Abstract
AIM: To study the protective effects of tumor necrosis factor α (TNF α ) antibody and ulinastatin on liver ischemic reperfusion in rats.
METHODS: One hundred and twenty male SD rats were randomly divided into four groups: Normal control group, ischemic group, TNFα antibody group and TNFα antibody + ulinastatin group. The animals were killed at 0, 3, 6, 9, 12 h after ischemia for 60 min and followed by reperfusion. Serum alanine aminotransferase (ALT), malondialdehyde (MDA) and liver histopathology were observed.
RESULTS: After ischemic reperfusion, the serum ALT and MDA were remarkably increased, and the hepatic congestion was obvious. Treatment of TNFα antibody and ulinastatin could significantly decrease serum ALT and MDA levels, and relieve hepatic congestion.
CONCLUSION: Ulinastatin and TNFα antibody can suppress the inflammatory reaction induced by hepatic ischemic reperfusion, and have protective effects on rat hepatic ischemic reperfusion injury.
INTRODUCTION
Liver ischemic reperfusion injury is induced when liver gets the retrieval of its blood perfusion or oxygen supply, and hepatic injury would aggravate due to ischemia and hypoxia injury[1-4]. Hepatic insufficiency or primary liver graft non-function can be caused by liver ischemic reperfusion injury after portal blockage, hemorrhagic shock or liver transplantation. As liver ischemic reperfusion is hard to be avoided in hepatic surgical practice and the existing prevention and cure methods are not satisfactory, research of the mechanisms and therapy on liver ischemic reperfusion becomes one of the hotspots in hepatic surgery[5,6].
Cytokines are polypeptides with extensive biological activities, and play important roles in the immunoloregulation. They prevent body from diseases and accelerate tissue rehabilitation. But on the other hand, too many cytokines can also lead to or aggravate tissue damages[7,8]. Recent researches have demonstrated that TNF α plays an important role in ischemic reperfusion injury of liver[9-12]. At the same time, ulinastatin has been applied in the clinical treatment of pancreatitis, shock and extracorporeal circulation because of its significant inhibitory effect on inflammation[13-15]. In the present study, we attempted to relieve ischemic reperfusion injury of liver by using TNF α antibody and ulinastatin, so as to provide experimental and theoretic bases for prevention and treatment of liver ischemic reperfusion injury.
MATERIALS AND METHODS
Animals
A total of 120 male Spargue-Dawfey (SD) rats weighing 230 ± 20 g, were obtained from Animal Research Center of Shaanxi Chinese Medical Institute, and fed with standard rat chow.
Drugs
Ulinastatin (Tianpu Co. Ltd., Guangdong, China) was diluted to 50 U/L by saline prior to use. TNF α monoclonal antibody (Jingmei Co. Ltd., Guangdong, China) was diluted 100 times by saline prior to use.
Experimental grouping
The rats were randomly divided into four groups. Group I: The control group, sham operation was performed, hepatic lobes of the rats were exposed without any treatment. Group II: Ischemic reperfusion injury group, in which blood stream of the rats’ liver lobes were blocked and then recovered after 60 min. Group III: TNF α antibody treatment group, in which TNF α antibody (2.0 mg/kg) was injected into the rats through dorsum veins of penis 5 min prior to reperfusion. Group IV: TNF α antibody and ulinastatin treatment group, in which both TNF α antibody (2.0 mg/kg) and ulinastatin (500 000 U/L, 0.5 mL) were simultaneously injected into the rats through dorsum veins of penis 5 min prior to reperfusion. Blood samples (2 mL) of all animals in each group were taken from hepatic superior and inferior vena cava at 0, 3, 6, 9 and 12 h after reperfusion. Then the rats were killed and liver samples were obtained.
Operation
The animals were intraabdominally anesthetized by pentobarbital sodium (30 mg/kg, 0.1 mL/10 g), and incised through median incision of the abdomen. After the liver pedicel between left and middle lobes of liver was exposed, ligaments between liver and septum transversum and abdominal wall were cut. The scatheless vascular clamp was used to block blood stream of portal veins and hepatic arteries of left and middle lobes of liver. After 60 min, the vascular clamp was released and blood stream recovered. So approximately seventy percent of liver was hypoxia, thus severe congestion of the mesentery vein was prevented. (Tables 1 and 2).
Table 1.
Levels of serum ALT in rats (U/L)
| Group | n |
Time of after reperfusion (h) |
||||
| 0 | 3 | 6 | 9 | 12 | ||
| Id | 6 | 40.52 ± 8.33 | 42.36 ± 3.71 | 43.19 ± 7.64 | 42.92 ± 5.18 | 42.66 ± 9.27 |
| II | 6 | 263.92 ± 16.90 | 315.61 ± 21.02 | 374.26 ± 19.56 | 289.11 ± 16.32 | 257.94 ± 27.41 |
| III | 6 | 238.73 ± 10.62 | 254.06 ± 13.78 | 273.17 ± 18.29 | 213.26 ± 26.54 | 172.53 ± 36.46 |
| IVb | 6 | 173.42 ± 15.33 | 189.08 ± 24.52 | 203.17 ± 23.19 | 175.36 ± 38.66 | 163.13 ± 32.27 |
bP < 0.01 vs other groups, dP < 0.01 vs group II and III.
Table 2.
Levels of serum MDA of rats (mmol/L)
| Group | n |
Time of after reperfusion (h) |
||||
| 0 | 3 | 6 | 9 | 12 | ||
| Id | 6 | 7.82 ± 0.17 | 7.91 ± 0.08 | 8.11 ± 0.13 | 8.04 ± 0.67 | 7.93 ± 0.41 |
| II | 6 | 13.81 ± 4.19 | 38.65 ± 4.73 | 46.36 ± 2.54 | 35.09 ± 5.12 | 24.09 ± 3.20 |
| III | 6 | 10.71 ± 2.52 | 18.23 ± 3.58 | 23.31 ± 4.49 | 16.83 ± 2.29 | 11.26 ± 3.16 |
| IVb | 6 | 9.12 ± 3.41 | 12.29 ± 6.83 | 15.18 ± 3.22 | 11.72 ± 4.15 | 9.42 ± 5.28 |
P < 0.01 vs group II and III,
P < 0.01 vs other groups.
Determination of ALT and MDA in serum
The blood samples in each group were poured into centrifuge tubes and the placement lasted for 20 min without shaking. After centrifugation at 2 000 r/min for 10 min, the sample serum was extracted and stored at -80 °C for determination.
ALT levels of sample serum were determined by an automatic biochemistry analyzer. MDA levels of sample serum were determined by the method introduced by Mourek et al[16], and the kit was purchased from Juli Biomedical Engineering Institute of Nanjing, China.
Pathological changes of liver
Fresh tissues of liver in each group were sampled. Haematoxylin-Eosin (HE) staining was performed on 100 g/L formaldehyde-fixed tissue sections. The histological patterns of the liver samples were observed under light microscope.
Statistical analysis
All the data were analyzed by Student’s t test and expressed as mean ± SD. P < 0.05 was considered statistically significant and P < 0.01 as very statistically significant.
RESULTS
ALT levels of sample serum
ALT levels of sample serum in each group were determined to assess the liver function of rats. The levels of ALT in ischemic reperfusion injury group at different time points were significantly higher than those in control (P < 0.01). The levels of ALT increased gradually after reperfusion, reached the peak 6 h after reperfusion, and then decreased mildly. The levels of ALT in TNFα antibody treatment group were remarkably lower than those in ischemic reperfusion injury group (P < 0.01). The levels of ALT in TNF α antibody and ulinastatin treatment group were lower than those in ischemic reperfusion injury group and TNFα antibody treatment group (P < 0.01).
MDA levels of sample serum
The oxygen-derived free radidicals induced lipid peroxidation reaction of polyvalent unsaturated fatty acid at plasmalemma, which developed lipid peroxidation products, such as MDA. So the MDA levels of sample serum showed the degree of lipid peroxidation injury of liver. The levels of MDA in ischemic reperfusion injury group at different time points were significantly higher than those in control (P < 0.01). The levels of MDA increased gradually after reperfusion, reached the peak 6 h after reperfusion, and then decreased. The levels of MDA in TNFα antibody treatment group were remarkably lower than those in ischemic reperfusion injury group (P < 0.01). The levels of MDA in TNFα antibody and ulinastatin treatment group were lower than those in ischemic reperfusion injury group and TNFα antibody treatment group (P < 0.01). (Figures 1 and 2).
Figure 1.
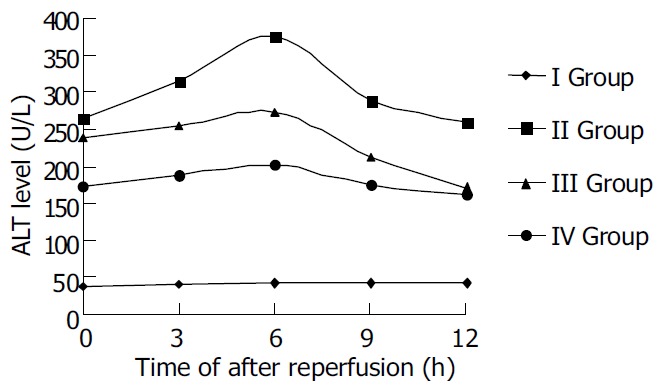
Serum ALT level after reperfusion in rats.
Figure 2.
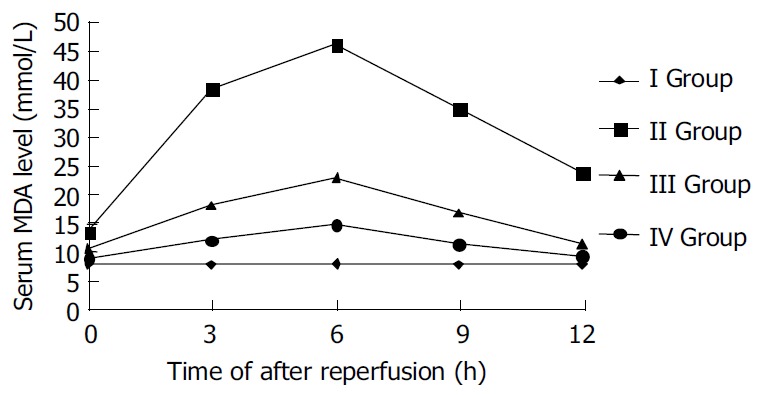
Serum MDA level after reperfusion in rats.
Histopathological observation
The blood stream in middle and left lobes of livers of rats was reperfused 1 h after blocked for 60 min. In ischemic reperfusion injury group, the middle and left lobes of livers were found to be swollen and faint. HE staining showed disorganized hepatic lobules and extensive hepatocytic edema with various degrees of vacuolation and lamellar necrosis. The liver tissues in TNFα antibody treatment group and in TNFα antibody + ulinastatin treatment group were found to insignificantly swollen and faint in appearance. HE staining showed a sprinkle of hepatocytic edema without vacuolation and lamellar necrosis. (Figures 3 and 4).
Figure 3.
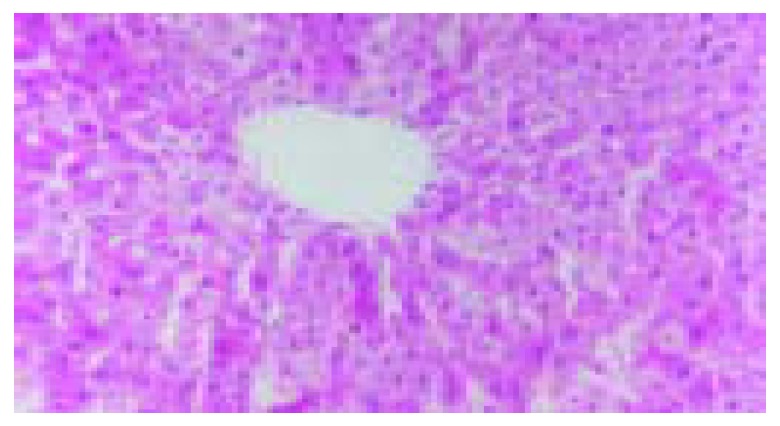
Disorganized hepatic lobules and extensive hepatocytic edema with various degrees of vacuolation and lamellar ne-crosis in ischemic reperfusion injury group.
Figure 4.
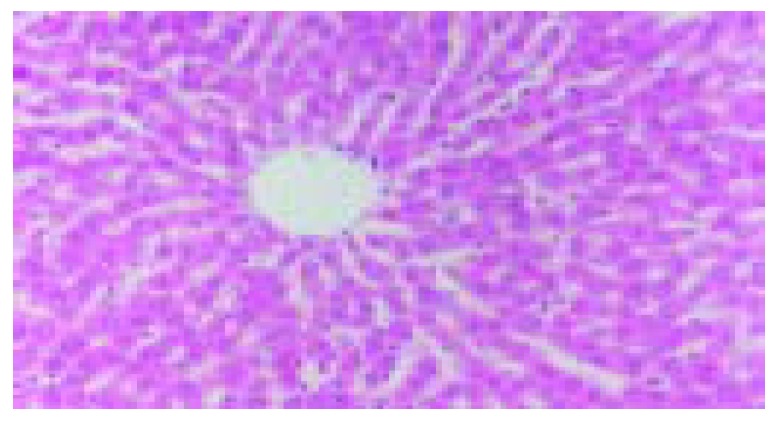
A sprinkle of hepatocytic edema without vacuola-tion and lamellar necrosis in TNFα antibody + ulinastatin treatment group.
DISCUSSION
Liver ischemic reperfusion injury can be frequently seen in surgical practice, and plays impotatnt roles in severe infection, trauma, shock, cardiorespiratory malfunction, organ transplantation, etc.. Ischemic reperfusion can cause a series of injuries on metabolism, structure, and function in hepatic tissues and cells, and even liver function failure. So it is one of the major factors influencing the prognosis, operative success and survival of patients.
Cytokines, are the soluble polypeptides excreted by immunocytes, and play important roles in immunological activation and inflammatory reaction. The study of cytokine effects on ischemic reperfusion injury in liver has become highlights at present[17-22]. TNFα is the polypeptide excreted by activated macrophages, endothelial cells, neutrophilic granulocytes and B lymphocytes, and plays an important role in inflammatory reaction. Liver possesses tremendous Kupffer cells (KC) that have the great potency to produce TNFα in human body. At the time of liver ischemia, for the blockage of ATP production, calcium pump of liver is in dysfunction, which causes intracellular calcium overload. Aggravated calcium overload can activate Kupffer cells. On the other hand, Kupffer cells have complement receptors, intracellular protein was released during ischemic reperfusion, thus activating complements, which can also activate Kupffer cells. The activated Kupffer cells excrete TNFα. Liver has plenty of TNFα receptors, and also is the major target organ of TNFα [23-26]. Some mechanisms were used to explain the liver damage caused by TNFα [27-30]. Firstly, TNFα could directly injure hepatocytes. Secondly, TNFα could activate neutrophilic granulocytes and mononuclear macrophages to express IL-1 and IL-6, and phosphatidase A2 that can decompose arachidonic acids. Inflammatory media were produced such as platelet active factor, leukotriene, and thromboxane A2. So the inflammatory reaction was aggravated. Thirdly, the toxic effect of TNFα on endothelial cells could induce the circulatory disorder of hepatic sinusoid, and TNFα could activate complement system that aggravates tissue damage by cytotoxicity. Finally, the oxygen-derived free radidicals induced by TNFα could facilitate oxidation explosion of neutrophilic granulocytes, which could also result in liver damage. Therefore, the way to block the production of TNFα in liver can be used to prevent liver from injury by TNFα .
Ulinastatin is one kind of glycoproteins containing 143 amino acids with molecular weight of 67 ku. It is the typical urine protease inhibitor isolated and purified from human urine. Ulinastatin has two active function domains which have a wide restrained zymogram with no overlapped region each other. So ulinastatin can inhibit many hydrolytic enzymes in one time, including trypsin, phospholipase A2, alidase and elastase. Moreover, the components of low molecular mass decomposed from ulinastatin can also inhibit hydrolytic enzymes strongly. On the other hand, ulinastatin could ameliorate the shock situation by blocking the production of myocardial depressant factors and stabilizing the membrane of lysosome[31-33]. Recent researches found that ulinastatin could block the release of inflammatory factors, prevent cascade reaction of cytokines, inhibit excessive activation of leukocytes and block vicious circles among cytokines, inflammatory factors and leukocytes. So when pancreatitis, shock or other severe infection occured, the action of multiple hydrolytic enzymes necessitated the use of ulinastatin, which can inhibit many hydrolytic enzymes at the same time and alleviate the vicious effects of multiple proteases on tissues and organs[34-36].
The present study was to verify the protective effect of TNFα antibody and ulinastatin on ischemic reperfusion injury of liver. The results showed that the levels of ALT and MDA in TNFα antibody and ulinastatin treatment group were remarkably decreased as compared with those in ischemic reperfusion injury group. Pathological changes of liver also demonstrated the significant protective effect of TNFα antibody and ulinastatin on ischemic reperfusion injury of liver. The present results suggest TNFα antibody and ulinastatin can effectively prevent ischemic reperfusion injury of liver. But further work is needed to make clear of their best concentrations, best ratio and their effect on immune system of the body.
Footnotes
Supported by the National Natural Science Foundation of China, No. 30070741
Edited by Zhang JZ and Wang XL Proofread by Xu FM
References
- 1.Zhu X, Qiu Y, Shi M, Ding Y. Matrine protects sinusoidal endothelial cells from cold ischemia and reperfusion injury in rat orthotopic liver transplantation. Ann Clin Lab Sci. 2003;33:216–225. [PubMed] [Google Scholar]
- 2.Zhou T, Chen JL, Song W, Wang F, Zhang MJ, Ni PH, Geng JG. Effect of N-desulfated heparin on hepatic/renal ischemia reperfusion injury in rats. World J Gastroenterol. 2002;8:897–900. doi: 10.3748/wjg.v8.i5.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jawan B, Goto S, Pan TL, Lai CY, Luk HN, Eng HL, Lin YC, Chen YS, Lan KM, Hsieh SW, et al. The protective mechanism of magnolol, a Chinese herb drug, against warm ischemia-reperfusion injury of rat liver. J Surg Res. 2003;110:378–382. doi: 10.1016/s0022-4804(03)00034-9. [DOI] [PubMed] [Google Scholar]
- 4.Teoh NC, Farrell GC. Hepatic ischemia reperfusion injury: pathogenic mechanisms and basis for hepatoprotection. J Gastroenterol Hepatol. 2003;18:891–902. doi: 10.1046/j.1440-1746.2003.03056.x. [DOI] [PubMed] [Google Scholar]
- 5.Fondevila C, Busuttil RW, Kupiec-Weglinski JW. Hepatic ischemia/reperfusion injury--a fresh look. Exp Mol Pathol. 2003;74:86–93. doi: 10.1016/s0014-4800(03)00008-x. [DOI] [PubMed] [Google Scholar]
- 6.Donckier V, Loi P, Closset J, Nagy N, Quertinmont E, Le Moine O, Devière J, Goldman M, Gelin M, Gianello P. Preconditioning of donors with interleukin-10 reduces hepatic ischemia-reperfusion injury after liver transplantation in pigs. Transplantation. 2003;75:902–904. doi: 10.1097/01.TP.0000056633.50445.AC. [DOI] [PubMed] [Google Scholar]
- 7.Banks WA, Farr SA, Morley JE. Entry of blood-borne cytokines into the central nervous system: effects on cognitive processes. Neuroimmunomodulation. 2003;10:319–327. doi: 10.1159/000071472. [DOI] [PubMed] [Google Scholar]
- 8.Trefzer U, Hofmann M, Sterry W, Asadullah K. Cytokine and anticytokine therapy in dermatology. Expert Opin Biol Ther. 2003;3:733–743. doi: 10.1517/14712598.3.5.733. [DOI] [PubMed] [Google Scholar]
- 9.Tsuchihashi S, Tamaki T, Tanaka M, Kawamura A, Kaizu T, Ikeda A, Kakita A. Pyrrolidine dithiocarbamate provides protection against hypothermic preservation and transplantation injury in the rat liver: the role of heme oxygenase-1. Surgery. 2003;133:556–567. doi: 10.1067/msy.2003.124. [DOI] [PubMed] [Google Scholar]
- 10.Dutkowski P, Wahl W, Winkelbach V, Watzka M, Krysiak M, Junginger T. Calcium prevents loss of glutathione and reduces oxidative stress upon reperfusion in the perfused liver. Int J Surg Investig. 2000;2:1–7. [PubMed] [Google Scholar]
- 11.Langdale LA, Kajikawa O, Frevert C, Liggitt HD. Sustained tolerance to lipopolysaccharide after liver ischemia-reperfusion injury. Shock. 2003;19:553–558. doi: 10.1097/01.shk.0000055238.25446.64. [DOI] [PubMed] [Google Scholar]
- 12.Chimalakonda AP, Mehvar R. Attenuation of Kupffer cell activation in cold-preserved livers after pretreatment of rats with methylprednisolone or its macromolecular prodrug. Pharm Res. 2003;20:1001–1008. doi: 10.1023/a:1024402121053. [DOI] [PubMed] [Google Scholar]
- 13.Masuda T, Sato K, Noda C, Ikeda KM, Matsunaga A, Ogura MN, Shimizu K, Nagasawa H, Matsuyama N, Izumi T. Protective effect of urinary trypsin inhibitor on myocardial mitochondria during hemorrhagic shock and reperfusion. Crit Care Med. 2003;31:1987–1992. doi: 10.1097/01.CCM.0000057037.44171.BA. [DOI] [PubMed] [Google Scholar]
- 14.Yano T, Anraku S, Nakayama R, Ushijima K. Neuroprotective effect of urinary trypsin inhibitor against focal cerebral ischemia-reperfusion injury in rats. Anesthesiology. 2003;98:465–473. doi: 10.1097/00000542-200302000-00028. [DOI] [PubMed] [Google Scholar]
- 15.Sato N, Endo S, Kimura Y, Ikeda K, Aoki K, Iwaya T, Akiyama Y, Noda Y, Saito K. Influence of a human protease inhibitor on surgical stress induced immunosuppression. Dig Surg. 2002;19:300–305. doi: 10.1159/000064578. [DOI] [PubMed] [Google Scholar]
- 16.Mourek J, Koudelová J. [Adrenergic tocolytics--their effects on lipoperoxidation in the brain] Ceska Gynekol. 1997;62:15–18. [PubMed] [Google Scholar]
- 17.Kato A, Gabay C, Okaya T, Lentsch AB. Specific role of interleukin-1 in hepatic neutrophil recruitment after ischemia/reperfusion. Am J Pathol. 2002;161:1797–1803. doi: 10.1016/S0002-9440(10)64456-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shinoda M, Shimazu M, Matsuda S, Wakabayashi G, Tanabe M, Hoshino K, Kamei S, Koyasu S, Kitajima M. c-Jun N-terminal kinase activation during warm hepatic ischemia/reperfusion injuries in a rat model. Wound Repair Regen. 2002;10:314–319. doi: 10.1046/j.1524-475x.2002.t01-1-10507.x. [DOI] [PubMed] [Google Scholar]
- 19.Cutrn JC, Perrelli MG, Cavalieri B, Peralta C, Rosell Catafau J, Poli G. Microvascular dysfunction induced by reperfusion injury and protective effect of ischemic preconditioning. Free Radic Biol Med. 2002;33:1200–1208. doi: 10.1016/s0891-5849(02)01017-1. [DOI] [PubMed] [Google Scholar]
- 20.Iwasaki Y, Tagaya N, Hattori Y, Yamaguchi K, Kubota K. Protective effect of ischemic preconditioning against intermittent warm-ischemia-induced liver injury. J Surg Res. 2002;107:82–92. doi: 10.1006/jsre.2002.6505. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi Y, Ganster RW, Gambotto A, Shao L, Kaizu T, Wu T, Yagnik GP, Nakao A, Tsoulfas G, Ishikawa T, et al. Role of NF-kappaB on liver cold ischemia-reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1175–G1184. doi: 10.1152/ajpgi.00515.2001. [DOI] [PubMed] [Google Scholar]
- 22.Zamora R, Vodovotz Y, Aulak KS, Kim PK, Kane JM, Alarcon L, Stuehr DJ, Billiar TR. A DNA microarray study of nitric oxide-induced genes in mouse hepatocytes: implications for hepatic heme oxygenase-1 expression in ischemia/reperfusion. Nitric Oxide. 2002;7:165–186. doi: 10.1016/s1089-8603(02)00104-0. [DOI] [PubMed] [Google Scholar]
- 23.Hines IN, Kawachi S, Harada H, Pavlick KP, Hoffman JM, Bharwani S, Wolf RE, Grisham MB. Role of nitric oxide in liver ischemia and reperfusion injury. Mol Cell Biochem. 2002;234-235:229–237. [PubMed] [Google Scholar]
- 24.Kim YI, Song KE, Ryeon HK, Hwang YJ, Yun YK, Lee JW, Chun BY. Enhanced inflammatory cytokine production at ischemia/reperfusion in human liver resection. Hepatogastroenterology. 2002;49:1077–1082. [PubMed] [Google Scholar]
- 25.Ben-Ari Z, Hochhauser E, Burstein I, Papo O, Kaganovsky E, Krasnov T, Vamichkim A, Vidne BA. Role of anti-tumor necrosis factor-alpha in ischemia/reperfusion injury in isolated rat liver in a blood-free environment. Transplantation. 2002;73:1875–1880. doi: 10.1097/00007890-200206270-00004. [DOI] [PubMed] [Google Scholar]
- 26.Kim YI, Hwang YJ, Song KE, Yun YK, Lee JW, Chun BY. Hepatocyte protection by a protease inhibitor against ischemia/reperfusion injury of human liver. J Am Coll Surg. 2002;195:41–50. doi: 10.1016/s1072-7515(01)01118-8. [DOI] [PubMed] [Google Scholar]
- 27.Kataoka M, Shimizu H, Mitsuhashi N, Ohtsuka M, Wakabayashi Y, Ito H, Kimura F, Nakagawa K, Yoshidome H, Shimizu Y, et al. Effect of cold-ischemia time on C-X-C chemokine expression and neutrophil accumulation in the graft liver after orthotopic liver transplantation in rats. Transplantation. 2002;73:1730–1735. doi: 10.1097/00007890-200206150-00007. [DOI] [PubMed] [Google Scholar]
- 28.Kato A, Edwards MJ, Lentsch AB. Gene deletion of NF-kappa B p50 does not alter the hepatic inflammatory response to ischemia/reperfusion. J Hepatol. 2002;37:48–55. doi: 10.1016/s0168-8278(02)00068-5. [DOI] [PubMed] [Google Scholar]
- 29.Peralta C, Perales JC, Bartrons R, Mitchell C, Gilgenkrantz H, Xaus C, Prats N, Fernández L, Gelpí E, Panés J, et al. The combination of ischemic preconditioning and liver Bcl-2 overexpression is a suitable strategy to prevent liver and lung damage after hepatic ischemia-reperfusion. Am J Pathol. 2002;160:2111–2122. doi: 10.1016/S0002-9440(10)61160-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harada N, Okajima K, Uchiba M, Katsuragi T. Ischemia/reperfusion-induced increase in the hepatic level of prostacyclin is mainly mediated by activation of capsaicin-sensitive sensory neurons in rats. J Lab Clin Med. 2002;139:218–226. doi: 10.1067/mlc.2002.121856. [DOI] [PubMed] [Google Scholar]
- 31.Itabashi K, Ito Y, Takahashi T, Ishii K, Sato K, Kakita A. Protective effects of urinary trypsin inhibitor (UTI) on hepatic microvasculature in hypotensive brain-dead rats. Eur Surg Res. 2002;34:330–338. doi: 10.1159/000063074. [DOI] [PubMed] [Google Scholar]
- 32.Lin SD, Endo R, Sato A, Takikawa Y, Shirakawa K, Suzuki K. Plasma and urine levels of urinary trypsin inhibitor in patients with acute and fulminant hepatitis. J Gastroenterol Hepatol. 2002;17:140–147. doi: 10.1046/j.1440-1746.2002.02676.x. [DOI] [PubMed] [Google Scholar]
- 33.Saitoh Y, Kaneda K, Murakawa M. The effect of ulinastatin pre-treatment on vecuronium-induced neuromuscular block in patients with hepatic cirrhosis. Anaesthesia. 2002;57:218–222. doi: 10.1046/j.0003-2409.2001.02468.x. [DOI] [PubMed] [Google Scholar]
- 34.Pugia MJ, Takemura T, Kuwajima S, Suzuki M, Cast TK, Profit JA, Schulman LS, Ohta Y, Lott JA. Clinical utility of a rapid test for uristatin. Clin Biochem. 2002;35:105–110. doi: 10.1016/s0009-9120(02)00288-6. [DOI] [PubMed] [Google Scholar]
- 35.Furukawa K, Kamimura T, Mahune Y, Ohota H, Yoshida T, Ishihara N, Tazaki K, Suzuki Y, Honda S, Ito K, et al. Two patients with severe alcoholic hepatitis ac-companied by hypercytokinemia and granulocytic hyperelastasemia, successfully treated by intravenous infusion of urinastarine Miradid. J Gastroenterol Hepatol. 2001;16:575–580. doi: 10.1046/j.1440-1746.2001.02466.x. [DOI] [PubMed] [Google Scholar]
- 36.Takada K, Komori M, Notoya A, Tomizawa Y, Ozaki M. Effect of ulinastatin on microcirculation during excessive hemorrhage using fluid therapy. In Vivo. 2003;17:129–135. [PubMed] [Google Scholar]


