Abstract
The human tyrosinase gene TYR is a multifunctional reporter gene with potential use in photoacoustic imaging (PAI), positron emission tomography (PET), and magnetic resonance imaging (MRI). We sought to establish and evaluate a reporter gene system using TYR under the control of the Tet-on gene expression system (gene expression induced by doxycycline [Dox]) as a multimodality imaging agent. We transfected TYR into human breast cancer cells (MDA-MB-231), naming the resulting cell line 231-TYR. Using non-transfected MDA-MB-231 cells as a control, we verified successful expression of TYR by 231-TYR after incubation with Dox using western blot, cellular tyrosinase activity, Masson-Fontana silver staining, and a cell immunofluorescence study, while the control cells and 231-TYR cells without Dox exposure revealed no TYR expression. Detected by its absorbance at 405 nm, increasing concentrations of melanin correlated positively with Dox concentration and incubation time. TYR expression by Dox-induced transfected cells shortened MRI T1 and T2 relaxation times. Photoacoustic signals were easily detected in these cells. 18F-5-fluoro-N-(2-[diethylamino]ethyl)picolinamide (18F-5-FPN), which targets melanin, quickly accumulated in Dox-induced 231-TYR cells. These show that TYR induction of melanin production is regulated by the Tet-on system, and TYR-containing indicator cells may have utility in multimodality imaging.
Molecular imaging has shown promise for non-invasive in vivo visualisation of cellular processes. In addition to the commonly used magnetic resonance imaging (MRI), positron emission-computed tomography (PET) and single photon emission-computed tomography (SPECT); optical bioluminescence, optical fluorescence, and photoacoustic imaging (PAI) are undergoing extensive investigation as potential modalities to diagnose and evaluate disease. Each imaging modality has its own strengths and weaknesses. Radionuclide-based imaging techniques (PET and SPECT) are highly sensitive, and PET is quantitatively robust, but they have relatively poor spatial resolution. MRI provides high-resolution images but suffers from low sensitivity. Optical imaging is limited by shallow tissue penetration1. Multimodality imaging usually combines two modalities to provide both structural and functional information. Examples include PET/CT, PET/MRI, and SPECT/CT. Research on the use of PET, SPECT, and MRI with immunofluorescence and photoacoustic imaging is ongoing2.
The design of molecular probes keeps direct and indirect imaging strategies in mind. Direct imaging images a labelled probe bound to a target, usually a protein. An example is the functional superparamagnetic iron oxide nanoparticles used to construct multifunctional nanostructures for PET/MRI or PET/near-infrared fluorescence (NIRF)/MRI3,4,5. This approach requires specific probes for each modality, and the multiple conjugations may affect the specificity of binding and imaging6. Indirect imaging is based on reporter genes, using a probe that specifically binds to the gene product. Commonly used reporter gene products include the thymidine kinase produced by herpes simplex virus type 1 (HSV1-tk) and the sodium iodide symporter (NIS) labelled with radiopharmaceuticals7,8,9,10, green fluorescent protein (GFP) and firefly luciferase (Fluc)11,12, used in fluorescence imaging, and ferritin and tyrosinase13,14, used in MRI. The indirect strategy often needs to fuse two, three, or even more reporter genes into cells. In our previous experiments, a triple-fused reporter gene (HSV1-tk, GFP and Fluc) was prepared for PET, fluorescence and bioluminescence imaging11. Gene fusion processes are difficult. Linkers, the distance between reporter genes, and the orientation of each reporter gene are the key factors. This has inspired a search for simpler probes.
Human tyrosinase (TYR), a key enzyme, catalyses the three most important steps in melanin production, which include oxidation of tyrosine to dopamine (DOPA), DOPA to dopaquinone, and 5, 6-dihydroxyindile to 5, 6-indolequinone15. Melanin production rate and yield correlate positively with TYR expression and activity16. After transduction of TYR into cells and encoding an active tyrosinase, melanin synthesis is activated. The advantage of melanin is its multiple properties that can be imaged with different modalities. Its wide absorption spectrum from the ultraviolet to near infrared enables its use in photoacoustic imaging17,18. Its affinity to iron can be as high as 16% of its own weight19. Ionised iron has high signal intensity on MRI T1-weighted images (T1WI), the intensity increasing with increasing ion concentration14. In addition, some studies have found that benzamide and its analogues specifically bind to melanin. Several radiopharmaceuticals, 125I-BZA, and 123/131I-IBZA (for SPECT imaging) have been developed for the diagnosis of melanoma20,21. Based on the same principle, some PET probes, such as (N-[2-(diethylamino) ethy1]-6-18F-fluoropicolinamide) (18F-MEL050), have demonstrated high and specific binding to melanin both in vitro and in vivo22. Another positron probe, 18F-5-fluoro-N-(2-[diethylamino]ethyl)picolinamide (18F-5-FPN), prepared by our group, has been shown to specifically target melanin in vitro and in vivo with high retention, affinity and favourable pharmacokinetics23. Potentially, using TYR, as a reporter gene, one could perform PAI, MRI, and PET or SPECT imaging. Previous studies have demonstrated that TYR can be used as a multifunctional reporter gene for PAI/MRI or PAI/MRI/PET imaging both in vitro and in vivo24,25.
In gene therapy and related gene studies26, it has been demonstrated that the timing and degree of gene expression with an activator substance is much better than the sustained expression of a gene product, as the sustained expression of exogenous genes or proteins may result in some unexpected adverse effects27. Since the advent of the Tet-off and Tet-on gene expression systems28,29, both have been widely used in various prokaryotic and eukaryotic models30,31. TYR, to act as a reporter gene, needs to be transfected and integrated into cells, and the Tet-on tetracycline gene induction system is widely used for inducible expression, as it can effectively control gene expression in vivo and in vitro using doxycycline (Dox) as the activator27,32.
We integrated a third-generation tetracycline-inducible gene expression system (Tet-On 3G®, Clontech™, TakaraBio, Otsu, Shiga, Japan) with TYR to establish a new reporter gene system (Fig. 1). The system was evaluated in vitro under the control of Dox for providing the feasibility of multimodality imaging.
Figure 1. Principle of the Tet-on inducible gene expression system regulating tyrosinase (TYR) gene expression using Dox.
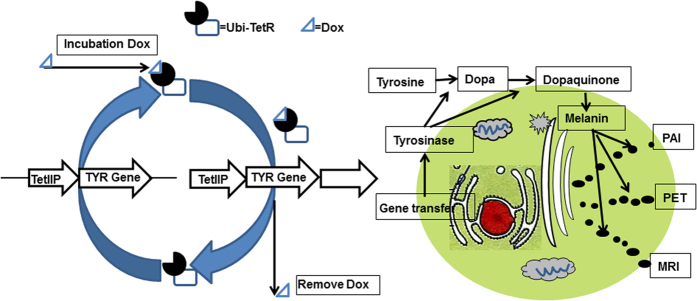
The TYR reporter gene is inserted into cells through the method of gene transfer. Gene expression product tyrosinase, the key enzyme, catalyses the process in melanin production. Melanin then serves as a multifunctional target for photoacoustic imaging (PAI), positron emission tomography (PET) and magnetic resonance imaging (MRI) multimodal imaging.
Results
Identification of tyrosine expression in different groups after Lenti-X tet-on 3G-TYR transduction
We successfully constructed the lentiviral vector Lenti-X Tet-On 3G-TYR, and selected a stable breast cancer cell line expressing TYR using puromycin. To measure the expression of the TYR gene in 231-TYR + Dox, 231-TYR and 231 cells, western blot was performed (Fig. 2A). We found that TYR was only successfully expressed in 231-TYR cells treated with Dox (231-TYR + Dox) and not in the control cells (231-TYR and 231 cells). Cellular tyrosinase activity was also assessed by measuring the amount of dopachrome. Figure 2B shows that the amount of dopachrome in 231-TYR + Dox cells increased over time, while no dopachrome was found in the control groups exposed to Dox. TYR activity in 231-TYR + Dox cells was significantly higher than that in the control cells (P < 0.05 for all time points). The 231-TYR + Dox, 231-TYR, and 231 cells were collected, and the melanin expression was estimated by visual inspection (Fig. 2C). An obvious black colour was visible in the 231-TYR + Dox cells, while the other cells just showed the colour of the culture medium. Melanin was also identified by Masson–Fontana silver staining, with coarse black particles only found in the 231-TYR + Dox cells (Fig. 2D).
Figure 2. Evaluation of the expression of TYR reporter in vitro.
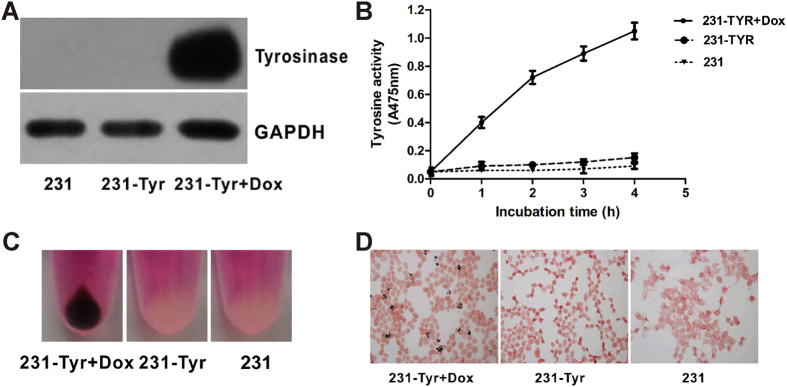
(A) Western blot assay of tyrosinase expression in 231-TYR+Dox, 231-TYR and 231 cells. GAPDH was used as the control. (B) Time-response tyrosinase activity curves in three groups. (C) Photos of the cell pellets from 231-TYR + Dox, 231-TYR and 231 cells. (D) Masson-Fontana silver staining in 231-TYR + Dox, 231-TYR and 231 cells.
Results of cell immunofluorescence studies
To further assess the expression of TYR, we performed immunofluorescence experiments. The immunofluorescence results in Fig. 3 demonstrate that TYR products were expressed by the 231-TYR + Dox cells, and not by the control cells.
Figure 3. Immunofluorescent staining of TYR in 231-TYR + Dox, 231-TYR and 231 cells. These cells were labelled for immunofluorescence with a TYR-specific monoclonal antibody (green) and their nuclei were counterstained with 4–6-diamidino-2-phenylindole (DAPI) (blue).
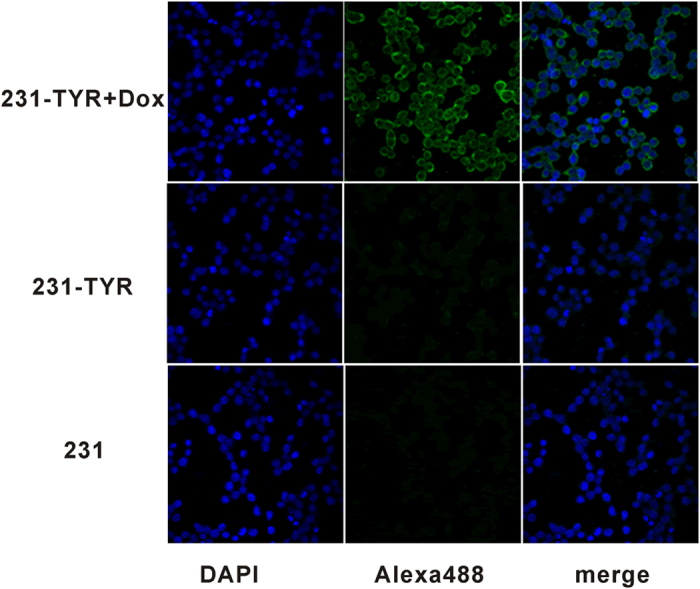
Representative images (×400) are shown.
Dox regulation of melanin production
We quantified the effect of Dox-induced TYR expression from the dosage and the duration of exposure to Dox in 231-TYR + Dox cells. As shown in Fig. 4A, the concentration of Dox and melanin yield was positively correlated, melanin production peaking at a concentration of Dox of 2000 ng/mL. Figure 4B displays the Dox-induced melanin yield in 231-TYR cells related to the length of time of Dox incubation, the melanin yield gradually increasing from 4 to 48 h, peaking at 48 h. Melanin began to decrease 4 h after the withdrawal of Dox and returned to normal levels at about 48 h (Fig. 4C). This suggests that Dox should be withdrawn in advance if we want to stop the effect of the reporter gene.
Figure 4.
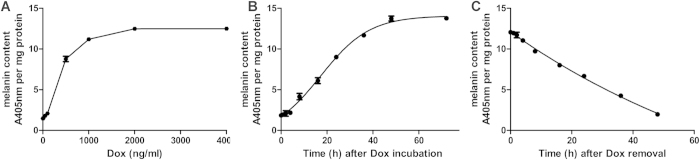
(A) Different concentrations of Dox regulate TYR expression in 231-TYR cell; (B) The incubation time of Dox affect the melanin production in 231-TYR cells; (C) the change of melanin content in 231-TYR cells after the withdrawal of Dox at different time.
Cell MRI
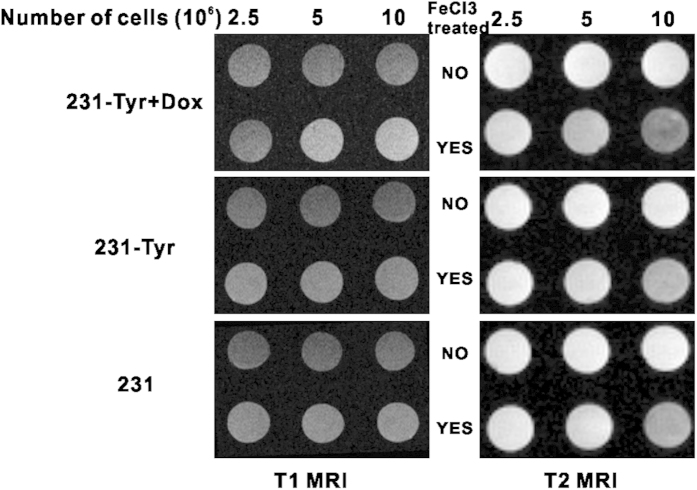
Different cell concentrations were used to study the sensitivity of MRI for detection of melanin (Fig. 5). We found that 231-TYR + Dox cells cultured with FeCl3-enriched medium displayed a much higher signal on T1-weighted images (T1WI), compared with 231-TYR and 231 cells (Fig. 5, left). The T1 relaxation times in msec of 231-TYR + Dox cells with the maximum concentration in the sample with and without FeCl3 were 1216.13 and 2470.91 msec, respectively, indication shortening of the T1 relaxation time by 50.78%. We also found that 231-TYR + Dox cells cultured in FeCl3-enriched medium displayed much lower signals on T2-weighted images (T2WI), compared with 231-TYR and 231cells (Fig. 5, right). T2 signal decreased with increasing number of 231-TYR + Dox cells. The T2 relaxation times in msec of 231-TYR + Dox cells with the maximum concentration in the sample with and without FeCl3 were 29.58 and 84.76 msec, respectively. The iron shortened the T2 relaxation time by 65.1%. The three cell lines cultured in medium without FeCl3-enrichment did not produce detectable T1 or T2-weighted signal, and the signals of the control cells with FeCl3 treatment only slightly increased and decreased T1 and T2 relaxation times, respectively.
Figure 5.
(A) T1 MRI (left) and T2 MRI (right) images of three concentrations of 231-TYR + Dox, 231-TYR and 231 cells pre-treated without (top row) or with (bottom row) FeCl3.
Cell PAI
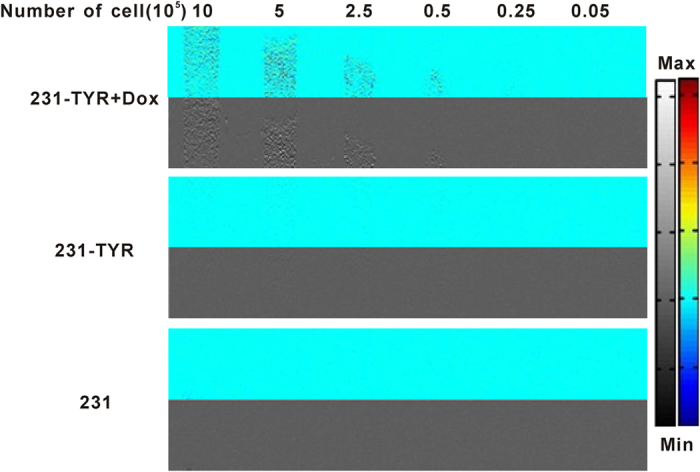
Figure 6 shows the photoacoustic signals of different concentrations of cells ranging from 1 × 105 to 2 × 107 /mL. The cell samples were located 2 mm below the surface of the gel phantoms, and the photoacoustic signals could be easily detected in 231-TYR + Dox cells with a cell concentration as low as 1 × 106/mL (5 × 104 231-TYR + Dox cells). The signal increased with increasing concentration of melanotic cells, while 231-TYR cells without Dox incubation and negative control 231 cells could not produce detectable photoacoustic signal even at 2 × 107/mL.
Figure 6. Photoacoustic images of the gel phantom with different concentrations of cells.
Cell uptake study of 18F-5-FPN
Uptake levels of 18F-5-FPN in 231-TYR + Dox, 231-TYR and 231 cells are shown in Fig. 7A. 18F-5-FPN quickly accumulated in 231-TYR + Dox cells, with uptake values 4.63 ± 0.24% at 60 min (n = 3). In comparison, no or rarely significant accumulation of 18F-5-FPN in 231-TYR and 231 cells was observed, with 60 min uptake values of 0.71 ± 0.06% and 0.60 ± 0.05%, respectively. As shown in Fig. 7B, the cell efflux study found that 18F-5-FPN efflux from both the 231-TYR + Dox and the contrast cells were most pronounced within the first 30 min; the values were determined to be 2.35% (30 min) for the 231-TYR + Dox cells, 0.18% and 0.24% (30 min) for the 231-TYR and 231 cells, respectively. After 30 min, cell uptake values were maintained with a relatively stable level. Administration of cold 19F-5-FPN inhibited the binding of 18F-5-FPN in 231-TYR + Dox cells in a concentration-dependent pattern shown in Fig. 7C, illustrating the specificity of binding of 18F-5-FPN to melanin in vitro.
Figure 7. Cell uptake studies of 18F-5-FPN.
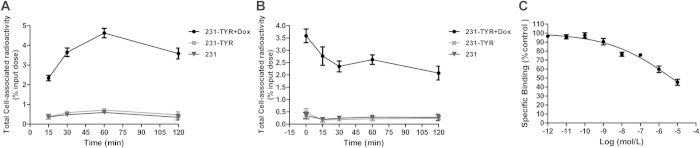
(A) Uptake of 18F-5-FPN in 231-TYR + Dox, 231-TYR and 231 cells after incubation with 18F-5-FPN at 37 °C for 30, 60 and 120 min. All results expressed as percentage of input dose as a result of triplicate measurements ± SD. (B) The 18F-5-FPN cell uptake efflux study performed in 231-TYR+Dox, 231-TYR and 231 cells at the serial time points. (C) competitive cell-binding assay of 19F-5-FPN to 231-TYR + Dox cells, all results expressed as percentage of cellular uptake inhibition ratio.
Discussion
In this study, we successfully constructed a lentiviral vector complex containing TYR as a reporter gene and used the Tet-on system to control its expression. After transducing TYR into the breast cancer cell line MDA-MB-231, a stable line expressing TYR (231-TYR) was established and screened. We verified that Dox induction could precisely regulate the expression of TYR. Further, we demonstrated that tyrosinase, as a multifunctional reporter gene product, could be used for MRI/PET/PAI multimodality imaging in vitro.
TYR has been used as a reporter gene for magnetic resonance imaging25. Most previous studies using TYR as a MRI reporter gene have analysed the changes in T1 signal; nonetheless, Fe (III) also has an impact on the T2 relaxation time. In this study, we observed that TYR changed the T1 and T2 relaxation times (Fig. 5), consistent with the signal changes observed in images of pigmented melanoma tumours33. Quantitative analysis revealed that the T2 relaxation time changed more than that of T1 in 231-TYR + Dox cells. Photoacoustic imaging (PAI) can be used for functional and molecular imaging with endogenous and exogenous contrast agents. Melanin is a common endogenous contrast agent34. In our study, photoacoustic signal changes were only detected in melanotic 231-TYR + Dox cells (Fig. 6). Signal detection was very sensitive, identifying signals from only 5 × 104 231-TYR + Dox cells. The sensitivity in our study was lower than the results of Qin et al.25, which may be related to difference in instrumentation or different levels of TYR expression. We prepared and evaluated 18F-5-fluoro-N-(2-[diethylamino]ethyl)picolinamide (18F-5-FPN), which has a high affinity with melanin, in our previous study23. In this study, 18F-5-FPN specifically bound to the melanin in 231-TYR + Dox cells, and blocked with excess nonradioactive standards (Fig. 7), demonstrating the feasibility of TYR as a reporter gene for PET imaging.
In the three different imaging modalities, PAI has the highest sensitivity. However, when spatial resolution of 1 mm is necessary, its penetration is less than 5 cm because of the optical attenuation effect. In addition, ultrasound signals cannot penetrate hollow visci or lung tissue owing to the acoustic impedance effect. PET and MRI do not suffer from these limitations. MRI shows a characteristic signal pattern on T1WI and T2WI, with high spatial resolution. 18F-5-FPN for PET imaging of melanin/melanoma exhibits high specificity, and can provide functional information. Therefore, a single reporter gene for PAI/MRI/PET multimodality imaging could make up for each modality’s shortcomings.
Effective control of the time and level of gene expression is better than the sustained expression of gene in gene therapy. Sustained expression of exogenous genes or protein may result in adverse effects and receptor downregulation. The Tet-On 3G system consists of three parts including a regulating unit, reaction originals that connect with the TYR gene, and inducers. Tetracycline repressor factor (Tet repressor, TetR) and ubiquitin promoter (Ubi) compose the regulating unit; TetR is a repressor protein of Tetracycline inducible promoter (TetIIP). TetIIP, an inducible promoter, mediates expression of TYR gene. After TetR inhibition on TetIIP was released using Tet (Dox) combines with TetR, TetIIP will induce TYR gene expression. In our study, the TYR was only expressed in the presence of Dox in 231-TYR cells using western blot, Masson-Fontana silver staining, and immunofluorescence experiments (Figs 2 and 3). Additional studies of the dosage and differences in length of Dox exposure were conducted (Fig. 4). These results confirmed that the Tet-on system quickly responded to Dox, and it could excite the TYR gene expression reversibly, quantitatively and reproducibly.
We demonstrated the potential use of TYR for PAI/MRI/PET multimodality imaging in vitro. In the future, its potential as an in vivo probe for multimodal imaging should be investigated for the following reasons: (1) TYR is an endogenous highly biocompatible gene, with the potential for low measurable impact when transfected into amelanotic cells. (2) Dox is an attractive agent for inducing gene expression in vivo. (3) TYR encodes tyrosinase in the transfected cells, which is the key enzyme for synthesising melanin. Melanin is a polymer and contains multiple binding sites for paramagnetic iron ions, while simultaneously binding benzamide radiopharmaceuticals, making PET/MRI feasible. (4) Used as a multifunctional reporter gene for PAI/MRI/PET imaging, TYR may not only solve problems of spatial resolution and sensitivity, but may also enable imaging of microvessels involved in angiogenesis by Doppler photoacoustic tomography.
TYR also has potential as a therapeutic agent. Melanin, produced with tyrosine kinase expressed by TYR, significantly enhances the absorption of light in the near infrared, which is characterised by low absorption and maximum light penetration in tissues. Stritzker et al.35 used a near-infrared laser to specifically transfer energy to melanin. The transferred energy converted to thermal energy, which then heated the melanin-producing cells to a high temperature, causing protein denaturation and cell death. In addition, benzamide and its analogues have been labelled with radionuclides to irradiate melanomas. The resulting low transient uptake in the excretory organs has been promising. These data indicate that systemic radionuclide therapy using benzamides for the therapy of pigmented melanoma is of considerable potential36,37. TYR transfection of tumours, causing them to synthesise melanin which is subsequently irradiated by radiolabelled benzamides, may be an effective method of unsealed source therapy.
Conclusions
We successfully demonstrated that transfected human TYR can induce the production of melanin in amelanotic cells, and the gene expression can be accurately regulated by the Tet-on system. A preliminary in vitro study suggests that TYR, as a single reporter gene, could change T1 and T2 relaxation times on MRI, the signals on PAI, and the accumulation of PET tracer, which suggests its feasibility for multimodality molecular imaging. Further studies in vivo are necessary.
Methods
Construction of the lentivirus vector complex containing TYR and the Tet-on system
cDNA encoding human TYR (NM_000372.3) in a pcDNA3.1 vector was kindly provided by Dr. Zhen Cheng of Stanford University. Tet-on 3G system used a GV308 vector (TetIIP-MCS-3FLAG-Ubi-TetR-IRES-Puromycin; 12.4 Kb; Gene Chem Co., Ltd, Shanghai, China). TYR DNA was amplified by a polymerase chain reaction (PCR) with primers flanking the TYR open reading frame with BamHI and NheI restriction enzyme sequences within the 5′ and 3′ primers, respectively, and it was purified using a gel extraction kit (Qiagen®, Tiangen Biotech Co., Ltd. Beijing, China). The purified TYR encoding the inserted cDNA and the GV308 vector were both digested with BamHI and NheI restriction enzymes (New England Biolabs, Inc., Ipswich MA, USA) and ligated together with DNA ligase (New England Biolabs). The ligation mixture was used to transform E. coli DH5a competent cells, which were plated on LB broth plates supplemented with puromycin and shaken for 24 h at 37 °C. Bacterial colonies and plasmid DNA were isolated from the resulting colonies. After the recombinant plasmid was identified by DNA sequencing and double restriction enzyme digestion, plasmid preparation (Maxiprep®, Qiagen) was performed, and the concentration of the plasmid was measured. Then, the recombinant plasmid DNA and liposomes were co-transfected into human embryonic kidney 293T cells. We collected the cell supernatant containing the lentiviral particles, concentrated it, and measured the virus titre. The recombinant expression vector was named Lenti-X Tet-on 3G-TYR and stored at −80 °C.
Establishing a stable cell line expressing TYR
The human breast cancer MDA-MB-231 cell line (No.Tchu155) was obtained from the Cell Bank of the Chinese Academy of Sciences. MDA-MB-231 cells were grown in Leibovitz’s L-15 medium (L-15®; Gibco, Carlsbad CA, USA) supplemented with 10% (v/v) foetal bovine serum (Gibco). To establish a stable cell line expressing TYR, the MDA-MB-231 cells were seeded into 6-well plates at a density of 5 × 105 per well and incubated overnight. We co-transduced these cells with the lentivirus Lenti-X tet-on 3G-TYR (multiplicity of infection, MOI = 2) and a transfection enhancer polybrene (Gene Chem Co., Ltd, Shanghai, China), then the cells were replaced into the complete medium 10 h after the transduction. Seventy-two hours after transfection, cells were trypsinised and diluted to a 1000 cells/mL single-cell suspension, and seeded into 96-well plates by the limiting dilution method. After the cells adhered, we observed them carefully under a microscope, choosing and marking those holes observed to contain only 1–2 cells. The next day, these cells were cultured in L-15 medium with 10% FBS containing 1 μg/mL puromycin. The medium was changed every 2–3 days. Cells in dishes grew for several weeks until large cell colonies were visible. Dox (2 μg/mL) was added to each well containing colony cells as an inducer, and carefully observed under light microscopy. The colony with the darkest colour was considered to be capable of producing melanin and termed as 231-TYR cells. This colony was trypsinised from the 96-well plate, cultured and used for subsequent experiments.
Experimental and control groups
The cells were divided into three groups as follows: (1) 231-TYR cells treated with Dox named as 231-TYR + Dox, which was considered the experimental group; (2) 231-TYR cells with no treatment as one control and named as 231-TYR; (3) MDA-MB-231 cells without any treatment named as 231, which was another control.
TYR detection by western blot
The cells in the six-well plates were washed twice with cold PBS (0.01 M, pH 7.2) and dissolved in 300 μL radio-immunoprecipitation assay buffer containing protease inhibitors. The lysates were centrifuged at 12000 rpm (68.2 g) for 15 min at 4 °C, and the supernatants collected. Total cellular proteins (20 μg per lane) were resolved using 10% sodium dodecyl sulphate polyacrylamide gel electrophoresis (Bio-Rad®, Hercules CA, USA) and were transferred to a nitrocellulose filter membrane (Bio-Rad). The membranes were blocked at 4 °C for 1 h in triethanolamine buffered saline solution (TBST) supplemented with 5% non-fat milk. After a brief rinse, the membranes were incubated overnight at 4 °C in TBST containing 5% bovine serum albumin with the primary antibody diluted in TBST (tyrosinase monoclonal antibody, 1:500), (Sigma Chemical Corporation, St. Louis, MO, USA). A glyceraldehyde-3-phosphate dehydrogenase (GAPDH, 1:1000 Santa Cruz Biotechnology, Santa Cruz CA, USA) polyclonal antibody was used as an internal control protein. The blots were washed three times with TBST for 10 min, followed by 1-h incubation with a horseradish peroxidase-conjugated anti-mouse IgG antibody (1:2000, Santa Cruz) at room temperature. The antigen-antibody peroxidase complex was visualised using enhanced chemiluminescence reagents (ECL®, Amersham Biotechnology, Piscataway NJ, USA) according to the manufacturer’s protocol.
Assessment of cellular tyrosinase activity
The sample preparation procedure was the same as that described in the western blot assay. After quantifying the protein levels, the concentration of samples was adjusted to 0.5 μg/μL. The tyrosinase activity was measured as per the published protocols with some modifications25. The experiment was conducted in a 96-well flat-bottom plate. Each well contained 50 μL of cell lysate and 50 μL of 2 mg/mL 3-(3,4-dihydroxyphenyl)-L-alanine (L-DOPA). The mixture was incubated at 37 °C. The absorbance of the reaction mixtures was measured using a plate reader (Omega Bio-tek, Doraville CA, USA) at 475 nm at 1, 2, 3, and 4 h.
Masson–Fontana silver staining
The three groups of cells were grown overnight in a 6-well plate at a density of 5 × 105 per well with sterilised coverslips. The cells adhering to the coverslips were fixed in iced acetone at 4 °C for 10 min, and then dried at room temperature. The melanin pigment content of these cells was visualised with Masson–Fontana silver staining as per the published protocol38. First, the coverslips were rinsed with PBS, then incubated with silver ammonia solution in the dark at 56 °C for 35–40 min. After rinsing in distilled water, the cells were quickly incubated with sodium thiosulfate solution for 1 min. Finally, the coverslips were incubated with neutral red staining solution for 5 min, sealed, and observed under a microscope (Nikon Eclipse 90i; Kawasaki, Kanagawa, Japan). Noticeable black particles could be seen in the 231 TYR + Dox cells.
Cell immunofluorescence study
The sample preparation procedure was the same as that for the Masson–Fontana silver staining. The coverslips were rinsed with PBS, blocked with 1% bull serum albumin and incubated with a primary antibody (mouse anti-TYR, diluted 1:500; Sigma) overnight at 4 °C. After rinsing in PBS, the cells were incubated with a diluted secondary antibody (Alexa Fluor 488-labelled goat anti-rabbit IgG, diluted 1:200, Beyotime, Beijing, China) at 37 °C for 60 min. Finally, the coverslips were incubated with 4–6-diamidino-2-phenylindole (DAPI; Beyotime) for 5 min, sealed with an agent resistant to quenching, and observed under a confocal microscope (LSM 710: Zeiss, Oberkochen, Germany).
Measurement of melanin content in 231-TYR cells regulated by Dox
A sample of the 231-TYR cells was digested, re-suspended and cultured in flasks overnight. Then, these cells were incubated with Dox in serial concentrations (10–4000 ng/mL) at 37 °C for 48 h. Melanin content of these cells was measured as described previously with some modifications39. The cultured cells were harvested and washed with PBS. They were incubated in 500 μL of 1 N NaOH in an 80 °C water bath for 2 h, then the solution was mixed. After determination of protein content, protein concentration was adjusted to 0.5 μg/μL, and the extracts were then transferred into 96-well plates in triplicate with 50-μL aliquots. The relative melanin content of samples was determined by measuring their absorbance at 405 nm. Results were expressed as absorbance of 405 nm per mg protein.
The incubation time with Dox was investigated to assess for any effect on melanin production in 231-TYR cells. After the cells were cultured in flasks overnight, they were placed in fresh medium containing Dox (2 μg/mL), then continually incubated for 0, 1, 2, 4, 8, 16, 24, 36, 48, and 72 h. Melanin content at different incubation times was measured as described previously.
To assess the impact of Dox on TYR expression, the changes in melanin content after withdrawing Dox at different times were studied in 231-TYR+Dox cells. We cultured the 231-TYR cells with medium containing Dox (2 μg/mL) for 48 h, then replaced the medium with fresh medium without Dox. The cells were digested and collected for determination of melanin content after Dox was removed at 0, 1, 2, 4, 8, 16, 24, 36, and 48 h.
Cell MRI
Cell phantoms were prepared as follows25: the 96-well PCR plates were embedded in a cuboid container filled with 1% UltraPureTM agarose gel (Invitrogen, Carlsbad, CA, USA). After solidification, the tubes were pulled out, and then the bottoms of the resulting holes were filled with 100 μL of 1% agarose. Different concentrations of cells (100 μL, ranging from 2.5 × 107/mL to 1 × 108/mL) suspended in 1% agarose were layered into the middle part of the holes, and then the surface of the phantom was covered with thin 1% agarose gel. MRI was performed using a Bruker 4.7 T/30 cm MRI Imaging System (Bruker Instrument Co., Ltd, Karlsruhe, Germany) with a 72-mm Agilent® radiofrequency (RF) coil. The imaging protocol consisted of axial T1- and T2-weighted fast spin echo (FSE) sequences. T1WI was acquired with the following parameters: repetition time (TR): 350 ms; echo time (TE):11 ms; field of view (FOV): 4.0 × 4.0; matrix size: 256 × 256; slice thickness: 1 mm. T2WI was acquired with the following parameters: repetition time (TR): 2000 ms; echo time (TE): 36 ms; field of view (FOV): 4.0 × 4.0; matrix size: 256 × 256; slice thickness: 1 mm. Image analysis was performed using Paravison operational software.
Cell PAI
Agarose phantoms were prepared using the PCR tubes. The bottoms of the tubes were filled with 1% agarose gel in distilled water (150 μL). After being cooled down, different concentrations of cells (50 μL) ranging from 1 × 105/mL to 2 × 107/mL suspended in 1% agarose were filled into the middle part of the tubes, then the tops of the tubes were filled with 1% agarose. An acoustic-resolution photoacoustic microscopy system independently manufactured by the National Laboratory for Optoelectronics, Huazhong University of Science and Technology (Wuhan, China) was used to acquire photoacoustic images with a laser at excitation wavelength of 532 nm, a focal depth of 6 mm, pulse width of 6 ns and pulse repetition of 30 Hz.
Cell uptake studies of 18F-5-FPN
Preparation of 18F-5-FPN was conducted with the same protocol as described in our previous study23. The cellular uptake studies were performed in all experimental and control groups (231-TYR + Dox, 231-TYR, and 231 cells). Cells at a density of 1 × 105 per well were seeded in 24-well plates and incubated overnight. Then, the cells were incubated with 0.2 mL of medium containing 37 kBq (0.5 pM) of 18F-5-FPN at 37 °C. At 30, 60, or 120 min after incubation, the medium was removed and cells were washed three times with PBS (pH 7.4) and lysed with 1 N NaOH for 5 min at room temperature. The radioactivity of the cell lysate was measured by a gamma counter (2470, WIZARD; PerkinElmer, Waltham MA, USA). For the cell efflux study, these cells were implanted into plates overnight. 18F-5-FPN (37 kBq, 0.5 pM) was added to each properly and incubated for 2 h at 37 °C. After being washed twice with PBS, the cells were incubated in a culture medium for 15, 30, 60 or 120 min. Then, the cells were lysed with 1 N NaOH. For the blocking study, 1 × 105 231-TYR + Dox cells were seeded overnight and were incubated at 37 °C for 1 h with 18F-5-FPN (37 kBq, 0.5 pM) in the presence of 100 μL standards 19F-5-FPN (10−12 to 10−5 M). Then, the cells were washed and the radioactivity measured as with the celluar uptake study.
Statistical analysis
Quantitative data were expressed as mean ± standard deviation (SD). Means were compared using one-way ANOVA and the Student’s t-test with P < 0.05 indicating statistical significance.
Additional Information
How to cite this article: Feng, H. et al. TYR as a multifunctional reporter gene regulated by the Tet-on system for multimodality imaging: an in vitro study. Sci. Rep. 5, 15502; doi: 10.1038/srep15502 (2015).
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81371626), the Natural Science Foundation of Hubei Province of China for Distinguished Young Scholars (No. 2010CDA094), and Foundation of Huazhong University of Science and Technology (2014XJGH007). We thank Dr. Fang Fang at the Wuhan Institute of Physics and Mathematics, Chinese Academy of Magnetic Resonance Centre, for her kind help in Cell MRI imaging. We also thank Dr. Xiaoquan Yang and Dr. Kai Zhao at School of Life Science and Technology, Huazhong University of Science and Technology, for their kind help in Cell PAI images.
Footnotes
Author Contributions X.L. coneived and designed the study, analyzed and interpretated the data, drafted the article and revised it critically for important intellectual content. H.F. acquired all data, analyzed and interpretated the data and drafted the article. X.X. prepared PET probe and acquired the data of cell uptake studies. C.L. acquired the data of cell MRI images. Y.S. acquired the data of cell uptake studies. C.Q. acquired the data of cell PAI images and interpretated of data. Y.Z. contributed to the conception of the study.
References
- Massoud T. F. & Gambhir S. S. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev 17, 545–580 (2003). [DOI] [PubMed] [Google Scholar]
- Xing Y., Zhao J., Conti P. S. & Chen K. Radiolabeled nanoparticles for multimodality tumor imaging. Theranostics 4, 290–306 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J. et al. PET/NIRF/MRI triple functional iron oxide nanoparticles. Biomaterials 31, 3016–3022 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. C. et al. Facile preparation of a hybrid nanoprobe for triple-modality optical/PET/MR imaging. Small 6, 2863–2868 (2010). [DOI] [PubMed] [Google Scholar]
- Stelter L. et al. Modification of aminosilanized superparamagnetic nanoparticles: feasibility of multimodal detection using 3T MRI, small animal PET, and fluorescence imaging. Mol Imaging Biol 12, 25–34 (2010). [DOI] [PubMed] [Google Scholar]
- Higuchi T. et al. Combined reporter gene PET and iron oxide MRI for monitoring survival and localization of transplanted cells in the rat heart. J Nucl Med 50, 1088–1094 (2009). [DOI] [PubMed] [Google Scholar]
- Zhang Y., Yang Y. & Cai W. Multimodality Imaging of Integrin alpha(v)beta(3) Expression. Theranostics 1, 135–148 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjuvajev J. G. et al. Imaging the expression of transfected genes in vivo. Cancer Res 55, 6126–6132 (1995). [PubMed] [Google Scholar]
- Likar Y. et al. A new pyrimidine-specific reporter gene: a mutated human deoxycytidine kinase suitable for PET during treatment with acycloguanosine-based cytotoxic drugs. J Nucl Med 51, 1395–1403 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponomarev V. et al. A human-derived reporter gene for noninvasive imaging in humans: mitochondrial thymidine kinase type 2. J Nucl Med 48, 819–826 (2007). [DOI] [PubMed] [Google Scholar]
- Pei Z. et al. Multimodality molecular imaging to monitor transplanted stem cells for the treatment of ischemic heart disease. PLoS One 9, e90543 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M. et al. Whole-body optical imaging of green fluorescent protein-expressing tumors and metastases. Proc Natl Acad Sci USA 97, 1206–1211 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deans A. E. et al. Cellular MRI contrast via coexpression of transferrin receptor and ferritin. Magn Reson Med 56, 51–59 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissleder R. et al. MR imaging and scintigraphy of gene expression through melanin induction. Radiology 204, 425–429 (1997). [DOI] [PubMed] [Google Scholar]
- Körner A. & Pawelek J. Mammalian tyrosinase catalyzes three reactions in the biosynthesis of melanin. Science 217, 1163–1165 (1982). [DOI] [PubMed] [Google Scholar]
- Cánovas F. G., García-Carmona F., Sánchez J. V., Pastor J. L. & Teruel J. A. The role of pH in the melanin biosynthesis pathway. J Biol Chem 257, 8738–8744 (1982). [PubMed] [Google Scholar]
- Zonios G. et al. Melanin absorption spectroscopy: new method for noninvasive skin investigation and melanoma detection. J Biomed Opt 13, 014017 (2008). [DOI] [PubMed] [Google Scholar]
- Zhang Y., Hong H. & Cai W. Photoacoustic imaging. Cold Spring Harb Protoc 9, 1015–1025 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enochs W. S., Petherick P., Bogdanova A., Mohr U. & Weissleder R. Paramagnetic metal scavenging by melanin: MR imaging. Radiology 204, 417–423 (1997). [DOI] [PubMed] [Google Scholar]
- Michelot J. M. et al. Synthesis and evaluation of new iodine-125 radiopharmaceuticals as potential tracers for malignant melanoma. J Nucl Med 32, 1573–1580 (1991). [PubMed] [Google Scholar]
- Moins N. et al. 123I-N-(2-diethylaminoethyl)-2-iodobenzamide: a potential imaging agent for cutaneous melanoma staging. Eur J Nucl Med Mol Imaging 29, 1478–1484 (2002). [DOI] [PubMed] [Google Scholar]
- Liu H. et al. Development of 18F-labeled picolinamide probes for PET imaging of malignant melanoma. J Med Chem 56, 895–901 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng H. et al. Imaging malignant melanoma with 18F-5-FPN. Eur J Nucl Med Mol Imaging. 2015 Aug 11. [Epub ahead of print] 10.1007/s00259-015-3134-2 (2015). [DOI] [PubMed] [Google Scholar]
- Paproski R. J., Forbrich A. E., Wachowicz K., Hitt M. M. & Zemp R. J. Tyrosinase as a dual reporter gene for both photoacoustic and magnetic resonance imaging. Biomed Opt Express 2, 771–780 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C. et al. Tyrosinase as a multifunctional reporter gene for Photoacoustic/MRI/PET triple modality molecular imaging. Sci Rep 3, 1490 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G. et al. Therapeutic gene expression in transduced mesenchymal stem cells can be monitored using a reporter gene. Nucl Med Biol 39, 1243–1250 (2012). [DOI] [PubMed] [Google Scholar]
- Xia X., Ayala M., Thiede B. R. & Zhang S. C. In vitro- and in vivo-induced transgene expression in human embryonic stem cells and derivatives. Stem Cells 26, 525–533 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen M. & Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA 89, 5547–5551 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen M. et al. Transcriptional activation by tetracyclines in mammalian cells. Science 268, 1766–1769 (1995). [DOI] [PubMed] [Google Scholar]
- Kistner A. et al. Doxycycline-mediated quantitative and tissue-specific control of gene expression in transgenic mice. Proc Natl Acad Sci USA 93, 10933–10938 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massie B. et al. Inducible overexpression of a toxic protein by an adenovirus vector with a tetracycline-regulatable expression cassette. J Virol 72, 2289–2296 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X. et al. Transient, inducible, placenta-specific gene expression in mice. Endocrinology 153, 5637–5644 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofue K. et al. MR imaging of hepatic metastasis in patients with malignant melanoma: evaluation of suspected lesions screened at contrast-enhanced CT. Eur J Radiol 81, 714–718 (2012). [DOI] [PubMed] [Google Scholar]
- Kircher M. F. et al. A brain tumor molecular imaging strategy using a new triple-modality MRI-photoacoustic-Raman nanoparticle. Nat Med 18, 829–834 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stritzker J. et al. Vaccinia virus-mediated melanin production allows MR and optoacoustic deep tissue imaging and laser-induced thermotherapy of cancer. Proc Natl Acad Sci USA 110, 3316–3320 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mier W. et al. Radiopharmaceutical therapy of patients with metastasized melanoma with the melanin-binding benzamide 131I-BA52. J Nucl Med 55, 9–14 (2014). [DOI] [PubMed] [Google Scholar]
- Ballard B. et al. In vitro and in vivo evaluation of melanin-binding decapeptide 4B4 radiolabeled with 177Lu, 166Ho, and 153Sm radiolanthanides for the purpose of targeted radionuclide therapy of melanoma. Cancer Biother Radiopharm 26, 547–556 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candan G. et al. Combining poly-arginine with the hydrophobic counter-anion 4-(1-pyrenyl)-butyric acid for protein transduction in transdermal delivery. Biomaterials 33, 6468–6475 (2012). [DOI] [PubMed] [Google Scholar]
- Ookubo N. et al. The transdermal inhibition of melanogenesis by a cell-membrane-permeable peptide delivery system based on poly-arginine. Biomaterials 35, 4508–4516 (2014). [DOI] [PubMed] [Google Scholar]