Abstract
Population-based studies have shown an inverse association between dietary menaquinones (MK-n, vitamin K2) intake, coronary calcification and CHD risk, suggesting a potential role of vitamin K in vascular health. To date, the effects of increased menaquinone intake on (markers of) vascular health have been investigated using predominantly food supplements. Dairy products contain many essential nutrients and can serve as a good matrix for food fortification in order to support health. We were therefore interested to study the effects of a menaquinone-fortified yogurt drink (menaquinone as menaquinone-7 (MK-7); 28 µg MK-7/yogurt drink) on vitamin K status and markers of vascular health. The yogurt drink was also fortified with n-3 PUFA, vitamin D, vitamin C, Ca and Mg to support vascular and/or general health. Healthy men (n 32) and postmenopausal women (n 28) with a mean age of 56 (sd 5) years received either basic or fortified yogurt drink twice per d for 12 weeks. MK-7 was efficiently absorbed from the fortified yogurt drink. Levels of circulating MK-7 were significantly increased from 0·28 to 1·94 ng/ml. In accordance, intake of the fortified yogurt drink improved vitamin K status, as measured by significant decreases in uncarboxylated osteocalcin and desphospho-uncarboxylated matrix Gla-protein. No effects were, however, seen on markers of inflammation, endothelial dysfunction and lipid metabolism. In summary, consumption of a yogurt drink fortified with low doses of among others MK-7 for 3 months significantly improved vitamin K status in a healthy population.
Key words: Food fortification, Vitamin K status, n-3 PUFA, Vascular health
Abbreviations: 25-OH-D, 25-hydroxyvitamin D; cOC, carboxylated osteocalcin; dp-cMGP, desphospho-carboxylated matrix Gla-protein; dp-ucMGP, desphospho-uncarboxylated matrix Gla-protein; MGP, matrix Gla-protein; MK-n, menaquinone-n; OC, osteocalcin; ucOC, uncarboxylated osteocalcin; VCAM, vascular cell adhesion molecule
Several population-based studies have shown an inverse association between dietary menaquinone-n (MK-n; vitamin K2) intake and CHD risk, suggesting a potential role of vitamin K in vascular health(1–3). Vitamin K serves as a cofactor for γ-glutamate carboxylase, promoting the post-translational conversion of glutamate residues into γ-carboxyglutamate (Gla) in so-called Gla-proteins(4–6). The Gla-residues confer Ca-binding properties needed for the proper functioning of these proteins. Of all known Gla-proteins, osteocalcin (OC, synthesised by osteoblasts) and matrix Gla-protein (MGP, synthesised primarily by vascular smooth muscle cells) are investigated most extensively. During vitamin K insufficiency, carboxylation proceeds to a lesser extent, resulting in the release of Gla-proteins in the circulation as undercarboxylated species. Circulating uncarboxylated OC (ucOC) and desphospho-uncarboxylated MGP (dp-ucMGP) are recognised markers for bone and vascular vitamin K status, respectively. Remarkably, substantial fractions of OC and MGP circulate as uncarboxylated species in most healthy adults(7–10), suggesting that vitamin K insufficiency is widespread in Western society. High levels of ucOC form an independent risk predictor for bone fracture and low bone mineral density(11–16). High levels of dp-ucMGP were found especially in subjects at increased risk for CVD(17,18) and have been associated with arterial calcification and cardiovascular mortality(19,20). OC and MGP carboxylation can be improved by increased vitamin K intake by diet(21,22) and supplements(7,8,23).
Dietary forms of vitamin K are phylloquinone (vitamin K1) and the group of menaquinones. Food sources of phylloquinone are green vegetables and several plant oils(24–26), whereas menaquinones are primarily found in meat and egg yolk (short-chain MK-4) and in fermented foods, like cheese and curd (MK-7 to MK-10, also referred to as long-chain menaquinones)(26). Whereas all forms of vitamin K appear to be initially associated with TAG-rich lipoproteins, the long-chain menaquinones are also associated with LDL. These data suggest that the menaquinones have different transport pathways and distribution, and they are therefore thought to be the most adequate form of vitamin K to supply extra-hepatic tissues, such as bone and the arterial wall.
In earlier studies on the beneficial effects of increased vitamin K intake, supplements (tablets, capsules) were used almost invariably. Dairy products contain many essential nutrients with recognised health benefits(27) and can serve as an ideal matrix for food fortification in order to support health. For this study, we fortified a yogurt drink with menaquinones (in the form of MK-7) and n-3 PUFA (EPA + DHA(28–30)) to support heart health. Vitamin D, vitamin C, Ca and Mg were also added to support general health. First, we studied absorption of vitamins from the fortified yogurt drink. Next, we investigated effects of the fortified yogurt drink on markers of vitamin K status, inflammation, endothelial dysfunction and lipid metabolism. We hypothesised that the fortified yogurt has beneficial effects on vitamin K status and markers of cardiovascular health.
Experimental methods
Study design
Healthy men and postmenopausal women between 45 and 65 years of age were recruited from the southern region of Limburg, the Netherlands, through advertisements in local newspapers. Exclusion criteria were: <2 years postmenopausal, BMI <20 and >30 kg/m2, hypertension, hypercholesterolaemia, metabolic or gastrointestinal diseases, chronic degenerative or inflammatory diseases, diabetes mellitus, coagulation disorders, cows’ milk allergy and/or lactose intolerance, abuse of drugs and/or alcohol, use of corticosteroids, oral anticoagulants, blood pressure-lowering medication, and/or cholesterol-lowering medication, use of vitamin K supplements, and high dietary intake of vitamin K (assessed by interviews and questionnaires). If subjects were eligible for inclusion, blood was taken at a screening visit in order to select participants with normal or low vascular vitamin K status (dp-ucMGP values >150 pmol/l). After a final health check (interviews and questionnaires), sixty participants (thirty-two men and twenty-eight women) were included in the study and randomly assigned (computer-generated random permutation procedure with stratification for sex) to receive either a basic yogurt drink (control group) or a fortified yogurt drink twice per d for 12 weeks. Both the participants and the study personnel were blinded as to the composition of the drinks (double-blind design). In the period starting at 2 weeks before the start of the study until the final blood sampling, participants were asked to restrict their intake of vitamin K-rich foods. Participants visited the research site at baseline and after 4, 8 and 12 weeks for blood sampling, and for measurements of body weight and height (at baseline). They were instructed to report any signs of illness, medication used and any deviations from the study protocol. In addition, subjects were urged not to change their level of physical exercise or alcohol consumption during the study.
Ethics statement
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Medical Ethics Committee of the Maastricht University Medical Centre (Maastricht, The Netherlands). Written informed consent was obtained from all subjects. Trial registration code: clinicaltrials.gov: NCT01672099.
Study products
The yogurt drinks (250 ml/container) were provided in two batches by FrieslandCampina. The yogurts were coded and subsequently delivered directly to VitaK (Maastricht, The Netherlands) as complete ready-to-use end-products. During visits at the research site or during home visits, study products were handed out to the study participants every 2 weeks. Leftovers had to be returned to assess compliance. The basic yogurt drink was manufactured according to standard procedures (Table 1). The fortified yogurt drink was enriched with MK-7 and n-3 PUFA to support vascular health (Table 1). In addition, various basic nutrients were added to support general health: vitamin D, vitamin C, Ca and Mg. The chosen amounts represent 15 % of the recommended dietary intake. The participants consumed two 250 ml yogurt drinks daily; one drink during breakfast and one during dinner. We chose a daily dose of MK-7 (56 µg) that is consistent with the lower level in the commercially available MK-7 supplements, i.e. 45–200 µg, and suggested to beneficially affect bone and heart health. A study duration of 12 weeks was chosen based on earlier findings showing significant effects on OC and MGP carboxylation with nutritional doses of MK-7(7). We used pure, natural, minimally processed, non-encapsulated marine oil rich in long-chain n-3 PUFA as EPA + DHA. The active biological compound MK-7 (MenaQ7) was supplied by NattoPharma. To verify the stability of MK-7, three samples of the study products were analysed at the start and end of the intervention period. The MK-7 content of the fortified yogurt drinks was stable over time.
Table 1.
Study product composition
| Per 100 ml | Standard drinking yogurt | Fortified drinking yogurt |
|---|---|---|
| DM (%) | 13·7 | 14·4 |
| Protein (%) | 2·6 | 2·5 |
| Milk fat (%) | 1·0 | 1·1 |
| Saccharides (%) | 5·3 | 5·3 |
| Vitamin K2 (MenaQ7) (μg) | Below detection limit | 11·65* |
| Fish oil (EPA + DHA) (mg) | <2 | 36·3* |
| Vitamin D3 (IU) | <0·1 | 0·37* |
| Ascorbic acid (mg) | 0·6 | 23·5* |
| Ca (mg) | 77 | 108* |
| Mg (mg) | 7·5 | 58* |
15 % of the recommended dietary intake.
Blood sampling
Fasting venous blood was collected by venepuncture for the preparation of serum and plasma (Vacutainer; Greiner Bio-One BV). All blood samples were drawn between 08·00 and 11·00 hours by experienced research nurses. For plasma preparation, blood (10 ml) was collected in citrate tubes, centrifuged at 3000 g for 15 min, and plasma was divided into aliquots and stored at −80°C until analysis. For serum preparation, blood (10 ml) was allowed to clot for 30 min at room temperature, centrifuged, and the serum was stored at −80°C.
Circulating markers
Circulating MK-7 levels were measured at baseline and after 12 weeks with a standard HPLC technique using a C18 reversed-phase column and fluorometric detection after post-column electrochemical reduction(26). Serum ucOC and carboxylated OC (cOC) concentrations were determined with commercial dual-antibody ELISA tests (Takara Shuzo Co. Ltd). Plasma dp-ucMGP and desphospho-carboxylated MGP (dp-cMGP) levels were measured by in-house dual-antibody ELISA tests(17). An in-house control plasma pool was run on all ELISA plates. To minimise inter-assay variation, the different time-point samples of each subject were analysed on the same ELISA plate, in ascending order of randomisation. In addition to measurements at baseline and at 12 weeks, these markers for vitamin K status were also measured after 4 and 8 weeks of the 12-week intervention period in order to determine more precisely when a significant effect was achieved.
Markers of inflammation, endothelial dysfunction and lipid metabolism were measured with commercial immunoassays at baseline and at the end of the intervention. The following markers were measured: vascular cell adhesion molecule (VCAM), intercellular adhesion molecule and E-selectin (R&D Systems Europe); serum amyloid A, vascular endothelial growth factor, IL-6 and TNF-α (Invitrogen); von Willebrand factor (Abnova); secretory type-II phospholipase A-2 (Wuhan USCN Business Co.). C-reactive protein (inflammation marker), total cholesterol and TAG (markers of lipid metabolism) were measured in serum using fully enzymic techniques on a clinical chemistry analyser. HDL-cholesterol was measured with the use of a homogeneous colorimetric technique. LDL-cholesterol was calculated by the Friedewald formula(31). Circulating vitamin D (25-hydroxyvitamin D; 25-OH-D) was measured by the automated IDS-iSYS method (IDS plc). Vitamin C (ascorbic acid) levels were measured by a commercial kit (Abcam).
All samples were measured in duplicate. Within- and between-assay precisions were <5 and <10 %, respectively, for all analytical procedures.
Statistics
The primary outcome measure was circulating dp-ucMGP (vascular vitamin K status). Based on preliminary estimates of the standard deviation in ucMGP in healthy subjects, we determined that thirty participants were required in each group to have a statistical power of 90 % to detect a 15 % difference between treatment groups while allowing for a withdrawal of 10 %. The distribution of variables was analysed by normality tests, boxplots and histograms. Serum amyloid A, IL-6, secretory type-II phospholipase A2 and C-reactive protein were log-transformed. Other variables are presented as means with standard deviations or standard errors (Fig. 1). The baseline characteristics of both treatment groups were compared with a Fisher's exact test for categorical variables (sex and smoking) and an independent-samples t test for continuous variables. Statistical significance is reached if P < 0·0024 (Bonferroni correction for multiple comparisons, i.e. twenty-one different circulating markers). Correlation analyses at baseline were performed using the Pearson test (two-sided significance level P < 0·05). The repeated-measurements ANOVA was used to test between-group and within-group differences for changes of markers of vitamin K status (dp-ucMGP and ucOC) at the different time points. The P value was adapted for multiple comparisons between three time points (i.e. absolute changes after 4, 8 and 12 weeks), meaning that P < 0·017 was considered significant. Multivariate linear regression analysis was performed to test for associations between treatment and the markers of interest, which were treated as dependent variables (i.e. end values after 12 weeks of circulating vitamins, and markers of vitamin K status, inflammation, endothelial dysfunction and lipid metabolism). The corresponding baseline value and the treatment regimen (standard/fortified yogurt) were included as independent variables and age, BMI and TAG were included as potential confounders. Analysis of the markers of lipid metabolism was performed with age and BMI as confounder. P < 0·0024 was considered significant (Bonferroni correction). The potential confounders sex and smoking were not included in the analyses, because they were equally divided over the two treatment groups (see Table 2) and did not contribute to the results. Statistics were performed using SPSS for Windows, version 19 (SPSS Inc.).
Fig. 1.
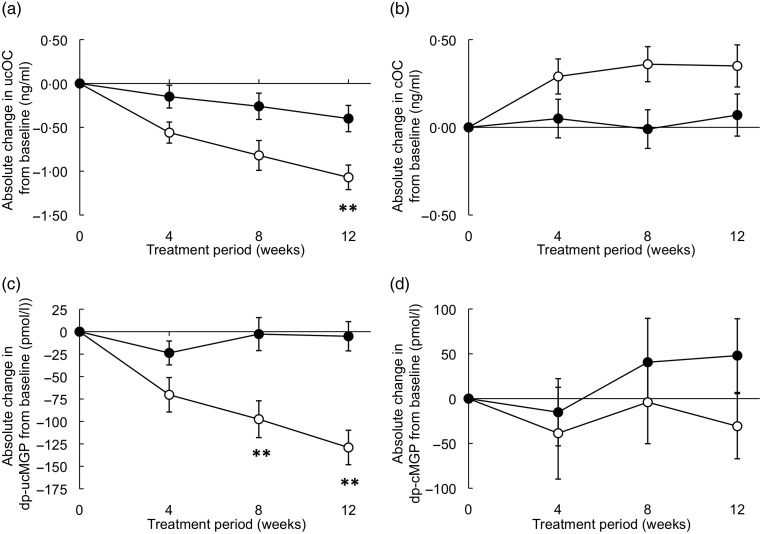
Effects of 12-week consumption of a fortified yogurt drink on markers of vitamin K status. Participants received twice per d either a basic yogurt drink (control group; ●) or a fortified yogurt drink (fortified with menaquinone-7, EPA + DHA, vitamin D, vitamin C, calcium and magnesium; ○) for 12 weeks. (a) Uncarboxylated osteocalcin (ucOC); (b) carboxylated osteocalcin (cOC); (c) desphospho-uncarboxylated matrix Gla-protein (dp-ucMGP); (d) desphospho-carboxylated matrix Gla-protein (dp-cMGP). Values are means (n 56), with standard errors represented by vertical bars. ** Mean value was significantly different from that for the control group (P < 0·017; correction for multiple comparisons, one-way ANOVA).
Table 2.
Baseline characteristics of the total study group and of the groups consuming the standard yogurt or the fortified yogurt
(Mean values and standard deviations; numbers of subjects and percentages)
| Total group (n 56) | Standard yogurt (n 29) | Fortified yogurt (n 27) | P* | ||||
|---|---|---|---|---|---|---|---|
| Mean | sd | Mean | sd | Mean | sd | ||
| Sex (n) | 0·49 | ||||||
| Male | 30 | 15 | 15 | ||||
| Female | 26 | 14 | 12 | ||||
| Age (years) | 56 | 5 | 56 | 5 | 56 | 5 | 0·76 |
| Weight (kg) | 75 | 13 | 73 | 13 | 79 | 12 | 0·08 |
| BMI (kg/m2) | 26 | 3 | 25 | 3 | 26 | 2 | 0·33 |
| Smoking | 0·59 | ||||||
| n | 10 | 5 | 5 | ||||
| % | 18 | 17 | 19 | ||||
| Vitamins | |||||||
| MK-7 (ng/ml) | 0·25 | 0·24 | 0·21 | 0·21 | 0·28 | 0·28 | 0·29 |
| Vitamin C (ng/ml) | 41·4 | 13·2 | 43·4 | 14·6 | 39·2 | 11·4 | 0·22 |
| 25-OH-D (ng/ml) | 37·6 | 10·8 | 39·9 | 7·6 | 35·0 | 13·1 | 0·09 |
| Vitamin K status | |||||||
| ucOC (ng/ml) | 3·51 | 1·75 | 3·47 | 1·60 | 3·57 | 1·93 | 0·83 |
| cOC (ng/ml) | 5·00 | 1·31 | 5·04 | 1·29 | 4·96 | 1·34 | 0·81 |
| dp-ucMGP (pmol/l) | 442 | 187 | 405 | 157 | 481 | 211 | 0·13 |
| dp-cMGP (pmol/l) | 1812 | 425 | 1781 | 342 | 1847 | 508 | 0·57 |
| Markers of inflammation | |||||||
| IL-6 (pg/ml)† | 0·77 | 0·63 | 0·67 | 0·35 | 0·88 | 0·82 | 0·62 |
| sPLA-2 (pg/ml)† | 3681 | 4137 | 3594 | 3044 | 3776 | 5124 | 0·30 |
| vWF (pg/ml) | 1007 | 329 | 962 | 288 | 1056 | 367 | 0·29 |
| TNF-α (pg/ml) | 1·24 | 0·67 | 1·10 | 0·49 | 1·54 | 0·74 | 0·022 |
| CRP (mg/l)† | 1·50 | 1·44 | 1·63 | 1·66 | 1·35 | 1·16 | 0·50 |
| Markers of endothelial dysfunction | |||||||
| VEGF (pg/ml) | 238 | 131 | 244 | 154 | 233 | 104 | 0·76 |
| VCAM (ng/ml) | 728 | 347 | 647 | 213 | 816 | 436 | 0·07 |
| ICAM (ng/ml) | 216 | 41·6 | 207 | 45 | 225 | 37 | 0·12 |
| SAA (μg/ml)† | 18 | 16 | 19 | 19 | 16 | 13 | 0·88 |
| E-selectin (pg/ml) | 29 | 13 | 30 | 14 | 29 | 11 | 0·97 |
| Markers of lipid metabolism | |||||||
| tCHOL (mmol/l) | 5·89 | 1·03 | 5·91 | 1·06 | 5·87 | 1·02 | 0·88 |
| LDL (mmol/l) | 3·84 | 0·95 | 3·82 | 0·95 | 3·85 | 0·97 | 0·91 |
| HDL (mmol/l) | 1·39 | 0·43 | 1·42 | 0·45 | 1·36 | 0·42 | 0·62 |
| TAG (mmol/l) | 1·48 | 0·67 | 1·43 | 0·61 | 1·47 | 0·67 | 0·87 |
MK-7, menaquinone-7; 25-OH-D, 25-hydroxyvitamin D; ucOC, uncarboxylated osteocalcin; cOC, carboxylated osteocalcin; dp-ucMGP, desphospho-uncarboxylated matrix Gla-protein; dp-cMGP, desphospho-carboxylated matrix Gla-protein; sPLA-2, secretory type-II phospholipase A2; vWF, von Willebrand factor; CRP, C-reactive protein; VEGF, vascular endothelial growth factor; VCAM, vascular cell adhesion molecule; ICAM, intercellular adhesion molecule; SAA, serum amyloid A; tCHOL, total cholesterol.
P values of the between-group analysis of both treatment groups at baseline (Fisher's exact test for categorical data (sex and smoking) and the independent-samples t tests for continuous data). No significant differences were found (multiple comparisons: P < 0·0024).
Analysis between the subjects consuming the standard yogurt and consuming the fortified yogurt was performed after logarithmic transformation of the values.
Results
Baseline characteristics
Baseline characteristics are shown in Table 2. Of the sixty participants, four dropped out (two men and two women) during the study: three participants (n 2 in fortified yogurt group, n 1 in the control group) disliked the study product and one participant (n 1 in fortified yogurt group) was no longer eligible for inclusion because of starting oral anticoagulant treatment. The results are therefore given for the fifty-six participants who completed the study. Because no between-group differences were found between men and women, except for TAG which was higher in men than in women (1·73 (sd 0·70) mmol/l and 1·19 (sd 0·51) mmol/l, respectively; P = 0·002), data of men and women were combined in both treatment groups. No significant differences were found between the treatment groups.
At baseline, dp-ucMGP and dp-cMGP correlated significantly with the inflammation marker IL-6 (r 0·321 and r 0·308, respectively; P < 0·05) and with markers of endothelial dysfunction von Willebrand factor (r 0·395 and r 0·393; P < 0·01) and VCAM (r 0·399 and r 0·653; P < 0·001). Circulating ucOC and cOC did not correlate with markers of vascular health.
Absorption of vitamins
Increased levels of circulating MK-7 (as compared with baseline) were observed after consumption of the fortified yogurt drink during 12 weeks (mean difference: 1·66 (sd 0·96) ng/ml). This increase differed significantly from the change in the control group (mean difference: −0·01 (sd 0·22) ng/ml; P < 0·0001). After adjustment for baseline MK-7 values, age, BMI and TAG, the end difference in plasma MK-7 concentrations between the treatment groups remained significant (Table 3). The water-soluble vitamin C was well absorbed, which can be seen from the unchanged levels (from 43·5 (sd 14·4) to 43·6 (sd 18·2) ng/ml; P = 0·952) in the control group compared with the significant increase (from 39·1 (sd 11·7) to 46·1 (sd 13·0) ng/ml; P = 0·002) in the group using the fortified yogurt drink. For the fat-soluble vitamin D the data are compromised by the fact that – probably due to seasonal effects – in the control group circulating 25-OH-D decreased significantly from 39·9 (sd 7·6) to 31·1 (sd 6·2) ng/ml (P < 0·0001); the concomitant decrease in the fortified group (from 35·0 (sd 13·1) to 32·5 (sd 11·2) ng/ml; P = 0·081) was much lower, but whether this was the result of vitamin D absorption from the yogurt drink cannot be concluded from the present data. The end values of circulating vitamin C and 25-OH-D between both treatment groups showed no significant differences (independent-samples t test: P = 0·57 and P = 0·55, respectively). However, regression analysis, adjusted for age, BMI and TAG, resulted in a significant increase of vitamin C (P = 0·048) and 25-OH-D (P = 0·005) levels after 12 weeks’ consumption of fortified yogurt compared with consumption of the standard yogurt (Table 3). After correction for multiple comparisons the significance was lost for vitamin C, but the difference between both yogurts in 25-OH-D was still borderline significant.
Table 3.
Effect of 12-week consumption of fortified yogurt compared with standard yogurt on markers for vitamin K status, inflammation, endothelial dysfunction and lipid metabolism*
(β-Coefficients and 95 % confidence intervals)
| β | 95 % CI | P† | |
|---|---|---|---|
| Vitamins | |||
| MK-7 (ng/ml) | 0·85 | 0·29, 1·40 | 0·004 |
| Vitamin C (ng/ml) | 5·79 | 0·04, 11·54 | 0·048 |
| 25-OH-D (ng/ml) | 3·75 | 1·17, 6·33 | 0·005 |
| Vitamin K status | |||
| ucOC (ng/ml) | −0·70 | −1·10, −0·29 | 0·001 |
| cOC (ng/ml) | 0·20 | −0·16, 0·57 | 0·27 |
| dp-ucMGP (pmol/l) | −96 | −146, −46 | <0·0001 |
| dp-cMGP (pmol/l) | −66 | −174, 42 | 0·23 |
| Markers of inflammation | |||
| IL-6 (pg/ml)‡ | −0·08 | −0·25, 0·08 | 0·30 |
| sPLA-2 (pg/ml)‡ | −0·03 | −0·15, 0·09 | 0·63 |
| vWF (pg/ml) | 66 | −31, 162 | 0·18 |
| TNF-α (pg/ml) | −0·15 | −0·51, 0·20 | 0·38 |
| CRP (mg/l)‡ | 0·13 | −0·02, 0·29 | 0·10 |
| Markers of endothelial dysfunction | |||
| VEGF (pg/ml) | −0·8 | −20·2, 18·6 | 0·93 |
| VCAM (ng/ml) | 1·4 | −70·9, 73·6 | 0·97 |
| ICAM (ng/ml) | 5·6 | −6·6, 17·7 | 0·36 |
| SAA (μg/ml)‡ | −0·02 | −0·17, 0·12 | 0·76 |
| E-selectin (pg/ml) | −0·71 | −4·23, 2·81 | 0·69 |
| Markers of lipid metabolism | |||
| tCHOL (mmol/l) | −0·08 | −0·45, 0·29 | 0·68 |
| LDL (mmol/l) | −0·09 | −0·41, 0·24 | 0·61 |
| HDL (mmol/l) | −0·07 | −0·17, 0·03 | 0·18 |
| TAG (mmol/l) | 0·11 | −0·07, 0·29 | 0·23 |
MK-7, menaquinone-7; 25-OH-D, 25-hydroxyvitamin D; ucOC, uncarboxylated osteocalcin; cOC, carboxylated osteocalcin; dp-ucMGP, desphospho-uncarboxylated matrix Gla-protein; dp-cMGP, desphospho-carboxylated matrix Gla-protein; sPLA-2, secretory type-II phospholipase A2; vWF, von Willebrand factor; CRP, C-reactive protein; VEGF, vascular endothelial growth factor; VCAM, vascular cell adhesion molecule; ICAM, intercellular adhesion molecule; SAA, serum amyloid A; tCHOL, total cholesterol.
The effect of the fortified yogurt on the dependent variable (end-value of the markers) was adjusted for the baseline values of the concomitant variable, age, BMI and TAG. The effects on markers of lipid metabolism were adjusted for age and BMI.
P values of the multivariate regression analysis were regarded statistically significant if P < 0·0024 (Bonferroni correction for multiple comparisons).
Analysis between the subjects consuming the standard yogurt and consuming the fortified yogurt was performed after logarithmic transformation of the values.
Markers of vitamin K status
Both ucOC and dp-ucMGP decreased during the 12 weeks’ consumption of the fortified yogurt, although only the decrease in dp-ucMGP was significant (repeated-measures, within-subject analysis: P = 0·011). Significant between-group differences were found for circulating dp-ucMGP already after 8 weeks (P = 0·008), but 12 weeks of intake were needed to significantly decrease ucOC levels (P = 0·001).
Circulating ucOC decreased by 33 (sd 18) % after consuming the fortified yogurt for 12 weeks, compared with −7 (sd 26) % in the group drinking the standard yogurt (P < 0·0001). Circulating dp-ucMGP decreased by 24 (sd 13) % in the group consuming the fortified yogurt drink, whereas dp-ucMGP levels remained unchanged in the group with the standard yogurt (−1 (sd 28) %; P < 0·0001, see Fig. 1(a), 1(c)). Changes in circulating cOC and dp-cMGP levels did not differ between the two treatment groups (Fig. 1(b), 1(d)). Multivariate linear regression analysis showed that after adjusting for age, BMI and TAG the difference between the treatment groups remained significant for ucOC (P = 0·001) and dp-ucMGP (P < 0·0001), even after correction for multiple comparisons (Table 3).
Circulating markers of inflammation, endothelial dysfunction and lipid metabolism
Markers of inflammation, endothelial dysfunction and lipid metabolism were not affected after consuming the fortified yogurt drink. Also after adjusting for the potential confounders age, BMI and TAG none of the circulating markers reached the level of significance (Table 3).
Discussion
In the present paper, we report that yogurt forms an excellent matrix for food fortification with vitamin K since MK-7 was well absorbed and transported to its target tissues, including bone and arteries. This is the first time that a dose of vitamin K lower than the RDA (Commission Directive 2008/100/EC) is shown to be able to significantly improve vitamin K status (as measured by significant decreases in circulating ucOC and dp-ucMGP) in healthy adults. We previously showed a significant improvement of vitamin K status in healthy children after intake of MK-7 supplements (daily dose of 45 µg). This dose however – when taking into account body weight – equals 150 µg for adults. Vascular vitamin K status (as measured by circulating dp-ucMGP) correlated at baseline with markers for inflammation and endothelial dysfunction. During the relatively short intervention period, however, these markers for vascular health were not changed by consumption of the fortified yogurt drink.
Most intervention studies with vitamin K published today have used food supplements rather than fortified foods, and the decreases in ucOC and dp-ucMGP ranged between 20 and 85 % depending on the vitamin K dose(7–10,23,32–41). While phylloquinone was supplemented at high doses (0·5–1·0 mg/d), effects of MK-7 intervention were demonstrated at lower intake levels: steady-state levels for plasma MK-7 of 6 ng/ml were shown at 150 µg/d with a significant effect on OC carboxylation(42). Furthermore, Theuwissen et al. showed an increase in circulating MK-7 from 0·4 to 1·5 ng/ml at an intake of 90 µg/d accompanied by significant decreases in ucOC and dp-ucMGP (30 and 34 %, respectively)(7). At lower intakes (10 to 45 µg/d), no significant effects were observed. Next to supplements, MK-7-enriched yogurt was used to supply participants with an extra 100 µg MK-7/d and after 12 months of treatment the ucOC:cOC ratio decreased by about 50 %(32). Unfortunately, results on circulating MK-7 were lacking. In our current study, circulating MK-7 had increased from 0·28 to 1·94 ng/ml already after 12 weeks, leading to significant decreases in circulating ucOC and dp-ucMGP (33 and 24 %, respectively). These figures are comparable with those obtained with supplements containing 90 µg MK-7(7), and demonstrate that a yogurt drink is an excellent matrix for fortification with MK-7. The added fish oil may have facilitated absorption and/or efficacy of the lipophilic MK-7 accounting for the observed circulating MK-7 levels, because it is well known that vitamin K absorption is stimulated by concomitant fat intake and bile secretion(43). Whether specific components in the fortified yogurt drink, such as the fish oil or the dairy product matrix itself, influenced the bioavailability of MK-7 needs further investigation.
Since low dietary menaquinone intake has been associated with vascular calcification and mortality, we have investigated baseline associations between markers of vitamin K status and vascular health. We used circulating dp-ucMGP and ucOC as markers of vitamin K status of the vasculature and bone, respectively. Significant correlations were found between plasma dp-ucMGP levels and markers for low-grade inflammation (IL-6) and endothelial dysfunction (VCAM and von Willebrand factor). Circulating ucOC did not correlate with markers of vascular health. Observational studies have shown associations between various measures of vitamin K status (as determined by plasma phylloquinone, phylloquinone intake, (%) ucOC, and dp-ucMGP) and inflammatory markers(8,44–46). Remarkably, in our study the other measured MGP form, i.e. dp-cMGP, also correlated with the vascular markers.
Finally, we addressed the question whether the fortified yogurt drink may have beneficial effects on the markers for vascular health. This turned out not to be the case. Both vitamin K and fish oil have been linked to suppression of inflammation, but no clear underlying mechanisms have been found so far. Regarding vitamin K, long-term phylloquinone supplementation at 0·5 mg/d showed no effects on circulating cytokines, despite beneficial effects on vascular health as measured by decreased progression of coronary arterial calcification in elderly. In contrast, 3-month MK-4 administration at 45 mg/d significantly decreased C-reactive protein and matrix metalloproteinase-3 levels in female rheumatoid arthritis (RA) patients(47). Difference in dosage or the inflammatory state of the participants (healthy v. RA) may account for the diverging results. On the other hand, our study may lack sufficient power to detect effects on inflammatory markers since we based our power calculations on changes in vitamin K status. Regarding fish oil, the latest review by Rangel-Huerta et al.(48) concludes that dietary n-3 PUFA are associated with lower levels of inflammatory markers in CVD and other chronic diseases. Fish oil supplementation had generally no effect on inflammatory markers in the studies of healthy participants. Fish oil supplementation was found to have only isolated effects on plasma intercellular adhesion molecule (P = 0·05; 2·1 g/d for 8 weeks)(49) and VCAM levels (P < 0·05; 1 g/d for 12 weeks)(50). The authors indicate – as mentioned for vitamin K – that the lack of an anti-inflammatory effect may be due to the lower serum levels in healthy subjects, minimising the possibility of their reduction. Compared with the dosages described (range 0·4–23·6 g/d; doses >5 g/d through infusion), we used a relatively low daily dose of n-3 PUFA which may also contribute to the null findings.
Our study has some limitations. First, no results were available on the background diet of the participants. We collected information on their intake of vitamin K-containing food products, but not on their general dietary habits. Second, our study population was limited to healthy elderly, which makes extrapolation to other population groups difficult. Finally, the fact that besides MK-7 other nutrients were added to the fortified yogurt means that it remains unclear whether the effect of the intervention on vitamin K status would have been different if only MK-7 had been added.
Conclusion
We have demonstrated that consuming a yogurt drink fortified with low doses of among others vitamin K (MK-7, daily dose of 56 µg), vitamins C and D, and n-3 PUFA (EPA + DHA, daily dose of 0·2 g) significantly improves vitamin K status. Inflammatory biomarkers and lipid markers had, however, not changed after drinking the yogurt for 12 weeks. The importance of n-3 PUFA for menaquinone absorption needs further investigation.
Acknowledgements
This study was supported by FrieslandCampina (Amersfoort, The Netherlands). R. M. L. Z. contributed to the design of the study and the preparation of the manuscript. FrieslandCampina received support from NL Innovatie (grant number: FND10001).
C. V. contributed to the design of the study, conduct of the study, interpretation of findings and the preparation of the manuscript. M. H. J. K. performed the statistical analyses and helped write the manuscript. K. J. T. contributed to the conduct of the study. L. A. J. L. M. B. and E. T. contributed to the analysis of data, interpretation of findings and preparation of the manuscript. R. M. L. Z. contributed to the design of the study and the preparation of the manuscript. All authors approved the final manuscript.
There were no conflicts of interest.
References
- 1.Geleijnse JM, Vermeer C, Grobbee DE, et al. (2004) Dietary intake of menaquinone is associated with a reduced risk of coronary heart disease: the Rotterdam Study. J Nutr 134, 3100–3105. [DOI] [PubMed] [Google Scholar]
- 2.Beulens JW, Bots ML, Atsma F, et al. (2009) High dietary menaquinone intake is associated with reduced coronary calcification. Atherosclerosis 203, 489–493. [DOI] [PubMed] [Google Scholar]
- 3.Gast GC, de Roos NM, Sluijs I, et al. (2009) A high menaquinone intake reduces the incidence of coronary heart disease. Nutr Metab Cardiovasc Dis 19, 504–510. [DOI] [PubMed] [Google Scholar]
- 4.Vermeer C (1990) γ-Carboxyglutamate-containing proteins and the vitamin K-dependent carboxylase. Biochem J 266, 625–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suttie JW (1993) Synthesis of vitamin K-dependent proteins. FASEB J 7, 445–452. [DOI] [PubMed] [Google Scholar]
- 6.Furie B, Bouchard BA & Furie BC (1999) Vitamin K-dependent biosynthesis of γ-carboxyglutamic acid. Blood 93, 1798–1808. [PubMed] [Google Scholar]
- 7.Theuwissen E, Cranenburg EC, Knapen MH, et al. (2012) Low-dose menaquinone-7 supplementation improved extra-hepatic vitamin K status, but had no effect on thrombin generation in healthy subjects. Br J Nutr 108, 1652–1657. [DOI] [PubMed] [Google Scholar]
- 8.Shea MK, O'Donnell CJ, Vermeer C, et al. (2011) Circulating uncarboxylated matrix Gla-protein is associated with vitamin K nutritional status, but not coronary artery calcium, in older adults. J Nutr 141, 1529–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brugè F, Bacchetti T, Principi F, et al. (2011) Olive oil supplemented with menaquinone-7 significantly affects osteocalcin carboxylation. Br J Nutr 106, 1056–1062. [DOI] [PubMed] [Google Scholar]
- 10.Dalmeijer GW, van der Schouw YT, Magdeleyns E, et al. (2012) The effect of menaquinone-7 supplementation on circulating species of matrix Gla-protein. Atherosclerosis 225, 397–402. [DOI] [PubMed] [Google Scholar]
- 11.Szulc P, Chapuy MC, Meunier PJ, et al. (1996) Serum undercarboxylated osteocalcin is a marker of the risk of hip fracture in elderly women. J Clin Invest 9, 1769–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szulc P, Arlot M, Chapuy MC, et al. (1994) Serum undercarboxylated osteocalcin correlates with hip bone mineral density in elderly women. J Bone Miner Res 9, 1591–1595. [DOI] [PubMed] [Google Scholar]
- 13.Booth SL, Broe KE, Peterson JW, et al. (2004) Associations between vitamin K biochemical measures and bone mineral density in men and women. J Clin Endocrinol Metab 89, 4904–4909. [DOI] [PubMed] [Google Scholar]
- 14.Vergnaud P, Garnero P, Meunier PJ, et al. (1997) Undercarboxylated osteocalcin measured with a specific immunoassay predicts hip fracture in elderly women: the EPIDOS study. J Clin Endocrinol Metab 82, 719–724. [DOI] [PubMed] [Google Scholar]
- 15.Knapen MHJ, Nieuwenhuijzen Kruseman AC, Wouters RSME, et al. (1998) Correlation of serum osteocalcin fractions with bone mineral density in women during the first 10 years after menopause. Calc Tissue Int 63, 375–379. [DOI] [PubMed] [Google Scholar]
- 16.Luukinen H, Käkönen SM, Pettersson K, et al. (2000) Strong prediction of fractures among older adults by the ratio of carboxylated to total serum osteocalcin. J Bone Miner Res 15, 2473–2478. [DOI] [PubMed] [Google Scholar]
- 17.Cranenburg EC, Vermeer C, Koos R, et al. (2008) The circulating inactive form of matrix Gla protein (ucMGP) as a biomarker for cardiovascular calcification. J Vasc Res 45, 427–436. [DOI] [PubMed] [Google Scholar]
- 18.Cranenburg EC, Koos R, Schurgers LJ, et al. (2010) Characterisation and potential diagnostic value of circulating matrix Gla protein (MGP) species. Thromb Haemost 104, 811–822. [DOI] [PubMed] [Google Scholar]
- 19.Rennenberg RJ, de Leeuw PW, Kessels AG, et al. (2010) Calcium scores and matrix Gla-protein levels: association with vitamin K status. Eur J Clin Invest 40, 344–349. [DOI] [PubMed] [Google Scholar]
- 20.Ueland T, Dahl CP, Gullestad L, et al. (2011) Circulating levels of non-phosphorylated undercarboxylated matrix Gla-protein are associated with disease severity in patients with chronic heart failure. Clin Sci 121, 119–127. [DOI] [PubMed] [Google Scholar]
- 21.Sokoll LJ, Booth SL, O'Brien ME, et al. (1997) Changes in serum osteocalcin, plasma phylloquinone, and urinary γ-carboxyglutamic acid in response to altered intakes of dietary phylloquinone in human subjects. Am J Clin Nutr 65, 779–784. [DOI] [PubMed] [Google Scholar]
- 22.Booth SL, O'Brien-Morse ME, Dallal GE, et al. (1999) Response of vitamin K status to different intakes and sources of phylloquinone-rich foods: comparison of younger and older adults. Am J Clin Nutr 70, 368–377. [DOI] [PubMed] [Google Scholar]
- 23.Westenfeld R, Krueger T, Schlieper G, et al. (2012) Effect of vitamin K2 supplementation on functional vitamin K deficiency in hemodialysis patients: a randomized trial. Am J Kidney Dis 59, 186–195. [DOI] [PubMed] [Google Scholar]
- 24.Booth SL, Sadowski JA, Weihrauch JL, et al. (1993) Vitamin K1 (phylloquinone) content of foods: a provisional table. J Food Comp Anal 6, 109–120. [Google Scholar]
- 25.Shearer MJ & Bolton-Smith C (2000) The U.K. food data-base for vitamin K and why we need it. Food Chem 68, 213–218. [Google Scholar]
- 26.Schurgers LJ & Vermeer C (2000) Determination of phylloquinone and menaquinones in food. Effect of food matrix on circulating vitamin K concentrations. Haemostasis 30, 298–307. [DOI] [PubMed] [Google Scholar]
- 27.Gaucheron F (2011) Milk and dairy products: a unique micronutrient combination. J Am Coll Nutr 30, 400S–409S. [DOI] [PubMed] [Google Scholar]
- 28.Kromhout D, Bosschieter EB & de Coulander CL (1987) The inverse relation between fish consumption and 2-year mortality from coronary heart disease. Lancet i, 177–183. [DOI] [PubMed] [Google Scholar]
- 29.Burr ML, Fehily AM, Gilbert JF, et al. (1989) Effects of changes in fat, fish and fibre intakes on death and myocardial reinfarction. Lancet ii, 757–761. [DOI] [PubMed] [Google Scholar]
- 30.Kris-Etherton PM, Harris WS & Appel LJ (2003) Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Arterioscler Thromb Vasc Biol 23, 151–152. [DOI] [PubMed] [Google Scholar]
- 31.Friedewald WT, Levy RI & Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18, 499–502. [PubMed] [Google Scholar]
- 32.Kanellakis S, Moschonis G, Tenta R, et al. (2012) Changes in parameters of bone metabolism in postmenopausal women following a 12-month intervention period using dairy products enriched with calcium, vitamin D, and phylloquinone (vitamin K1) or menaquinone-7 (vitamin K2): the Postmenopausal Health Study II. Calc Tissue Int 90, 251–262. [DOI] [PubMed] [Google Scholar]
- 33.Braam LA, Knapen MH, Geusens P, et al. (2003) Vitamin K1 supplementation retards bone loss in postmenopausal women between 50 and 60 years of age. Calc Tissue Int 73, 21–26. [DOI] [PubMed] [Google Scholar]
- 34.Bolton-Smith C, McMurdo ME, Paterson CR, et al. (2007) Two-year randomized controlled trial of vitamin K1 (phylloquinone) and vitamin D3 plus calcium on the bone health of older women. J Bone Miner Res 22, 509–519. [DOI] [PubMed] [Google Scholar]
- 35.Booth SL, Dallal G, Shea MK, et al. (2008) Effect of vitamin K supplementation on bone loss in elderly men and women. J Clin Endocrinol Metab 93, 1217–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Emaus N, Gjesdal CG, Almas B, et al. (2009) Vitamin K2 supplementation does not influence bone loss in early menopausal women: a randomised double-blind placebo-controlled trial. Osteoporos Int 21, 1731–1740. [DOI] [PubMed] [Google Scholar]
- 37.Knapen MH, Schurgers LJ & Vermeer C (2007) Vitamin K2 supplementation improves hip bone geometry and bone strength indices in postmenopausal women. Osteoporos Int 18, 963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bugel S, Sorensen AD, Hels O, et al. (2007) Effect of phylloquinone supplementation on biochemical markers of vitamin K status and bone turnover in postmenopausal women. Br J Nutr 97, 373–380. [DOI] [PubMed] [Google Scholar]
- 39.Cheung AM, Tile L, Lee Y, et al. (2008) Vitamin K supplementation in postmenopausal women with osteopenia (ECKO trial): a randomized controlled trial. PLoS Med 5, e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Binkley N, Harke J, Krueger D, et al. (2009) Vitamin K treatment reduces undercarboxylated osteocalcin but does not alter bone turnover, density, or geometry in healthy postmenopausal North American women. J Bone Miner Res 24, 983–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Summeren MJ, Braam LA, Lilien MR, et al. (2009) The effect of menaquinone-7 (vitamin K2) supplementation on osteocalcin carboxylation in healthy prepubertal children. Br J Nutr 102, 1171–1178. [DOI] [PubMed] [Google Scholar]
- 42.Schurgers LJ, Teunissen KJ, Hamulyak K, et al. (2007) Vitamin K-containing dietary supplements: comparison of synthetic vitamin K1 and natto-derived menaquinone-7. Blood 109, 3279–3283. [DOI] [PubMed] [Google Scholar]
- 43.Shearer MJ, Fu X & Booth SL (2012) Vitamin K nutrition, metabolism, and requirements: current concepts and future research. Adv Nutr 3, 182–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shea MK, Dallal GE, Dawson-Hughes B, et al. (2008) Vitamin K, circulating cytokines, and bone mineral density in older men and women. Am J Clin Nutr 88, 356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shea MK, Booth SL, Massaro JM, et al. (2008) Vitamin K and vitamin D status: associations with inflammatory markers in the Framingham Offspring Study. Am J Epidemiol 167, 313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Juanola-Falgarona M, Salas-Salvadó J, Estruch R, et al. (2013) Association between dietary phylloquinone intake and peripheral metabolic risk markers related to insulin resistance and diabetes in elderly subjects at high cardiovascular risk. Cardiovasc Diabetol 12, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ebina K, Shi K, Hirao M, et al. (2013) Vitamin K2 administration is associated with decreased disease activity in patients with rheumatoid arthritis. Mod Rheumatol 23, 1001–1007. [DOI] [PubMed] [Google Scholar]
- 48.Rangel-Huerta OD, Aguilera CM, Mesa MD, et al. (2012) Omega-3 long-chain polyunsaturated fatty acids supplementation on inflammatory biomarkers: a systematic review of randomized clinical trials. Br J Nutr 107, S159–S170. [DOI] [PubMed] [Google Scholar]
- 49.Yusof HM, Miles E & Calder P (2008) Influence of very long-chain n-3 fatty acids on plasma markers of inflammation in middle-aged men. Prostaglandins Leukot Essent Fatty Acids 78, 219–228. [DOI] [PubMed] [Google Scholar]
- 50.Thies F, Miles E, Nebe-von-Caron G, et al. (2001) Influence of dietary supplementation with long-chain n-3 or n-6 polyunsaturated fatty acids on blood inflammatory cell populations and functions and on plasma soluble adhesion molecules in healthy adults. Lipids 36, 1186–1193. [DOI] [PubMed] [Google Scholar]