Abstract
Serrated polyps of the colorectum form a group of related lesions which include aberrant crypt foci (ACF), conventional hyperplastic polyps, mixed (admixed) polyps, serrated adenomas and sessile serrated adenomas. In recent years the molecular differences between these morphologically similar lesions have been highlighted, and their differing biological potential has been realised. In particular, the sessile serrated adenoma has become recognised as the precursor lesion to a group of sporadic colorectal carcinomas characterised by morphological and molecular features distinct from conventional adenomas. These recent findings have challenged the long held paradigm that all colorectal carcinomas arise via the traditional adenoma-carcinoma sequence. In addition, they present a major challenge for the early detection and management of colorectal cancer, which is no longer regarded as a homogeneous entity.
Keywords: Serrated adenoma, Serrated neoplasia, Serrated adenocarcinoma, Hyperplastic polyps, Microsatellite instability
INTRODUCTION
Our understanding of the pathogenesis of colorectal carcinoma has seen a paradigm shift in recent years, mainly due to the emergence of a group of serrated lesions as precursors to a molecularly distinct group of colorectal carcinomas. This has challenged the long held view that the majority of colorectal carcinomas arise from conventional adenomas.
Serrated polyps of the colorectum form a group of morphologically related lesions which include aberrant crypt foci (ACF), conventional hyperplastic polyps, mixed (admixed) polyps, serrated adenomas and sessile serrated adenomas. While these lesions share some histological features, they differ significantly on the molecular level, and more importantly, are biologically distinct. The prototype of serrated lesions is the hyperplastic polyp. Since its recognition as a pathological entity, different from adenomas, hyperplastic polyps have been regarded as innocuous, non-neoplastic lesions with no malignant potential[1,2]. This dogmatic view remained unchanged for many years despite several reports documenting occurrence of cancer in “hyperplastic polyps” or frequent incidence of colorectal cancer in patients with numerous “hyperplastic” polyps[3]. Eventually the employment of molecular techniques for research into colorectal cancer provided data which changed our view on this adenoma-carcinoma sequence, and a new pathway of colorectal cancer development called the “serrated pathway” was recognised and accepted. The “serrated pathway” story would not be complete without recognition of its end point malignant lesion-the “serrated adenocarcinoma”. The existence of serrated adenocarcinomas as a distinct entity was supported by molecular data obtained from detailed microarray studies and correlated with conventional histomorphology. Reproducible histopathological criteria have now been proposed for this new subtype of colorectal cancer (CRC), which accounts for a substantial proportion of CRCs.
Hyperplastic polyps
Hyperplastic polyps are typically small, smooth, sessile lesions most commonly encountered in the distal colon and rectum of patients usually older than 40 years of age[4,5]. Morphologically, hyperplastic polyps have numerous crypts with a convoluted luminal pattern and immature proliferative cells in the lower portions. Serrations tend to be restricted to the superficial portions of the crypts, which contain cytologically bland mature cell types with variable amounts of mucin. Hyperplastic polyps have an expanded but otherwise normal proliferative zone, with mitoses restricted mainly to the basal portions of the crypt epithelium. An attempt has been made to subdivide hyperplastic polyps based on the morphological features of the mature cells, however this subclassification is not yet routinely reported[6,7].
Traditional serrated adenomas
It was recognised as early as 1984 that there were some colonic polyps which demonstrated features of both hyperplastic polyps and adenomatous polyps, which at the time were designated as “mixed hyperplastic adenomatous polyps”[8]. Some years later a review of 110 of these lesions sought to identify their characteristics, and the term “serrated adenoma” was proposed to identify this particular group of polyps[9]. Their definition of this lesion was quite broad, encompassing any serrated lesion with cytological dysplasia. This was reappraised by Torlakovic and colleagues[7], who identified a subgroup of “traditional serrated adenomas” as distinct from sessile serrated adenomas, which are described below. According to their schema, traditional serrated adenomas are polyps with a complex serrated architecture which demonstrate characteristic cytological features of central, elongated nuclei, mild pseudostratification and distinctly eosinophilic cytoplasm. They are often pedunculated and may demonstrate a villiform architecture under low power examination[6,7]. The epithelial cells of a traditional serrated adenoma differ from those of a conventional adenoma, which typically display more prominent nuclear hyperchromasia and more basophilic cytoplasm[6]. These polyps are rare, constituting less than 1% of all colorectal polyps, and have a predilection for the sigmoid colon and rectum. They usually adopt a polypoid configuration and are readily visible endoscopically[10].
Sessile serrated adenomas
The existence of an aggressive variant of hyperplastic polyp with malignant potential emerged in studies from the Jass group, who questioned the dogmatic adenoma-carcinoma sequence as the exclusive route to development of colorectal cancer. A subset of hyperplastic-like polyps called “sessile serrated polyps” occurring mainly in the right colon of middle aged females was shown to have an increased risk of malignant transformation[11]. The currently used term describing this polyp type is “sessile serrated adenoma”, which was recently coined by Torlakovic and colleagues[7]. Sessile serrated adenomas are typically sessile, right-sided in location and tend to be large (Figure 1). These polyps display alterations of the proliferation zone which produce characteristic architectural abnormalities. The crypts are often dilated at their base, and may extend laterally parallel to the muscularis mucosae (Figure 2). Occasionally the crypts herniate through the muscularis mucosae, producing an appearance which may be confused with invasive tumour (Figure 3). The proliferation zone is asymmetrical, and mitoses can be found in the upper portions of the crypts. Serration is often seen at the base of the crypts, as are cells showing mature differentiation, such as goblet cells or gastric foveolar cells. Subtle cytological atypia may be seen, but is not required for the diagnosis, which rests primarily on architectural features.
Figure 1.
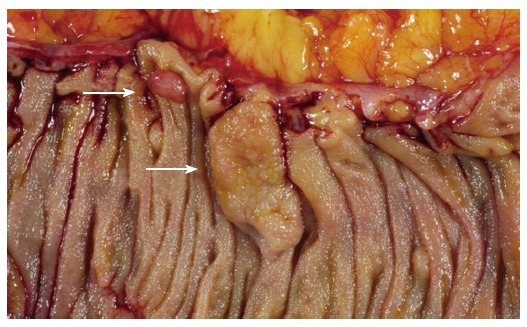
Sessile serrated adenoma (lower arrow). These polyps tend to be large, sessile and situated on the crest of the mucosal folds. This appearance contrasts with that of a typical adenomatous polyp (upper arrow).
Figure 2.
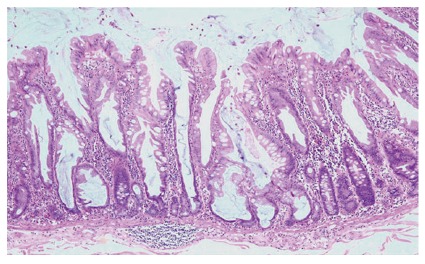
Low power appearance of a sessile serrated adenoma. Serration is seen to the base of the crypts, which are dilated and may show growth parallel to the muscularis mucosae (HE, x 20).
Figure 3.
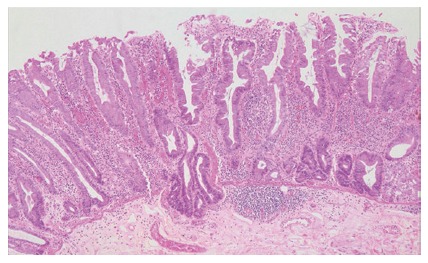
The crypts of a sessile serrated adenoma may herniate through the muscularis mucosae, producing an appearance which may be confused with invasive tumour (HE, x 20).
Serrated polyps and cancer
Over recent years a body of evidence has accumulated suggesting that some serrated polyps have a predisposition for malignant transformation. In their original series Longacre et al[9] found that 37% of their “serrated adenomas” contained foci of significant dysplasia, and 10% contained areas of intramucosal carcinoma. In a large series of colorectal carcinomas 5.8% were associated with an adjacent serrated adenoma[12]. Of these, half were found in the caecum. In addition to these findings, a condition of multiple serrated polyps, known as hyperplastic polyposis (Figure 4), is now recognised to carry an increased risk of colorectal carcinoma. There were early case reports of malignancy coexisting in patients with this condition[3,13], and approximately half of all reported cases up to that time were associated with adenocarcinoma[14]. The morphology of the polyps in this condition fits the criteria for sessile serrated adenomas[7,14], and it has been suggested that the condition be renamed “serrated adenomatous polyposis” to reflect the now recognised risk for carcinoma[15].
Figure 4.
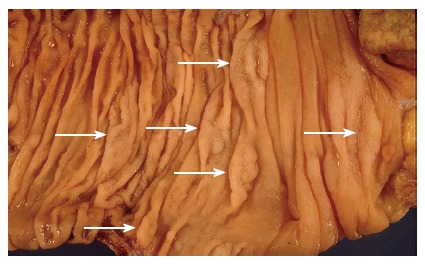
An example of hyperplastic polyposis, demonstrating numerous sessile serrated adenomas (arrows).
Serrated adenocarcinoma
The existence of a distinct subset of serrated adeno-carcinomas was anticipated after the recognition of serrated precursor lesions, and indeed recently the morphological characteristics of these tumours have been described. Serrated adenocarcinomas show a superficial resemblance to traditional hyperplastic polyps, however glandular serration in itself is a non-specific feature, and a number of other criteria have been recognised. These features include prominent mucin production, abundant eosinophilic cytoplasm, retained polarity and chromatin condensation around the periphery of the nucleus. Less well differentiated tumours show a more trabecular growth pattern, but the cytological details are the same[16-18]. More recent DNA expression studies using microarrays have demonstrated that serrated adenocarcinomas cluster into a molecular entity distinct from conventional adenocarcinoma, providing molecular support for their classification as a distinct subtype[18,19]. The suggestion that these tumours may show a different biological potential still needs to be validated, and the reproducibility of these initial studies remains to be seen.
Molecular characteristics of serrated neoplasia
A subset of colorectal carcinomas show high levels of DNA replication errors in repeated nucleotide sequences, or microsatellites, within the genome, usually within non-coding regions. This microsatellite instability (MSI) results from underlying mismatch repair gene deficiency[20-22], and tumours showing high levels of microsatellite instability (MSI-H tumours) have distinctive clinical and pathological features[23-25]. This can occur in a familial context with germ line mutations in mismatch repair genes, and produces a syndrome known as Hereditary Non-Polyposis Colorectal Carcinoma (HNPCC), or Lynch Syndrome. In addition, approximately 10%-15% of sporadic colorectal carcinomas also show microsatellite instability, usually secondary to hypermethylation of the promoter region in the MLH1 gene.
Evidence of microsatellite instability has been found in 29% of hyperplastic polyps, 53% of serrated adenomas and 83% of “mixed” polyps[26]. Within this study the “mixed” polyps groups comprised polyps within which there were an admixture of hyperplastic, serrated adenomatous or tubular adenomatous epithelial patterns. In a subset of these mixed polyps, evidence of the same mutation was found in hyperplastic areas and adjacent neoplastic areas, suggesting a clonal relationship[26]. In addition, the hyperplastic areas of the mixed polyps showed a greatly increased rate of microsatellite instability when compared to the rate in pure hyperplastic polyps. Sessile serrated adenomas have been shown to have loss of expression of the mismatch repair protein MLH1[7] (Figure 5), prompting the suggestion that a majority of the “mixed” polyps in the previous study[26] may actually have been sessile serrated adenomas, perhaps with areas of tubular adenoma[15].
Figure 5.
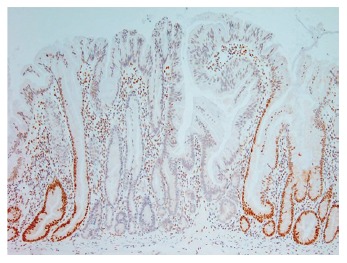
Immunohistochemical staining for the mismatch repair protein MLH1, demonstrating “clonal” loss of expression in a sessile serrated adenoma (x 20).
Demonstration of increased expression of secretory mucins MUC2 and MUC5 in MSI-H cancers and identical mucin and DNA changes in serrated colorectal polyps, was seen as a strong support for serrated polyps being a precursor lesion for at least microsatellite unstable sporadic cancers[27,28]. A comparison of polyps from resection specimens with MSI-H cancers to those with microsatellite stable carcinomas found significantly more serrated polyps in association with MSI cancers[29]. In addition, a high proportion of these polyps showed loss of MLH1 protein, which was also seen in the synchronous carcinomas. These changes were seen to be able to precede the development of histological dysplasia, and it was suggested that they represented early changes in a progression from hyperplastic polyps through “serrated adenomas” to invasive carcinoma[29]. Other authors have retrospectively analysed the illustrations provided and have suggested that the “hyperplastic polyps” of this study actually have morphological features more akin to sessile serrated adenomas[15].
A somewhat similar study evaluated 106 “hyperplastic-like” polyps that had arisen in the same sites as subsequent microsatellite unstable colorectal carcinomas[30]. As a group these polyps were larger than control polyps, and showed expanded crypt proliferation zones, crypt basilar dilation, serration within the basilar regions and maturational changes[30]. Again, these features were remarkably similar to the morphology of sessile serrated adenomas.
Taken together, these results have led to the suggestion that sessile serrated adenomas in particular may represent the precursor lesion to sporadic MSI carcinomas[15]. An important molecular feature of these carcinomas is the methylation of multiple regions of cytosine-guanosine dinucleotides, or CpG islands, within the promoter regions of genes. This is known as the CpG island methylator phenotype (CIMP), and has the effect of downregulating gene expression[31]. The genetic instability characterising many of these tumours is a direct result of promoter methylation silencing the expression of MLH1[32-34]. Methylation at this and other genetic loci is uncommon in typical hyperplastic polyps, but is commonly found in the setting of hyperplastic polyposis[35,36]. More frequent methylation has also been reported to be associated with larger size[36] and proximal location[37] of serrated polyps, both of which are features of sessile serrated adenomas, and O’Brien et al[37] found significantly more promoter methylation in polyps with “abnormal proliferation” when compared to other serrated polyps.
BRAF is a member of the RAF family of serine/threonine kinases, and acts as a signal transducer for growth factors within the RAS-RAF-MAP kinase pathway[38]. Activating mutations in BRAF have been identified in a subset of colorectal carcinomas[39,40], and are particularly associated with sporadic tumours showing microsatellite instability. KRAS is another signal transducer that acts in tandem with BRAF in the same signalling pathway. KRAS and BRAF mutations appear to be mutually exclusive within both polyps and carcinomas[41-43], with KRAS mutations more typically associated with the classic adenoma-carcinoma sequence[42,44,45]. BRAF mutations were found to be much more frequent in sessile serrated adenomas and mixed polyps when compared to traditional hyperplastic polyps, and are rarely found in conventional adenomas[45,46]. The same studies have shown a strong association between BRAF mutations and a CIMP-high phenotype in both serrated polyps and carcinomas. Conversely, KRAS mutations were only found in 7% of sessile serrated adenomas, compared to 44% of large traditional adenomas[45].
Towards a molecular classification of bowel cancer
For many years colorectal carcinoma was thought to be a relatively homogeneous entity. Loss of the gene APC was understood to initiate neoplastic growth in the form of adenomatous polyps. Further mutations, typically via chromosomal deletions, drove the process to malignant transformation, with mutation of KRAS and TP53 prominent among these[44]. However, studies which examined the genetic changes in MSI cancers found that while a proportion of these had APC mutations, this seemed to be a later event in tumour genesis, with the mismatch repair deficit occurring earlier in the sequence[47,48]. Subsequent studies separated sporadic MSI cancers from those occurring in a familial setting, and found that APC or KRAS mutations were uncommon in the sporadic tumours[49,50]. These findings suggested that there was an alternative route to colorectal carcinogenesis involving loss of mismatch repair genes as an initial event, a position which has been strengthened by the emergence of sessile serrated adenomas as likely precursor lesions to the sporadic group, forming the “serrated neoplasia” pathway[10,27]. Indeed, this pathway may begin to be responsible for a greater proportion of colorectal carcinomas diagnosed in the future, as screening and early intervention efforts reduce the incidence of cancers arising from traditional adenomas. Indeed, it has been suggested that an apparent increase in incidence of right sided colon cancers (“shift to the right”) might be a result of existing surveillance strategies being orientated towards eradication of conventional adenomas while hyperplastic-like polyps, occurring most frequently in the right colon, have not been subjected to similar policy[51-53].
Jass has attempted to integrate this new molecular understanding of colorectal carcinoma by suggesting a new classification scheme combining both molecular and clinical features[17]. He proposes a scheme recognising five separate groups. Two groups are characterised by high levels of promoter methylation, mutations in BRAF and microsatellite instability. These tumours arise from serrated polyps, and tend to show chromosomal stability. He separates the groups on the level of microsatellite instability. A further two groups show low or absent levels of promoter methylation, KRAS mutations, low or absent microsatellite instability and chromosomal instability. These tumours arise in traditional adenomas and may be sporadic or familial. A fifth group comprises tumours associated with HNPCC, with negative promoter methylation, BRAF mutation negative, stable chromosomes and high levels of microsatellite instability. These groupings can be related to certain morphological features. Serrated adenocarcinomas, as described above, tend to show microsatellite instability, and are more likely to fall within the first two groups. Poor differentiation, intraepithelial lymphocytes and lack of “dirty” necrosis are additional features associated with MSI tumours, both sporadic as well as those associated with HNPCC[17,24,54]. In particular, a subgroup of carcinomas associated with MSI is characterised by good circumscription, poor differentiation, closely packed trabeculae or sheets, lack of nuclear pleomorphism and lymphocyte infiltration[24]. These tumours, known as medullary carcinomas, are particularly important to recognise as they carry a good prognosis which belies their lack of differentiation[17,24,55].
Implications for management
Making recommendations for management is currently hampered by the recent changes in terminology and a lack of data in a number of aspects related to the sessile serrated adenoma. As it is a new entity, not previously diagnosed by pathologists, there is no data regarding the sensitivity of screening for faecal occult blood. This will be a critical issue for further studies to address. In addition, these predominantly sessile lesions may not be as readily detected by colonoscopy as they have a tendency to flatten against the mucosa when the bowel is inflated. Newer procedures such as virtual colonoscopy will also need to be assessed - our local experience, while limited, does indicate that the sessile serrated adenoma can be successfully detected with this technique (Figure 6).
Figure 6.
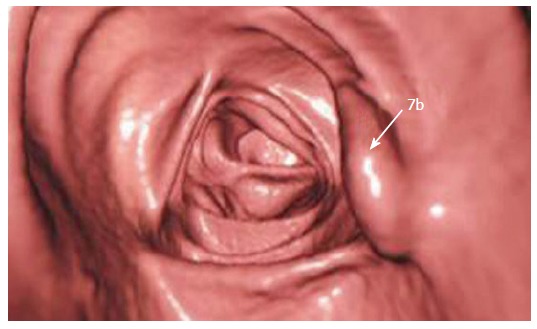
Virtual colonoscopic image demonstrating a sessile serrated adenoma (arrow).
As outlined above, the critical histological features identifying a sessile serrated adenoma are largely architectural, and are thus best appreciated when most of the polyp is submitted. Small, superficial biopsies may be difficult to distinguish from a traditional hyperplastic polyp. In this scenario, the pathologist is greatly aided by the provision of adequate clinical information, in particular the site, size and multiplicity of the lesions. The provision of this information can be aided by making endoscopy findings available to the reporting pathologist. Where feasible, photographs of the endoscopic appearance of the lesion can also be invaluable.
Once a sessile serrated adenoma has been identified, their recurrence rate and the rate of progression to carcinoma remain to be determined. When a sessile serrated adenoma is seen in conjunction to a carcinoma there is typically a transition zone of adenomatous epithelium, which tends to adopt a morphology more akin to traditional adenomas, typically with a villous architecture[15], and microsatellite instability is more frequently found in dysplastic foci of the serrated polyps in patients with hyperplastic polyposis[56]. These lesions have been referred to as mixed hyperplastic polyp/villous adenomas, but perhaps a term such as “sessile serrated adenoma in transition” may more accurately reflect the biological potential of this lesion. Irrespective of the presence or absence of cytological dysplasia within these polyps, the patients harbouring these lesions should be managed in a similar way to patients with conventional adenomas. A series of proposed management guidelines incorporating this concept have recently been published[6,15].
A further complication to the assessment of these polyps lies in the suggestion that in some sessile serrated adenomas the dysplasia may resemble the cytological atypia seen in serrated adenocarcinoma, with round vesicular nuclei, prominent nuclear membranes and cytoplasmic eosinophilia[17]. In addition, Jass and colleagues have described “fusion” polyps, which combine the molecular features of both serrated and traditional adenomatous pathways[57]. Such polyps show frequent loss of KRAS or BRAF, as well as loss of expression of the DNA repair gene MGMT, and the suggestion is that these polyps may in fact be particularly aggressive[57].
CONCLUSION
A “serrated neoplasia” pathway has now emerged as distinct from the more traditional adenoma-carcinoma sequence. Key to this pathway is extensive DNA methylation, silencing of mismatch repair genes and BRAF mutation. A precursor lesion appears to have been identified in the form of the sessile serrated adenoma, and a major challenge now is to facilitate the early detection of these polyps and to determine the best management strategy once they have been identified. Future classifications of colorectal carcinoma are likely to be based on a combination of clinical, morphological and molecular features, and represent a second major challenge. What is certain is that the role of the pathologist will now become central for the analysis of the molecular features of these lesions as well as the morphology.
ACKNOWLEDGMENTS
The authors would like to acknowledge Mr A Hunter of the Royal Adelaide Hospital for provision of the virtual colonoscopic image.
Footnotes
Supported by The Cancer & Bowel Research Trust, Australia
S- Editor Ma N L- Editor Roberts SE E- Editor Liu Y
References
- 1.Morson B. Poyps and cancer of the large bowel. West J Med. 1976;125:93–99. [PMC free article] [PubMed] [Google Scholar]
- 2.Morson BC. Precancerous lesions of the colon and rectum. Classification and controversial issues. JAMA. 1962;179:316–321. doi: 10.1001/jama.1962.03050050006002. [DOI] [PubMed] [Google Scholar]
- 3.Jeevaratnam P, Cottier DS, Browett PJ, Van De Water NS, Pokos V, Jass JR. Familial giant hyperplastic polyposis predisposing to colorectal cancer: a new hereditary bowel cancer syndrome. J Pathol. 1996;179:20–25. doi: 10.1002/(SICI)1096-9896(199605)179:1<20::AID-PATH538>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 4.Estrada RG, Spjut HJ. Hyperplastic polyps of the large bowel. Am J Surg Pathol. 1980;4:127–133. doi: 10.1097/00000478-198004000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Goldman H, Ming S, Hickock DF. Nature and significance of hyperplastic polyps of the human colon. Arch Pathol. 1970;89:349–354. [PubMed] [Google Scholar]
- 6.Snover DC. Serrated polyps of the large intestine. Semin Diagn Pathol. 2005;22:301–308. doi: 10.1053/j.semdp.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Torlakovic E, Skovlund E, Snover DC, Torlakovic G, Nesland JM. Morphologic reappraisal of serrated colorectal polyps. Am J Surg Pathol. 2003;27:65–81. doi: 10.1097/00000478-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Urbanski SJ, Kossakowska AE, Marcon N, Bruce WR. Mixed hyperplastic adenomatous polyps--an underdiagnosed entity. Report of a case of adenocarcinoma arising within a mixed hyperplastic adenomatous polyp. Am J Surg Pathol. 1984;8:551–556. [PubMed] [Google Scholar]
- 9.Longacre TA, Fenoglio-Preiser CM. Mixed hyperplastic adenomatous polyps/serrated adenomas. A distinct form of colorectal neoplasia. Am J Surg Pathol. 1990;14:524–537. doi: 10.1097/00000478-199006000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Jass JR. Serrated route to colorectal cancer: back street or super highway? J Pathol. 2001;193:283–285. doi: 10.1002/1096-9896(200103)193:3<283::AID-PATH799>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 11.Jass JR. Hyperplastic-like polyps as precursors of microsatellite-unstable colorectal cancer. Am J Clin Pathol. 2003;119:773–775. doi: 10.1309/UYN7-0N9W-2DVN-9ART. [DOI] [PubMed] [Google Scholar]
- 12.Mäkinen MJ, George SM, Jernvall P, Mäkelä J, Vihko P, Karttunen TJ. Colorectal carcinoma associated with serrated adenoma--prevalence, histological features, and prognosis. J Pathol. 2001;193:286–294. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH800>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 13.Bengoechea O, Martínez-Peñuela JM, Larrínaga B, Valerdi J, Borda F. Hyperplastic polyposis of the colorectum and adenocarcinoma in a 24-year-old man. Am J Surg Pathol. 1987;11:323–327. doi: 10.1097/00000478-198704000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Torlakovic E, Snover DC. Serrated adenomatous polyposis in humans. Gastroenterology. 1996;110:748–755. doi: 10.1053/gast.1996.v110.pm8608884. [DOI] [PubMed] [Google Scholar]
- 15.Snover DC, Jass JR, Fenoglio-Preiser C, Batts KP. Serrated polyps of the large intestine: a morphologic and molecular review of an evolving concept. Am J Clin Pathol. 2005;124:380–391. doi: 10.1309/V2EP-TPLJ-RB3F-GHJL. [DOI] [PubMed] [Google Scholar]
- 16.Tuppurainen K, Mäkinen JM, Junttila O, Liakka A, Kyllönen AP, Tuominen H, Karttunen TJ, Mäkinen MJ. Morphology and microsatellite instability in sporadic serrated and non-serrated colorectal cancer. J Pathol. 2005;207:285–294. doi: 10.1002/path.1850. [DOI] [PubMed] [Google Scholar]
- 17.Jass JR. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology. 2007;50:113–130. doi: 10.1111/j.1365-2559.2006.02549.x. [DOI] [PubMed] [Google Scholar]
- 18.Mäkinen MJ. Colorectal serrated adenocarcinoma. Histopathology. 2007;50:131–150. doi: 10.1111/j.1365-2559.2006.02548.x. [DOI] [PubMed] [Google Scholar]
- 19.Laiho P, Kokko A, Vanharanta S, Salovaara R, Sammalkorpi H, Järvinen H, Mecklin JP, Karttunen TJ, Tuppurainen K, Davalos V, et al. Serrated carcinomas form a subclass of colorectal cancer with distinct molecular basis. Oncogene. 2007;26:312–320. doi: 10.1038/sj.onc.1209778. [DOI] [PubMed] [Google Scholar]
- 20.Ionov Y, Peinado MA, Malkhosyan S, Shibata D, Perucho M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature. 1993;363:558–561. doi: 10.1038/363558a0. [DOI] [PubMed] [Google Scholar]
- 21.Aaltonen LA, Peltomäki P, Leach FS, Sistonen P, Pylkkänen L, Mecklin JP, Järvinen H, Powell SM, Jen J, Hamilton SR. Clues to the pathogenesis of familial colorectal cancer. Science. 1993;260:812–816. doi: 10.1126/science.8484121. [DOI] [PubMed] [Google Scholar]
- 22.Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993;260:816–819. doi: 10.1126/science.8484122. [DOI] [PubMed] [Google Scholar]
- 23.Kim H, Jen J, Vogelstein B, Hamilton SR. Clinical and pathological characteristics of sporadic colorectal carcinomas with DNA replication errors in microsatellite sequences. Am J Pathol. 1994;145:148–156. [PMC free article] [PubMed] [Google Scholar]
- 24.Jass JR, Do KA, Simms LA, Iino H, Wynter C, Pillay SP, Searle J, Radford-Smith G, Young J, Leggett B. Morphology of sporadic colorectal cancer with DNA replication errors. Gut. 1998;42:673–679. doi: 10.1136/gut.42.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michael-Robinson JM, Biemer-Hüttmann A, Purdie DM, Walsh MD, Simms LA, Biden KG, Young JP, Leggett BA, Jass JR, Radford-Smith GL. Tumour infiltrating lymphocytes and apoptosis are independent features in colorectal cancer stratified according to microsatellite instability status. Gut. 2001;48:360–366. doi: 10.1136/gut.48.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iino H, Jass JR, Simms LA, Young J, Leggett B, Ajioka Y, Watanabe H. DNA microsatellite instability in hyperplastic polyps, serrated adenomas, and mixed polyps: a mild mutator pathway for colorectal cancer? J Clin Pathol. 1999;52:5–9. doi: 10.1136/jcp.52.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jass JR. Serrated adenoma and colorectal cancer. J Pathol. 1999;187:499–502. doi: 10.1002/(SICI)1096-9896(199904)187:5<499::AID-PATH309>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 28.Biemer-Hüttmann AE, Walsh MD, McGuckin MA, Ajioka Y, Watanabe H, Leggett BA, Jass JR. Immunohistochemical staining patterns of MUC1, MUC2, MUC4, and MUC5AC mucins in hyperplastic polyps, serrated adenomas, and traditional adenomas of the colorectum. J Histochem Cytochem. 1999;47:1039–1048. doi: 10.1177/002215549904700808. [DOI] [PubMed] [Google Scholar]
- 29.Hawkins NJ, Ward RL. Sporadic colorectal cancers with microsatellite instability and their possible origin in hyperplastic polyps and serrated adenomas. J Natl Cancer Inst. 2001;93:1307–1313. doi: 10.1093/jnci/93.17.1307. [DOI] [PubMed] [Google Scholar]
- 30.Goldstein NS, Bhanot P, Odish E, Hunter S. Hyperplastic-like colon polyps that preceded microsatellite-unstable adenocarcinomas. Am J Clin Pathol. 2003;119:778–796. doi: 10.1309/DRFQ-0WFU-F1G1-3CTK. [DOI] [PubMed] [Google Scholar]
- 31.Ahuja N, Mohan AL, Li Q, Stolker JM, Herman JG, Hamilton SR, Baylin SB, Issa JP. Association between CpG island methylation and microsatellite instability in colorectal cancer. Cancer Res. 1997;57:3370–3374. [PubMed] [Google Scholar]
- 32.Kane MF, Loda M, Gaida GM, Lipman J, Mishra R, Goldman H, Jessup JM, Kolodner R. Methylation of the hMLH1 promoter correlates with lack of expression of hMLH1 in sporadic colon tumors and mismatch repair-defective human tumor cell lines. Cancer Res. 1997;57:808–811. [PubMed] [Google Scholar]
- 33.Cunningham JM, Christensen ER, Tester DJ, Kim CY, Roche PC, Burgart LJ, Thibodeau SN. Hypermethylation of the hMLH1 promoter in colon cancer with microsatellite instability. Cancer Res. 1998;58:3455–3460. [PubMed] [Google Scholar]
- 34.Toyota M, Ahuja N, Ohe-Toyota M, Herman JG, Baylin SB, Issa JP. CpG island methylator phenotype in colorectal cancer. Proc Natl Acad Sci USA. 1999;96:8681–8686. doi: 10.1073/pnas.96.15.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan AO, Issa JP, Morris JS, Hamilton SR, Rashid A. Concordant CpG island methylation in hyperplastic polyposis. Am J Pathol. 2002;160:529–536. doi: 10.1016/S0002-9440(10)64872-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wynter CV, Walsh MD, Higuchi T, Leggett BA, Young J, Jass JR. Methylation patterns define two types of hyperplastic polyp associated with colorectal cancer. Gut. 2004;53:573–580. doi: 10.1136/gut.2003.030841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Brien MJ, Yang S, Clebanoff JL, Mulcahy E, Farraye FA, Amorosino M, Swan N. Hyperplastic (serrated) polyps of the colorectum: relationship of CpG island methylator phenotype and K-ras mutation to location and histologic subtype. Am J Surg Pathol. 2004;28:423–434. doi: 10.1097/00000478-200404000-00001. [DOI] [PubMed] [Google Scholar]
- 38.Peyssonnaux C, Eychène A. The Raf/MEK/ERK pathway: new concepts of activation. Biol Cell. 2001;93:53–62. doi: 10.1016/s0248-4900(01)01125-x. [DOI] [PubMed] [Google Scholar]
- 39.Yuen ST, Davies H, Chan TL, Ho JW, Bignell GR, Cox C, Stephens P, Edkins S, Tsui WW, Chan AS, et al. Similarity of the phenotypic patterns associated with BRAF and KRAS mutations in colorectal neoplasia. Cancer Res. 2002;62:6451–6455. [PubMed] [Google Scholar]
- 40.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 41.Rajagopalan H, Bardelli A, Lengauer C, Kinzler KW, Vogelstein B, Velculescu VE. Tumorigenesis: RAF/RAS oncogenes and mismatch-repair status. Nature. 2002;418:934. doi: 10.1038/418934a. [DOI] [PubMed] [Google Scholar]
- 42.Chan TL, Zhao W, Leung SY, Yuen ST. BRAF and KRAS mutations in colorectal hyperplastic polyps and serrated adenomas. Cancer Res. 2003;63:4878–4881. [PubMed] [Google Scholar]
- 43.Yang S, Farraye FA, Mack C, Posnik O, O'Brien MJ. BRAF and KRAS Mutations in hyperplastic polyps and serrated adenomas of the colorectum: relationship to histology and CpG island methylation status. Am J Surg Pathol. 2004;28:1452–1459. doi: 10.1097/01.pas.0000141404.56839.6a. [DOI] [PubMed] [Google Scholar]
- 44.Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- 45.O'Brien MJ, Yang S, Mack C, Xu H, Huang CS, Mulcahy E, Amorosino M, Farraye FA. Comparison of microsatellite instability, CpG island methylation phenotype, BRAF and KRAS status in serrated polyps and traditional adenomas indicates separate pathways to distinct colorectal carcinoma end points. Am J Surg Pathol. 2006;30:1491–1501. doi: 10.1097/01.pas.0000213313.36306.85. [DOI] [PubMed] [Google Scholar]
- 46.Kambara T, Simms LA, Whitehall VL, Spring KJ, Wynter CV, Walsh MD, Barker MA, Arnold S, McGivern A, Matsubara N, et al. BRAF mutation is associated with DNA methylation in serrated polyps and cancers of the colorectum. Gut. 2004;53:1137–1144. doi: 10.1136/gut.2003.037671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Konishi M, Kikuchi-Yanoshita R, Tanaka K, Muraoka M, Onda A, Okumura Y, Kishi N, Iwama T, Mori T, Koike M, et al. Molecular nature of colon tumors in hereditary nonpolyposis colon cancer, familial polyposis, and sporadic colon cancer. Gastroenterology. 1996;111:307–317. doi: 10.1053/gast.1996.v111.pm8690195. [DOI] [PubMed] [Google Scholar]
- 48.Huang J, Papadopoulos N, McKinley AJ, Farrington SM, Curtis LJ, Wyllie AH, Zheng S, Willson JK, Markowitz SD, Morin P, et al. APC mutations in colorectal tumors with mismatch repair deficiency. Proc Natl Acad Sci USA. 1996;93:9049–9054. doi: 10.1073/pnas.93.17.9049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salahshor S, Kressner U, Pâhlman L, Glimelius B, Lindmark G, Lindblom A. Colorectal cancer with and without microsatellite instability involves different genes. Genes Chromosomes Cancer. 1999;26:247–252. [PubMed] [Google Scholar]
- 50.Jass JR, Biden KG, Cummings MC, Simms LA, Walsh M, Schoch E, Meltzer SJ, Wright C, Searle J, Young J, et al. Characterisation of a subtype of colorectal cancer combining features of the suppressor and mild mutator pathways. J Clin Pathol. 1999;52:455–460. doi: 10.1136/jcp.52.6.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cucino C, Buchner AM, Sonnenberg A. Continued rightward shift of colorectal cancer. Dis Colon Rectum. 2002;45:1035–1040. doi: 10.1007/s10350-004-6356-0. [DOI] [PubMed] [Google Scholar]
- 52.Mensink PB, Kolkman JJ, Van Baarlen J, Kleibeuker JH. Change in anatomic distribution and incidence of colorectal carcinoma over a period of 15 years: clinical considerations. Dis Colon Rectum. 2002;45:1393–1396. doi: 10.1007/s10350-004-6431-6. [DOI] [PubMed] [Google Scholar]
- 53.Thörn M, Bergström R, Kressner U, Sparén P, Zack M, Ekbom A. Trends in colorectal cancer incidence in Sweden 1959-93 by gender, localization, time period, and birth cohort. Cancer Causes Control. 1998;9:145–152. doi: 10.1023/a:1008826109697. [DOI] [PubMed] [Google Scholar]
- 54.Greenson JK, Bonner JD, Ben-Yzhak O, Cohen HI, Miselevich I, Resnick MB, Trougouboff P, Tomsho LD, Kim E, Low M, et al. Phenotype of microsatellite unstable colorectal carcinomas: Well-differentiated and focally mucinous tumors and the absence of dirty necrosis correlate with microsatellite instability. Am J Surg Pathol. 2003;27:563–570. doi: 10.1097/00000478-200305000-00001. [DOI] [PubMed] [Google Scholar]
- 55.Jessurun J, Romero-Guadarrama M, Manivel JC. Medullary adenocarcinoma of the colon: clinicopathologic study of 11 cases. Hum Pathol. 1999;30:843–848. doi: 10.1016/s0046-8177(99)90146-6. [DOI] [PubMed] [Google Scholar]
- 56.Jass JR, Iino H, Ruszkiewicz A, Painter D, Solomon MJ, Koorey DJ, Cohn D, Furlong KL, Walsh MD, Palazzo J, et al. Neoplastic progression occurs through mutator pathways in hyperplastic polyposis of the colorectum. Gut. 2000;47:43–49. doi: 10.1136/gut.47.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jass JR, Baker K, Zlobec I, Higuchi T, Barker M, Buchanan D, Young J. Advanced colorectal polyps with the molecular and morphological features of serrated polyps and adenomas: concept of a 'fusion' pathway to colorectal cancer. Histopathology. 2006;49:121–131. doi: 10.1111/j.1365-2559.2006.02466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


