Abstract
AIM: To investigate the expression of PTEN/MMAC1/TEP1 and vascular endothelial growth factor (VEGF), their roles in biologic behavior and angiogenesis and their association in gastric cancer.
METHODS: Immunohistochemical staining was used to evaluate the expression of PTEN, VEGF and microvascular density (MVD) on paraffin-embedded sections in 70 patients with primary gastric cancer and 24 patients with chronic superficial gastritis (CSG). Expression of PTEN, VEGF and MVD were compared with clinicopathological features of gastric cancer. The relationship between expression of PTEN, VEGF and MVD as well as the relationship between PTEN and VEGF expression in caner cells were investigated.
RESULTS: PTEN expression significantly decreased (t = 3.98, P < 0.01) whereas both VEGF expression and MVD significant increased (t = 4.29 and 4.41, respectively, both P < 0.01) in gastric cancer group compared with CSG group. PTEN expression was significantly down-regulated (t = 1.95, P < 0.05) whereas VEGF expression (t = 2.37, P < 0.05) and MVD (t = 3.28, P < 0.01) was significantly up-regulated in advanced gastric cancer compared with early-stage gastric cancer. PTEN expression in gastric cancer showed a negative association with lymph node metastasis (t = 3.91, P < 0.01), invasion depth (t = 1.95, P < 0.05) and age (t = 4.69, P < 0.01). MVD in PTEN-negative gastric cancer was significantly higher than that in PTEN-positive gastric cancer (t = 3.69, P < 0.01), and there was a negative correlation between PTEN expression and MVD (γ = -0.363, P < 0.05). VEGF expression was positively associated with invasion depth (especially with serosa invasion, t = 4.69, P < 0.01), lymph node metastasis (t = 2.31, P < 0.05) and TNM stage (t = 3.04, P < 0.01). MVD in VEGF-positive gastric cancer was significantly higher than that in VEGF-negative gastric cancer (t = 4.62, P < 0.01), and there was a positive correlation between VEGF expression of and MVD (γ = 0.512, P < 0.05). VEGF expression in PTEN-negative gastric cancer was significantly stronger than that in PTEN-positive gastric cancer (t = 2.61, P < 0.05), and there was a significantly negative correlation between the expression of VEGF and PTEN (γ = -0.403, P < 0.05).
CONCLUSION: Our results imply that inactivation of PTEN gene and over-expression of VEGF contribute to the neovascularization and progression of gastric cancer. PTEN-related angiogenesis might be attributed to its up-regulation of VEGF expression. PTEN and VEGF could be used as the markers reflecting the biologic behaviors of tumor and viable targets in therapeutic approaches to inhibit angiogenesis of gastric cancers.
INTRODUCTION
Gastric cancer is one of the tumors with a relatively high incidence and mortality in the digestive system. Although many advanced measures have been taken to improve the outcome of patients suffering from gastric cancer, up to now, there has been no radical progress in reducing its incidence and mortality. This could be due to the fact that the underlying mechanism in tumorigenesis and progression of gastric cancer is still poorly understood. Many studies have demonstrated that tumor suppressor genes, such as p53, play an important role in oncogenesis and progression of various malignancies. Recently, many investigators have been interested in the role of PTEN/MMAC1/TEP1, a novel tumor suppressor gene, located on chromosome band 10q 23.3. Accumulated evidence has suggested that inactivation of PTEN/MMAC1/TEP1 gene was implicated in the carcinogenesis and progression of various tumors[1-10]. Loss of PTEN expression was dominantly attributed to the inactive alteration of PTEN gene, including mutations, deletions, loss of heterozygosity (LOH) and promoter methylation in various malignancies[11,12], meaning that the expressive intensity of PTEN could almost embody the status of PTEN gene.
Several studies have strongly implied that PTEN was associated with tumor-induced angiogenesis[13-16]. However, less information regarding the role of PTEN in gastric cancer, to our knowledge, was available. The present study was designed to investigate the role of PTEN in tumorigenesis and progression of gastric cancer and its association with angiogenesis and VEGF expression.
MATERIALS AND METHODS
Subjects
Surgical specimens of 70 patients with histologically confirmed primary gastric adenocarcinoma and 24 patients with chronic superficial gastritis and duodenal ulcer were obtained from Department of General Surgery, Affiliated Hospital, Luzhou Medical College and Department of General Surgery, West China Hospital, Sichuan University in 1996-2000. There were 56 male and 14 female patients in gastric cancer group with age range from 31 to 66 (mean 45) years while there were 21 male and three female patients in chronic superficial gastritis group with age range from 28 to 43 (mean 35) years. All patients with gastric cancer had received radical resection or palliative surgical treatments, but no one had ever accepted chemotherapy and radiotherapy as well as biotherapy before operation.
Evaluation of clinicopathological features
In gastric cancer group, 18 tumors were categorized histologically as well-differentiation and 52 poor-differentiations. Eight tumors were categorized as early-stage gastric cancer and 62 advanced gastric cancer. Thrifty two patients had serosa invasion, and 46 had lymph node metastasis. According to the criteria set out by the Union of International Caner Commission (new TNM stage, UICC for gastric cancer, 1985), 20 tumors were categorized as stage I, 16 stage II , 30 stage III and four stage IV.
Antibodies and reagents
Rabbit anti-PTEN polyclonal antibody, rabbit ant-VEGF polyclonal antibody, mouse anti-CD34 monoclonal antibody and streptavidin-biotin-peroxidase (KIT-9710, UltraSensitive S-P for mouse or rabbit) as well as DAB reagents were all purchased from Maixin Corporation (Fuzhou, Fujian province, China).
Immunohistochemical staining
Five-micron paraffin-embedded sections were dewaxed in xylene, dehydrated in ethanol. Endogenous peroxidase activity was blocked by incubation of samples in a 3% solution of hydrogen peroxide in methanol and heated in pressure-cooker for 50 s to retrieve antigens. After washing with PBS, the samples were incubated for 60 min with primary antibody against CD34, PTEN or VEGF at 37 °C, for 60 min with the biotin-conjugated second antibody and for 10 min with the third antibody streptavidin-peroxidase at room temperature, and then the immunoreactive products were stained with DAB and counter-stained with methyl green subsequently. PBS was used instead of the primary antibodies for negative controls.
Evaluation of PTEN and VEGF immunostaining
PTEN and VEGF protein expression in benign and malignant gastric epithelium cells was assessed according to the score graded as the percentage of positive immunoreactive cells and the score graded as positive immunoreactive intensity, and the sum of both was used to reflect the level of PTEN and VEGF protein expression. The score graded as percentage of positive immunoreactive cells was defined as follows: < 10% as 0, 10%-25% as 1, 25%-50% as 2, and ≥ 50% as 3. The score graded as positive immunoreactive intensity was defined as follows: Negative stain (equal to background) as 0, weak positive stain (weak yellow) as 1, positive stain (yellow) as 2, and strong positive stain (brown) as 3. The sum of scores less than or equal to 2 (≤ 2) was defined as negative PTEN (PTEN-) or VEGF (VEGF-) protein expression, and more than or equal to 3 (≥ 3) as positive PTEN (PTEN+) or VEGF (VEGF+) expression.
Microvascular density counting
Microvascular density (MVD) was determined according to the criterion introduced by Weidner[17]: Any separated single vascular endothelium cell or cluster of endothelium cells and microvascular tube with diameter less than 8 erythrocytes were counted. Briefly, the stained sections were screened at × 100 magnifications under a light microscope (Olympus) to identify four regions with the highest number of microvessels, which were then counted at × 200 magnifications, and the average was used to reflect MVD.
Statistical analysis
All results were expressed as mean ± SD. Statistic software SPSS 11.5 for Windows was used to analyze the results using Student t test and Pearson correlation analysis. The accepted level of significance was P < 0.05 (Two-tailed).
RESULTS
Characteristics and comparison of PTEN and VEGF expression and MVD in gastric cancer with those in chronic superficial gastritis
PTEN and VEGF expression were demonstrated to localize in cytoplasm of gastric glandular epithelium and tumor cells (Figure 1, Figure 2). In gastric cancer, the positive immunoreactive signal in invasive front region was weaker for PTEN, but stronger for VEGF and microvessels (Figure 3) than that in the fundic region of tumor. There was a significant down-regulation of PTEN expression and a significant up-regulation of both VEGF and MVD in gastric cancer in comparison with those in chronic superficial gastritis (Table 1).
Figure 1.
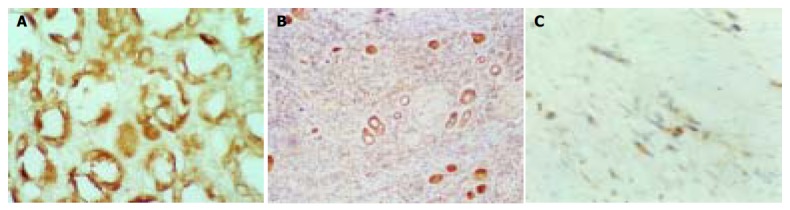
Expression of PTEN. A: Strongly positive expression in bening gastric glandular epithelium cells (Immunohistochemical staining, S-P × 400). B: Positive expression in moderately differentiated cancer cells (Immunohistochemical staining, S-P × 100). C: Weakly positive expression in poorly differentiated cancer cells. (Immunohistochemical staining, S-P × 400).
Figure 2.
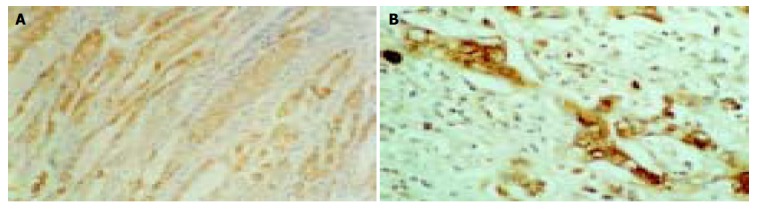
Expression of VEGF. A: Weakly positive expression in benign gastric glandular epithelium cells. B: Strongly positive expression in poorly differentiated cancer cells. (Immunohistochemical staining, S-P × 400).
Figure 3.
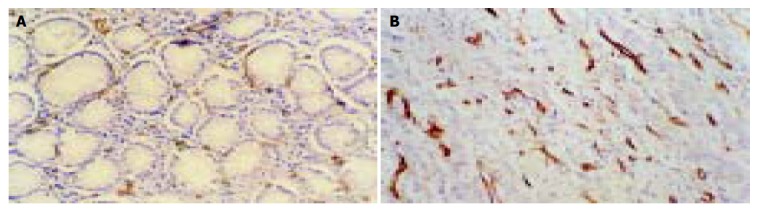
MVD in benign/malignant gastric tissues. A: Lessand weakly stained microvessel labeled by CD34 was regularly distributed among the benign gastric epithelium gland (Immunohistochemical staining, S-P × 400). B: Increased and strongly stained microvessel labeled by CD34 was irregularly infiltrated in poorly differentiated adenocarcinoma (Immunohistochemical staining, S-P × 200).
Table 1.
Comparison in PTEN and VEGF expression and MVD between chronic superficial gastritis (CSG) group and gastric cancer (GC) group (mean ± SD)
| Groups | n | PTEN | VEGF | MVD |
| CSG | 24 | 3.13 ± 2.3 | 1.25 ± 1.16 | 29.6 ± 9.9 |
| GC | 70 | 1.34 ± 1.75b | 2.94 ± 1.80b | 48.3 ± 19.9b |
P < 0.01 vs CSG group.
Association of PTEN and VEGF expression and MVD with clinicopathological profiles in gastric cancer
As showed in Table 2, The down-regulation of PTEN expression was closely associated with the older patients (> 35 years), invasion depth and lymphatic metastasis of tumor, and a downtrend of PTEN expression was observed with the increase of invasion depth of tumor, especially in advanced stage of gastric cancer (≥ T2) (Table 2). The up-regulation of both VEGF expression and MVD were significantly associated with the invasion depth, lymphatic metastasis and TNM stage, but none of them was associated with the histological differentiation of gastric cancer (Table 2).
Table 2.
Association of PTEN and VEGF expression and MVD with clinicopathologic profiles in gastric cancer (mean ± SD)
| Variables | n | PTEN | VEGF | MVD |
| Age (yr) | ||||
| ≤ 35 | 22 | 2.64 ± 1.81 | 2.77 ± 1.96 | 46.23 ± 17.61 |
| >35 | 48 | 0.83 ± 1.33b | 3.02 ± 1.76 | 49.20 ± 20.05 |
| Gender | ||||
| Male | 56 | 1.32 ± 1.69 | 3.00 ± 1.91 | 48.45 ± 20.39 |
| Female | 14 | 1.71 ± 1.82 | 2.71 ± 1.73 | 47.54 ± 12.72 |
| Differentiation | ||||
| Well | 18 | 1.56 ± 1.82 | 3.33 ± 1.88 | 45.94 ± 17.08 |
| Poor | 52 | 1.35 ± 1.69 | 2.81 ± 1.86 | 49.07 ± 20.72 |
| Invasion depth | ||||
| T1 | 8 | 2.50 ± 1.73 | 1.5 ± 1.91 | 27.94 ± 12.1 |
| ≥ T2 | 62 | 1.26 ± 1.69a | 3.13 ± 1.82a | 50.9 ± 19.31b |
| < T3 | 38 | 1.47 ± 1.81 | 2.08 ± 1.20 | 39.6 ± 16.6 |
| ≥ T3 | 32 | 1.31 ± 1.66 | 3.97 ± 1.15a | 58.6 ± 18.9b |
| LN metastasis | ||||
| Positive | 46 | 0.87 ± 1.36 | 3.57 ± 1.77 | 53.9 ± 18.2 |
| Negative | 24 | 2.42 ± 1.93b | 1.74 ± 2.07a | 37.5 ± 19.3b |
| TNM Stage | ||||
| I + II | 36 | 1.83 ± 1.81 | 2.51 ± 1.29 | 39.6 ± 18.3 |
| III + IV | 34 | 0.97 ± 1.55 | 3.41 ± 1.18b | 57.5 ± 17.7b |
P < 0.05,
P < 0.01 vs different variables.
Correlation of PTEN and VEGF expression with MVD in gastric cancer
MVD was significantly higher in PTEN negative (n = 54) than in PTEN positive gastric cancer (n = 16) (52.7 ± 19.6 vs 33.4 ± 13.2, t = 3.69, P < 0.01), and significantly higher in VEGF positive (n = 52) than in VEGF negative gastric cancer (n = 18) (52.71 ± 19.59 vs 29.78 ± 12.9, t = 4.62, P < 0.001). There was a significantly negative correlation between MVD and the intensity of PTEN expression (γ = -0.363, P < 0.05) and a positive correlation between MVD and the intensity of VEGF expression (γ = 0.512, P < 0.01).
Correlation between VEGF and PTEN expression
The intensity of VEGF expression was significantly higher in PTEN negative than in PTEN positive gastric cancer (3.18 ± 1.53 vs 2.15 ± 2.19, t = 2.61, P < 0.05). Furthermore, There was a significantly negative correlation between VEGF and PTEN expression (γ = -0.403, P < 0.05).
DISCUSSION
PTEN/MMAC1/TEP1-encoding product, a dual-specificity protein-phospholipid phosphatase, which was involved in regulation of a variety of signal transduction pathways through dephosphorylation, including down-regulation of the activity of the focal adhesion kinase (FAK) to inhibit cell adhesion, invasion and metastasis[18-20], disabling of phosphatidylinositol 3-kinase (PI3’k)/Akt signal pathway to accelerate cell apoptosis and inhibit cell proliferation and inhibition of MAPK signal pathway to restrain cell differentiation[21-23]. Some studies[18-20,24-27] have demonstrated that anti-sense or deletion of PTEN gene significantly up-regulates the ability of cell proliferation, adhesion and migration, accompanied by increased activity of FAK and PI3’k/Akt, whereas over-expression of PTEN protein in wild-type PTEN transfected cells is detected, with a resultant cell cycle arrest, increased cell apoptosis, decreased potential in cell mitosis, proliferation, adhesion and migration, and the concomitant decrease of FAK and PI3’k/Akt activities, suggesting that PTEN regulates negatively the growth, invasion and metastasis of tumors, and then the inactivation of PTEN greatly contributes to the tumorigenesis and progression of tumors.
So far, little information on the expression and role of PTEN gene in gastric cancer is available. Recently, Byun et al[28] showed an abnormally low expression with only in 36% (20/55) gastric cancer tissues and 33% (5/15) cell lines while none of 71 cases with non-cancer tissues showed a decreased expression. The LOH of PTEN gene reached at 47% (14/30) and was closely linked to low expression of PTEN mRNA in gastric cancer. Furthermore, the rate of LOH was significantly higher in advanced gastric cancers (63%) than that in early-stage tumors (18%), and in poorly differentiated tumors (69%) than in well-or moderately differentiated tumors (29%). Fei et al[29] compared PTEN expression in 26 gastric cancer, 21 first-degree relatives of gastric cancer patients and 12 healthy individuals by RT-PCR and immunohistochemistry, and found that PTEN expression was significantly decreased in gastric cancer group and the first-degree relatives group compared with those in matched non-malignant gastric tissues and healthy control group. Kang et al[30] screened 310 cases with gastric carcinoma, and found that 62 cases lost PTEN expression, and the loss of PTEN expression was linked to the promoter methylation of PTEN gene, which was significantly associated with tumor depth and size, lymphatic invasion, advanced stage, pTNM stage, and patients’ survival, implying that loss of PTEN expression is involved in the pathogenesis of gastric cancer. Nevertheless, there are some discrepant and conflict findings. One study from Japan did not find any mutation and promoter methylation of PTEN gene as well as the alteration of mRNA in gastric cancer cell lines and primary tumor tissues[31]. Another study showed that the mutation rate of PTEN gene was not significantly increased in human advanced gastric cancer[32], suggesting that PTEN does not participate in gastric carcinogenesis and progression as a tumor suppressor gene. In current study, PTEN expression was only slightly decreased in early gastric cancer cells but significantly decreased in advanced gastric cancer cells, compared with that in benign gastric epithelial cells. PTEN expression decreased as the invasion density increased, and lower PTEN expression was observed in gastric cancer with lymph node invasion and in TNM III/IV stage. Our results were very similar to those reported by Kang et al[30], suggesting that loss of PTEN expression is a relatively later molecule event in the pathogenesis of gastric cancer, and thus plays more important role in the progression than in oncogenesis of gastric cancer. The malignant gastric epithelial cells with loss of PTEN expression may hold the characteristics with a high aggressive and metastatic potential, and thus PTEN can be considered as an objective and reliable marker reflecting the pathobiological behaviors of gastric cancer. Moreover, Lee et al[33] showed that loss of PTEN expression was significantly associated with poor gastric carcinoma prognosis. In addition, we also observed a significantly decreased expression of PTEN protein in the older patients (> 35 years) with gastric cancer compared with the younger patients, implying that PTEN may be more implicated in the gastric carcinogenesis in the elder. In other words, there might hold dissimilar tumorigenetic mechanisms between the older and younger patients with gastric cancer.
Angiogenesis is prerequisite for progressing tumor growth, invasion and metastasis. MVD in tumor tissue is unanimously considered as a better parameter to reflect the level of the neovascularization of tumor. VEGF, one of the most powerful pro-angiogenic factors, is dominantly involved in all the process including vascular endothelial cell mitosis, proliferation, adhesion and migration. VEGF expression is induced in a hypoxia-inducible factor alpha (HIF-1alpha)-dependent way through activation of the PI3 kinase signaling pathway[34]. VEGF and MVD are all involved in prognosis in various carcinomas. Our results revealed a significantly increase in MVD and VEGF expression in gastric cancer cells, and both the VEGF expression and MVD were significantly associated with the invasion depth, lymphatic metastasis and pTNM stage. Furthermore, MVD was significantly correlated with the intensity of VEGF expression. These results suggest VEGF is dominantly involved in the neovascularization of gastric cancer, and thus facilitates the tumor’s growth, invasion and metastasis.
Several studies[13-16] have suggested that PTEN is implicated in the regulation of tumor’s angiogenesis, and loss of PTEN expression is closely associated with the increased neovascularization in various malignancies in vitro and in vivo. However, information on the relationship between PTEN protein expression and neovascularization in gastric cancer is scarce. Zheng et al[35-38] recently showed MVD was negatively related to PTEN expression in gastric cancer. In the present study, we also observed that MVD in PTEN negative gastric cancer was markedly higher than that in PTEN positive gastric cancer, and there was a significantly negative correlation between MVD and PTEN expression in gastric cancer tissue, implying that loss of PTEN expression is highly implicated in the neovascularization of tumor in gastric cancer.
The mechanism of PTEN-related angiogenesis is not well known. Recent in vitro studies have suggested that loss of PTEN expression significantly up-regulates VEGF expression via modulation of HIF-1alpha expression and VEGF-mediated pro-angiogenic signaling through PI3’K/Akt-dependent signaling transduction pathway to enhance the anti-apoptotic, proliferative, and chemotactic activity of endothelial cells and the ability of tube formation. Jiang et al[39-41] screened the expression of PTEN and HIF-1alpha mRNA and VEGF protein in human colorectal tumor tissues, and observed a negative correlation of PTEN expression with HIF-1alpha (γ = -0.36, P < 0.05) and VEGF (γ = -0.48, P < 0.05) and a positive correlation between VEGF and HIF-1alpha (γ = 0.71, P < 0.01). In this study, we also found a significantly increase in VEGF expression in PTEN negative gastric cancer compared with PTEN positive gastric cancer, and that the intensity of VEGF expression was negatively associated with PTEN expression. Taking all these findings together, we postulate that VEGF is a key downstream molecule for PTEN function in carcinogenesis and progression in a wide range of human carcinomas including gastric cancer. Therefore, inactivation of PTEN gene could contribute to gastric tumor progression by directly functioning on tumor cells to enhance the ability of growth, anti-apoptotic, invasion and metastasis through up-regulation of PI3’K/Akt signaling transduction pathway, by up-regulating VEGF expression in tumor cells, which enhances the activity of tumor-derived angiogenesis and functions on vascular endothelial cells to increase angiogenesis in tumor tissues. Based on these data, it is concluded that PTEN and VEGF are reliable targets in the therapeutic approach for the inhibition of angiogenesis in gastric cancer.
Footnotes
Edited by Xia HHX Proofread by Xu FM
References
- 1.Sato N, Tsunoda H, Nishida M, Morishita Y, Takimoto Y, Kubo T, Noguchi M. Loss of heterozygosity on 10q23.3 and mutation of the tumor suppressor gene PTEN in benign endometrial cyst of the ovary: possible sequence progression from benign endometrial cyst to endometrioid carcinoma and clear cell carcinoma of the ovary. Cancer Res. 2000;60:7052–7056. [PubMed] [Google Scholar]
- 2.Kondo K, Yao M, Kobayashi K, Ota S, Yoshida M, Kaneko S, Baba M, Sakai N, Kishida T, Kawakami S, et al. PTEN/MMAC1/TEP1 mutations in human primary renal-cell carcinomas and renal carcinoma cell lines. Int J Cancer. 2001;91:219–224. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1034>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 3.Mutter GL, Lin MC, Fitzgerald JT, Kum JB, Baak JP, Lees JA, Weng LP, Eng C. Altered PTEN expression as a diagnostic marker for the earliest endometrial precancers. J Natl Cancer Inst. 2000;92:924–930. doi: 10.1093/jnci/92.11.924. [DOI] [PubMed] [Google Scholar]
- 4.Davies MP, Gibbs FE, Halliwell N, Joyce KA, Roebuck MM, Rossi ML, Salisbury J, Sibson DR, Tacconi L, Walker C. Mutation in the PTEN/MMAC1 gene in archival low grade and high grade gliomas. Br J Cancer. 1999;79:1542–1548. doi: 10.1038/sj.bjc.6690246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubin MA, Gerstein A, Reid K, Bostwick DG, Cheng L, Parsons R, Papadopoulos N. 10q23.3 loss of heterozygosity is higher in lymph node-positive (pT2-3,N+) versus lymph node-negative (pT2-3,N0) prostate cancer. Hum Pathol. 2000;31:504–508. doi: 10.1053/hp.2000.6713. [DOI] [PubMed] [Google Scholar]
- 6.Guanti G, Resta N, Simone C, Cariola F, Demma I, Fiorente P, Gentile M. Involvement of PTEN mutations in the genetic pathways of colorectal cancerogenesis. Hum Mol Genet. 2000;9:283–287. doi: 10.1093/hmg/9.2.283. [DOI] [PubMed] [Google Scholar]
- 7.Kimura F, Watanabe J, Hata H, Fujisawa T, Kamata Y, Nishimura Y, Jobo T, Kuramoto H. PTEN immunohistochemical expression is suppressed in G1 endometrioid adenocarcinoma of the uterine corpus. J Cancer Res Clin Oncol. 2004;130:161–168. doi: 10.1007/s00432-003-0517-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung MJ, Jung SH, Lee BJ, Kang MJ, Lee DG. Inactivation of the PTEN gene protein product is associated with the invasiveness and metastasis, but not angiogenesis, of breast cancer. Pathol Int. 2004;54:10–15. doi: 10.1111/j.1440-1827.2004.01576.x. [DOI] [PubMed] [Google Scholar]
- 9.Kato H, Fujimura M, Kumabe T, Ishioka C, Kanamaru R, Yoshimoto T. PTEN gene mutation and high MIB-1 labeling index may contribute to dissemination in patients with glioblastoma. J Clin Neurosci. 2004;11:37–41. doi: 10.1016/j.jocn.2002.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Dicuonzo G, Angeletti S, Garcia-Foncillas J, Brugarolas A, Okrouzhnov Y, Santini D, Tonini G, Lorino G, De Cesaris M, Baldi A. Colorectal carcinomas and PTEN/MMAC1 gene mutations. Clin Cancer Res. 2001;7:4049–4053. [PubMed] [Google Scholar]
- 11.Sano T, Lin H, Chen X, Langford LA, Koul D, Bondy ML, Hess KR, Myers JN, Hong YK, Yung WK, et al. Differential expression of MMAC/PTEN in glioblastoma multiforme: relationship to localization and prognosis. Cancer Res. 1999;59:1820–1824. [PubMed] [Google Scholar]
- 12.Idoate MA, Soria E, Lozano MD, Sola JJ, Panizo A, de Alava E, Manrique M, Pardo-Mindán FJ. PTEN protein expression correlates with PTEN gene molecular changes but not with VEGF expression in astrocytomas. Diagn Mol Pathol. 2003;12:160–165. doi: 10.1097/00019606-200309000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Hsu SC, Volpert OV, Steck PA, Mikkelsen T, Polverini PJ, Rao S, Chou P, Bouck NP. Inhibition of angiogenesis in human glioblastomas by chromosome 10 induction of thrombospondin-1. Cancer Res. 1996;56:5684–5691. [PubMed] [Google Scholar]
- 14.Giri D, Ittmann M. Inactivation of the PTEN tumor suppressor gene is associated with increased angiogenesis in clinically localized prostate carcinoma. Hum Pathol. 1999;30:419–424. doi: 10.1016/s0046-8177(99)90117-x. [DOI] [PubMed] [Google Scholar]
- 15.Jiang BH, Zheng JZ, Aoki M, Vogt PK. Phosphatidylinositol 3-kinase signaling mediates angiogenesis and expression of vascular endothelial growth factor in endothelial cells. Proc Natl Acad Sci U S A. 2000;97:1749–1753. doi: 10.1073/pnas.040560897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wen S, Stolarov J, Myers MP, Su JD, Wigler MH, Tonks NK, Durden DL. PTEN controls tumor-induced angiogenesis. Proc Natl Acad Sci U S A. 2001;98:4622–4627. doi: 10.1073/pnas.081063798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weidner N, Folkman J, Pozza F, Bevilacqua P, Allred EN, Moore DH, Meli S, Gasparini G. Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma. J Natl Cancer Inst. 1992;84:1875–1887. doi: 10.1093/jnci/84.24.1875. [DOI] [PubMed] [Google Scholar]
- 18.Tamura M, Gu J, Matsumoto K, Aota S, Parsons R, Yamada KM. Inhibition of cell migration, spreading, and focal adhesions by tumor suppressor PTEN. Science. 1998;280:1614–1617. doi: 10.1126/science.280.5369.1614. [DOI] [PubMed] [Google Scholar]
- 19.Cai T, Lei QY, Wang LY, Zha XL. TGF-beta 1 modulated the expression of alpha 5 beta 1 integrin and integrin-mediated signaling in human hepatocarcinoma cells. Biochem Biophys Res Commun. 2000;274:519–525. doi: 10.1006/bbrc.2000.3177. [DOI] [PubMed] [Google Scholar]
- 20.Zhang LN, Yu Q, Wang LY, Jin JW, Zha XL. [The effects of PTEN gene on migration and FAK phosphorylation of SMMC-7721 human hepatocarcinoma cell line] Shengwu Huaxue Yu Shengwuwuli Xubao (Shanghai) 2003;35:161–166. [PubMed] [Google Scholar]
- 21.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 22.Besson A, Robbins SM, Yong VW. PTEN/MMAC1/TEP1 in signal transduction and tumorigenesis. Eur J Biochem. 1999;263:605–611. doi: 10.1046/j.1432-1327.1999.00542.x. [DOI] [PubMed] [Google Scholar]
- 23.Waite KA, Eng C. Protean PTEN: form and function. Am J Hum Genet. 2002;70:829–844. doi: 10.1086/340026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Persad S, Attwell S, Gray V, Delcommenne M, Troussard A, Sanghera J, Dedhar S. Inhibition of integrin-linked kinase (ILK) suppresses activation of protein kinase B/Akt and induces cell cycle arrest and apoptosis of PTEN-mutant prostate cancer cells. Proc Natl Acad Sci U S A. 2000;97:3207–3212. doi: 10.1073/pnas.060579697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakurada A, Hamada H, Fukushige S, Yokoyama T, Yoshinaga K, Furukawa T, Sato S, Yajima A, Sato M, Fujimura S, et al. Adenovirus-mediated delivery of the PTEN gene inhibits cell growth by induction of apoptosis in endometrial cancer. Int J Oncol. 1999;15:1069–1074. doi: 10.3892/ijo.15.6.1069. [DOI] [PubMed] [Google Scholar]
- 26.Ghosh AK, Grigorieva I, Steele R, Hoover RG, Ray RB. PTEN transcriptionally modulates c-myc gene expression in human breast carcinoma cells and is involved in cell growth regulation. Gene. 1999;235:85–91. doi: 10.1016/s0378-1119(99)00206-1. [DOI] [PubMed] [Google Scholar]
- 27.Saito Y, Swanson X, Mhashilkar AM, Oida Y, Schrock R, Branch CD, Chada S, Zumstein L, Ramesh R. Adenovirus-mediated transfer of the PTEN gene inhibits human colorectal cancer growth in vitro and in vivo. Gene Ther. 2003;10:1961–1969. doi: 10.1038/sj.gt.3302100. [DOI] [PubMed] [Google Scholar]
- 28.Byun DS, Cho K, Ryu BK, Lee MG, Park JI, Chae KS, Kim HJ, Chi SG. Frequent monoallelic deletion of PTEN and its reciprocal associatioin with PIK3CA amplification in gastric carcinoma. Int J Cancer. 2003;104:318–327. doi: 10.1002/ijc.10962. [DOI] [PubMed] [Google Scholar]
- 29.Fei G, Ebert MP, Mawrin C, Leodolter A, Schmidt N, Dietzmann K, Malfertheiner P. Reduced PTEN expression in gastric cancer and in the gastric mucosa of gastric cancer relatives. Eur J Gastroenterol Hepatol. 2002;14:297–303. doi: 10.1097/00042737-200203000-00015. [DOI] [PubMed] [Google Scholar]
- 30.Kang YH, Lee HS, Kim WH. Promoter methylation and silencing of PTEN in gastric carcinoma. Lab Invest. 2002;82:285–291. doi: 10.1038/labinvest.3780422. [DOI] [PubMed] [Google Scholar]
- 31.Sato K, Tamura G, Tsuchiya T, Endoh Y, Sakata K, Motoyama T, Usuba O, Kimura W, Terashima M, Nishizuka S, et al. Analysis of genetic and epigenetic alterations of the PTEN gene in gastric cancer. Virchows Arch. 2002;440:160–165. doi: 10.1007/s004280100499. [DOI] [PubMed] [Google Scholar]
- 32.Wang JY, Huang TJ, Chen FM, Hsieh MC, Lin SR, Hou MF, Hsieh JS. Mutation analysis of the putative tumor suppressor gene PTEN/MMAC1 in advanced gastric carcinomas. Virchows Arch. 2003;442:437–443. doi: 10.1007/s00428-003-0803-5. [DOI] [PubMed] [Google Scholar]
- 33.Lee HS, Lee HK, Kim HS, Yang HK, Kim WH. Tumour suppressor gene expression correlates with gastric cancer prognosis. J Pathol. 2003;200:39–46. doi: 10.1002/path.1288. [DOI] [PubMed] [Google Scholar]
- 34.Sodhi A, Montaner S, Miyazaki H, Gutkind JS. MAPK and Akt act cooperatively but independently on hypoxia inducible factor-1alpha in rasV12 upregulation of VEGF. Biochem Biophys Res Commun. 2001;287:292–300. doi: 10.1006/bbrc.2001.5532. [DOI] [PubMed] [Google Scholar]
- 35.Zheng S, Han MY, Xiao ZX, Peng JP, Dong Q. Clinical significance of vascular endothelial growth factor expression and neovascularization in colorectal carcinoma. World J Gastroenterol. 2003;9:1227–1230. doi: 10.3748/wjg.v9.i6.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Korkolopoulou P, Konstantinidou AE, Kavantzas N, Patsouris E, Pavlopoulos PM, Christodoulou P, Thomas-Tsagli E, Davaris P. Morphometric microvascular characteristics predict prognosis in superficial and invasive bladder cancer. Virchows Arch. 2001;438:603–611. doi: 10.1007/s004280100400. [DOI] [PubMed] [Google Scholar]
- 37.Terlikowski S, Lenczewski A, Sulkowska M, Famulski W, Sulkowski S, Kulikowski M. Tissue expression of VEGF as a prognostic factor in early cervical squamous cell carcinoma. Folia Histochem Cytobiol. 2001;39 Suppl 2:112–113. [PubMed] [Google Scholar]
- 38.Cianchi F, Palomba A, Messerini L, Boddi V, Asirelli G, Perigli G, Bechi P, Taddei A, Pucciani F, Cortesini C. Tumor angiogenesis in lymph node-negative rectal cancer: correlation with clinicopathological parameters and prognosis. Ann Surg Oncol. 2002;9:20–26. doi: 10.1245/aso.2002.9.1.20. [DOI] [PubMed] [Google Scholar]
- 39.Gomez-Manzano C, Fueyo J, Jiang H, Glass TL, Lee HY, Hu M, Liu JL, Jasti SL, Liu TJ, Conrad CA, et al. Mechanisms underlying PTEN regulation of vascular endothelial growth factor and angiogenesis. Ann Neurol. 2003;53:109–117. doi: 10.1002/ana.10396. [DOI] [PubMed] [Google Scholar]
- 40.Koul D, Shen R, Garyali A, Ke LD, Liu TJ, Yung WK. MMAC/PTEN tumor suppressor gene regulates vascular endothelial growth factor-mediated angiogenesis in prostate cancer. Int J Oncol. 2002;21:469–475. [PubMed] [Google Scholar]
- 41.Huang J, Kontos CD. PTEN modulates vascular endothelial growth factor-mediated signaling and angiogenic effects. J Biol Chem. 2002;277:10760–10766. doi: 10.1074/jbc.M110219200. [DOI] [PubMed] [Google Scholar]


