Abstract
The clinical management of metastatic (stage IV) colorectal cancer (CRC) is a common challenge faced by surgeons and physicians. The last decade has seen exciting developments in the management of CRC, with significant improvements in prognosis for patients diagnosed with stage IV disease. Treatment options have expanded from 5-fluorouracil alone to a range of pharmaceutical and interventional therapies, improving survival, and providing a cure in selected cases. Enhanced understanding of the biologic pathways most important in colorectal carcinogenesis has led to a new generation of drugs showing promise in advanced disease. It is hoped that in the near future the treatment paradigm of metastatic CRC will be analogous to that of a chronic illness, rather than a rapidly terminal condition. This overview discusses the epidemiology of advanced CRC and currently available therapeutic options including medical, surgical, ablative and novel modalities in the management of metastatic colorectal cancer.
Keywords: Colorectal cancer, Metastases, Chemo-therapy, Oncology, Biological therapies
INTRODUCTION
In most Western societies, colorectal cancer (CRC) is the second most common cause of cancer-related death[1]. Worldwide, over 500 000 deaths per year are attributable to colorectal cancer[2]. Approximately 35% of patients have stage IV (M1, metastatic) disease at presentation and 20%- 50% with stage II or III disease will progress to stage IV. With the introduction of new therapies and improved surgical techniques the death rate continues to decline at approximately 1.8% per year. The five-year survival rate for stage IV disease overall, remains approximately 10%[1].
The common sites of metastasis are liver, peritoneum and lung. Approximately 50% of patients with stage IV disease will develop liver metastases[3]. Rectal cancer metastasizes to the lung as commonly as the liver[4]. Cerebral metastases are uncommon; CRC is responsible for around 5% of brain metastases and generally occurs at a late stage in the disease[5,6]. Peritoneal carcinomatosis may occur from transmural spread of the primary malignancy or perforation at diagnosis and is associated with a poor prognosis[7].
DIAGNOSIS
Computerized tomography[8] may be used as part of the diagnostic workup of abdominal symptoms or as part of routine surveillance after curative treatment for stage II and III colorectal cancer. It has been shown that surveillance CT imaging carries a survival benefit in this setting, compared with less intensive follow-up[4]. The 2005 American Society of Clinical Oncology (ASCO) guidelines recommend annual chest and abdominal CT scans for the first 3 years after primary treatment for patients at moderate to high risk of recurrence and for whom surgical excision of metastases with curative intent would be appropriate[9].
Positron emission tomography (PET) is increasingly utilized when potentially curative resection is under consideration. Combining PET with contrast-enhanced CT, compared with CT alone, increases the likelihood of finding extrahepatic metastases (89% vs 64%), new liver metastases after previous liver resection (100% vs 50%), and recurrences at the site of resection of the primary tumour (93% vs 53%)[10]. PET, particularly when used with CT, aids in accurate selection of patients for resection of metastases.
Serum carcino-embryonic antigen (CEA) is a useful biomarker for surveillance after treatment of stage II and III colorectal cancer; however, 20% of colorectal cancers do not express CEA[11]. CEA is most sensitive in detecting hepatic metastases, but is less likely to be elevated with isolated pulmonary metastases[9]. The ASCO 2005 guidelines recommend 3 monthly CEA testing for at least 3 years after diagnosis[9].
MANAGEMENT OF ISOLATED METASTASES
Curative surgery
The 5-year cure rate after resection of liver metastases without extra-hepatic disease is up to 40%, and even more in some series[12]. Improved surgical techniques and chemotherapy response rates have led to an increased number of patients being considered for resection. It is estimated that at least 20% of persons with liver metastases may be suitable to undergo resection with curative intent[13]. The suitability for resection of liver metastases is related to location of disease as resection must leave adequate viable liver (at least 30%), while avoiding major vascular structures as well as absence of extrahepatic disease, usually determined by CT and PET.
Risk factors for reduced survival following hepatic resection include a liver resection margin < 1 cm and multiple versus single metastatic deposits[14]. As an aid to choosing the most appropriate management plan for patients presenting with liver metastases, a computer program (Oncosurge) has been constructed following a comprehensive literature review of treatment options[15]. This tool will be useful for clinicians to assess resectability of patients with liver metastases, in conjunction with discussions in a multidisciplinary setting including experienced hepatobiliary surgeons.
Isolated pulmonary metastases may be considered for surgical resection in patients fit enough for thoracotomy. While operative mortality is just over 1%, 5-year survival rates are approximately 27%[16], with up to 36.9% reported by one series for solitary metastasis (19.3% for 2 metastases and 7.7% for > 2)[17]. One small study comparing 12 patients who underwent pulmonary resection within 3 mo of hepatic resection, to 9 who did not have the lung metastases resected with a 3 year survivals of 60% vs 31%[18]. Although small series, these data suggest that pulmonary metastectomy should be considered even after hepatic resection. In retrospective analyses, elevated pre-operative serum CEA was an adverse predictor of survival after pulmonary metastectomy[16,17,19].
The benefit of administering chemotherapy prior to (neo-adjuvant) or after (adjuvant) metastectomy is not yet fully established. Neoadjuvant chemotherapy may downstage tumours to make surgery feasible and more successful and may also aid in control of micrometastatic disease. There have been large studies demonstrating successful resection after neoadjuvant chemotherapy for 15%-20% of liver metastases previously deemed unresectable[20,21]. Disease progression during chemotherapy is indicative of a very poor prognosis regardless of resection[22]. Although complete response of metastases on imaging may occur, without surgery recurrence is the rule. Thus, current practice is to administer 2-3 mo of neoadjuvant combination chemotherapy with repeat imaging to assess response, followed by surgery if appropriate. Upcoming Phase III trials of pre- and post-operative FOLFOX4 chemotherapy versus surgery alone, may help to form an evidence base for decisions in this area[23].
Hepatic arterial infusion chemotherapy (HAI)
HAI relies on the preferential blood supply of metastases from the hepatic artery and the dual blood supply of the liver with the portal vein[25]. Higher drug levels are delivered at the sites of metastatic disease. The most common agent used is the 5FU analogue floxuridine (FUDR). The most serious side effects are biliary sclerosis or catheter-related complications, both of which can be fatal[26].
Until recently, trials comparing HAI to systemic chemotherapy for liver metastases had demonstrated improved response rates, but survival benefits were less clear[26-28]. A recent Phase III trial of 135 patients comparing HAI (FUDR, leucovorin (LV) and dexamethasone) to systemic chemotherapy (5FU and LV) showed a 24.4 mo vs 20 mo (P = 0.0034) median overall survival benefit favouring HAI. Time to extrahepatic progression, conversely, was 7.7 mo vs 14.8 mo favouring systemic chemotherapy (P = 0.029) [29]. As more efficacious agents (oxaliplatin, irinotecan) are now used in treatment of metastatic CRC, it is unclear as to whether superiority of HAI in such studies would be maintained when using these newer agents as comparators. A Cochrane review of HAI chemotherapy after resection or ablation of liver metastases secondary to CRC, found no significant overall survival advantage for HAI; as such this is not currently a recommended intervention after liver resection[30].
Radiofrequency ablation (RFA)
RFA uses high frequency alternating current creating ionic agitation and heat, resulting in cell death by coagulation necrosis[31]. Percutaneous, laparoscopic, and open surgical techniques may be employed. Patients who are eligible generally have fewer than five metastases, < 5 cm in diameter and clear of major blood vessels[32]. One recent study of 135 patients found a median survival of 28.9 mo after RFA for surgically unresectable liver metastases[31]. RFA can be considered for lung metastases, where thoracotomy is not indicated (surgically unresectable or the patient is medically unfit). Multiple lesions can be treated in one procedure. The RAPTURE trial of RFA for lung metastases from a variety of malignancies, presented 2 year follow-up data in 2005. Of 53 patients with CRC, 72% remain cancer free at 2 years[33]. A Phase II study of 55 patients with metastatic CRC undergoing RFA demonstrated a 2-year disease-free survival rate of 57% and median overall survival of 33 mo[34]. The major complication of lung RFA is pneumothorax (up to 43% in one study)[35].
Selective internal radiation therapy (SIRT)
SIR-Spheres® are biocompatible radio-active microspheres containing yttrium-90 which emits beta radiation. This Australian invention[36] delivers up to 40 times more radiation to liver metastases than would be possible using conventional radiotherapy[37], and has shown benefits in liver metastases from both breast and colorectal cancer as well as primary hepatocellular carcinoma (Figure 1) . A hepatic arterial catheter is used to deliver the microspheres, which may be combined with HAI or intravenous chemotherapy. A Phase III trial of 74 patients comparing HAI plus SIRT to HAI alone found a 44% vs 17.6% partial and complete response rate (P = 0.01), with a 15.9 mo vs 9.7 mo time to progression (P = 0.001), favouring the combination[38]. This technology appears promising for the future.
Figure 1.
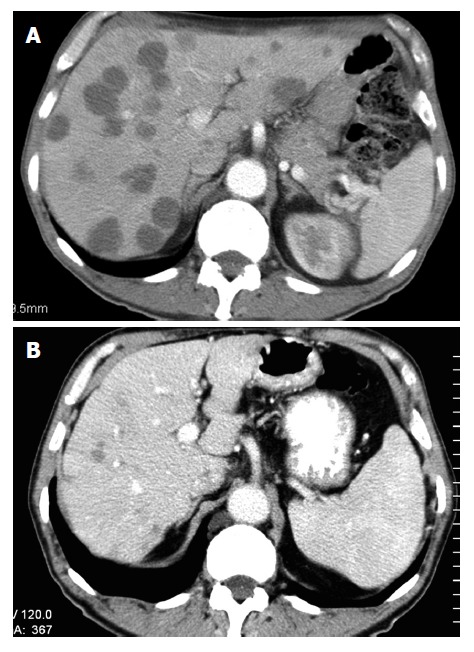
Response of hepatic colorectal cancer metastases treated with SIR spheres. A: Pre-treatment; B: At 12 wk post after intrahepatic infusion of SIR spheres.
SYSTEMIC CHEMOTHERAPY
Please refer to Tables 1 and 2 for details of chemotherapy acronyms, regimens and key Phase III trials, which are discussed below.
Table 1.
Common chemotherapy regimens for metastatic colorectal cancer
| Regimen | Description Cycle length | |
| 5FU Mayo[93,94] | 5-FU 425 mg/m2/d D1-5 | 4 wk |
| LV 20 mg/m2/d D 1-5 | ||
| 5FU Roswell Park[45] | 5-FU 500 mg/m2/d weekly × 6 | 8 wk |
| LV 500 mg/m2/d weekly × 6 | ||
| LVFU2[95] | 5FU 400 mg/m2 bolus D1 and D2 | 2 wk |
| (de Gramont) | LV 200 mg/m2 D1 and D2 | |
| 5FU 600 mg/m2 CIVI 22 h D1 and D2 | ||
| LV5FU2 (AIO)[96] | LV 500 mg/m2 D1 weekly × 6 | 8 wk |
| 5FU 2300-2600 mg/m2 CIVI 24 h D1 weekly × 6 | ||
| Capecitabine[48] | 1250 mg/m2 BD, D1-14 | 3 wk |
| FOLFOX4[53] | Oxaliplatin 85 mg/m2 D1 | 2 wk |
| LV 200 mg/m2 D1 and D2 | ||
| 5FU 400 mg/m2 D1 and D2 | ||
| 5FU 600 mg/m2 CIVI 22 h D1 and D2 | ||
| FOLFOX6[97] | Oxaliplatin 100 mg/m2 D1 | 2 wk |
| LV 400 mg/m2 D1 | ||
| 5FU 400 mg/m2 D1 | ||
| 5FU 2400-3000 mg/m2 CIVI 46 h (D1, D2) | ||
| bFOL[98] | Oxaliplatin 85 mg/m2 D1, D15 | 4 wk |
| LV 20 mg/m2 D1, D8, D15 | ||
| 5FU 500 mg/m2 D1, D8, D15 | ||
| FUFOX[99] | Oxaliplatin 85 mg/m2 D1, 15, 29; | 8 wk |
| LV 20 mg/m2 D1, 8, 15, 22, 29; | ||
| 5FU 500 mg/m2 D 1, 8, 15, 22, 29 | ||
| FLOX[100] | Oxaliplatin 85 mg/m2 D1 wk 1, 3, 5 | 8 wk |
| 5FU 500 mg/m2 bolus weekly wk 1-6 | ||
| LV 500 mg/m2 bolus weekly wk 1-6 | ||
| FOLFIRI[63] | Irinotecan 180 mg/m2 D1, | 2 wk |
| LV 200 mg/m2 D1 and D2, | ||
| 5FU 400 mg/m2 bolus D1 and D2, | ||
| 5FU 600 mg/m2 CIVI 22h D1 and D2 | ||
| IFL[62] | Irinotecan 100-125 mg/m2 weekly × 4 wk | 6 wk |
| LV 20 mg/m2 weekly × 4 wk, | ||
| 5FU 400-500 mg/m2 bolus weekly × 4 wk | ||
| XELOX[54] | Oxaliplatin 130 mg/m2 D1 | 3 wk |
| Capecitabine 1 g/m2 BD D1-14 | ||
| CAPOX[55] | Capecitabine 1 g/m2 BD D1-14 | 3 wk |
| Oxaliplatin 70 mg/m2 D1, D8 | ||
| XELIRI[64] | Irinotecan 200-250 mg/m2 D1 | 3 wk |
| Capecitabine 1 g/m2 BD D1-14 | ||
| CAPIRI[65] | Capecitabine 1 g/m2 BD D1-14 | 3 wk |
| Irinotecan 100 mg/m2 D1, D8 | ||
| FOLFOXIRI [91,101] | Irinotecan 125-175 mg/m2 D1 | 2 wk |
| Oxaliplatin 85-100 mg/m2 D1 | ||
| LV 200 mg/m2 D1 | ||
| 5FU 400 mg/m2 bolus D1 | ||
| 5FU 3200 mg/m2 CIVI 48 h | ||
D1: day 1; LV: leucovorin; CIVI: continuous intravenous infusion; BD: twice daily; d: daily.
Table 2.
Key phase III trials in metastatic colorectal cancer
| Author/trial | n | Arms | RR (%) | TTP (mo) | OS (mo) |
| Van Cutsem[48] | 602 | Capecitabine | 18.90 | 5.2 | 13.2 |
| 5FU/LV Mayo | 15.00 | 4.7 | 12.1 | ||
| (P NA) | (P = 0.65 NS) | (P = 0.33 NS) | |||
| De Gramont[53] | 420 | FOLFOX4 | 50.70 | 9 | 16.2 |
| LV5FU2 | 22.30 | 6.2 | 14.7 | ||
| (P = 0.0001) | (P = 0.0003) | (P = 0.12 NS) | |||
| Hochster | 147 | FOLFOX | 41 | 8.7 | 19.2 |
| TREE-1[56] | bFOL | 20 | 6.9 | 17.9 | |
| CapeOx | 27 | 5.9 | 17.2 | ||
| (P NA) | (P NA) | (P NA) | |||
| Hochster | 213 | FOLFOX + bevacizumab | 52 | 9.9 | 26 |
| TREE-2[56] | bFOL + bevacizumab | 39 | 8.3 | 20.7 | |
| CapeOx + bevacizumab | 46 | 10.3 | 27 | ||
| (P NA) | (P NA) | (P NA) | |||
| Fuchs | 430 | FOLFIRI | 46.60 | 7.6 | 23.1 |
| BICC-Ca[66] | mIFL | 41.90 | 5.8 | 17.6 | |
| (P = 0.10) | |||||
| CAPIRI | 38 | 5.5 | 18.9 | ||
| (P NA) | (P NA) | (P = 0.19 NS) | |||
| Fuchs | 117 | FOLFIRI + bevacizumab | 54.40 | 9.9 | NR |
| BICC-C 2[66] | mIFL + bevacizumab | 53.30 | 8.3 | 18.7 | |
| (P NA) | (P NA) | ||||
| Saltz[62] | 683 | IFL | 39 | 7 | 14.8 |
| 5FU/LV (Mayo) | 21 | 4.3 | 12.6 | ||
| (P < 0.001) | (P = 0.004) | (P = 0.04) | |||
| Irinotecan | |||||
| Douillard[63] | 387 | FOLFIRI | 35 | 6.7 | 17.4 |
| LV5FU2 (AIO) | 22 | 4.4 | 14.1 | ||
| (P =0.005) | (P < 0.001) | (P = 0.031) | |||
| Tournigand[88] | 220 | FOLFIRI ≥ FOLFOX6 | 56 | 8.5 | 21.5 |
| GERCOR | FOLFOX6 ≥ FOLFIRI | 54 | 8 | 20.6 | |
| (P = NS) | (P = 0.26 NS) | (P = 0.99 NS) | |||
| Goldberg[89] | 795 | FOLFOX4 | 45 | 8.7 | 19.5 |
| N9741 | IFL | 31 | 6.9 | 15 | |
| (P = 0.002) | (P = 0.0014) | (P = 0.0001) | |||
| IROX | 35 | 6.5 | 17.4 | ||
| (P = 0.03) | (P = 0.001) | (P = 0.09 NS) | |||
| Colucci[90] | 360 | FOLFIRI | 31 | 7 | 14 |
| FOLFOX4 | 34 | 7 | 15 | ||
| (P = 0.60 NS) | (P = NS) | (P = 0.28 NS) | |||
| Ross[71] | 200 | Mitomycin C + infusional 5FU | 54 | 7.9 | 14 |
| Infusional 5FU | 38 | 5.4 | 15 | ||
| (P = 0.024) | (P = 0.033) | (P = NS) | |||
| Souglakos et al[91] | 283 | FOLFOXIRI | 43 | 8.4 | 21.5 |
| FOLFIRI | 33.6 | 6.9 | 19.5 | ||
| (P = 0.168 NS) | (P = 0.17 NS) | (P = 0.337 NS) | |||
| Falcone [92] | 244 | FOLFOXIRI | 66 | 9.8 | 22.6 |
| FOLFIRI | 41 | 6.9 | 16.7 | ||
| (P = 0.0002) | (P = 0.0006) | (P = 0.032) | |||
| Hurwitz[24] | 813 | IFL + bevacizumab | 44.8 | 10.6 | 20.3 |
| IFL | 34.8 | 6.2 | 15.6 | ||
| (P = 0.004) | (P < 0.001) | (P < 0.001) | |||
| Giantonio[78] | 822 | FOLFOX4 + bevacizumab | 22 | 7.2 | 12.9 |
| ECOGE 3200 | FOLFOX4 | 9 | 4.8 | 10.8 | |
| (P < 0.0001) | (P = 0.0018) | ||||
| Bevacizumab | Discontinued | ||||
| Cunningham[81] | 329 | Cetuximab + irinotecan | 22.9 | 4.1 | 8.6 |
| BOND-1 | Irinotecan | 10.8 | 1.5 | 6.9 | |
| (P = 0.007) | (P < 0.001) | (P = 0.48 NS) | |||
| BOND-2[102] | 74 | Cetuximab + irinotecan + bevacizumab | 38 | 8.5 | n/a |
| cetuximab + bevacizumab | |||||
| 23 | 6.9 | n/a | |||
| Peeters[87] | 463 | Panitumumab | Improved PFS | Approx 6.5 m both arms | |
| BSC | (HR 0.54 P < 0.0001) | (P = NS) |
NS: Not significant; BSC: Best supportive care; NA: Not available. aIn the BICC-C trial all arms were also randomized to +/- celecoxib, which neither improved efficacy nor toxicity of chemotherapy.
5-Fluorouracil and calcium leucovorin
The anti-metabolite 5-fluorouracil (5FU) was the mainstay of chemotherapy for metastatic colorectal cancer in the latter half of the 20th century[39]. The addition of intravenous calcium leucovorin (folinic acid, LV) stabilizes the binding of 5FU to thymidylate synthase, enhancing the inhibition of DNA synthesis[40]. Combination therapy was demonstrated in a meta-analysis of nineteen trials to improve response rate and overall survival over 5FU alone[41]. High dose LV does not appear to convey any survival advantage over lower doses[42,43]. The combination provides a median survival of approximately 12 mo compared with 6 mo for supportive care alone[42]. Until the last decade, 5FU and LV were the only active agents in common use for metastatic CRC. Levamisole, an immunomodulatory agent, was initially combined with 5FU-based regimens, but was later abandoned after no survival benefit was shown in the adjuvant setting[44,45].
Despite numerous documented regimens employing either bolus or infusional 5FU, minimal overall survival advantages have been shown for any one although the response rate and progression free survival appear better with infusional schedules, and one meta-analysis suggested a slight survival advantage for infusional over bolus 5FU (12.1 vs 11.3 m, P = 0.04)[46]. The side effect profiles differ; infusions of 5FU cause more diarrhoea and hand-foot syndrome (erythema, dryness and cracking of palms and soles) while bolus 5FU carries a higher incidence of haematological toxicity. 5FU/LV is used alone in patients who are intolerant or have contra-indications to more complex regimens.
Capecitabine (Xeloda®)
This oral pro-drug is converted to 5FU in three enzymatic steps including thymidine phosphorylase, a tumour-associated angiogenic factor, theoretically resulting in increased concentration at the site of metastases[47]. Capecitabine is at least equivalent in efficacy to bolus 5FU in metastatic colorectal cancer[48]. The toxicity profile is similar to infusional 5FU with diarrhoea and hand-foot syndrome of some degree in up to 50%-60% of clinical trial subjects[49], often requiring a dose reduction. Although many patients prefer to use an oral form of chemotherapy rather than attending hospital for intravenous 5FU-based chemotherapy, compliance must be assured when such therapy is used.
Tegafur is an oral 5FU pro-drug given in combination with uracil (UFT) and oral leucovorin. Phase III data comparing UFT/LV with bolus 5FU/LV in previously untreated metastatic CRC demonstrated equivalent overall survival (12.4 m vs 13.4 m, P = 0.630 NS) with less diarrhoea and myelosuppression than bolus 5FU[50].
Oxaliplatin (Eloxatin®)
This platinum-based agent works by forming platinum-DNA adducts, thus blocking DNA replication[51]. Although it has minimal single-agent activity, it is synergistic with 5FU in the treatment of metastatic CRC[52]. A large Phase III trial of 420 patients compared FOLFOX4 to LV5FU2 (Table 1) and demonstrated improved response rate (50.7% vs 22.3%, P = 0.0001) and progression-free survival (9.0 mo vs 6.2 mo, P = 0.0003), but overall survival was not statistically significantly different[53], possibly attributable to patients in the control arm later receiving oxaliplatin, thus obscuring any survival benefit. Newer trials have combined capecitabine with oxaliplatin (CAPOX or XELOX), with phase II data demonstrating a 19.5 mo median overall survival[54,55]. TREE-1 (Table 2), a small Phase III trial, compared CAPOX and two other regimens combining oxaliplatin with 5FU and found equivalent overall survival (OS) in each arm[56].
Oxaliplatin’s main toxicity is sensory peripheral neuropathy of two types-an acute, temporary cold related dysaesthesia; and a chronic cumulative persistent sensory neuropathy which is dose-limiting and may be irreversible[57]. Up to 90% of patients experience some form of acute neurotoxicity[58] and 10%-15% chronic neuropathy[59]. While most fully recover after a median time of 13 wk[60], in the MOSAIC study which employed the FOLFOX4 regimen, 29% of patients still had some degree of neurotoxicity 12 mo after cessation of therapy[57].
Irinotecan (CPT11, Camptosar®)
Irinotecan, a camptothecin derivative, inhibits Topoisomerase I, impeding DNA uncoiling causing double-stranded DNA breaks[61]. A Phase III trial of 683 patients compared IFL (Table 1) with single agent irinotecan or bolus 5FU/LV (Mayo regimen). Response rate, progression free survival and median overall survival (14.8 mo vs 12.6 mo, P = 0.04) were all improved with IFL[62]. Another Phase III trial randomized 387 patients to FOLFIRI versus LV5FU2 (Table 1). This trial also demonstrated a higher overall survival (median 17.4 mo vs 14.1 mo, P = 0.031), favouring the irinotecan arm[63]. The combination of capecitabine with irinotecan (CAPIRI or XELIRI) in Phase II studies suggest comparable activity to FOLFIRI with a 16-19 mo median overall survival[64,65]; however, recent data from a phase III trial found a trend towards superior response rate and overall survival with FOLFIRI over CAPIRI (OS 23.1 m vs 18.9 m P = 0.19 NS)[66]. The main dose-limiting side effect is diarrhoea, experienced in over 50% of patients in these studies. This was of Grade 3 or 4 severity in 22%-44% of those treated.
Mitomycin C
This antineoplastic antibiotic, isolated from Streptomyces caespitosis, is activated to become an alkylating agent in vivo, cross-linking and inhibiting DNA synthesis and function[67]. It demonstrates single-agent activity in metastatic CRC[68,69], but is accompanied by a significant risk of neutropenia and a small risk of haemolytic-uraemic syndrome[70]. A randomised study of 200 patients showed a 54% vs 38% response rate in patients receiving mitomycin C and infusional 5FU, compared with 5FU alone (P = 0.024); overall survival was equivalent[71]. Irinotecan added to mitomycin C, in 41 patients who had progressed on 5FU, showed a median overall survival of 11.9 mo[72]. More recently, a phase II study of 36 patients demonstrated efficacy for mitomycin C and capecitabine in irinotecan-refractory metastatic CRC, with a 15.2% response rate and median overall survival of 9.3 mo[73].
Targeted therapies
Inhibitors of circulating growth and angiogenic factors, their cell surface receptors, and corresponding intracellular tyrosine kinases are increasingly used combined with or as an alternative to chemotherapy. For metastatic CRC, two agents have entered routine clinical practice: the monoclonal antibodies bevacizumab and cetuximab.
Bevacizumab (Avastin®)
Bevacizumab is a humanised monoclonal antibody targeting vascular endothelial growth factor (VEGF), an angiogenic factor over-expressed in approximately 50% of colorectal cancers[74]. The antibody-bound form of VEGF is unable to bind to its cell surface receptor, preventing activation of an intracellular tyrosine kinase pathway which regulates cell proliferation, angiogenesis, and cell survival[75]. Bevacizumab in combination with chemotherapy, is now regarded as appropriate 1st-line therapy for metastatic CRC. After a preliminary Phase II study suggested that bevacizumab had efficacy in combination with 5FU/LV in metastatic CRC[76], a Phase III trial with 813 previously untreated patients randomized to IFL +/- bevacizumab further demonstrated activity. A significant overall survival advantage was seen favouring the experimental arm (20.3 mo vs 15.6 mo, P < 0.001)[24]. An analysis of 3 trials using 5FU/LV +/- bevacizumab demonstrated a 17.9 mo vs 14.6 mo (P = 0.008) overall survival advantage with the combination compared with 5FU-based treatment alone[77]. In the three arm ECOG 3200 study, FOLFOX4 +/- bevacizumab was compared with bevacizumab alone in 822 patients with previously treated metastatic CRC. The bevacizumab-alone arm was discontinued due to inferiority at an interim analysis. An overall survival advantage (12.9 mo vs 10.8 mo, P = 0.0024) as well as significant response rates and progression-free survival benefits were seen in the FOLFOX4 plus bevacizumab arm[78]. The TREE-2 study (Table 2) added bevacizumab to three different oxaliplatin-containing regimens and found improved response rates and time to progression when added to all three[56]. The toxicities of bevacizumab when added to chemotherapy alone include hypertension (22% vs 8.3% overall and 11% vs 2% requiring treatment), bleeding or thrombosis, in particular arterial thrombotic events (CVA, AMI, TIA, angina), were increased (5% vs 2.5%), proteinuria in 26% and gastro-intestinal perforation in 1.5% or 6/393[24]. VEGF is involved in wound healing, which may explain the last complication.
Cetuximab (Erbitux®)
This chimeric monoclonal antibody, targeting the extracellular domain of the epidermal growth factor receptor (EGFR or HER-1), has demonstrated activity in metastatic CRC. Although the EGFR gene is over-expressed or upregulated in 60%-80% of colorectal cancers[79], cetuximab response in CRC appears independent of EGFR expression[80,81]. In a phase II study (128 patients) adding cetuximab to irinotecan after failure of irinotecan alone, a 22.5% response rate was obtained[82]. In the BOND-1 study of 329 participants refractory to irinotecan, randomised to cetuximab and irinotecan or cetuximab alone, a significantly higher response rate and median time to progression for the combination was seen, although overall survival was no different[81]. The above trials explored the use of cetuximab in previously treated patients. Its role in 1st-line treatment; and also in combination with oxaliplatin-based regimens, is yet to be fully elucidated. The main side effect is an acneiform skin rash in up to 89%[83]; the degree of skin reaction may correlate with response rate[80,84]. Diarrhoea is relatively common; and allergic reactions may occur, possibly related to the mouse component of the antibody[85].
Panitumumab
This antibody also targets the EGFR, but in contrast to cetuximab, it is derived from the XenoMouse, a transgenic mouse which produces fully humanized antibodies[86]. A Phase III trial comparing panitumumab with best supportive care in 463 patients with CRC after progression on irinotecan and oxaliplatin, showed 8% vs 0% partial responses and 28% vs 10% with stable disease. No overall survival benefit has been seen to date[87]. Rash of some degree occurs in over 90% of patients, and hypomagnesaemia in 38% of patients in this trial, but allergic reactions appear uncommon.
Which agent and which combination?
Decisions as to the best choice of therapy are based on performance status, co-morbidities, and the preferences of the individual. The optimal combination and sequencing of therapeutic agents in the metastatic setting is unknown. A randomized study of FOLFIRI followed by FOLFOX6 at progression, or the reverse sequence, demonstrated equivalent time to first progression (8.5 mo vs 8.0 mo P = 0.26) and median overall survival (21.5 mo vs 20.6 mo, P = 0.99)[88]. A Phase III study in 795 persons compared FOLFOX4 with IFL and IROX (irinotecan and oxaliplatin). All outcome measures were better for the FOLFOX4 regimen with median survival 19.5 mo (vs 15 mo for IFL P = 0.0001 and 17.4 mo for IROXP = 0.09 NS)[89]. Because oxaliplatin was not available commercially in the US at the time, the difference in overall survival may have been accentuated by differential access to second-line treatment for those in the two arms of the trial. Additionally, only the FOLFOX4 arm used infusional 5FU, which may have contributed to its advantage. In fact, phase III data from a study of 360 persons comparing FOLFIRI and FOLFOX4 (both using infusional 5FU), demonstrated no difference in response rate, time to progression and OS between the two arms[90]. A recent Phase III study comparing FOLFOXIRI (Tables 1 and 2) to FOLFIRI in 283 participants demonstrated more toxic side effects but no difference in outcomes with the triple combination[91]. A further phase III trial compared FOLFOXIRI with FOLFIRI in 244 persons and found a statistically significant overall survival advantage of 22.6 mo vs 16.7 mo (P = 0.032) for the triplet arm with increased but manageable toxicities[92]. In practice, most fit patients will receive a number of therapeutic agents for management of metastatic disease, including 5FU, capecitabine, irinotecan, oxaliplatin, cetuximab and bevicuzimab, as overall survival benefits continue to improve (Figure 2).
Figure 2.
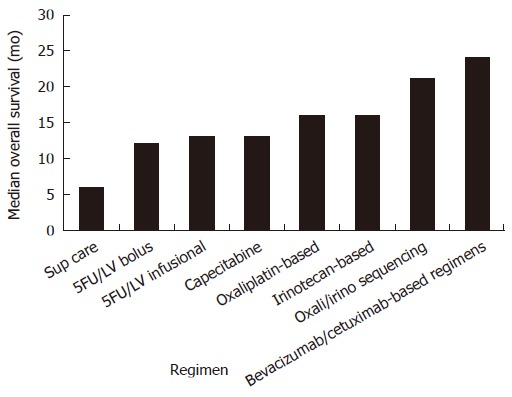
Survival benefits of chemotherapy regimens in metastatic colorectal cancer.
CONCLUSION
The management of metastatic colorectal cancer in the twenty-first century is becoming increasingly complex, with the development of innovative new therapies and further scope for combinations of active agents. In addition, there have been significant advances in surgical and other ablative and local techniques, and it seems certain that targeted therapies will become a major component of the management of colorectal cancer Overall, these gains in the last decade are beginning to impact on survival and quality of life for people affected with this devastating disease, and there is hope that terminal metastatic colorectal cancer may one day become a rarity.
Footnotes
S- Editor Ma N L- Editor Rippe RA E- Editor Ma WH
References
- 1.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 2.Boyle P, Ferlay J. Mortality and survival in breast and colorectal cancer. Nat Clin Pract Oncol. 2005;2:424–425. doi: 10.1038/ncponc0288. [DOI] [PubMed] [Google Scholar]
- 3.Borner MM. Neoadjuvant chemotherapy for unresectable liver metastases of colorectal cancer--too good to be true? Ann Oncol. 1999;10:623–626. doi: 10.1023/a:1008353227103. [DOI] [PubMed] [Google Scholar]
- 4.Chau I, Allen MJ, Cunningham D, Norman AR, Brown G, Ford HE, Tebbutt N, Tait D, Hill M, Ross PJ, et al. The value of routine serum carcino-embryonic antigen measurement and computed tomography in the surveillance of patients after adjuvant chemotherapy for colorectal cancer. J Clin Oncol. 2004;22:1420–1429. doi: 10.1200/JCO.2004.05.041. [DOI] [PubMed] [Google Scholar]
- 5.Uskent ND. Survival from the precocious brain metastasis of the colon cancer. Turk J Cancer. 2003;33:154–157. [Google Scholar]
- 6.Ruelle A, Gambini C, Macchia G, Andrioli G. Brain metastasis from colon cancer. Case report showing a clinical and CT unusual appearance. J Neurosurg Sci. 1987;31:33–36. [PubMed] [Google Scholar]
- 7.DeVita VHS, Rosenberg S. Cancer - Principles and Practice of Oncology. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2005. p. 2240. [Google Scholar]
- 8.Committee. ACNCCGR. Guidelines for the Prevention, Early Detection and Management of Colorectal Cancer. The Cancer Council Australia and Australian Cancer Network; 2005: p. 94. [Google Scholar]
- 9.Desch CE, Benson AB, Somerfield MR, Flynn PJ, Krause C, Loprinzi CL, Minsky BD, Pfister DG, Virgo KS, Petrelli NJ. Colorectal cancer surveillance: 2005 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2005;23:8512–8519. doi: 10.1200/JCO.2005.04.0063. [DOI] [PubMed] [Google Scholar]
- 10.Lonneux M, Reffad AM, Detry R, Kartheuser A, Gigot JF, Pauwels S. FDG-PET improves the staging and selection of patients with recurrent colorectal cancer. Eur J Nucl Med Mol Imaging. 2002;29:915–921. doi: 10.1007/s00259-002-0802-9. [DOI] [PubMed] [Google Scholar]
- 11.Tobaruela E, Enriquez JM, Diez M, Camunas J, Muguerza J, Granell J. Evaluation of serum carcinoembryonic antigen monitoring in the follow-up of colorectal cancer patients with metastatic lymph nodes and a normal preoperative serum level. Int J Biol Markers. 1997;12:18–21. doi: 10.1177/172460089701200104. [DOI] [PubMed] [Google Scholar]
- 12.Taylor I, Mullee MA, Campbell MJ. Prognostic index for the development of liver metastases in patients with colorectal cancer. Br J Surg. 1990;77:499–501. doi: 10.1002/bjs.1800770508. [DOI] [PubMed] [Google Scholar]
- 13.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318; discussion 318-321. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor M, Forster J, Langer B, Taylor BR, Greig PD, Mahut C. A study of prognostic factors for hepatic resection for colorectal metastases. Am J Surg. 1997;173:467–471. doi: 10.1016/s0002-9610(97)00020-2. [DOI] [PubMed] [Google Scholar]
- 15.Poston GJ, Adam R, Alberts S, Curley S, Figueras J, Haller D, Kunstlinger F, Mentha G, Nordlinger B, Patt Y, et al. OncoSurge: a strategy for improving resectability with curative intent in metastatic colorectal cancer. J Clin Oncol. 2005;23:7125–7134. doi: 10.1200/JCO.2005.08.722. [DOI] [PubMed] [Google Scholar]
- 16.Girard P, Baldeyrou P, Grunenwald D. Lung metastases from colorectal cancer: results of surgery. Presse Med. 1995;24:1028–1032. [PubMed] [Google Scholar]
- 17.McAfee MK, Allen MS, Trastek VF, Ilstrup DM, Deschamps C, Pairolero PC. Colorectal lung metastases: results of surgical excision. Ann Thorac Surg. 1992;53:780–785; discussion 785-786;. doi: 10.1016/0003-4975(92)91435-c. [DOI] [PubMed] [Google Scholar]
- 18.Labow DM, Buell JE, Yoshida A, Rosen S, Posner MC. Isolated pulmonary recurrence after resection of colorectal hepatic metastases--is resection indicated? Cancer J. 2002;8:342–347. doi: 10.1097/00130404-200207000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Girard P, Ducreux M, Baldeyrou P, Rougier P, Le Chevalier T, Bougaran J, Lasser P, Gayet B, Ruffié P, Grunenwald D. Surgery for lung metastases from colorectal cancer: analysis of prognostic factors. J Clin Oncol. 1996;14:2047–2053. doi: 10.1200/JCO.1996.14.7.2047. [DOI] [PubMed] [Google Scholar]
- 20.Delaunoit T, Alberts SR, Sargent DJ, Green E, Goldberg RM, Krook J, Fuchs C, Ramanathan RK, Williamson SK, Morton RF, et al. Chemotherapy permits resection of metastatic colorectal cancer: experience from Intergroup N9741. Ann Oncol. 2005;16:425–429. doi: 10.1093/annonc/mdi092. [DOI] [PubMed] [Google Scholar]
- 21.Adam R, Vibert E, Pitombo M. Induction chemotherapy and surgery of colorectal liver metastases. Bull Cancer. 2006;93 Suppl 1:S45–S49. [PubMed] [Google Scholar]
- 22.Adam R, Pascal G, Castaing D, Azoulay D, Delvart V, Paule B, Levi F, Bismuth H. Tumor progression while on chemotherapy: a contraindication to liver resection for multiple colorectal metastases? Ann Surg. 2004;240:1052–1061; discussion 1061-1064;. doi: 10.1097/01.sla.0000145964.08365.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gruenberger T. Tumor response to pre-operative chemo-therapy (CT) with FOLFOX-4 for resectable colorectal cancer liver metastases (LM). Interim results of EORTC Intergroup randomized phase III study 40983. Proceedings of the American Society of Clinical Oncology, 2006 June 2-6; Atlanta GA, USA. Vol 24 (no.18S), Part I, Abstract 3500, 146s; [Google Scholar]
- 24.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 25.Breedis C, Young G. The blood supply of neoplasms in the liver. Am J Pathol. 1954;30:969–977. [PMC free article] [PubMed] [Google Scholar]
- 26.Lorenz M, Müller HH. Randomized, multicenter trial of fluorouracil plus leucovorin administered either via hepatic arterial or intravenous infusion versus fluorodeoxyuridine administered via hepatic arterial infusion in patients with nonresectable liver metastases from colorectal carcinoma. J Clin Oncol. 2000;18:243–254. doi: 10.1200/JCO.2000.18.2.243. [DOI] [PubMed] [Google Scholar]
- 27.Allen-Mersh TG, Earlam S, Fordy C, Abrams K, Houghton J. Quality of life and survival with continuous hepatic-artery floxuridine infusion for colorectal liver metastases. Lancet. 1994;344:1255–1260. doi: 10.1016/s0140-6736(94)90750-1. [DOI] [PubMed] [Google Scholar]
- 28.Rougier P, Laplanche A, Huguier M, Hay JM, Ollivier JM, Escat J, Salmon R, Julien M, Roullet Audy JC, Gallot D. Hepatic arterial infusion of floxuridine in patients with liver metastases from colorectal carcinoma: long-term results of a prospective randomized trial. J Clin Oncol. 1992;10:1112–1118. doi: 10.1200/JCO.1992.10.7.1112. [DOI] [PubMed] [Google Scholar]
- 29.Kemeny NE, Niedzwiecki D, Hollis DR, Lenz HJ, Warren RS, Naughton MJ, Weeks JC, Sigurdson ER, Herndon JE, Zhang C, et al. Hepatic arterial infusion versus systemic therapy for hepatic metastases from colorectal cancer: a randomized trial of efficacy, quality of life, and molecular markers (CALGB 9481) J Clin Oncol. 2006;24:1395–1403. doi: 10.1200/JCO.2005.03.8166. [DOI] [PubMed] [Google Scholar]
- 30.Nelson RL, Freels S. A systematic review of hepatic artery chemotherapy after hepatic resection of colorectal cancer metastatic to the liver. Dis Colon Rectum. 2004;47:739–745. doi: 10.1007/s10350-003-0113-7. [DOI] [PubMed] [Google Scholar]
- 31.Berber E, Pelley R, Siperstein AE. Predictors of survival after radiofrequency thermal ablation of colorectal cancer metastases to the liver: a prospective study. J Clin Oncol. 2005;23:1358–1364. doi: 10.1200/JCO.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 32.Poston GJ. Radiofrequency ablation of colorectal liver metastases: where are we really going? J Clin Oncol. 2005;23:1342–1344. doi: 10.1200/JCO.2005.10.911. [DOI] [PubMed] [Google Scholar]
- 33.Lenconi R. Radiofrequency Ablation of Pulmonary Tumors Response Evaluation (RAPTURE) Trial: 2-Year Survival Outcomes. Proceedings of the Society of Interventional Radiology 30th Annual Meeting, 2005 April; New Orleans, USA. Abstract #506; [Google Scholar]
- 34.Yan TD, King J, Glenn D, Steinke K, Morris DL. Percutaneous radiofrequency ablation of inoperable pulmonary metastases from colorectal cancer. Proceedings of the American Society of Clinical Oncology, 2006 June 2-6, Atlanta GA, USA. Vol 24 (no,18S), Part I, Abstract 3502, 146s. [Google Scholar]
- 35.Steinke K, King J, Glenn D, Morris DL. Radiofrequency ablation (RFA) of lung metastases from colorectal cancer (CRC)-one-year follow-up. Radiologe. 2004;44:687–692. doi: 10.1007/s00117-004-1071-3. [DOI] [PubMed] [Google Scholar]
- 36.Sirtex Medical, Australia Available from: www.sirtexmedical.com.
- 37.Lim L, Gibbs P, Yip D, Shapiro JD, Dowling R, Smith D, Little A, Bailey W, Liechtenstein M. Prospective study of treatment with selective internal radiation therapy spheres in patients with unresectable primary or secondary hepatic malignancies. Intern Med J. 2005;35:222–227. doi: 10.1111/j.1445-5994.2005.00789.x. [DOI] [PubMed] [Google Scholar]
- 38.Gray B, Van Hazel G, Hope M, Burton M, Moroz P, Anderson J, Gebski V. Randomised trial of SIR-Spheres plus chemotherapy vs. chemotherapy alone for treating patients with liver metastases from primary large bowel cancer. Ann Oncol. 2001;12:1711–1720. doi: 10.1023/a:1013569329846. [DOI] [PubMed] [Google Scholar]
- 39.Heidelberger C, Chaudhuri NK, Danneberg P, Mooren D, Griesbach L, Duschinsky R, Schnitzer RJ, Pleven E, Scheiner J. Fluorinated pyrimidines, a new class of tumour-inhibitory compounds. Nature. 1957;179:663–666. doi: 10.1038/179663a0. [DOI] [PubMed] [Google Scholar]
- 40.Petrelli N, Douglass HO, Herrera L, Russell D, Stablein DM, Bruckner HW, Mayer RJ, Schinella R, Green MD, Muggia FM. The modulation of fluorouracil with leucovorin in metastatic colorectal carcinoma: a prospective randomized phase III trial. Gastrointestinal Tumor Study Group. J Clin Oncol. 1989;7:1419–1426. doi: 10.1200/JCO.1989.7.10.1419. [DOI] [PubMed] [Google Scholar]
- 41.Zhang ZG, Harstrick A, Rustum YM. Modulation of fluoropyrimidines: role of dose and schedule of leucovorin administration. Semin Oncol. 1992;19:10–15. [PubMed] [Google Scholar]
- 42.Ychou M, Fabbro-Peray P, Perney P, Marçais O, Gouze C, Ribard D, Bons-Rosset F, Heran B, Veyrac M, Blanc F. A prospective randomized study comparing high- and low-dose leucovorin combined with same-dose 5-fluorouracil in advanced colorectal cancer. Am J Clin Oncol. 1998;21:233–236. doi: 10.1097/00000421-199806000-00004. [DOI] [PubMed] [Google Scholar]
- 43.Jäger E, Heike M, Bernhard H, Klein O, Bernhard G, Lautz D, Michaelis J, Meyer zum Büschenfelde KH, Knuth A. Weekly high-dose leucovorin versus low-dose leucovorin combined with fluorouracil in advanced colorectal cancer: results of a randomized multicenter trial. Study Group for Palliative Treatment of Metastatic Colorectal Cancer Study Protocol 1. J Clin Oncol. 1996;14:2274–2279. doi: 10.1200/JCO.1996.14.8.2274. [DOI] [PubMed] [Google Scholar]
- 44.Dencausse Y, Hartung G, Sturm J, Kopp-Schneider A, Hagmüller E, Wojatschek C, Lindemann H, Fritze D, Queisser W. Adjuvant chemotherapy in stage III colon cancer with 5-fluorouracil and levamisole versus 5-fluorouracil and leucovorin. Onkologie. 2002;25:426–430. doi: 10.1159/000067436. [DOI] [PubMed] [Google Scholar]
- 45.Haller DG, Catalano PJ, Macdonald JS, O'Rourke MA, Frontiera MS, Jackson DV, Mayer RJ. Phase III study of fluorouracil, leucovorin, and levamisole in high-risk stage II and III colon cancer: final report of Intergroup 0089. J Clin Oncol. 2005;23:8671–8678. doi: 10.1200/JCO.2004.00.5686. [DOI] [PubMed] [Google Scholar]
- 46.Efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal cancer. Meta-analysis Group In Cancer. J Clin Oncol. 1998;16:301–308. doi: 10.1200/JCO.1998.16.1.301. [DOI] [PubMed] [Google Scholar]
- 47.Papamichael D. The use of thymidylate synthase inhibitors in the treatment of advanced colorectal cancer: current status. Oncologist. 1999;4:478–487. [PubMed] [Google Scholar]
- 48.Van Cutsem E, Hoff PM, Harper P, Bukowski RM, Cunningham D, Dufour P, Graeven U, Lokich J, Madajewicz S, Maroun JA, et al. Oral capecitabine vs intravenous 5-fluorouracil and leucovorin: integrated efficacy data and novel analyses from two large, randomised, phase III trials. Br J Cancer. 2004;90:1190–1197. doi: 10.1038/sj.bjc.6601676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoff PM, Cassidy J, Schmoll HJ. The evolution of fluoropyrimidine therapy: from intravenous to oral. Oncologist. 2001;6 Suppl 4:3–11. doi: 10.1634/theoncologist.6-suppl_4-3. [DOI] [PubMed] [Google Scholar]
- 50.Douillard JY, Hoff PM, Skillings JR, Eisenberg P, Davidson N, Harper P, Vincent MD, Lembersky BC, Thompson S, Maniero A, et al. Multicenter phase III study of uracil/tegafur and oral leucovorin versus fluorouracil and leucovorin in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2002;20:3605–3616. doi: 10.1200/JCO.2002.04.123. [DOI] [PubMed] [Google Scholar]
- 51.Raymond E, Buquet-Fagot C, Djelloul S, Mester J, Cvitkovic E, Allain P, Louvet C, Gespach C. Antitumor activity of oxaliplatin in combination with 5-fluorouracil and the thymidylate synthase inhibitor AG337 in human colon, breast and ovarian cancers. Anticancer Drugs. 1997;8:876–885. doi: 10.1097/00001813-199710000-00009. [DOI] [PubMed] [Google Scholar]
- 52.Rothenberg ML, Oza AM, Bigelow RH, Berlin JD, Marshall JL, Ramanathan RK, Hart LL, Gupta S, Garay CA, Burger BG, et al. Superiority of oxaliplatin and fluorouracil-leucovorin compared with either therapy alone in patients with progressive colorectal cancer after irinotecan and fluorouracil-leucovorin: interim results of a phase III trial. J Clin Oncol. 2003;21:2059–2069. doi: 10.1200/JCO.2003.11.126. [DOI] [PubMed] [Google Scholar]
- 53.de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 54.Cassidy J, Tabernero J, Twelves C, Brunet R, Butts C, Conroy T, Debraud F, Figer A, Grossmann J, Sawada N, et al. XELOX (capecitabine plus oxaliplatin): active first-line therapy for patients with metastatic colorectal cancer. J Clin Oncol. 2004;22:2084–2091. doi: 10.1200/JCO.2004.11.069. [DOI] [PubMed] [Google Scholar]
- 55.Chang DZ, Abbruzzese JL. Capecitabine plus oxaliplatin vs infusional 5-fluorouracil plus oxaliplatin in the treatment of colorectal cancer. Pro: The CapeOx regimen is preferred over FOLFOX. Clin Adv Hematol Oncol. 2005;3:400–404. [PubMed] [Google Scholar]
- 56.Hochster HS, Hart LL, Ramanathan RK, Hainsworth JD, Hedrick EE, Childs BH. Safety and efficacy of oxaliplatin/fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer (mCRC): Final analysis of the TREE-Study. J Clin Oncol. 2006;24 suppl 18:S3510. doi: 10.1200/JCO.2007.15.4138. [DOI] [PubMed] [Google Scholar]
- 57.André T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan P, Bridgewater J, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 58.Cersosimo RJ. Oxaliplatin-associated neuropathy: a review. Ann Pharmacother. 2005;39:128–135. doi: 10.1345/aph.1E319. [DOI] [PubMed] [Google Scholar]
- 59.Gamelin E, Gamelin L, Bossi L, Quasthoff S. Clinical aspects and molecular basis of oxaliplatin neurotoxicity: current management and development of preventive measures. Semin Oncol. 2002;29:21–33. doi: 10.1053/sonc.2002.35525. [DOI] [PubMed] [Google Scholar]
- 60.Cassidy J, Misset JL. Oxaliplatin-related side effects: characteristics and management. Semin Oncol. 2002;29:11–20. doi: 10.1053/sonc.2002.35524. [DOI] [PubMed] [Google Scholar]
- 61.Hsiang YH, Liu LF. Identification of mammalian DNA topoisomerase I as an intracellular target of the anticancer drug camptothecin. Cancer Res. 1988;48:1722–1726. [PubMed] [Google Scholar]
- 62.Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta N, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343:905–914. doi: 10.1056/NEJM200009283431302. [DOI] [PubMed] [Google Scholar]
- 63.Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041–1047. doi: 10.1016/s0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- 64.Delord JP, Pierga JY, Dieras V, Bertheault-Cvitkovic F, Turpin FL, Lokiec F, Lochon I, Chatelut E, Canal P, Guimbaud R, et al. A phase I clinical and pharmacokinetic study of capecitabine (Xeloda) and irinotecan combination therapy (XELIRI) in patients with metastatic gastrointestinal tumours. Br J Cancer. 2005;92:820–826. doi: 10.1038/sj.bjc.6602354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim TW, Kang WK, Chang HM, Park JO, Ryoo BY, Ahn JS, Zang DY, Lee KH, Kang YK, Kim SR, et al. Multicenter phase II study of oral capecitabine plus irinotecan as first-line chemotherapy in advanced colorectal cancer: a Korean Cancer Study Group trial. Acta Oncol. 2005;44:230–235. doi: 10.1080/02841860510029590. [DOI] [PubMed] [Google Scholar]
- 66.Fuchs C, Marshall J, Mitchell E, Wierzbicki R, Ganju V, Jeffery M, Schultz J, Richards D, Wang B, Morrison M. A randomized trial of first-line irinotecan/fluoropymidine combinations with or without celecoxib in metastatic colorectal cancer (BICC-C) J Clin Oncol. 2006;24 suppl 18:S3506. [Google Scholar]
- 67.Haskell C. Cancer Treatment. 5th ed. WB Saunders Co, 2001: pp. 181–182. [Google Scholar]
- 68.Hartmann JT, Kanz L, Bokemeyer C. Phase II study of continuous 120-hour-infusion of mitomycin C as salvage chemotherapy in patients with progressive or rapidly recurrent gastrointestinal adenocarcinoma. Anticancer Res. 2000;20:1177–1182. [PubMed] [Google Scholar]
- 69.Anderson N, Lokich J, Moore C, Bern M, Coco F. A dose-escalation phase II clinical trial of infusional mitomycin C for 7 days in patients with advanced measurable colorectal cancer refractory or resistant to 5-fluorouracil. Cancer Invest. 1999;17:586–593. doi: 10.3109/07357909909032844. [DOI] [PubMed] [Google Scholar]
- 70.Zakarija A, Bennett C. Drug-induced thrombotic microangiopathy. Semin Thromb Hemost. 2005;31:681–690. doi: 10.1055/s-2005-925474. [DOI] [PubMed] [Google Scholar]
- 71.Ross P, Norman A, Cunningham D, Webb A, Iveson T, Padhani A, Prendiville J, Watson M, Massey A, Popescu R, et al. A prospective randomised trial of protracted venous infusion 5-fluorouracil with or without mitomycin C in advanced colorectal cancer. Ann Oncol. 1997;8:995–1001. doi: 10.1023/a:1008263516099. [DOI] [PubMed] [Google Scholar]
- 72.Yamada Y, Shirao K, Hyodo I, Arai Y, Denda T, Ambo T, Ohtsu A. Phase II study of biweekly irinotecan and mitomycin C combination therapy in patients with fluoropyrimidine-resistant advanced colorectal cancer. Cancer Chemother Pharmacol. 2003;52:125–130. doi: 10.1007/s00280-003-0604-1. [DOI] [PubMed] [Google Scholar]
- 73.Lim DH, Park YS, Park BB, Ji SH, Lee J, Park KW, Kang JH, Lee SH, Park JO, Kim K, et al. Mitomycin-C and capecitabine as third-line chemotherapy in patients with advanced colorectal cancer: a phase II study. Cancer Chemother Pharmacol. 2005;56:10–14. doi: 10.1007/s00280-004-0963-2. [DOI] [PubMed] [Google Scholar]
- 74.Lee JC, Chow NH, Wang ST, Huang SM. Prognostic value of vascular endothelial growth factor expression in colorectal cancer patients. Eur J Cancer. 2000;36:748–753. doi: 10.1016/s0959-8049(00)00003-4. [DOI] [PubMed] [Google Scholar]
- 75.Mulcahy MF, Benson AB. Bevacizumab in the treatment of colorectal cancer. Expert Opin Biol Ther. 2005;5:997–1005. doi: 10.1517/14712598.5.7.997. [DOI] [PubMed] [Google Scholar]
- 76.Kabbinavar F, Hurwitz HI, Fehrenbacher L, Meropol NJ, Novotny WF, Lieberman G, Griffing S, Bergsland E. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:60–65. doi: 10.1200/JCO.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 77.Kabbinavar FF, Hambleton J, Mass RD, Hurwitz HI, Bergsland E, Sarkar S. Combined analysis of efficacy: the addition of bevacizumab to fluorouracil/leucovorin improves survival for patients with metastatic colorectal cancer. J Clin Oncol. 2005;23:3706–3712. doi: 10.1200/JCO.2005.00.232. [DOI] [PubMed] [Google Scholar]
- 78.Giantonio B. High-dose bevacizumab improves survival when combined with FOLFOX4 in previously treated advanced colorectal cancer: Results from the Eastern Cooperative Oncology Group (ECOG) study E3200. Proceedings of the Americal Society of Clinical Oncology Annual Meeting 2005 May 13-17; Orlando FL, USA. Vol 23 (Suppl. 16S), Abstract 2; [Google Scholar]
- 79.Porebska I, Harlozińska A, Bojarowski T. Expression of the tyrosine kinase activity growth factor receptors (EGFR, ERB B2, ERB B3) in colorectal adenocarcinomas and adenomas. Tumour Biol. 2000;21:105–115. doi: 10.1159/000030116. [DOI] [PubMed] [Google Scholar]
- 80.Saltz LB, Meropol NJ, Loehrer PJ, Needle MN, Kopit J, Mayer RJ. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol. 2004;22:1201–1208. doi: 10.1200/JCO.2004.10.182. [DOI] [PubMed] [Google Scholar]
- 81.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 82.Saltz L, Rubin M, Hochster H. Cetuximab (IMC-C225) plus irinotecan (CPT-11) is active in CPT-11-refractory colorectal cancer (CRC) that expresses epidermal growth factor receptor (EGFR). Proceedings of the American Society of Clinical Oncology; 2001 May 12-15, San Francisco CA, USA. Vol 20 (3a), Abstract 7. [Google Scholar]
- 83.Vincenzi B, Santini D, Russo A, Silletta M, Gavasci M, Battistoni F, Di Cuonzo G, Rocci L, Gebbia N, Tonini G. Angiogenesis modifications related with cetuximab plus irinotecan as anticancer treatment in advanced colorectal cancer patients. Ann Oncol. 2006;17:835–841. doi: 10.1093/annonc/mdl031. [DOI] [PubMed] [Google Scholar]
- 84.Chong G, Cunningham D. The role of cetuximab in the therapy of previously treated advanced colorectal cancer. Semin Oncol. 2005;32:S55–S58. doi: 10.1053/j.seminoncol.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 85.Chung KY, Saltz LB. Antibody-based therapies for colorectal cancer. Oncologist. 2005;10:701–709. doi: 10.1634/theoncologist.10-9-701. [DOI] [PubMed] [Google Scholar]
- 86.Green LL. Antibody engineering via genetic engineering of the mouse: XenoMouse strains are a vehicle for the facile generation of therapeutic human monoclonal antibodies. J Immunol Methods. 1999;231:11–23. doi: 10.1016/s0022-1759(99)00137-4. [DOI] [PubMed] [Google Scholar]
- 87.Peeters M, Van Cutsem E, Siena S, et al. A phase 3, multicenter, randomized controlled trial (RCT) of panitumumab plus best supportive care (BSC) vs BSC alone in patients (pts) with metastatic colorectal cancer (mCRC) Proceedings of the American Association for Cancer Research (AACR); 2006 Apr 1-5; Washington DC, USA. Abstract CP-1 Available from URL: http://aacr/org. [Google Scholar]
- 88.Tournigand C, André T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229–237. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 89.Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC, Alberts SR. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22:23–30. doi: 10.1200/JCO.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 90.Colucci G, Gebbia V, Paoletti G, Giuliani F, Caruso M, Gebbia N, Cartenì G, Agostara B, Pezzella G, Manzione L, et al. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell'Italia Meridionale. J Clin Oncol. 2005;23:4866–4875. doi: 10.1200/JCO.2005.07.113. [DOI] [PubMed] [Google Scholar]
- 91.Souglakos J, Androulakis N, Syrigos K, Polyzos A, Ziras N, Athanasiadis A, Kakolyris S, Tsousis S, Kouroussis Ch, Vamvakas L, et al. FOLFOXIRI (folinic acid, 5-fluorouracil, oxaliplatin and irinotecan) vs FOLFIRI (folinic acid, 5-fluorouracil and irinotecan) as first-line treatment in metastatic colorectal cancer (MCC): a multicentre randomised phase III trial from the Hellenic Oncology Research Group (HORG) Br J Cancer. 2006;94:798–805. doi: 10.1038/sj.bjc.6603011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Falcone A, Masi G, Brunetti I. The triplet combination of irinotecan, oxaliplatin and 5FU/LVThe triplet combination of irinotecan, oxaliplatin and 5FU/LV (FOLFOXIRI) vs the doublet of irinotecan and 5FU/LV (FOLFIRI) as first-line treatment of metastatic colorectal cancer (MCRC): Results of a randomized phase III trial by the Gruppo Oncologico Nort Obest (G.O.N.O.). Proceedings of the American Society of Clinical Oncology; 2006 June 2-6, Atlanta GA, USA. Vol 24 (no,18S), Part I, Abstract 3513, 149s. [Google Scholar]
- 93.O'Connell MJ. A phase III trial of 5-fluorouracil and leucovorin in the treatment of advanced colorectal cancer. A Mayo Clinic/North Central Cancer Treatment Group study. Cancer. 1989;63:1026–1030. doi: 10.1002/1097-0142(19890315)63:6+<1026::aid-cncr2820631307>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 94.O'Connell MJ, Mailliard JA, Kahn MJ, Macdonald JS, Haller DG, Mayer RJ, Wieand HS. Controlled trial of fluorouracil and low-dose leucovorin given for 6 months as postoperative adjuvant therapy for colon cancer. J Clin Oncol. 1997;15:246–250. doi: 10.1200/JCO.1997.15.1.246. [DOI] [PubMed] [Google Scholar]
- 95.de Gramont A, Louvet C, André T, Tournigand C, Raymond E, Molitor JL, Krulik M. [Modulation of 5-fluorouracil with folinic acid in advanced colorectal cancers. Groupe d'étude et de recherche sur les cancers de l'ovaire et digestifs (GERCOD)] Rev Med Interne. 1997;18 Suppl 4:372s–378s. doi: 10.1016/s0248-8663(97)83768-x. [DOI] [PubMed] [Google Scholar]
- 96.Ychou M, Duffour J, Pinguet F, Kramar A, Joulia JM, Topart D, Bressolle F. Individual 5FU-dose adaptation schedule using bimonthly pharmacokinetically modulated LV5FU2 regimen: a feasibility study in patients with advanced colorectal cancer. Anticancer Res. 1999;19:2229–2235. [PubMed] [Google Scholar]
- 97.Maindrault-Goebel F, Louvet C, André T, Carola E, Lotz JP, Molitor JL, Garcia ML, Gilles-Amar V, Izrael V, Krulik M, et al. Oxaliplatin added to the simplified bimonthly leucovorin and 5-fluorouracil regimen as second-line therapy for metastatic colorectal cancer (FOLFOX6). GERCOR. Eur J Cancer. 1999;35:1338–1342. doi: 10.1016/s0959-8049(99)00149-5. [DOI] [PubMed] [Google Scholar]
- 98.Hochster H, Chachoua A, Speyer J, Escalon J, Zeleniuch-Jacquotte A, Muggia F. Oxaliplatin with weekly bolus fluorouracil and low-dose leucovorin as first-line therapy for patients with colorectal cancer. J Clin Oncol. 2003;21:2703–2707. doi: 10.1200/JCO.2003.02.071. [DOI] [PubMed] [Google Scholar]
- 99.Moehler M, Hoffmann T, Hildner K, Siebler J, Galle PR, Heike M. Weekly oxaliplatin, high-dose folinic acid and 24h-5-fluorouracil (FUFOX) as salvage therapy in metastatic colorectal cancer patients pretreated with irinotecan and folinic acid/5-fluorouracil regimens. Z Gastroenterol. 2002;40:957–964. doi: 10.1055/s-2002-36156. [DOI] [PubMed] [Google Scholar]
- 100.Sørbye H, Dahl O. Nordic 5-fluorouracil/leucovorin bolus schedule combined with oxaliplatin (Nordic FLOX) as first-line treatment of metastatic colorectal cancer. Acta Oncol. 2003;42:827–831. doi: 10.1080/02841860310018972. [DOI] [PubMed] [Google Scholar]
- 101.Masi G, Allegrini G, Cupini S, Marcucci L, Cerri E, Brunetti I, Fontana E, Ricci S, Andreuccetti M, Falcone A. First-line treatment of metastatic colorectal cancer with irinotecan, oxaliplatin and 5-fluorouracil/leucovorin (FOLFOXIRI): results of a phase II study with a simplified biweekly schedule. Ann Oncol. 2004;15:1766–1772. doi: 10.1093/annonc/mdh470. [DOI] [PubMed] [Google Scholar]
- 102.Saltz LB, Lenz HJ, Hochster H. Randomized phase II trial of cetuximab/bevacizumab/irinotecan (CBI) versus cetuximab/bevacizumab (CB) in irinotecan-refractory colorectal cancer. J Clin Oncol. 2005;23 suppl:S248. doi: 10.1200/JCO.2007.12.0949. [DOI] [PubMed] [Google Scholar]


