Abstract
AIM: To study the pathway of apoptosis in chronic liver disease and the role of mitochondria in programmed cell death.
METHODS: Liver biopsy specimens from 72 cases of chronic hepatitis and 29 cases of post hepatitis cirrhosis were studied. The pro-apoptotic protein Fas, FasL, Bax and the anti-apoptotic protein Bcl-2, Bcl-xL, Bcl-2α were studied immunohistochemically by SP method. Specimens from 15 cases of chronic hepatitis and post hepatitis cirrhosis were examined for their ultramicrostructures with special attention to their mitochondrial changes. Specimens from 3 normal adults (demised in traffic accidents) were used as control.
RESULTS: The expression of proapoptotic proteins (Fas, FasL, Bax) in hepatocytes was significantly higher in the chronic hepatitis group than in the cirrhosis group (P < 0.001). In the study of ultramicrostructure 364 hepatocytes were examined, from 12 cases of chronic hepatitis (including 10 mild cases, 1 moderate case and 1 severe case). Out of 364 hepatocytes 40 (11.0%) hepatocytes were found with various kinds of destruction in their mitochondria. Rupture of the outer membrane of mitochondria and the leakage of matrix from the intermembrane space were definitely demonstrated. The ultramicrostructural changes of mitochondria in the chronic hepatitis group were statistically higher than that in normal adults control group (χ2=4.32, P < 0.05).
CONCLUSION: The result of the study was in support of the current view that the apoptotic process in chronic hepatitis patients were largely along the intrinsic pathway (mitochondrial pathway), given that the intrinsic and extrinsic pathways could interlinked (converged) at some point on their progression, also it is impossible at present to exclude the possibility that the two pathways could be chosen by hepatocytes in parallel simultaneously.
INTRODUCTION
Fas receptor induced hepatocellular apoptosis has long been implicated in the pathogenesis of viral hepatitis and potentially other liver diseases. The apoptosis is initiated by binding of the Fas ligand expressed on the surface of lymphocytes to the Fas receptor on the hepatocytes. After initiation the apoptotic process, the cascade reaction of caspases and eventually to the DNA fragmentation and cell death continued[4,5]. In addition to the above extrinsic pathway it was recognized recently that apoptosis can occur independent of cell surface receptors. Apoptosis can occur through the activation of p53 by DNA damage. Activation of p53 leads to translocation of proapoptotic protein Bax to the mitochondria and form pores at the outer mitochondrial membranes. The formation of pores induces the release of cytochrome c and other proteins from the intermembrane space. The released cytochrome c and other protein then activated other caspases and eventually lead to apoptosis (intrinsic pathway). In this study we tried to find out the apoptosis pathway in chronic hepatitis. We studied it by immunohistochemical method and ultramicrostructural examination of mitochondrial changes.
MATERIALS AND METHODS
Liver biopsy specimens from 72 cases of chronic hepatitis of more than one year and 29 cases of post-hepatitis cirrhosis were studied immunohistochemically, out of them 12 cases of chronic hepatitis, 3 cases post-hepatitis cirrhosis and 3 normal adults, were examined by electronmicroscopy for the ultramicrostructurs of apoptotic hepatocytes. Criteria of clinical and histological diagnoses were carried out in accordance with the consensus protocols published by the 10th Congress of Viral Hepatitis of China. The histological activity of hepatitis was assessed by the histological activity index (HAI) of Knodell et al[9] The intensity of expression in immunohistochemical assay was divided into (-), (+), (++) and (+ + +) in this study[10,11].
Serum markers of HBV infection and anti-HCV antibody were detected by ELISA. Liver biopsy specimens were examined after hematoxylin and eosin staining. The immunohistochemical examination was carried out by streptoavidin peroxidase conjugation. Mouse anti-human Bcl-2, Bcl-XL monoclonal antibody and rabbit anti-human Fas and rabbit anti-human FasL polyclonal antibodies were obtained from Santo Cruz (USA). Mouse anti-Bcl-2α monoclonal antibody was obtained from Maixin Bio company. Ultramicrostructure of mitochondria was studied by EM of Hitachi H-600 (Japan). The ultramicrostructure changes of mitochondria in specimens from 15 cases of chronic hepatitis and post hepatitis cirrhosis were examined with special attention to their mitochondrial changes. Specimens from 3 normal adults (demised in traffic accidents) were used as control. At least 15 hepatocytes in each section were observed.
The statistics was carried out by χ2 with MINITAB software
RESULTS
Immunohistochemical findings
Both the expressions of proapoptotic proteins (Fas, FasL, Bax) and antiapoptotic proteins (Bcl-2, Bcl-2α , Bcl-XL) were found mainly in cytoplasm and membranes of hepatocytes. Some expressions could also be found in cytoplasma of epithelial cells of the biliary canaliculus. In 101 cases of chronic liver disease the proapoptotic protein expression was significant higher than the antiapoptotic protein expression. The proapoptotic proteins Fas, FasL, Bax were 87.5%, 94.4% and 91.7% respectively. the antiapoptotic proteins Bcl-2, Bcl-XL, Bcl-2α were 56.9%, 61.1% and 38.9% (P < 0.001) respectively in the chronic hepatitis group (Figure 1, Figure 2, Table 1, Table 2, Table 3).
Figure 1.
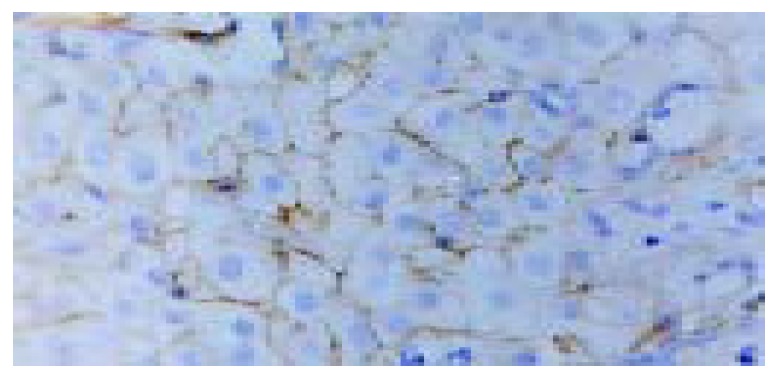
Expression of Fas in hepatocytes of chronic hepatitis (SP, original magnification: × 400).
Figure 2.

Bcl-2 expression on cell membranes and cytoplasma of hepatocytes of chronic hepatitis (SP, original magnification: × 400).
Table 1.
Positive expression of apoptotic proteins in the liver specimens of chronic liver disease (%)
| n | Fas | FasL | Bax | Bcl-2 | Bcl-XL | Bcl-2α | |
| CH | 72 | 63 (87.5) | 68 (94.4) | 66 (91.7) | 41 (56.9) | 44 (61.1) | 28 (38.9) |
| LC | 29 | 24 (82.8) | 27 (93.1) | 25 (86.2) | 20 (69.0) | 21 (72.4) | 13 (44.8) |
CH: Chronic hepatitis group. A: Fas: Bcl-2 χ2 = 16.754, B: Fas: Bcl-XL χ2 = 13.131; C: Fas: Bcl-2α χ2 = 36.575, D: FasL: Bcl-2 χ2 = 27.517; E: FasL: Bcl-XL χ2 = 23.143, F: FasL: Bcl-2α χ2 = 50.00; G: Bax: Bcl-2 χ2 = 22.733, H: Bax: Bcl-XL χ2 = 18.635; I: Bax: Bcl-2α χ2 = 44.242; I: P < 0.001; J: Bcl-2: Bcl-2αχ2 = 8.472, K: Bcl- XL: Bcl-2αχ2 = 7.111; J-K P < 0.01. LC: Liver Cirrhosis group. L: FasL: Bcl-2α χ2 = 15.789; M: Bax: Bcl-2αχ2 = 10.898; I-M: P < 0.001; N: Fas: Bcl-2αχ2 = 9.032, P < 0.01; O: FasL: Bcl-2 χ2 = 5.497; P: FasL: Bcl- XL χ2 = 4.35; O-P: P < 0.05.
Table 2.
Degree of expression of proapoptotic proteins on the liver specimens of chronic liver disease (%)
| n |
Fas |
FasL |
Bax |
|||||||
| (+) | (++) | (+++) | (+) | (++) | (+++) | (+) | (++) | (+++) | ||
| CH | 72 | 32 (44.4) | 29 (40.3) | 2 (2.8) | 29 (40.3) | 34 (47.2) | 5 (6.9) | 26 (36.1) | 24 (33.3) | 16 (22.2) |
| LC | 29 | 13 (44.8) | 9 (31.0) | 2 (6.9) | 8 (27.6) | 15 (51.7) | 4 (13.8) | 11 (37.9) | 14 (48.3) | 0 |
Table 3.
Degree of expression of anti-apoptotic proteins on the liver specimens of chronic liver disease (%)
| n |
Bcl-2 |
Bcl-XL |
Bcl-2α |
|||||||
| (+) | (++) | (+++) | (+) | (++) | (+++) | (+) | (++) | (+++) | ||
| CH | 72 | 21 (29.2) | 13 (18.1) | 7 (2.8) | 27 (37.5) | 10 (13.9) | 7 (9.2) | 25 (34.7) | 3 (4.2) | 0 |
| LC | 29 | 12 (41.4) | 6 (20.7) | 2 (6.9) | 12 (41.4) | 7 (24.1) | 2 (6.9) | 8 (27.6) | 9 (10.4) | 2 (6.9) |
Untramicrostructure findings
The ultramicrostructures of mitochondria in 12 cases of chronic hepatitis (including 10 mild cases, 1 moderate case and 1 severe case) were studied. Among the 364 hepatocytes examined, 40 hepatocytes (11.0%) were found with distinct abnormality in their mitochondria (Table 4). Changes of the mitochondria include disappearance of criastae, rupture of the outer membranes (Figure 3, Figure 4), leakage of mitochondrial matrix,“herniation” of matrix from the ruptured membranes (Figure 5, Figure 6).
Table 4.
Apoptosis and mitochondria of the liver specimens of chronic liver disease
| Code No. |
HAI Score |
Fas | Fasl | Bax | Bcl-2 | Bcl-2α | Bcl-χ l | A | B | A/B % | |||
| G | S | HAI | |||||||||||
| 1 | CH-mild | 1 | 0 | 1 | ++ | ++ | ++ | ++ | + | - | 3 | 20 | 15.0 |
| 2 | CH-mild | 1 | 0 | 4 | + | ++ | +++ | +++ | - | + | 2 | 26 | 7.7 |
| 4 | CH-mild | 1 | 1 | 3 | ++ | + | + | + | - | ++ | 2 | 26 | 7.7 |
| 7 | CH-mild | 1 | 0 | 6 | ++ | +++ | ++ | ++ | - | ++ | 4 | 36 | 11.2 |
| 9 | CH-mild | 1 | 0 | 2 | + | +++ | - | - | - | +++ | 6 | 25 | 24.0 |
| 10 | CH-mild | 1 | 0 | 1 | ++ | + | - | - | - | ++ | 5 | 41 | 12.2 |
| 11 | CH-mild | 2 | 0 | 3 | + | ++ | - | - | + | - | 2 | 37 | 5.4 |
| 13 | CH-mild | 1 | 0 | 2 | - | + | - | - | - | + | 1 | 31 | 3.2 |
| 14 | CH-mild | 1 | 0 | 1 | ++ | - | - | - | - | - | 1 | 15 | 6.7 |
| 15 | CH-mild | 1 | 0 | 3 | ++ | + | - | - | - | + | 4 | 31 | 12.9 |
| 3 | CH-MOD | 2 | 0 | 8 | ++ | + | + | + | + | + | 8 | 40 | 20.0 |
| 5 | CH-SEV | 4 | 15 | 15 | ++ | +++ | - | - | - | - | 2 | 36 | 5.6 |
| 6 | LC | 3 | 12 | 12 | + | + | +++ | +++ | - | ++ | 0 | 15 | |
| 8 | LC | 4 | 15 | 15 | - | - | + | + | - | +++ | 1 | 24 | 4.2 |
| 12 | LC | 4 | 8 | 8 | + | + | - | - | - | ++ | 5 | 34 | 14.7 |
MOD: Moderate; SEV: Severe; G: Grade; S: Stage. A: Number of hepatocytes with abnormal mitochondrial; B: Total number of hepatocytes examined by EM.
Figure 3.
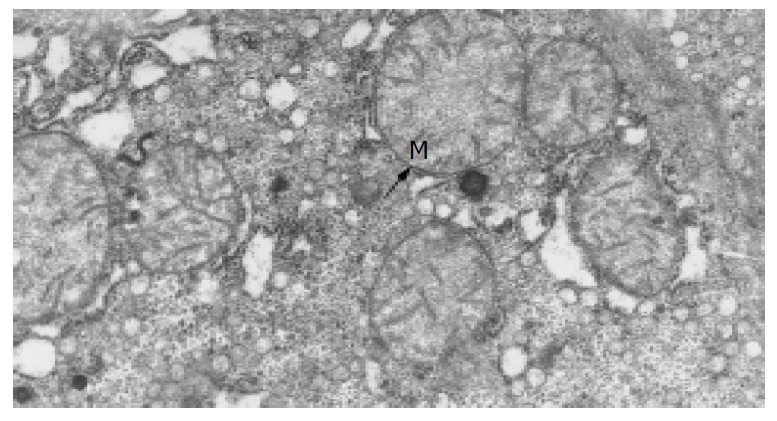
Normal mitochondria in normal adults (Trans-EM, original magnification: × 26 000).
Figure 4.
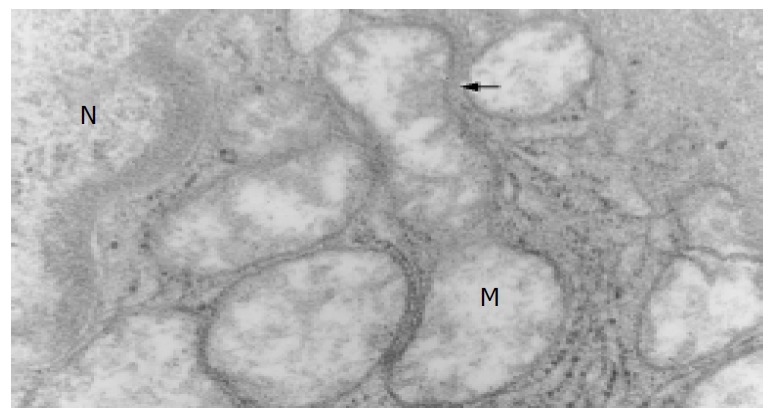
Membrane rupture, disappearance of crista due to apoptosis (Trans-EM, original magnification: × 25 000).
Figure 5.

Herniation of matrix from ruptured membranes of apoptotic mitochondria (Trans-EM, original magnification: × 20 000).
Figure 6.
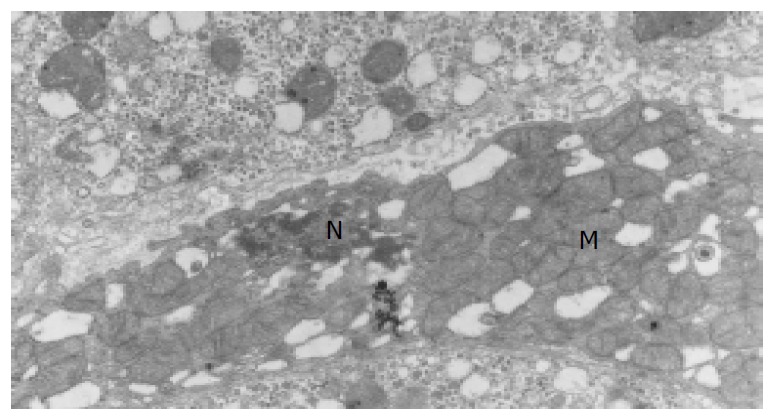
Degeneration and concentration of apoptotic mito-chondria (Trans-EM, original magnification: × 15 000).
DISCUSSION
This study demonstrated that the proapoptotic proteins in chronic hepatitis were significantly higher than the antiapoptotic proteins. This was in strong contrast to many tumor cells whose antiapoptotic proteins were higher than proapoptotic proteins. It was also noted that in the 12 cases of chronic hepatitis patients of more than one year the outer mitochondrial membrane damages or ruptures were also in strong contrast to the normal adults control. Recent investigations inclined to support the view that the apoptosis of hepatocytes may be taken the intrinsic pathway (or mitochondrial pathway)[12-15]. In view of the prominent mitochondrial changes seen in this study the author was highly in favor of the current view that the apoptosis of hepatocytes in chronic hepatitis may begin from the injured DNA which activates the p53 protein to translocate the proapoptotic protein Bax to the mitochondrial and form pores at the outer mitochondrial membrane to release the cytochrome c to the cytosol. This begining lead to the complete death of the hepatocytes[16].
The result was considered consistent with the current view that the apoptosis in chronic hepatitis was a comparatively long persistent phenomenon which took place mainly along the intrinsic pathway (mitochondria pathway), although it was impossible at present to exclude the possibility that the intrinsic and extrinsic could take place in parallel simultaneously. Further study of the apoptotic pathway of chronic hepatitis would be helpful in finding out the effective methods of interrupting the progressive course of the disease.
ACKNOWLEDGEMENTS
We would like to express thanks to Professor Yao- Xuan Huang for directions.
Footnotes
Supported by the National Natural Science Foundation of China, No. 39770660 and the Beijing Military Command Foundation, No. 95B008 and the Social Development Program of Lianyungang City, No. SH 0210
Edited by Wang XL Proofread by Chen WW and Xu FM
References
- 1.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 2.Ravi R, Bedi A. Requirement of BAX for TRAIL/Apo2L-induced apoptosis of colorectal cancers: synergism with sulindac-mediated inhibition of Bcl-x(L) Cancer Res. 2002;62:1583–1587. [PubMed] [Google Scholar]
- 3.Zheng HC, Sun JM, Wei ZL, Yang XF, Zhang YC, Xin Y. Expression of Fas ligand and caspase-3 contributes to formation of immune escape in gastric cancer. World J Gastroenterol. 2003;9:1415–1420. doi: 10.3748/wjg.v9.i7.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenstein S, Ghias K, Krett NL, Rosen ST. Mechanisms of glucocorticoid-mediated apoptosis in hematological malignancies. Clin Cancer Res. 2002;8:1681–1694. [PubMed] [Google Scholar]
- 5.Tsujimoto Y. Bcl-2 family of proteins-Life-or-death. Switch Clin Immunol. 2001;36:266–273. [Google Scholar]
- 6.Adrain C, Martin SJ. The mitochondrial apoptosome: a killer unleashed by the cytochrome seas. Trends Biochem Sci. 2001;26:390–397. doi: 10.1016/s0968-0004(01)01844-8. [DOI] [PubMed] [Google Scholar]
- 7.Capaldi RA. The changing face of mitochondrial research. Trends Biochem Sci. 2000;25:212–214. doi: 10.1016/s0968-0004(00)01584-x. [DOI] [PubMed] [Google Scholar]
- 8.Feldmann G, Haouzi D, Moreau A, Durand-Schneider AM, Bringuier A, Berson A, Mansouri A, Fau D, Pessayre D. Opening of the mitochondrial permeability transition pore causes matrix expansion and outer membrane rupture in Fas-mediated hepatic apoptosis in mice. Hepatology. 2000;31:674–683. doi: 10.1002/hep.510310318. [DOI] [PubMed] [Google Scholar]
- 9.Desmet VJ. Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW, Wollman J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis [Hepatology 1981; 1: 431-435] J Hepatol. 2003;38:382–386. doi: 10.1016/s0168-8278(03)00005-9. [DOI] [PubMed] [Google Scholar]
- 10.Chen NL, Bai L, Deng T, Zhang C, Chen H. Study on pathway of apoptosis in chronic liver disease. Zhonghua Chuanranbing Zazhi. 2003;21:122–124. [Google Scholar]
- 11.Chen N, Deng T, Chen P, Li L. [The regulation of apoptosis by Bcl-2, bcl-X(L), Bcl-2alpha and Bax in chronic liver disease] Zhonghua Neike Zazhi. 2000;39:808–810. [PubMed] [Google Scholar]
- 12.Jaeschke H, Bajt ML. Regulation of apoptotic signaling pathways in hepatocytes in vivo. Hepatology. 2003;37:942–945. doi: 10.1002/hep.510370432. [DOI] [PubMed] [Google Scholar]
- 13.Fang DC. Genetic instability in the development of gastric cancer. Shijie Huaren Xiaohua Zazhi. 2003;11:1–5. [Google Scholar]
- 14.Newmeyer DD, Ferguson-Miller S. Mitochondria: releasing power for life and unleashing the machineries of death. Cell. 2003;112:481–490. doi: 10.1016/s0092-8674(03)00116-8. [DOI] [PubMed] [Google Scholar]
- 15.Green DR. Overview: apoptotic signaling pathways in the immune system. Immunol Rev. 2003;193:5–9. doi: 10.1034/j.1600-065x.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- 16.Rathmell JC, Thompson CB. Pathways of apoptosis in lymphocyte development, homeostasis, and disease. Cell. 2002;109 Suppl:S97–107. doi: 10.1016/s0092-8674(02)00704-3. [DOI] [PubMed] [Google Scholar]


