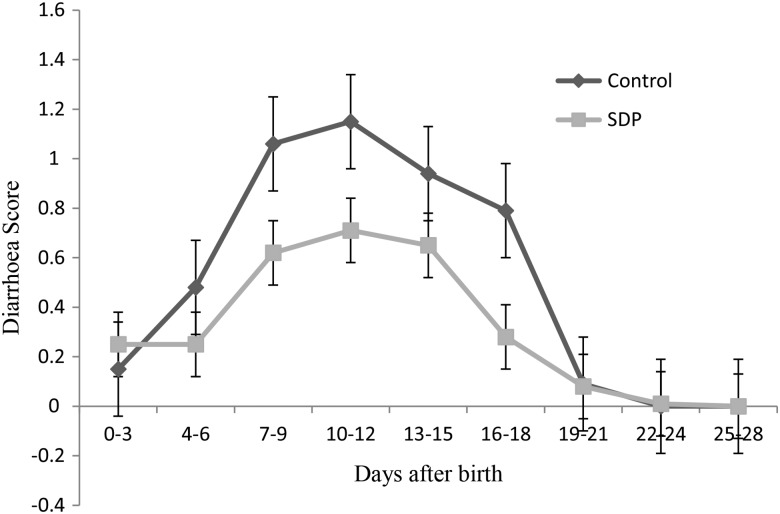
There was a day effect on piglet diarrhoea score (P < 0·05; Fig. 1). Piglets suckling the SDP-supplemented sows had a lower diarrhoea score during the lactation period compared with those suckling the basal-fed sows (P < 0·05; Fig. 1).
Fig. 1.
Differences in piglet diarrhoea score over time during days 0–3, 4–6, 7–9, 10–12, 13–15, 16–18, 19–21, 22–24 and 25–28 d post-birth. Diarrhoea score is measured on a scale from 0 to 3: (0) no diarrhoea; (1) slight; (2) middle; (3) acute(24). (■), Group supplemented with seaweed-derived polysaccharides (SDP) containing laminarin and fucoidan; (♦), control group. Values are means, with standard errors represented by vertical bars. There were SDP (P = 0·010) and time (P < 0·001) effects. There was no SDP × time interaction (P > 0·10).
Sow faecal and piglet colonic microbiota population
Sows supplemented with SDP had reduced Enterobacteriaceae log GCN/g of faeces on the expected parturition day compared with those fed the basal diet (P < 0·05; Table 3). Sow Lactobacillus spp. log GCN/g of faeces did not differ between dietary treatments (P > 0·10; Table 3).
Table 3.
Effect of maternal dietary treatment on selected microbiota gene numbers
(Least-square mean values with their standard errors)
| Control | SDP | sem | P | |
|---|---|---|---|---|
| Sows – expected farrowing date (log GCN/g faeces)* | ||||
| Lactobacillus spp. | 8·97 | 8·68 | 0·14 | 0·153 |
| Enterobacteriaceae | 8·55 | 7·76 | 0·24 | 0·031 |
| LER | 1·06 | 1·14 | 0·03 | 0·102 |
| 0 h – immediately after birth (log GCN/g colonic digesta)† | ||||
| Lactobacillus spp. | 6·91 | 7·01 | 0·12 | 0·591 |
| Enterobacteriaceae | 7·59 | 7·87 | 0·14 | 0·204 |
| LER | 0·91 | 0·89 | 0·01 | 0·342 |
| 48 h after birth (log GCN/g colonic digesta)† | ||||
| Lactobacillus spp. | 9·53 | 8·58 | 0·40 | 0·119 |
| Enterobacteriaceae | 10·01 | 10·34 | 0·30 | 0·453 |
| LER | 0·96 | 0·84 | 0·05 | 0·122 |
| Weaning (log GCN/g colonic digesta)† | ||||
| Lactobacillus spp. | 9·70 | 9·95 | 0·39 | 0·663 |
| Enterobacteriaceae | 9·14 | 9·08 | 0·43 | 0·921 |
| LER | 1·08 | 1·11 | 0·07 | 0·748 |
SDP, seaweed-derived polysaccharides containing laminarin and fucoidan; GCN, gene copy number; LER, ratio of Lactobacillus spp. to Enterobacteriaceae.
n 10 sows per treatment group.
n 8 piglets per treatment group.
Piglet Lactobacillus spp. and Enterobacteriaceae log GCN/g of colon digesta at birth (0 h), 48 h after birth and weaning did not differ between maternal dietary treatments (P > 0·10; Table 3).
Piglet small-intestinal morphology
The VH and the CD in the ileum showed a numerical increase from birth to weaning (VH average: 143 v. 406 µm, and CD average: 26 v. 126 µm at birth and weaning, respectively). Piglet small-intestinal morphology at birth and 48 h after birth did not differ between maternal dietary treatments (P > 0·10; Table 4). There was a positive linear effect of piglet birth weight on the VH in the ileum (R2 0·53; P < 0·001) and jejunum (R2 0·41; P < 0·01). There was also a positive linear effect of piglet BW at 48 h after birth on the VH in the jejunum (R2 0·41; P < 0·01).
Table 4.
Effect of maternal dietary treatment on small-intestinal morphology of piglets
(Least-square mean values with their standard errors)
| Control | SDP | sem | P | |
|---|---|---|---|---|
| 0 h – immediately after birth* | ||||
| Duodenum | ||||
| VH (μm) | 174 | 165 | 16·70 | 0·694 |
| CD (μm) | 24 | 23 | 1·36 | 0·499 |
| VH:CD | 7·3 | 7·3 | 0·71 | 0·999 |
| Jejunum | ||||
| VH (μm) | 186 | 176 | 16·32 | 0·674 |
| CD (μm) | 24 | 26 | 1·31 | 0·283 |
| VH:CD | 7·7 | 7·1 | 0·68 | 0·500 |
| Ileum | ||||
| VH (μm) | 136 | 154 | 16·71 | 0·465 |
| CD (μm) | 25 | 28 | 1·36 | 0·165 |
| VH:CD | 5·6 | 5·5 | 0·71 | 0·946 |
| 48 h after birth* | ||||
| Duodenum | ||||
| VH (μm) | 229 | 235 | 24·70 | 0·871 |
| CD (μm) | 30 | 29 | 2·46 | 0·756 |
| VH:CD | 7·6 | 8·2 | 0·78 | 0·600 |
| Jejunum | ||||
| VH (μm) | 179 | 225 | 24·45 | 0·188 |
| CD (μm) | 37 | 32 | 2·45 | 0·223 |
| VH:CD | 5·3 | 7·0 | 0·77 | 0·129 |
| Ileum | ||||
| VH (μm) | 160 | 198 | 24·58 | 0·277 |
| CD (μm) | 38 | 38 | 2·47 | 0·880 |
| VH:CD | 4·2 | 5·3 | 0·78 | 0·355 |
| Weaning* | ||||
| Duodenum | ||||
| VH (μm) | 465 | 465 | 23·60 | 0·984 |
| CD (μm) | 136 | 118 | 11·39 | 0·257 |
| VH:CD | 3·7 | 4·1 | 0·30 | 0·353 |
| Jejunum | ||||
| VH (μm) | 317 | 454 | 23·60 | <0·001 |
| CD (μm) | 93 | 121 | 11·39 | 0·089 |
| VH:CD | 3·6 | 3·8 | 0·30 | 0·707 |
| Ileum | ||||
| VH (μm) | 347 | 466 | 23·60 | 0·001 |
| CD (μm) | 144 | 108 | 11·39 | 0·032 |
| VH:CD | 2·5 | 4·4 | 0·30 | <0·001 |
SDP, seaweed-derived polysaccharides containing laminarin and fucoidan; VH, villus height; CD, crypt depth.
n 8 piglets per treatment group.
At weaning, piglets suckling the SDP-supplemented sows had increased VH in the jejunum and ileum compared with those suckling the basal-fed sows (P < 0·05). Piglets suckling the SDP-supplemented sows had reduced CD in the ileum compared with those suckling the basal-fed sows (P < 0·05). Piglets suckling the SDP-supplemented sows had an increased VH:CD ratio in the ileum compared with those suckling the basal-fed sows (P < 0·05). There was a positive linear effect of piglet BW at weaning on the VH in the duodenum (R2 0·35; P < 0·05).
Nutrient transporter gene expression
GLUT1, GLUT2 and PEPT1 are highly expressed at birth (relative abundance average: 1·38, 1·85 and 1·76 for GLUT1, GLUT2 and PEPT1, respectively), followed by a decline to the end of lactation (relative abundance average: 0·65, 0·41 and 0·43 for GLUT1, GLUT2 and PEPT1, respectively). The gene expression of FAPB2 and SGLT1 was lowly expressed at birth (relative abundance average: 1·04 and 0·83 for FABP2 and SGLT1, respectively), followed by an increase to the end of lactation (relative abundance average: 1·91 and 1·79 for FABP2 and SGLT1, respectively).
Nutrient transporter gene expression in ileum tissue of piglets at birth did not differ between maternal dietary treatments (P > 0·10; Table 5). At 48 h after birth, the gene expression of SGLT1 was down-regulated in the ileum of piglets suckling the SDP-supplemented sows compared with those suckling the basal-fed sows (P < 0·05; Table 5).
Table 5.
Effect of maternal dietary treatment on the normalised relative abundance of nutrient transporter gene expression in ileal tissue of piglets
(Least-square mean values with their standard errors)
| Control | SDP | sem | P | |
|---|---|---|---|---|
| 0 h – immediately after birth* | ||||
| PEPT1 | 1·68 | 1·86 | 0·25 | 0·626 |
| FABP2 | 0·97 | 1·12 | 0·25 | 0·676 |
| SGLT1 | 0·92 | 0·74 | 0·10 | 0·253 |
| GLUT1 | 1·41 | 0·99 | 0·25 | 0·255 |
| GLUT2 | 1·97 | 1·71 | 0·38 | 0·646 |
| 48 h after birth* | ||||
| PEPT1 | 1·79 | 2·00 | 0·27 | 0·593 |
| FABP2 | 1·12 | 1·27 | 0·30 | 0·732 |
| SGLT1 | 1·12 | 0·80 | 0·11 | 0·050 |
| GLUT1 | 1·73 | 1·38 | 0·38 | 0·536 |
| GLUT2 | 2·18 | 1·78 | 0·40 | 0·501 |
| Weaning* | ||||
| PEPT1 | 1·71 | 0·43 | 0·35 | 0·025 |
| FABP2 | 1·78 | 2·05 | 0·65 | 0·783 |
| SGLT1 | 1·42 | 2·23 | 0·44 | 0·226 |
| GLUT1 | 0·98 | 0·65 | 0·06 | 0·003 |
| GLUT2 | 1·76 | 0·41 | 0·36 | 0·025 |
SDP, seaweed-derived polysaccharides containing laminarin and fucoidan; PEPT, peptide transporter; FABP, fatty acid binding protein; SGLT, sodium–glucose-linked transporter; GLUT, glucose transporter.
n 8 piglets per treatment group.
At weaning, the gene expression of PEPT1, GLUT1 and GLUT2 was down-regulated in the ileum of piglets suckling the SDP-supplemented sows compared with those suckling the basal-fed sows (P < 0·05; Table 5). There was a negative linear effect of VH on GLUT1 gene expression in the ileum of piglets (R2 0·36; P < 0·05).
Inflammatory cytokine gene expression in the ileum
Inflammatory cytokine gene expression in ileum tissue of piglets at birth and 48 h after birth did not differ between maternal dietary treatments (P > 0·10; Table 6).
Table 6.
Effect of maternal dietary treatment on piglet ileal transcriptional response of genes related to the immune response
(Least-square mean values with their standard errors)
| Control | SDP | sem | P | |
|---|---|---|---|---|
| 0 h – immediately after birth* | ||||
| FOXP3 | 1·23 | 1·81 | 0·23 | 0·108 |
| IFN-γ | 1·09 | 1·62 | 0·18 | 0·102 |
| IL-1 | 0·92 | 0·90 | 0·13 | 0·907 |
| IL-6 | 1·19 | 1·17 | 0·18 | 0·926 |
| IL-8 | 4·71 | 4·33 | 0·42 | 0·598 |
| IL-10 | 1·43 | 2·09 | 0·32 | 0·173 |
| IL-12A(p35) | 0·76 | 1·02 | 0·09 | 0·101 |
| IL-17R | 0·45 | 0·51 | 0·05 | 0·476 |
| TGF-β1 | 1·18 | 1·50 | 0·18 | 0·210 |
| TNF-α | 2·02 | 2·86 | 0·44 | 0·210 |
| 48 h after birth* | ||||
| FOXP3 | 1·03 | 1·59 | 0·39 | 0·329 |
| IFN-γ | 1·45 | 1·39 | 0·47 | 0·934 |
| IL-1 | 1·31 | 0·97 | 0·45 | 0·609 |
| IL-6 | 1·60 | 1·85 | 0·47 | 0·713 |
| IL-8 | 2·04 | 2·81 | 0·77 | 0·491 |
| IL-10 | 1·58 | 1·64 | 0·36 | 0·902 |
| IL-12A(p35) | 1·19 | 1·15 | 0·22 | 0·911 |
| IL-17R | 1·46 | 1·00 | 0·22 | 0·156 |
| TGF-β1 | 1·46 | 1·24 | 0·30 | 0·605 |
| TNF-α | 1·22 | 1·69 | 0·30 | 0·288 |
| Weaning* | ||||
| FOXP3 | 1·24 | 1·73 | 0·17 | 0·101 |
| IFN-γ | 0·91 | 3·22 | 0·37 | 0·001 |
| IL-1 | 0·99 | 2·85 | 0·16 | <0·001 |
| IL-6 | 1·12 | 0·64 | 0·09 | 0·003 |
| IL-8 | 1·15 | 0·77 | 0·13 | 0·050 |
| IL-10 | 0·82 | 0·29 | 0·08 | <0·001 |
| IL-12A (p35) | 0·97 | 1·93 | 0·13 | <0·001 |
| IL-17R | 1·16 | 0·35 | 0·11 | <0·001 |
| TGF-β1 | 1·07 | 2·23 | 0·13 | <0·001 |
| TNF-α | 0·93 | 1·66 | 0·07 | <0·001 |
SDP, seaweed-derived polysaccharides containing laminarin and fucoidan; FOXP3, forkhead box P3; IFN-γ, interferon γ; TGF-β1, transforming growth factor β1.
n 8 piglets per treatment group.
There was a positive linear effect of piglet birth weight on IL-10 gene expression in the ileum (R2 0·41; P < 0·01). There was a negative linear effect of TNF-α gene expression on GLUT1 gene expression in the ileum of piglets at birth (0 h) (R2 < 0·31; P < 0·05). There was a negative linear effect of piglet BW at 48 h after birth on TNF-α (R2 0·36; P < 0·05) and IL-10 (R2 < 0·38; P < 0·01) gene expression in the ileum.
At weaning, the gene expression of IL-1, IL-12A (p35), IFN-γ, TGF-β1 and TNF-α was up-regulated and the gene expression of IL-10, IL-17R, IL-6 and IL-8 was down-regulated in the ileum of piglets suckling the SDP-supplemented sows compared with those suckling the basal-fed sows (P < 0·05; Table 6). There was a negative linear effect of TNF-α gene expression on GLUT1 (R2 0·51; P < 0·01), GLUT2 (R2 0·41; P < 0·01) and PEPT1 (R2 0·43; P < 0·01) gene expression in the ileum of piglets.
Inflammatory cytokine gene expression following an ex vivo lipopolysaccharide challenge in the colon
Inflammatory cytokine gene expression in piglet colon tissue following an ex vivo LPS challenge at birth did not differ between maternal dietary treatments (P > 0·10) (Table 7). The LPS challenge up-regulated the gene expression of IL-6, IL-8, IL-10 and TNF-α and down-regulated the gene expression of FOXP3, IL-1, IL-12A (p35), IL-17R, IFN-γ and TGF-β1 in the colon tissue of piglets at birth (P < 0·05) (Table 7).
Table 7.
Effect of maternal dietary treatment on piglet colonic transcriptional response genes related to the immune response following an ex vivo lipopolysaccharide (LPS) challenge
(Least-square mean values and pooled standard errors)
| SDP | LPS | P** | |||||
|---|---|---|---|---|---|---|---|
| No | Yes | No | Yes | sem* | SDP | LPS | |
| 0 h – immediately after birth† | |||||||
| FOXP3 | 0·66 | 0·54 | 0·81 | 0·40 | 0·06 | 0·153 | <0·001 |
| IFN-γ | 0·61 | 0·69 | 1·06 | 0·25 | 0·13 | 0·676 | <0·001 |
| IL-1 | 0·95 | 0·93 | 1·14 | 0·74 | 0·08 | 0·876 | 0·004 |
| IL-6 | 1·03 | 1·25 | 0·92 | 1·36 | 0·13 | 0·245 | 0·023 |
| IL-8 | 0·72 | 0·60 | 0·24 | 1·08 | 0·14 | 0·540 | <0·001 |
| IL-10 | 1·11 | 1·16 | 0·71 | 1·57 | 0·20 | 0·849 | 0·005 |
| IL-12A (p35) | 0·99 | 1·13 | 1·31 | 0·80 | 0·13 | 0·482 | 0·013 |
| IL-17R | 1·80 | 2·01 | 2·13 | 1·68 | 0·12 | 0·218 | 0·014 |
| TGF-β1 | 0·79 | 0·71 | 0·84 | 0·65 | 0·05 | 0·228 | 0·006 |
| TNF-α | 0·81 | 0·40 | 0·53 | 0·77 | 0·06 | 0·353 | 0·054 |
| 48 h after birth† | |||||||
| FOXP3 | 1·17 | 1·03 | 1·05 | 1·15 | 0·14 | 0·480 | 0·617 |
| IFN-γ | 1·77 | 1·51 | 1·63 | 1·64 | 0·53 | 0·729 | 0·993 |
| IL-1 | 2·16 | 1·30 | 1·41 | 2·05 | 0·46 | 0·196*** | 0·332 |
| IL-6 | 2·71 | 1·24 | 1·05 | 2·90 | 0·64 | 0·119*** | 0·052 |
| IL-8 | 1·75 | 0·97 | 1·21 | 1·52 | 0·39 | 0·170 | 0·573 |
| IL-10 | 2·06 | 1·43 | 1·43 | 2·06 | 0·51 | 0·390 | 0·390 |
| IL-12A (p35) | 1·13 | 1·06 | 1·05 | 1·14 | 0·12 | 0·666 | 0·619 |
| IL-17R | 1·11 | 1·04 | 1·13 | 1·02 | 0·13 | 0·687 | 0·529 |
| TGF-β1 | 1·13 | 1·06 | 1·08 | 1·11 | 0·11 | 0·628 | 0·873 |
| TNF-α | 1·51 | 1·30 | 1·40 | 1·41 | 0·29 | 0·619 | 0·990 |
| Weaning† | |||||||
| FOXP3 | 1·22 | 2·07 | 1·44 | 1·86 | 0·12 | <0·001 | 0·027 |
| IFN-γ | 1·64 | 2·96 | 2·03 | 2·57 | 0·40 | 0·028 | 0·348 |
| IL-1 | 1·24 | 1·06 | 1·42 | 0·88 | 0·22 | 0·574 | 0·101 |
| IL-6 | 0·98 | 0·33 | 0·64 | 0·67 | 0·13 | 0·002 | 0·901 |
| IL-8 | 1·92 | 1·84 | 0·93 | 2·83 | 0·41 | 0·892 | 0·003 |
| IL-10 | 2·01 | 1·71 | 0·80 | 2·92 | 0·39 | 0·588 | <0·001 |
| IL-12A (p35) | 1·38 | 1·47 | 1·18 | 1·67 | 0·27 | 0·811 | 0·207 |
| IL-17R | 1·20 | 1·00 | 1·01 | 1·19 | 0·10 | 0·199 | 0·240 |
| TGF-β1 | 0·95 | 1·25 | 1·12 | 1·08 | 0·10 | 0·048 | 0·827 |
| TNF-α | 1·55 | 1·72 | 1·08 | 2·19 | 0·36 | 0·738 | 0·040 |
SDP, seaweed-derived polysaccharides containing laminarin and fucoidan; FOXP3, forkhead box P3; IFN-γ, interferon γ; TGF-β1, transforming growth factor β1.
n 8 piglets per treatment group.
Pooled sem.
There was no SDP × LPS interaction (P > 0·10).
There was a SDP × LPS interaction (P < 0·05).
At 48 h after birth, there was an interactive effect between maternal dietary treatment and LPS challenge on the gene expression of IL-1 and IL-6 (P < 0·05) (Table 7). The gene expression of IL-1 and IL-6 was down-regulated in the LPS-challenged colon tissue of piglets suckling the SDP-supplemented sows compared with those suckling the basal-fed sows (P < 0·05). However, there was no difference in the gene expression of IL-1 and IL-6 in the unchallenged colon tissue between treatment groups (P > 0·10). The LPS challenge up-regulated the gene expression of IL-6 in the colonic tissue of piglets at weaning (P < 0·05) (Table 7).
At weaning, there was no maternal dietary treatment × LPS challenge interaction on cytokine gene expression (P > 0·10) (Table 7). The gene expression of FOXP3, IFN-γ and TGF-β1 was up-regulated and IL-6 was down-regulated in the colon tissue of piglets suckling the SDP-supplemented sows compared with those suckling the basal-fed sows (P < 0·05) (Table 7). The LPS challenge up-regulated the gene expression of FOXP3, IL-8, IL-10 and TNF-α in the colonic tissue of piglets at weaning (P < 0·05) (Table 7).
Discussion
The hypothesis of this study is that maternal SDP supplementation from day 83 of gestation would improve piglet BW at birth and modulate selected intestinal microbial populations, the inflammatory response and aspects of intestinal health of piglets at birth and during the suckling period, making them more immune competent to deal with post-weaning adversities. The positive response observed in piglets suckling the SDP-supplemented sows, such as down-regulation of pro-inflammatory cytokine gene expression, and improved intestinal morphology in the ileum, partially supports this hypothesis. Dietary supplementation of SDP increased the gestation length. Gestation length of supplemented animals was prolonged by 1 d compared with the basal-fed gilts. The sow's gestation length is an average of 114 d, with 85 % of farrowing concentrating between 114 and 116 d of gestation(35). Pig litters are unique: large litters at birth and the piglets are born relatively well developed. Much of this development takes place during the last few days of gestation(36). It seems likely that prolonged gestation would improve piglet maturation at birth, as the nutrient needs for fetal growth increase from day 69 of gestation(19), and the transference of nutrients to the fetus increases between days 90 and 100 of gestation(20). SDP contain sugars normally not present in plant dietary fibres(37,38). It was expected that these sugars, particularly laminarin, would provide nutrients to the fetus and consequently increase piglet birth weight. However, in the present study the increase in gestation length did not influence piglet BW at birth. There was also no effect of maternal dietary treatment on piglet BW at weaning. In agreement, Leonard et al.(17) reported that sow dietary supplementation of a similar SDP from day 109 of gestation did not favour the growth performance of piglets during the lactation period.
Maternal dietary SDP supplementation numerically increased colostrum IgG concentration (P = 0·07). This is probably due to immunomodulatory property of laminarin(39). Laminarin is absorbed from the ileum, as recent work by Heim et al.(25) showed that there was an increase in glucose transporters in the ileum following the ingestion of laminarin in weaned pigs. According to Bourne & Curtis(39), all colostral IgG is derived from the serum of the sow. The IgG uptake from serum is then mediated by specific Fc-dependent receptors on mammary epithelial cells(40,41). Previously, Leonard et al.(17) reported that maternal supplementation of a similar SDP from day 109 of gestation increased colostrum IgG concentration.
Aspects of intestinal health and immune status
The intestine is a major site of digestion, nutrient absorption, harbouring a complex microbiota and a highly evolved mucosal immune system(11,42). In the small intestine, the nutrients undergo a series of degradative steps carried out by digestive enzymes(43,44) that can then be efficiently absorbed via nutrient transporters expressed along the crypt–villus axis in the enterocytes(43). Under conventional practice, abrupt weaning is often associated with undesirable morphological changes in the small-intestinal architecture, which include villous atrophy and crypt hyperplasia(45,46). There was no effect of maternal dietary treatment on nutrient transporter gene expression in the ileum of newborn piglets (0 h). There was also no effect of maternal dietary treatment on small-intestinal morphology. There was a positive linear effect of piglet birth weight on VH in the ileum and jejunum. Maternal dietary supplementation of SDP resulted in a down-regulation of the gene expression of SGLT1 in the ileum of piglets at 48 h after birth, and PEPT1, GLUT1 and GLUT2 at weaning. It is still not clear why maternal dietary supplementation of SDP down-regulated protein and glucose transporter gene expression in the ileum of suckling piglets. First, it may be related to an increase in VH in the ileum of these piglets. According to the regression analysis, there was a negative relationship between VH and the gene expression of GLUT1 at weaning. However, in this study, only the mRNA abundance of these nutrient transporters was measured, and unfortunately the nutrient transporter proteins were not measured. The expression of SGLT1 is tightly linked to the villus architecture(47). Regulation of glucose transport by diet may involve increased transcription of SGLT1 mainly in crypt cells. As cells migrate to the villus, the mRNA is degraded, and transporter proteins are then inserted into the membrane, leading to increases in glucose transport about 1 d after an increase in carbohydrate levels. Endocrine factors regulate the development of intestinal brush-border enzymes during weaning, but do not regulate the development of intestinal glucose transporters(47). The absence of regulation of glucose transport in suckling animals that nurse on milk is due to animals consuming a diet that does not vary in carbohydrate composition(48). It is possible that SGLT1 in cells present in the small intestine since birth cannot be regulated, and dietary regulation begins only when these cells are eventually replaced. The difference in SGLT1 expression may have been due to difference in milk composition. Unfortunately, milk composition was not measured in this experiment. However, Leonard et al.(14) demonstrated that sow dietary supplementation of a similar SDP altered milk composition during lactation.
Second, the down-regulation of PEPT1 gene expression in the ileum of piglets suckling the SDP-supplemented sows at weaning may be attributed to the up-regulation of pro-inflammatory cytokine gene expression(49). This is supported by a negative relationship between TNF-α gene expression and PEPT1 gene expression in the ileum at weaning. According to Shu et al.(49), bacterial infections alter PEPT1 expression level in the intestine. Furthermore, in the current study, a panel of pro-inflammatory cytokines was up-regulated in the ileum of piglets suckling SDP-supplemented sows at weaning. Third, the down-regulation of nutrient transporter gene expression may be attributed to the polysaccharide fucoidan. Heim et al.(50) reported that when gilts were supplemented with fucoidan, from day 107 of gestation to weaning (day 24), the gene expression of SGLT1 was down-regulated in the ileum of piglets at weaning.
The present data also show that GLUT1, GLUT2 and PEPT1 are highly expressed at birth followed by a decline to the end of lactation. On the other hand, the gene expression of FAPB2 and SGLT1 was lowly expressed at birth, followed by an increase to the end of lactation. VH and CD in the ileum showed a numerical increase from birth to weaning. Interestingly, there was a positive linear effect of piglet BW at birth on VH in the ileum. However, no maternal dietary treatment effect was observed on piglet BW at birth and VH in the ileum. In addition, maternal dietary SDP supplementation positively changed piglet small-intestinal architecture at weaning (increased VH and VH:CD ratio). The VH:CD ratio is a useful criterion for assessing intestinal health and function(46). This increase in VH and the VH:CD ratio may be attributed to the reduced diarrhoea score during the lactation period. Reduction in VH and the VH:CD ratio have been associated with increased incidences of scouring in weaned pigs challenged with enterotoxigenic E. coli (18).
Immediately after birth the neonatal intestine is rapidly invaded by bacteria, originating from the mother and the environment(8) and this primary colonisation is important for the right development and programming of the animal's local and systemic immune system(5). Dietary provision of SDP reduced dam Enterobacteriaceae GCN/g faeces on the expected parturition day, indicating an antimicrobial property of SDP. Previous research indicated that supplementation of a similar SDP to sows decreased their faecal Enterobacteriaceae numbers at parturition(17). The reduction in sow Enterobacteriaceae GCN/g faeces may be attributed to the potential agglutination properties of the SDP. β-Glucans (laminarin) have the capacity to agglutinate certain bacterial species, thus inhibiting subsequent attachment and colonisation of epithelial mucosal surfaces(51). Furthermore, fucoidan possesses numerous biological functions, including antimicrobial properties(52). Demecková et al.(4) reported that sow faecal microbiota composition at farrowing influences subsequent bacterial colonisation of the neonatal intestinal tract. In the current study, lactobacilli were selected as bacterial indicators of beneficial bacteria, while Enterobacteriaceae were chosen based on their potential association with gastrointestinal disequilibrium(53). Piglets suckling the SDP-supplemented sows had reduced diarrhoea score during the lactation period. However, the decline in sow Enterobacteriaceae GCN/g faeces did not affect piglet Enterobacteriaceae GCN/g colonic digesta at birth, 48 h after birth and weaning. This reduction in diarrhoea score may be attributed to the immunomodulatory effects of SDP in enhancing the cellular and humoral immune function, as well as suppressing the E. coli population(17). Leonard et al.(17) demonstrated that sow SDP supplementation reduced piglet colonic E. coli numbers at weaning. A suppressed colonic E. coli number may ultimately alleviate the incidence and severity of diarrhoea. Unfortunately, E. coli GCN was not measured in the current study.
LPS is a component of the outer membrane of Gram-negative bacteria and a commonly used immunostimulant(54). To mimic an immunological stress and the response of pigs exposed to a microbial challenge, colon tissue of piglets was LPS challenged ex vivo at birth, 48 h after birth and weaning. The biological effects mediated by LPS are attributed to inflammatory cytokine synthesis and release by stimulated macrophages(55,56). The LPS challenge up-regulated the gene expression of a panel of pro-inflammatory cytokines at birth (IL-6, IL-8 and TNF-α), 48 h after birth (IL-6) and weaning (IL-8 and TNF-α), proving the LPS challenge worked.
The colonic tissue of piglets suckling the SDP-supplemented sows, following LPS challenge had IL-1 and IL-6 gene expression down-regulated at 48 h after birth. There are a number of ways these findings could be interpreted. Porcine epithelial cells respond to various pathogens with the production of TNF-α and IFN-γ which block pathogen replication and cytokines are also involved in recruitment and activation of immune cells(57). The fact that piglets suckling the basal fed sows displayed increased expression of IL-1 and IL-6 in response to LPS suggests that these animals retained a capacity to respond more adequately to infection relative to the piglets suckling SDP-supplemented sows. An alternative hypothesis is that piglets suckling the SDP-supplemented sows possess a number of inherent energy-saving mechanisms, one of which is exhibited as a reduced inflammatory response following infection, as a result of maternal feeding of SDP. While both pig populations had no differences in their pathogenic bacteria levels at 48 h, experimental infection would be necessary to establish if this reduction in cytokine expression results in a measurable decrease in their resistance to pathogenic bacteria.
At weaning, the gene expression of the anti-inflammatory cytokine TGF-β1 was up-regulated and the gene expression of the pro-inflammatory cytokines IL-6 and IL-8 was down-regulated in the ileum and colon tissues of piglets suckling the SDP-supplemented sows. IL-6 is produced by a number of cell types including macrophages, endothelial cells, B cells and mast cells, and is up-regulated in most, if not all inflammatory states(58). IL-8 is known to be regulated by inflammatory signals(59). IL-6 and IL-8 are cytokines classically involved in E. coli-caused diarrhoea(60). Down-regulation of the gene expression of these cytokines may be related to the reduced faecal scores observed in piglets suckling the SDP-supplemented sows. If the maternal SDP supplementation suppresses the secretion of pro-inflammatory cytokines, this could lower the activity of the immune system resulting in an energy-saving mechanism and increased growth performance during the suckling and post-weaning periods, as suggested by Li et al.(61).
There was a positive linear relationship between piglet BW at birth and the anti-inflammatory IL-10 gene expression in the ileum. IL-10 is considered a potent anti-inflammatory cytokine that strongly inhibits the production of pro-inflammatory cytokines(62). There was negative linear relationship between piglet BW at 48 h after birth and gene expression of the pro-inflammatory TNF-α in the ileum, enforcing the concept of maximising piglets’ ingestion of colostrum and milk in the first 48 h after birth.
A panel of pro-inflammatory cytokines was also up-regulated in the ileum and colon tissues of piglets suckling the SDP-supplemented sows at weaning. However, this pro-inflammatory response did not affect piglet growth performance during the lactation period. These cytokines play an important role in acute inflammation and are responsible for neutrophil recruitment and activation to the initial site of infection(63). β-Glucans can stimulate macrophages, neutrophils and natural killer cells, and could promote T cell-specific responses by induction of pro-inflammatory cytokines like IFN-γ, IL-8 and IL-12A (p35) from those cells(64–66). This pro-inflammatory response could be of particular importance because piglets often encounter presenting pathogens immediately post-weaning. Leonard et al.(17) reported that maternal SDP supplementation enhanced the expression of TNF-α in the ileum after an ex vivo LPS challenge at weaning.
Conclusion
Maternal dietary SDP supplementation down-regulated the gene expression of a panel of pro-inflammatory cytokines, classically involved in E. coli-caused diarrhoea in piglets at 48 h after birth and weaning, indicating an important immunomodulatory effect of SDP. Maternal SDP supplementation also had positive effect in the small-intestinal architecture of suckling piglets, represented by the increased VH in the ileum of piglets at weaning. Even though little positive effect was observed in nutrient transporter gene expression and the microbiota population of suckling piglets, the results provide new insights into the protective activity of maternal SDP supplementation, making the suckling piglets more immune competent to deal with pathogens commonly encountered during the immediate post-weaning period.
Acknowledgements
This study was supported financially by the Irish Research Council for Science, Engineering and Technology (IRCSET) and BioAtlantis Ltd. The IRCSET and BioAtlantis had no role in the design, analysis or writing of this article. G. H. designed the experiment, wrote the manuscript, collected samples and carried out laboratory analysis. J. V. O’. D. was the principal investigator responsible for the design of the experiment, supervision of data collection and statistical analysis and correction of the manuscript. T. S. designed the study. C. J. O’. S. contributed toward designing the study and laboratory analysis. D. N. D. and A. M. E. helped with data collection. K. T. contributed toward laboratory analysis. All the authors approved the final version submitted for publication. None of the authors had a financial or personal conflict of interest in relation to the present study.
References
- 1.Heim G, Mellagi APG, Bierhals T, et al. (2011) Absorption of IgG via colostrum in biological piglets and adopted piglets after cross-fostering. Arq Bras Med Vet Zoot 63, 1073–1078. [Google Scholar]
- 2.Le Dividich J, Rooke JA & Herpin P (2005) Nutritional and immunological importance of colostrum for the new-born pig. J Agric Sci 143, 469–485. [Google Scholar]
- 3.Zhang H, Malo C & Buddington RK (1997) Suckling induces rapid intestinal growth and changes in brush border digestive functions of newborn pigs. J Nutr 127, 418–426. [DOI] [PubMed] [Google Scholar]
- 4.Demecková V, Kelly D, Coutts AG, et al. (2002) The effect of fermented liquid feeding on the faecal microbiology and colostrum quality of farrowing sows. Int J Food Microbiol 79, 85–97. [DOI] [PubMed] [Google Scholar]
- 5.Yao K, Sun Z, Liu Z, et al. (2013) Development of the gastrointestinal tract in pigs In Nutritional and Physiological Functions of Amino Acids in Pigs, pp. 3–18 [Blachier F, Wu G and Yin Y, editors].Vienna: Springer Verlag. [Google Scholar]
- 6.Maxwell CV & Carter SD (2001) Feeding the weaned pig In Swine Nutrition, 2nd ed., pp. 692–715 [Lewis AJ and Southern LL, editors]. Boca Raton, FL: CRC Press. [Google Scholar]
- 7.Salmon H (1999) The mammary gland and neonate mucosal immunity. Vet Immun Immunopathol 72, 143–155. [DOI] [PubMed] [Google Scholar]
- 8.Kostantinov SR, Favier CF, Zhu WY, et al. (2004) Microbial diversity studies of the porcine gastrointestinal ecosystem during weaning transition. Anim Res 53, 317–324. [Google Scholar]
- 9.Xu RJ, Wang F & Zhang SH (2000) Postnatal adaptation of the gastrointestinal tract in neonatal pigs: a possible role of milk-borne growth factors. Livest Sci 66, 95–107. [Google Scholar]
- 10.Rome S, Barbot L, Windsor E, et al. (2002) The regionalization of PepT1, NBAT and EAAC1 transporters in the small intestine of rats are unchanged from birth to adulthood. J Nutr 132, 1009–1011. [DOI] [PubMed] [Google Scholar]
- 11.Takanashi N, Tomosada Y, Villena J, et al. (2013) Advanced application of bovine intestinal epithelial cell line for evaluating regulatory effect of lactobacilli against heat-killed enterotoxigenic Escherichia coli-mediated inflammation. BMC Microbiol 13, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cario E, Brown D, McKee M, et al. (2002) Commensal-associated molecular patterns induce selective Toll-like receptor trafficking from apical membrane to cytoplasmic compartments in polarized intestinal epithelium. Am J Pathol 160, 165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dibner JJ & Richards JD (2005) Antibiotic growth promoters in agriculture: history and mode of action. Poult Sci 84, 634–643. [DOI] [PubMed] [Google Scholar]
- 14.Leonard SG, Sweeney T, Bahar B, et al. (2010) Effect of maternal fish oil and seaweed extract supplementation on colostrum and milk composition, humoral immune response, and performance of suckled piglets. J Anim Sci 88, 2988–2997. [DOI] [PubMed] [Google Scholar]
- 15.Leonard SG, Sweeney T, Pierce KM, et al. (2010) The effects of supplementing the diet of the sow with seaweed extracts and fish oil on aspects of gastrointestinal health and performance of the weaned piglet. Br J Nutr 105, 549–560.20875191 [Google Scholar]
- 16.Leonard SG, Sweeney T, Bahar B, et al. (2011) Effects of dietary seaweed extract supplementation in sows and post-weaned pigs on performance, intestinal morphology, intestinal microflora and immune status. Br J Nutr 106, 688–699. [DOI] [PubMed] [Google Scholar]
- 17.Leonard SG, Sweeney T, Bahar B, et al. (2012) Effect of maternal seaweed extract supplementation on suckling piglet growth, humoral immunity, selected microflora, and immune response after an ex vivo lipopolysaccharide challenge. J Anim Sci 90, 505–514. [DOI] [PubMed] [Google Scholar]
- 18.Heim G, Sweeney T, O'Shea CJ, et al. (2014) Effect of maternal supplementation with seaweed extracts on growth performance and aspects of gastrointestinal health of newly weaned piglets after challenge with enterotoxigenic Escherichia coli K88. Br J Nutr 112, 1955–1965. [DOI] [PubMed] [Google Scholar]
- 19.McPherson RL, Ji F, Wu G, et al. (2004) Growth and compositional changes of fetal tissues in pigs. J Anim Sci 82, 2534–2540. [DOI] [PubMed] [Google Scholar]
- 20.Biensen NJ, Wilson ME & Ford SP (1998) The impact of either a Meishan or Yorkshire uterus on Meishan or Yorkshire fetal and placental development to days 70, 90, and 110 of gestation. J Anim Sci 76, 2169–2176. [DOI] [PubMed] [Google Scholar]
- 21.Lynch MB, Sweeney T, Callan JJ, et al. (2009) The effect of dietary Laminaria-derived laminarin and fucoidan on nutrient digestibility, nitrogen utilisation, intestinal microflora and volatile fatty acid concentration in pigs. J Sci Food Agric 90, 430–437. [DOI] [PubMed] [Google Scholar]
- 22.National Research Council (2012) Nutrient Requirements of Swine, 11th ed. Washington, DC: National Academies Press. [Google Scholar]
- 23.Heim G, Mellagi APG, Bierhals T, et al. (2012) Effects of cross-fostering within 24 h after birth on pre-weaning behaviour, growth performance and survival rate of biological and adopted piglets. Livest Sci 150, 121–127. [Google Scholar]
- 24.Stamati S, Alexopoulos C, Siochu A, et al. (2006) Probiosis in sows by administration of Bacillus toyoi spores during late pregnancy and lactation: effect on their health status/performance and on litter characteristics. Intern J Probiotics Prebiotics 1, 33–40. [Google Scholar]
- 25.Heim G, Walsh AM, Sweeney T, et al. (2014) Effect of seaweed derived laminarin and fucoidan, and zinc oxide on gut morphology, nutrient transporters, nutrient digestibility, growth performance and selected microbial populations in weaned pigs. Br J Nutr 111, 1577–1585. [DOI] [PubMed] [Google Scholar]
- 26.Usov AI, Smirnova GP & Klochkova NG (2001) Polysaccharides of algae: 55. Polysaccharide composition of several brown algae from Kamchatka. Russ J Bioorg Chem 27, 395–399. [DOI] [PubMed] [Google Scholar]
- 27.O'Shea CJ, Sweeney T, Bahar B, et al. (2012) Indices of gastrointestinal fermentation and manure emissions of growing–finishing pigs as influenced through singular or combined consumption of Lactobacillus plantarum and inulin. J Anim Sci 90, 3848–3857. [DOI] [PubMed] [Google Scholar]
- 28.Metzler-Zebeli BU, Hooda S, Pieper R, et al. (2010) Nonstarch polysaccharides modulate bacterial microbiota, pathways for butyrate production, and abundance of pathogenic Escherichia coli in the pig gastrointestinal tract. Appl Environ Microbiol 76, 3692–3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee C, Kim J, Shin SG, et al. (2006) Absolute and relative qPCR quantification of plasmid copy number in Escherichia coli. J Biotechnol 123, 273–280. [DOI] [PubMed] [Google Scholar]
- 30.Liu P, Piao XS, Thacker PA, et al. (2010) Chito-oligosaccharide reduces diarrhea incidence and attenuates the immune response of weaned pigs challenged with Escherichia coli K88. J Anim Sci 88, 3871–3879. [DOI] [PubMed] [Google Scholar]
- 31.Smith AG, O'Doherty JV, Reilly P, et al. (2011) The effects of laminarin derived from Laminaria digitata on measurements of gut health: selected bacterial populations, intestinal fermentation, mucin gene expression and cytokine gene expression in the pig. Br J Nutr 105, 669–677. [DOI] [PubMed] [Google Scholar]
- 32.Ryan MT, Collins CB, O'Doherty JV, et al. (2010) Selection of stable reference genes for quantitative real-time PCR in porcine gastrointestinal tissues. Livest Sci 133, 42–44. [Google Scholar]
- 33.Vandesompele J, De Preter K, Pattyn F, et al. (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3, research0034.1–research0034.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Statistical Analysis Systems Institute (1985) Statistical Analysis Systems, 6.12 ed. Cary, NC: SAS Institute, Inc. [Google Scholar]
- 35.Meredith MJ (1995) Pigs breeding and infertility In Animal Breeding and Infertility, pp. 278–353 [Meredith MJ, editor]. Oxford: Blackwell Science. [Google Scholar]
- 36.Hakkarainen J (1975) Developmental changes of protein, RNA, DNA, lipid, and glycogen in the liver, skeletal muscle and brain of the pig. Acta Vet Scand 59, Suppl., 1–198. [PubMed] [Google Scholar]
- 37.Lahaye M & Keaffer B (1997) Seaweed dietary fibres: structure, physico-chemical and biological properties relevant to intestinal physiology. Sci Aliments 17, 563–584. [Google Scholar]
- 38.MacArtain P, Gill CIR, Brooks M, et al. (2007) Nutritional value of edible seaweeds. Nutr Rev 65, 535–543. [DOI] [PubMed] [Google Scholar]
- 39.Bourne FJ & Curtis J (1973) The transfer of immunoglobins IgG, IgA and IgM from serum to colostrum and milk in the sow. Immunology 24, 157–162. [PMC free article] [PubMed] [Google Scholar]
- 40.Huang SC, Hu Z, Hasler-Rapacz J, et al. (1992) Preferential mammary storage and secretion of immunoglobulin γ (IgG) subclasses in swine. J Reprod Immunol 21, 15–28. [DOI] [PubMed] [Google Scholar]
- 41.Schnulle PM & Hurley WL (2003) Sequence and expression of the FcRn in the porcine mammary gland. Vet Immunol Immunopathol 91, 227–231. [DOI] [PubMed] [Google Scholar]
- 42.Lallès JP, Bosi P, Smidt H, et al. (2007) Weaning – a challenge to gut physiologists. Livest Sci 108, 82–93. [Google Scholar]
- 43.Daniel H (2004) Molecular and integrative physiology of intestinal peptide transport. Annu Rev Physiol 66, 361–384. [DOI] [PubMed] [Google Scholar]
- 44.Van Goudoever JB, Corpeleijn W, Riedijk M, et al. (2008) The impact of enteral insulin-like growth factor 1 and nutrition on gut permeability and amino acid utilization. J Nutr 138, 1829S–1833S. [DOI] [PubMed] [Google Scholar]
- 45.Cera KR, Mahan DC, Cross RF, et al. (1988) Effect of age, weaning and postweaning diet on small intestinal growth and jejunal morphology in young swine. J Anim Sci 66, 574–584. [DOI] [PubMed] [Google Scholar]
- 46.Pluske JR, Hampson DJ & Williams IH (1997) Factors influencing the structure and function of the small intestine in the weaned pig: a review. Livest Prod Sci 51, 215–236. [Google Scholar]
- 47.Ferraris RP (2001) Dietary and developmental regulation of intestinal sugar transport. Biochem J 360, 265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Toloza EM & Diamond J (1992) Ontogenetic development of nutrient transporters in rat intestine. Am J Physiol 263, G593–G604. [DOI] [PubMed] [Google Scholar]
- 49.Shu HJ, Takeda H, Shinzawa H, et al. (2002) Effect of lipopolysaccharide on peptide transporter 1 expression in rat small intestine and its attenuation by dexamethasone. Digestion 65, 21–29. [DOI] [PubMed] [Google Scholar]
- 50.Heim G, O'Shea CJ, Doyle DN, et al. (2015) Effect of maternal dietary supplementation of laminarin and fucoidan, independently or in combination, on pig growth performance and aspects of intestinal health. Anim Feed Sci Tech 204, 28–41. [Google Scholar]
- 51.Sweeney T, Collins C, Reilly P, et al. (2012). Effect of purified β-glucans derived from Laminaria digitata, Laminaria hyperborea and Saccharomyces cerevisiae on piglet performance, selected bacterial populations, volatile fatty acids and pro-inflammatory cytokines in the gastrointestinal tract of pigs. Br J Nutr 108, 1226–1234. [DOI] [PubMed] [Google Scholar]
- 52.Shibata H, Iimuro M, Uchiya N, et al. (2003) Preventive effects of Cladosiphon fucoidan against Helicobacter pylori infection in Mongolian gerbils. Helicobacter 8, 59–65. [DOI] [PubMed] [Google Scholar]
- 53.Nyachoti CM, Omogbenigun FO, Rademacher M, et al. (2006). Performance responses and indicators of gastrointestinal health in early-weaned pigs fed low-protein amino acid-supplemented diets. J Anim Sci 84, 125–134. [DOI] [PubMed] [Google Scholar]
- 54.Medvedev AE, Piao W, Shoenfelt J, et al. (2007) Role of TLR4 tyrosine phosphorylation in signal transduction and endotoxin tolerance. J Biol Chem 282, 16042–16053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johnson RW & von Borell E (1994) Lipopolysaccharide-induced sickness behavior in pigs is inhibited by pretreatment with indomethacin. J Anim Sci 72, 309–314. [DOI] [PubMed] [Google Scholar]
- 56.Johnson RW (1997) Inhibition of growth by pro-inflammatory cytokines: an integrated view. J Anim Sci 75, 1244–1255. [DOI] [PubMed] [Google Scholar]
- 57.Mair KH, Sedlak C, Käser T, et al. (2014) The porcine innate immune system: an update. Dev Comp Biol 45, 321–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dalrymple SA, Slattery R, Aud DM, et al. (1996) Interleukin-6 is required for a protective immune response to systemic Escherichia coli infection. Infec Immun 64, 3231–3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kasahara T, Mukaida N, Yamashita K, et al. (1991) IL-1 and TNF-α induction of IL-8 and monocyte chemotactic and activating factor (MCAF) mRNA expression in a human astrocytoma cell line. Immunology 74, 60–67. [PMC free article] [PubMed] [Google Scholar]
- 60.McLamb BL, Gibson AJ, Overman EL, et al. (2013) Early weaning stress in pigs impairs innate mucosal immune responses to enterotoxigenic E. coli challenge and exacerbates intestinal injury and clinical disease. PLOS ONE 8, e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li J, Li DF, Xing JJ, et al. (2006) Effects of β-glucans extracted from Saccharomyces cerevisiae on growth performance, and immunological and somatotropic responses of pigs challenged with Escherichia coli lipopolysaccharide. J Anim Sci 84, 2374–2381. [DOI] [PubMed] [Google Scholar]
- 62.Lauw FN, Pajkrt D, Hack CE, et al. (2000) Proinflammatory effects of IL-10 during human endotoxemia. J Immunol 165, 2783–2789. [DOI] [PubMed] [Google Scholar]
- 63.Girard F, Oswald IP, Taranu I, et al. (2005) Host immune status influences the development of attaching and effacing lesions in weaned pigs. Infect Immun 73, 5514–5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brown GD & Gordon S (2005) Immune recognition of fungal β-glucans. Cell Microbiol 7, 471–479. [DOI] [PubMed] [Google Scholar]
- 65.Xiao Z, Trincado CA & Murtaugh MPB (2004) Glucan enhancement of T cell IFN-γ response in swine. Vet Immunol Immunopathol 102, 315–320. [DOI] [PubMed] [Google Scholar]
- 66.Chaung HC, Huang TC, Yu JH, et al. (2009) Immunomodulatory effects of β-glucans on porcine aveolar macrophages and bone marrow haematopoietic cell-derived dendritic cells. Vet Immunol Immunopathol 131, 147–157. [DOI] [PubMed] [Google Scholar]
- 67.Sauvant D, Perez J-M & Tran G (editors) (2004) Tables of Composition and Nutritional Value of Feed Materials. Pigs, Poultry, Cattle, Sheep, Goats, Rabbits, Horses, Fish. Wageningen: Wageningen Academic Publishers. [Google Scholar]