Abstract
AIM: To investigate the effect of arsenic trioxide (As2O3) on expression of vascular endothelial growth factor receptor-1 (VEGFR-1, Flt-1) and VEGFR-2 (KDR) in human gastric tumor cells and proliferation of vascular endothelial cells.
METHODS: The solid tumor model was formed in nude mice with the gastric cancer cell line SGC-7901. The animals were treated with As2O3. Microvessel density (MVD) and expression of Flt-1 and KDR were detected by immunofluorescence laser confocal microscopy. SGC-7901 cells were treated respectively by exogenous recombinant human VEGF165 or VEGF165 + As2O3. Cell viability was measured by MTT assay. Cell viability of ECV304 cells was measured by MTT assay, and cell cycle and apoptosis were analyzed using flow cytometry.
RESULTS: The tumor growth inhibition was 30.33% and 50.85%, respectively, in mice treated with As2O3 2.5 and 5 mg/kg. MVD was significantly lower in arsenic-treated mice than in the control group. The fluorescence intensity levels of Flt-1 and KDR were significantly less in the arsenic-treated mice than in the control group. VEGF165 may accelerate growth of SGC7901 cells, but As2O3 may disturb the stimulating effect of VEGF165. ECV304 cell growth was suppressed by 76.51%, 71.09% and 61.49% after 48 h treatment with As2O3 at 0.5, 2.5 and 5 μmol/L, respectively. Early apoptosis in the As2O3-treated mice was 2.88-5.1 times higher than that in the controls, and late apoptosis was 1.17-1.67 times higher than that in the controls.
CONCLUSION: Our results showed that As2O3 delays tumor growth, inhibits MVD, down-regulates Flt-1 and KDR expression, and disturbs the stimulating effect of VEGF165 on the growth of SGC7901 cells. These results suggest that As2O3 might delay growth of gastric tumors through inhibiting the paracrine and autocrine pathways of VEGF/VEGFRs.
Keywords: Arsenic trioxide, Gastric tumor, Flt-1, Tumor growth inhibition
INTRODUCTION
Angiogenesis is recognized as playing a role in the pathophysiology of many human malignancies. It is an important factor in the progression and enlargement of solid neoplasms and is closely related to invasion and metastases[1-3]. Angiogenesis is influenced by a number of positive and negative regulatory factors, such as cytokines, extracellular matrix, and other cellular constituents including pericytes[4]. Among the factors contributing to angiogenesis, vascular endothelial growth factor (VEGF) is recognized as one of the most important molecules in the formation of new blood vessels. VEGF is a potent and specific mitogen for endothelial cells that activate the angiogenic switch in vivo and enhance vascular permeability. A variety of malignant human tumors are known to secrete VEGF, which has been correlated with the onset of angiogenesis in tumors[5-7]. Over-expression of VEGF is suggested to participate in the carcinogenic process[8]. VEGF binds to two distinct receptors on endothelial cells: VEGFR-1 (fms-like tyrosine kinase receptor 1, Flt-1) and VEGFR-2 (kinase insert domain containing receptor human homologue/fetal liver kinase 1 murine homologue, KDR/Flk-1)[9,10]. KDR is responsible for mitogenic signaling, and plays an important role in vasculogenesis and blood island formation, and Flt-1 regulates the assembly of endothelial cells and tissue factor production in endothelial cells[8]. Thus, it is suggested that inhibition of VEGF/VEGFR pathways may interrupt VEGF-induced angiogenesis. Recently, a few studies have demonstrated co-expression of VEGF and its receptors in tumor cells, which suggests that a VEGF autocrine pathway exists in tumor cells[11-14].
Arsenic is a common natural substance. Arsenic trioxide (As2O3) has shown substantial efficacy in treating both newly diagnosed and relapsed patients with acute promyelocytic leukemia (APL)[15]. Recent studies have shown that a wide variety of malignancies, including both hematological cancer and solid tumors derived from several tissue types, may be susceptible to therapy with As2O3[16-19]. These successes have prompted investigations to elucidate the mechanisms of action underlying these clinical responses. Emerging data suggest that arsenic induces apoptosis and inhibits tumor growth[15,16,20]. It has recently been reported that arsenic may inhibit angiogenesis[21-23]. We have recently shown in vivo and in vitro that As2O3 can inhibit VEGF expression and suppress angiogenesis and gastric tumor growth[24].
In the present work, we investigated further the effect of As2O3 on the expression of VEGF receptors Flt-1 and KDR in human gastric tumor cells and the proliferation of vascular endothelial cells. Our study demonstrated that As2O3 delayed tumor growth by inhibiting the paracrine and autocrine pathway of VEGF/VEGFRs.
MATERIALS AND METHODS
Animals and cells
Male Balb/c mice, 5-wk-old and weighing 19-21 g (from Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai, China) were used in this study. The mice were kept in a laminar-filtered airflow cabinet under pathogen-free conditions with a constant temperature of 22°C ± 2°C, relative humidity of 55% ± 5%, and 12-h dark/light cycles. All experiments were carried out according to the guidelines of the Laboratory Protocol of Animal Handling, Fourth Military Medical University. Human gastric cancer cell line SGC-7901 and ECV304, a cell line derived from human umbilical vessel endothelial cells, were purchased from the Animal Laboratory Centre, Fourth Military University.
Tumor xenografts in nude mice
SGC-7901 cells were cultured in RPMI 1640 medium supplemented with 100 mL/L fetal bovine serum (FBS) at 37°C in a 5 mL/L CO2 incubator. Thirty mice received subcutaneous injection in the right flank with 200 μL cell suspension containing 2 × 107 SGC-7901 cells. After 10 d, when established tumors of 0.2 cm-0.3 cm diameter were detected, drug administration was started.
Drug treatment
The animals were randomly divided into three groups of 10 animals each. Arsenious acid [H3AsO3 (As2O3 + 3H2O2⇔H3AsO3); Yida Pharmaceutical, Harbin, China] diluted with saline solution was injected intraperitoneally every day to the two treatment groups (2.5 and 5 mg/kg in 0.2 mL) and the same volume of saline solution was injected into the control group. After 10 d of treatment, three groups of mice were sacrificed and the tumor masses were removed. After the weight of tumor masses was measured, they were fixed in 40 g/L paraformaldehyde, and the frozen sections were prepared with a cryomicrotome (HM505E, Microm, Germany) for immunofluorescence analysis.
Tumor inhibition and regression
Tumor growth inhibition (TGI) was calculated by measuring tumor volumes before treatment, and 6 and 11 d after treatment. Tumor volume (cm3) was calculated using the formula: tumor volume = 1/2ab, where a is the long axis, and b the short axis. TGI (%) = (1 - Vt/Vc) × 100%, where Vt is the mean tumor volume of the arsenic-treated group, and Vc the mean tumor volume of the control group.
Microvessel density (MVD)
For identification of microvascular structure, frozen sections were cut and stained with rat anti-mouse CD31 (Biolegend, USA) and secondary antibody (goat anti-rat IgG conjugated TRITC). MVD was determined by confocal microscopy of tumor tissue sections. A single microvessel was defined as any immunofluorescent endothelial cell that was distinguished from adjacent tumor cells and other connective tissue elements. The microvessels were carefully counted in 20 fields (×400). The mean ± SE was expressed as the number of microvessels identified within the area.
Immunofluorescence for Flt-1 and KDR
The frozen sections were kept at room temperature for 30 min, incubated in distilled water and PBS for 5 min each, permeabilized in 1 g/L Triton-X-100 for 10 min, washed with PBS, blocked with 100 mL/L sheep serum (Sigma, USA) at 37°C for 20 min, incubated with the primary antibody (Flt-1 and KDR rabbit polyclonal antibodies; Lab Vision, Fremont, CA, USA) at 4°C overnight, washed with PBS, incubated with the secondary antibody (sheep anti-rabbit IgG conjugated FITC, diluted 1:100; Sigma) for 1h at 37°C, washed with PBS, and then examined by a TCS SP2 laser confocal microscope (Leica, Wetzlar, Germany).
For each group, several field images of Flt-1 or KDR were observed under confocal microscopy. The fluorescence intensity of each cell in the confocal fluorescence images was measured using the Leica Confocal analysis system, and the mean fluorescence intensity in a group of cells was then calculated.
Cell viability assay
ECV304 cells were seeded in a 96-well plate (2 × 103 cells/well). After 48 h seeding, cells were treated with As2O3 (0.5, 2.5 and 5 μmol/L) for 2 d, in three parallel wells each, and untreated cells served as a control. By 48 h, 20 μL MTT (15 mg/mL) was added to each well and incubated for a further 4 h. The medium was removed and 150 μL DMSO was added to each well. A492 was measured using a microculture reader. The percentage of viable cells was calculated as follows: (A of experimental group/A of control group) × 100%.
SGC-7901 cells were seeded in a 96-well plate (2 × 103 cells/well). After sedimentation, exogenous recombinant human VEGF165 (2.5, 5, 15 and 20 ng/mL) or VEGF165 (2.5, 5, 15 and 20 ng/mL) + 2.5 μmol/L As2O3 or VEGF165 (2.5, 5, 15 and 20 ng/mL) + 5 μmol/L As2O3 were added to the medium, in three parallel wells each, and cultured for a further 48 h. For control wells, an equal volume of medium was added. The methods for the MTT assay and calculation of the percentage of viable cells were the same as above.
Flow cytometry by annexin V-FITC conjugated with propidium iodide (PI) staining
ECV304 cells were seeded in a 24-well plate (1 × 106 cells/well). After 48 h seeding, cells were treated with As2O3 (0.5, 2.5 and 5 μmol/L) for 2 d, and untreated cells served as controls. By 48 h, the cells were washed twice with cold PBS and then resuspended in a binding buffer at a concentration of 1 × 106 cells/mL, and the 100 μL solution (1 × 105 cells) was transferred to 5-mL culture tubes. Five microliters of annexin V-FITC and 10 μL PI (μg/mL) were added to each 100-μL solution, and the cells were gently vortexed and incubated for 15 min at room temperature in the dark. The samples, to which 400 μL PBS was added, were analyzed by FACScalibur flow cytometer (BD Biosciences, USA). Early apoptosis was estimated by the relative amount of FITC+PI– cells.
Flow cytometry by PI staining
ECV304 cells were treated with As2O3 0.5, 2.5 or 5 μmol/L. By 48 h, the harvested cells were fixed with 1 mL of 750 mL/L cold ethanol at 4°C overnight, and then washed with PBS. The cells were incubated with RNAse and PI for 30 min in the dark. Cell cycle of samples was analyzed using FACSCalibur flow cytometry.
Statistical analysis
The data were represented as mean ± SD. Data were analyzed with SPSS 10.0 statistical software (SPSS, Chicago, IL, USA). Multiple statistical comparisons were performed using ANOVA in a multivariate linear model. The Student-Newman-Kewls test was used to assess differences between the treatment and control group. P < 0.05 was considered statistically significant.
RESULTS
As2O3 inhibition of growth of human gastric tumor xenografts
All 30 nude mice developed tumors 10 d after implantation of SGC-7901 cells. When drug administration was started, there was no significant difference in the tumor volume of the three groups (control group, 67 ± 45 mm3; 2.5 mg/kg As2O3 group, 63 ± 36 mm3; 5 mg/kg group, 66 ± 48 mm3, P > 0.05). Tumor volume in the three groups from 0, 6 and 11 d after treatment is shown in Figure 1. There were significant differences in the tumor volumes in the arsenic-treated groups (2.5 mg/kg, 696 ± 125 mm3 and 5 mg/kg, 491 ± 116 mm3) and control group (999 ± 338 mm3, P < 0.05). On the other hand, the tumor volume in the 5 mg/kg As2O3 group was significantly less than that in the 2.5 mg/kg group (P < 0.05). TGI was 30.33% (2.5 mg/kg group) and 50.85% (5 mg/kg group) after arsenic treatment.
Figure 1.
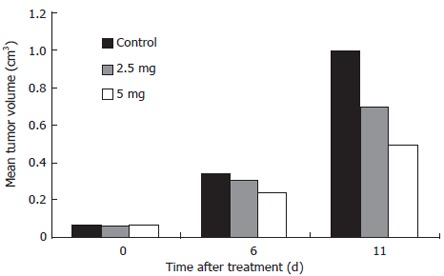
Arsenic trioxide inhibition gastric tumor xenografts growth.
As2O3 inhibition of tumor angiogenesis
Sections of tumors were stained for CD31 immuno-fluorescence to detect the number of endothelial cells (ECs) as a measure of tumor angiogenesis. MVD was significantly lower in the 2.5 mg/kg As2O3 group (9.32 ± 0.33 vs 5.36 ± 0.32, P < 0.01) and 5 mg/kg group (9.32 ± 0.33 vs 3.05 ± 0.24, P < 0.01) than in the control group. MVD was significantly lower in the 5 mg/kg group than in the 2.5 mg/kg group. These result demonstrated the decreased capillary density of the tumor after treatment with As2O3 (Figure 2).
Figure 2.

Effect of arsenic trioxide on tumor angiogenesis. Microvessel density (MVD) was measured with counting the fluorescence staining cell in high power field (400). Data are given as mean and SE.
Effect of As2O3 on Flt-1 and KDR expression in tumor xenografts
Expression of Flt-1 and KDR was confirmed by the presence of fluorescence-stained cytoplasm in the tumor cells. Stronger immunoreactivity to Flt-1 (Figure 3A) and KDR (Figure 4A) was found in all the SGC-7901 tumor xenografts of the control group. The weaker fluorescence intensity was observed in tumor cells of the mice treated with 2.5 mg/kg (Figures 3 and 4B) and 5 mg/kg (Figures 3 and 4C) As2O3. The expression of Flt-1 and KDR in tumor cells was significantly less in the arsenic-treated groups than in the control group (P < 0.001). Expression of Flt-1 and KDR in the 5 mg/kg As2O3 group was less than that in the 2.5 mg/kg group (Table 1).
Figure 3.
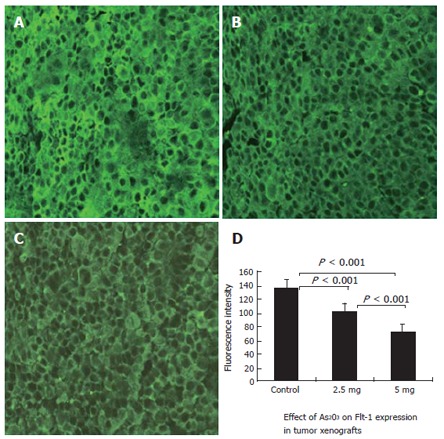
Effective inhibition of expression of Flt-1 (VEGFR-1) in the xenograft tumors by treatment with arsenic trioxide. Flt-1 expression was determined by Immunofluorescence staining and laser confocal microscope. Original magnification × 400. A: Control group; B: 2.5 mg/kg group; C: 5 mg/kg group; D: Quantitative analysis of fluorescence intensity in the three groups of tumors was measured using the Leica Confocal analysis system. Data are given as mean and SE.
Figure 4.
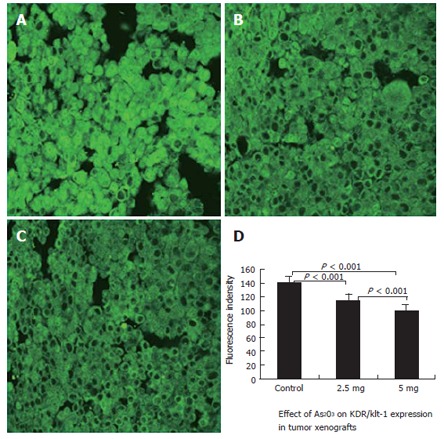
Effective inhibition of expression of KDR (VEGFR-2) in the xenograft tumors by treatment with arsenic trioxide. KDR expression was determined by Immunofluorescence staining and laser confocal microscopy. Original magnification × 400. A: Control group; B: 2.5 mg/kg group; C: 5 mg/kg group; D: Quantitative analysis of fluorescence intensity in the three groups of tumors was measured using the Leica Confocal analysis system. Data are given as mean and SE.
Table 1.
Effect of As2O3 on Flt-1 and KDR/Flk-1 expression in tumor xenografts (fluorescence intensity) (n = 10, mean ± SD)
| Group | Flt-1 | KDR/Flk-1 |
| Control | 137.41 ± 3.36 | 141.62 ± 1.66 |
| 2.5 mg/kg | 103.53 ± 2.23b | 114.99 ± 2.19b |
| 5 mg/kg | 72.17 ± 0.87bd | 101.11 ± 1.89bd |
P < 0.001, treatment groups vs control group;
P < 0.001, 5 mg/kg group vs 2.5 mg group.
Effect of As2O3 on VEGF-stimulated growth of SGC7901 tumor cells
SGC7901 cells were incubated with varying concentrations of VEGF165 or VEGF165 + 2.5 μmol/L As2O3 and varying concentrations of VEGF165 + 5 μmol/L As2O3. Its effects were measured using the MTT assay (Table 2). The results showed that VEGF165 may have accelerated the growth of SGC7901 cells, but As2O3 may have disturbed the stimulatory effect of VEGF165.
Table 2.
Effect of As2O3 on VEGF-stimulated growth of tumor cells (%)
|
As2O3 (ng/mL) |
|||||
| 2.5 | 5 | 10 | 15 | 20 | |
| VEGF165 | 105.79 | 106.62 | 100.97 | 107.59 | 109.38 |
| VEGF165 + 2.5 μmol/L As2O3 | 84.24 | 97.95 | 97.59 | 100.48 | 94.94 |
| VEGF165 + 5 μmol/L As2O3 | 76.87 | 68.55 | 83.74 | 85.06 | 84.94 |
Effect of As2O3 on proliferation of vascular endothelial cells
Cell viability was determined by the MTT assay (Figure 5). As2O3 inhibited the growth of ECV304 cells in a dose-dependent manner. Cell growth was suppressed by 76.51%, 71.09% and 61.49% after 48 h treatment with As2O3 at 0.5, 2.5 and 5 μmol/L, respectively.
Figure 5.
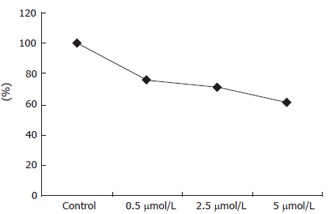
Effect of As2O3 on growth of ECV304 cells. The cell viability was analyzed by MTT assay.
Cell cycle and apoptosis of vascular endothelial cells induced by As2O3
Flow cytometry with only PI staining showed that the percentage of cells treated with As2O3 was higher in the sub-G1 period, lower in the sub-S period, and lower in the G2/G1 period when compared to the controls. Apoptotic cells were respectively 14.84% and 18.9% in the 2.5 and 5 μmol/L As2O3 groups (Table 3 and Figure 6). Early apoptotic cells, detected by flow cytometry with annexin V conjugated with PI staining, reached, respectively, 4.18, 5.36 and 7.4% in the 0.5, 2.5 and 5 μmol/L As2O3 groups (Figure 7). Early apoptosis in As2O3-treated groups was 2.88-5.1 times higher than that of the controls, and late apoptosis was 1.17-1.67 times than that of the controls.
Table 3.
Effect of As2O3 on cell cycle of ECV304 cells (%)
| Groups | G1 | G2/G1 | S | Apoptosis |
| Control | 94.24 | 2.05 | 5.76 | 0 |
| 0.5 μmol/L As2O3 | 96.17 | 1.81 | 2.59 | 0 |
| 2.5 μmol/L As2O3 | 96.15 | 1.91 | t0 | 14.84 |
| 5 μmol/L As2O3 | 96.96 | 1.84 | 0 | 18.90 |
Figure 6.
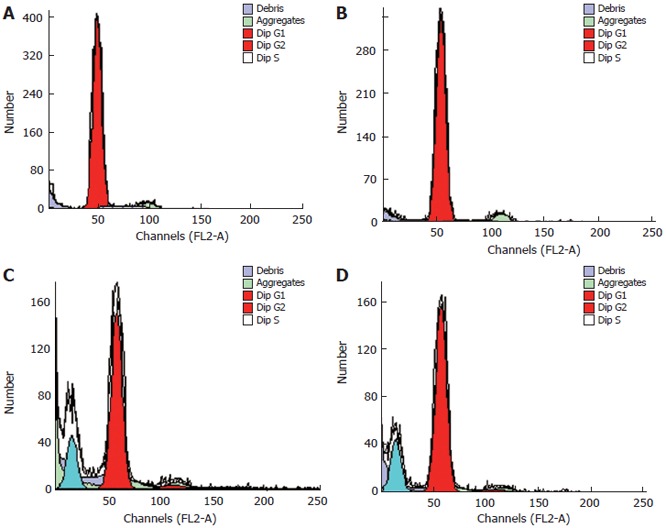
Effect of As2O3 on cell cycle of ECV304 cells. A: Control; B: 0.5 μmol/L As2O3; C: 2.5 μmol/L As2O3; D: 5 μmol/L As2O3.
Figure 7.
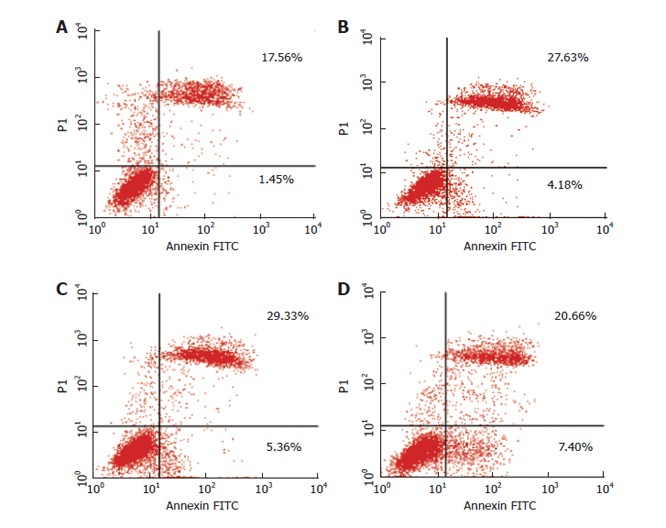
Induction of apoptosis in ECV304 with As2O3. Dual-stained with Annexin-V-FITC and PI and analyzed by flow cytometry. There was a dose-dependent increase in early apoptotic cells, as shown in the fourth quadrants plots. A: Control; B: 0.5 μmol/L As2O3; C: 2.5 μmol/L As2O3; D: 5 μmol/L As2O3.
DISCUSSION
Angiogenesis is critical for supporting the rapid growth of tumors. Angiogenesis inhibition is a promising therapeutic approach for the treatment of cancer. As2O3 has been demonstrated to induce complete remission in patients with APL without severe toxicity. More recently, in vitro studies have shown As2O3 apoptosis in other leukemia and solid tumor cells. As a novel anticancer agent, a few in vivo investigations of its efficacy on solid tumors have been carried out[19,21]. In this study, we observed the effect of As2O3 on growth and angiogenesis in a gastric cancer SGC7901 xenograft model. MVD was measured in tumor tissue by the amount of labeled CD31. The results revealed that a 10 d treatment with As2O3 resulted in tumor growth inhibition and MVD decrease. TGI was respectively 29.08% and 52.17% in mice treated with 2.5 and 5 mg/kg As2O3. MVD was significantly lower in tumor tissues in arsenic-treated mice than in the control groups. The results demonstrated that As2O3 inhibited gastric cancer growth in vivo and suppressed the formation of new blood vessels in cancer tissues. Anti-angiogenesis may be one of the mechanisms by which As2O3 inhibits gastric cancer growth.
ECs are the primary structured units of blood vessels. In recent years, ECs have been the target cell of some angiogenesis inhibitors that have been undergoing clinical trials. These inhibitors can selectively affect diverse EC functions that are related to angiogenesis, including activation, proliferation, migration, invasion and survival[25,26]. It has been reported that particles of realgar (As2S2) with an average diameter of 100-150 nm can induce EVCV-304 cell apoptosis[27]. We studied the effect of As2O3 on growth and proliferation of ECV304 cells. In our study, the MTT assay showed that As2O3 inhibited the growth of ECV304 cells in a dose-dependent manner. Flow cytometry assays with only PI staining showed that the percentage of the cells treated with As2O3 were higher in the sub-G1 period, lower in the sub-S period, and lower in the G2/G1 period when compared to the controls; and 14.84% and 18.9% of the cells treated with As2O3 at 2.5 and 5 μmol/L, respectively, were apoptotic. Early apoptosis in the As2O3 treated groups was 2.88-5.1 times higher than that in the controls, and late apoptosis was 1.17-1.67 times higher than that in the controls. These results showed that As2O3 inhibited the viability of ECV304 cells and arrested the cells in G1 phase, blocked or delayed their entry into S phase, disturbed DNA synthesis, and induced apoptosis in the G1 phase.
Tumor angiogenesis appears to be achieved by the overexpression of angiogenic agents within solid tumors that stimulate host vascular EC mitogenesis and possibly chemotaxis. In recent years, it has been widely shown that activity of VEGF is a key feature during tumor growth and angiogenesis[28-30]. In the course of occurrence and development of gastric cancer, angiogenesis plays a crucial role in which VEGF is believed to be the most important factor in neovascularization[31]. Some studies have shown that the serum level of VEGF is not only related to prognosis of gastric cancer, but also to treatment efficacy[32].
Both types of specific receptors for VEGF, Flt-1 and KDR, are commonly distributed in ECs. VEGF acts by binding to the receptors on ECs, and prompts EC proliferation. VEGF and its receptors are the most important pathways in tumor angiogenesis. Inhibition of VEGF/VEGFR pathways may suppress angiogenesis and tumor growth. However, the expression of VEGFR is not EC-specific, and recent emerging evidence has shown that VEGFRs are expressed in several types of non-endothelial cells, especially in tumor cells, which indicates that there is an autocrine pathway of VEGF in tumor cells. A report by Zhang et al[8] has shown that eight gastric cancer lines (RF-1, RF-48, NCI-87, NCI-SNU-1, NCI-SNU-5, NCI-SNU-16, AGS-1 and KATO-III) express VEGF, and six of these express both Flt-1 and KDR, and exogenous VEGF can stimulate the growth of KDR-positive tumor cells. These results suggest that VEGF acts not only as a paracrine factor on ECs, but also as an autocrine factor on tumor cells. VEGFR may play an important role in paracrine and autocrine pathways of VEGF, and VEGFR inhibitors can inhibit tumor angiogenesis and growth[8,9].
Our recent study[24] has demonstrated that As2O3 can inhibit expression of VEGF in gastric cancer. In the present study, we examined further the effect of As2O3 on VEGFR expression in vivo, and intended to confirm the anticancer activity of arsenic, which may block the paracrine and autocrine VEGF/VEGFR pathways, thereby delaying new tumor blood vessel formation and tumor cell growth. Our results showed that all of the Flt-1 and KDR expressed in the SGC-7901 tumor xenografts, and their expression in tumor cell control were higher than that in arsenic-treated mice. The fluorescence intensity levels of Flt-1 and KDR in tumor cells were significantly reduced in the arsenic-treated groups (P < 0.001). The fluorescence intensity levels of Flt-1 and KDR in the 5 mg/kg group were less than those in the 2.5 mg/kg group (P < 0.001). These results suggest that As2O3 can result in significant down-regulation of Flt-1 and KDR in a dose-dependent manner. The results of further experiments in vitro showed that exogenous VEGF165 could stimulate the growth of SGC7901 cells, and As2O3 may have disturbed the stimulatory effect of VEGF165. It indicates that the autocrine pathway of VEGF through VEGFRs is possible in gastric carcinoma, and As2O3 inhibits expression of Flt-1 and KDR in endothelial and tumor cells. As2O3 may block new blood vessel formation through the paracrine pathway and affect growth of tumor cells through the autocrine pathway of VEGF/VEGFRs, and delay tumor growth.
In conclusion, the results of our present study showed that As2O3 delayed growth of human gastric tumor xenografts in nude mice, decreased MVD in tumor tissues, inhibited proliferation of vascular ECs and induced their apoptosis, which resulted in down-regulation of Flt-1 and KDR expression in tumor tissues in a dose-dependent manner. Further results indicate that exogenous VEGF165 can stimulate the growth of SGC7901 cells, and As2O3 may disturb the stimulatory effect of VEGF165. These results suggest that As2O3 might delay growth of gastric tumor through inhibiting the paracrine and autocrine pathway of VEGF/VEGFRs.
COMMENTS
Background
Angiogenesis is an important factor in the progression and enlargement of solid neoplasms and is in close relation to invasion and metastases. Inhibition of angiogenesis may lead to control of tumor growth and metastasis, therefore, antiangiogenesis is a promising therapeutic approach for treatment of cancer. VEGF is recognized as one of the most important molecules in the formation of new blood vessels. VEGF binds to two distinct receptors on ECs: Flt-1(VEGFR-1) and KDR (VEGFR-2). Thus, it is suggested that inhibition of VEGF/VEGFR pathways may interrupt VEGF-induced angiogenesis.
Research frontiers
As2O3 shows substantial efficacy in treating both newly diagnosed and relapsed patients with APL. Recent studies have shown that a wide variety of malignancies, including both hematological cancer and solid tumors derived from several tissue types, may be susceptible to therapy with As2O3. These successes have prompted investigations to elucidate the mechanisms of action underlying these clinical responses. Emerging data suggest that arsenic induces apoptosis, and inhibits tumor growth and angiogenesis. We have recently shown in vivo and in vitro that As2O3 can inhibit VEGF expression and suppress angiogenesis and gastric tumor growth. In the present work, we investigated further the effect of As2O3 on expression of VEGF receptors Flt-1 and KDR in human gastric tumor cells and proliferation of vascular ECs.
Innovations and breakthroughs
Our results showed that As2O3 delayed growth of human gastric tumor xenografts in nude mice and decreased MVD in tumor tissues, inhibited proliferation of vascular ECs and induced their apoptosis, which resulted in down-regulation of Flt-1 and KDR/Flk-1 expression in tumor tissues in a dose-dependent manner. VEGF165 could stimulate the growth of SGC7901 cells, and As2O3 may have disturbed the stimulatory effect of VEGF165. These results suggest that As2O3 might delay growth of gastric tumor through inhibiting the paracrine and autocrine pathway of VEGF/VEGFRs.
Applications
The studies suggest that As2O3 might delay growth of gastric tumor through inhibiting the paracrine and autocrine pathway of VEGF/VEGFRs. One of the mechanisms by which As2O3 inhibits tumors growth is anti-angiogenesis through inhibiting VEGF/VEGFRs. Our results suggest that As2O3 might be used to treat tumors.
Terminology
VEGF is recognized as one of the most important factors in the formation of new blood vessels. As2O3 is a common natural substance.
Peer review
This is an excellent paper in terms of scientific rigor, and it is well-written. The experiments were well-designed and well-executed.
Footnotes
Supported by The Science Fund of the Second Affiliated Hospital of the Medical College, No. 2003-YL-35
S- Editor Liu Y L- Editor Kerr C E- Editor Wang HF
References
- 1.Folkman J. Angiogenesis and apoptosis. Semin Cancer Biol. 2003;13:159–167. doi: 10.1016/s1044-579x(02)00133-5. [DOI] [PubMed] [Google Scholar]
- 2.Dekel Y, Koren R, Kugel V, Livne PM, Gal R. Significance of angiogenesis and microvascular invasion in renal cell carcinoma. Pathol Oncol Res. 2002;8:129–132. doi: 10.1007/BF03033722. [DOI] [PubMed] [Google Scholar]
- 3.Edel MJ, Harvey JM, Papadimitriou JM. Comparison of vascularity and angiogenesis in primary invasive mammary carcinomas and in their respective axillary lymph node metastases. Clin Exp Metastasis. 2000;18:695–702. doi: 10.1023/a:1013139022051. [DOI] [PubMed] [Google Scholar]
- 4.Ferrara N. Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: therapeutic implications. Semin Oncol. 2002;29:10–14. doi: 10.1053/sonc.2002.37264. [DOI] [PubMed] [Google Scholar]
- 5.Cascinu S, Staccioli MP, Gasparini G, Giordani P, Catalano V, Ghiselli R, Rossi C, Baldelli AM, Graziano F, Saba V, et al. Expression of vascular endothelial growth factor can predict event-free survival in stage II colon cancer. Clin Cancer Res. 2000;6:2803–2807. [PubMed] [Google Scholar]
- 6.Shen GH, Ghazizadeh M, Kawanami O, Shimizu H, Jin E, Araki T, Sugisaki Y. Prognostic significance of vascular endothelial growth factor expression in human ovarian carcinoma. Br J Cancer. 2000;83:196–203. doi: 10.1054/bjoc.2000.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Benedetto M, Starzec A, Vassy R, Perret GY, Crépin M, Kraemer M. Inhibition of epidermoid carcinoma A431 cell growth and angiogenesis in nude mice by early and late treatment with a novel dextran derivative. Br J Cancer. 2003;88:1987–1994. doi: 10.1038/sj.bjc.6600985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang H, Wu J, Meng L, Shou CC. Expression of vascular endothelial growth factor and its receptors KDR and Flt-1 in gastric cancer cells. World J Gastroenterol. 2002;8:994–998. doi: 10.3748/wjg.v8.i6.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciardiello F, Bianco R, Caputo R, Caputo R, Damiano V, Troiani T, Melisi D, De Vita F, De Placido S, Bianco AR, et al. Antitumor activity of ZD6474, a vascular endothelial growth factor receptor tyrosine kinase inhibitor, in human cancer cells with acquired resistance to antiepidermal growth factor receptor therapy. Clin Cancer Res. 2004;10:784–793. doi: 10.1158/1078-0432.ccr-1100-03. [DOI] [PubMed] [Google Scholar]
- 10.Griffioen AW, Molema G. Angiogenesis: potentials for pharmacologic intervention in the treatment of cancer, cardiovascular diseases, and chronic inflammation. Pharmacol Rev. 2000;52:237–268. [PubMed] [Google Scholar]
- 11.Lee YK, Bone ND, Strege AK, Shanafelt TD, Jelinek DF, Kay NE. VEGF receptor phosphorylation status and apoptosis is modulated by a green tea component, epigallocatechin-3-gallate (EGCG), in B-cell chronic lymphocytic leukemia. Blood. 2004;104:788–794. doi: 10.1182/blood-2003-08-2763. [DOI] [PubMed] [Google Scholar]
- 12.Jackson MW, Roberts JS, Heckford SE, Ricciardelli C, Stahl J, Choong C, Horsfall DJ, Tilley WD. A potential autocrine role for vascular endothelial growth factor in prostate cancer. Cancer Res. 2002;62:854–859. [PubMed] [Google Scholar]
- 13.Dias S, Hattori K, Zhu Z, Heissig B, Choy M, Lane W, Wu Y, Chadburn A, Hyjek E, Gill M, et al. Autocrine stimulation of VEGFR-2 activates human leukemic cell growth and migration. J Clin Invest. 2000;106:511–521. doi: 10.1172/JCI8978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masood R, Cai J, Zheng T, Smith DL, Hinton DR, Gill PS. Vascular endothelial growth factor (VEGF) is an autocrine growth factor for VEGF receptor-positive human tumors. Blood. 2001;98:1904–1913. doi: 10.1182/blood.v98.6.1904. [DOI] [PubMed] [Google Scholar]
- 15.Slack JL, Waxman S, Tricot G, Tallman MS, Bloomfield CD. Advances in the management of acute promyelocytic leukemia and other hematologic malignancies with arsenic trioxide. Oncologist. 2002;7 Suppl 1:1–13. doi: 10.1634/theoncologist.7-suppl_1-1. [DOI] [PubMed] [Google Scholar]
- 16.Miller WH Jr, Schipper HM, Lee JS, Singer J, Waxman S. Mechanisms of action of arsenic trioxide. Cancer Res. 2002;62:3893–3903. [PubMed] [Google Scholar]
- 17.Munshi NC, Tricot G, Desikan R, Badros A, Zangari M, Toor A, Morris C, Anaissie E, Barlogie B. Clinical activity of arsenic trioxide for the treatment of multiple myeloma. Leukemia. 2002;16:1835–1837. doi: 10.1038/sj.leu.2402599. [DOI] [PubMed] [Google Scholar]
- 18.Waxman S, Anderson KC. History of the development of arsenic derivatives in cancer therapy. Oncologist. 2001;6 Suppl 2:3–10. doi: 10.1634/theoncologist.6-suppl_2-3. [DOI] [PubMed] [Google Scholar]
- 19.Murgo AJ. Clinical trials of arsenic trioxide in hematologic and solid tumors: overview of the National Cancer Institute Cooperative Research and Development Studies. Oncologist. 2001;6 Suppl 2:22–28. doi: 10.1634/theoncologist.6-suppl_2-22. [DOI] [PubMed] [Google Scholar]
- 20.Davison K, Mann KK, Miller WH Jr. Arsenic trioxide: mechanisms of action. Semin Hematol. 2002;39:3–7. doi: 10.1053/shem.2002.33610. [DOI] [PubMed] [Google Scholar]
- 21.Shen ZY, Shen J, Chen MH, Wu XY, Wu MH, Zeng Y. The inhibition of growth and angiogenesis in heterotransplanted esophageal carcinoma via intratumoral injection of arsenic trioxide. Oncol Rep. 2003;10:1869–1874. [PubMed] [Google Scholar]
- 22.Roboz GJ, Dias S, Lam G, Lane WJ, Soignet SL, Warrell RP Jr, Rafii S. Arsenic trioxide induces dose- and time-dependent apoptosis of endothelium and may exert an antileukemic effect via inhibition of angiogenesis. Blood. 2000;96:1525–1530. [PubMed] [Google Scholar]
- 23.Griffin RJ, Lee SH, Rood KL, Stewart MJ, Lyons JC, Lew YS, Park H, Song CW. Use of arsenic trioxide as an antivascular and thermosensitizing agent in solid tumors. Neoplasia. 2000;2:555–560. doi: 10.1038/sj.neo.7900123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao YF, Liu SX, Wu DD, Chen X, Ren LF. Inhibitory effect of arsenic trioxide on angiogenesis and expression of vascular endothelial growth factor in gastric cancer. World J Gastroenterol. 2006;12:5780–5786. doi: 10.3748/wjg.v12.i36.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griffioen AW, Damen CA, Mayo KH, Barendsz-Janson AF, Martinotti S, Blijham GH, Groenewegen G. Angiogenesis inhibitors overcome tumor induced endothelial cell anergy. Int J Cancer. 1999;80:315–319. doi: 10.1002/(sici)1097-0215(19990118)80:2<315::aid-ijc23>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 26.Kong HL, Crystal RG. Gene therapy strategies for tumor antiangiogenesis. J Natl Cancer Inst. 1998;90:273–286. doi: 10.1093/jnci/90.4.273. [DOI] [PubMed] [Google Scholar]
- 27.Deng Y, Xu H, Huang K, Yang X, Xie C, Wu J. Size effects of realgar particles on apoptosis in a human umbilical vein endothelial cell line: ECV-304. Pharmacol Res. 2001;44:513–518. doi: 10.1006/phrs.2001.0885. [DOI] [PubMed] [Google Scholar]
- 28.Hamma-Kourbali Y, Starzec A, Vassy R, Martin A, Kraemer M, Perret G, Crépin M. Carboxymethyl benzylamide dextran inhibits angiogenesis and growth of VEGF-overexpressing human epidermoid carcinoma xenograft in nude mice. Br J Cancer. 2003;89:215–221. doi: 10.1038/sj.bjc.6601029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bruns CJ, Liu W, Davis DW, Shaheen RM, McConkey DJ, Wilson MR, Bucana CD, Hicklin DJ, Ellis LM. Vascular endothelial growth factor is an in vivo survival factor for tumor endothelium in a murine model of colorectal carcinoma liver metastases. Cancer. 2000;89:488–499. [PubMed] [Google Scholar]
- 30.Fu JX, Wang W, Bai X, Wang L, Zhu ZL, Chen ZX, Ruan CG. [Coexpression of vascular endothelial growth factor and its receptors in human tumor cell lines] Ai Zheng. 2002;21:1217–1221. [PubMed] [Google Scholar]
- 31.Konno H, Baba M, Tanaka T, Kamiya K, Ota M, Oba K, Shoji A, Kaneko T, Nakamura S. Overexpression of vascular endothelial growth factor is responsible for the hematogenous recurrence of early-stage gastric carcinoma. Eur Surg Res. 2000;32:177–181. doi: 10.1159/000008760. [DOI] [PubMed] [Google Scholar]
- 32.Keyes KA, Mann L, Cox K, Treadway P, Iversen P, Chen YF, Teicher BA. Circulating angiogenic growth factor levels in mice bearing human tumors using Luminex Multiplex technology. Cancer Chemother Pharmacol. 2003;51:321–327. doi: 10.1007/s00280-003-0572-5. [DOI] [PubMed] [Google Scholar]


