Abstract
AIM: To investigate the chemopreventive efficacy of the Indian medicinal plant Acanthus ilicifolius L Acanthaceae in a transplantable Ehrlich ascites carcinoma (EAC)-bearing murine model.
METHODS: Male Swiss albino mice were divided into four groups: Group A was the untreated normal control; Group B was the EAC control mice group that received serial, intraperitoneal (ip) inoculations of rapidly proliferating 2 x 105 viable EAC cells in 0.2 mL of sterile phosphate buffered saline; Group C was the plant extract-treated group that received the aqueous leaf extract (ALE) of the plant at a dose of 2.5 mg/kg body weight by single ip injections, once daily for 10, 20 and 30 consecutive days following tumour inoculation (ALE control); and Group D was the EAC + ALE- treatment group. The chemopreventive potential of the ALE was evaluated in a murine model by studying various biological parameters and genotoxic markers, such as tumour cell count, mean survival of the animals, haematological indices, hepatocellular histology, immunohistochemical expression of liver metallothionein (MT) protein, sister-chromatid exchanges (SCEs), and DNA alterations.
RESULTS: Treatment of the EAC-bearing mice with the ALE significantly (P < 0.001) reduced viable tumour cell count by 68.34% (228.7 x 106 ± 0.53) when compared to EAC control mice (72.4 x 106 ± 0.49), and restored body and organ weights almost to the normal values. ALE administration also increased (P < 0.001) mean survival of the hosts from 35 ± 3.46 d in EAC control mice to 83 ± 2.69 d in EAC + ALE-treated mice. Haematological indices also showed marked improvement with administration of ALE in EAC-bearing animals. There was a significant increase in RBC count (P < 0.001), hemoglobin percent (P < 0.001), and haematocrit value (P < 0.001) from 4.3 ± 0.12, 6.4 ± 0.93, and 17.63 ± 0.72 respectively in EAC control mice to 7.1 ± 0.13, 12.1 ± 0.77, and 30.23 ± 0.57 respectively in EAC + ALE-treated group, along with concurrent decrement (P < 0.001) in WBC count from 18.8 ± 0.54 in EAC control to 8.4 ± 0.71 in EAC + ALE. Furthermore, treatment with ALE substantially improved hepatocellular architecture and no noticeable neoplastic lesions or foci of cellular alteration were observed. Daily administration of the ALE was found to limit liver MT expression, an important marker of cell proliferation with concomitant reduction in MT immunoreactivity (62.25 ± 2.58 vs 86.24 ± 5.69, P < 0.01). ALE was also potentially effective in reducing (P < 0.001) the frequency of SCEs from 14.94 ± 2.14 in EAC control to 5.12 ± 1.16 in EAC + ALE-treated group. Finally, in comparison to the EAC control, ALE was able to suppress in vivo DNA damage by abating the generations of ‘tailed’ DNA by 53.59% (98.65 ± 2.31 vs 45.06 ± 1.14, P < 0.001), and DNA single-strand breaks (SSBs) by 38.53% (3.14 ± 0.31 vs 1.93 ± 0.23, P < 0.01) in EAC-bearing murine liver.
CONCLUSION: Our data indicate that, ALE is beneficial in restoring haematological and hepatic histological profiles and in lengthening the survival of the animals against the proliferation of ascites tumour in vivo. Finally, the chemopreventive efficacy of the ALE is manifested in limiting MT expression and in preventing DNA alterations in murine liver. The promising results of this study suggest further investigation into the chemopreventive mechanisms of the medicinal plant A. ilicifolius in vivo and in vitro.
Keywords: Acanthus ilicifolius, Chemoprevention, DNA strand-breaks, Ehrlich ascites carcinoma, Haematological indices, Medicinal plants, Metallothionein, Sister-chromatid exchange, Transplantable tumour.
INTRODUCTION
Acanthus ilicifolius Linn., popularly known as “Harkach Kanta” belongs to the family Acanthaceae, has typical spinose margins on its evergreen leaves and stipular spines at stem nodes. The common name of the plant is Holy Leaved Acanthus. It is a gregarious, sparingly branched, evergreen shrub, 0.6-1.5 meters in height, common in the tidal swamps of creeks and rivers along the east and west coasts. The leaves are oblong or elliptic, pinnately toothed, acute or truncate and glabrous; its flowers are blue, sessile in opposite pairs and are in terminal crowded or interrupted spikes; the capsules are oblong, 2.5 cm long and are brown; the seeds are broad-ovate, compressed and are 0.6 cm in diameter. It is a plant of marshy habitat distributed widely throughout the mangroves of India including west coasts, Meghalaya and the Andamans and different parts of the Asian countries like Singhal, Burma, China, Thailand etc. The plant grows luxuriously by the side of the Ganges in Sunderbans, Hoogly, Howrah and 24 Parganas in West Bengal. The shrub is also planted as a sand-binder along the banks of tidal rivers and lakes. It is a folklore medicinal plant used mainly against rheumatism, paralysis, asthma and snake-bites. A decoction of the plant with sugar candy and cumin is used in dyspepsia with acid eructations. It is also considered to be a diuretic, and is used as a cure for dropsy and bilious swellings. In Goa, the leaves are employed as an emollient fomentation in rheumatism and neuralgia[1]. The leaves are bruised and soaked in water for external application and are also used as an expectorant. The analgesic, anti-inflammatory[2], and leishmanicidal[3] properties of A ilicifolius have been documented, whilst Babu et al have reported the antioxidant and hepatoprotective properties of the plant[4].
The propensity of cancer cells to show multiple genetic mutations underscores the concept that the carcinogenic process progresses by the accumulation of discrete genetic alterations. Evidence suggests that genomic instability may provide the driving force behind the genetic plasticity characteristic of cancer cells resulting in DNA damage, gene mutation, sister-chromatid exchanges (SCEs)[5], chromosomal aberrations (CAs), and cellular transformation[6,7]. Since SCEs data alone do not necessarily provide either a quantitative or a qualitative estimate of gross structural chromosomal damage, the SCEs assay should be regarded as a complement to rather than a substitute for CAs analysis. Earlier methods for detecting DNA unwinding in alkali have required physical separation of single- from double-stranded DNA using a hydroxyapatite column[8], specific nuclease digestion and precipitation or filter binding[9]. Moreover, radio-labeling of cells was required for detection of the small amounts of DNA involved. In cells where radio-labeling was not feasible, sensitive fluorimetric methods were substituted to permit detection and quantitation of DNA after column or filter separation. We have used a sensitive technique of Fluorimetric Analysis of DNA Unwinding (FADU) according to our established protocol[10] for the estimation of single-strand breaks (SSBs) in vivo. Besides FADU, single cell gel electrophoresis (SCGE) or the Comet assay, in particular the alkaline version of the assay, has also become a popular method for sensitive analysis, detection, and quantitation of DNA damage. Damage is detected as DNA strand-breaks, alkali-labile damage, and excision repair sites in individual interphase cells[11].
Our team workers visited the Sunderban area and gathered some information from the local people who used the plant as drugs for the regression of tumour growth with satisfactory results. Since the plant is easily available and information on the effect of this medicinal plant in a tumour-bearing animal model is not yet explored, it has encouraged us to proceed with the following objectives: firstly, to monitor the effect of its aqueous leaf extract (ALE) on mean survival time, liver histology, metallothionein (MT) expression, and haematological status, since the latter plays an important role in immunological and pathophysiological states of the animal; and secondly, to examine the antigenotoxic effects of the ALE in preventing hepatic DNA damage and SCEs and thereby chemoprotection of cells, particularly those undergoing drastic multiplication when transplanted as ascites cells within the peritoneal cavity of the host.
MATERIALS AND METHODS
Animals
Closed colony inbred male Swiss albino mice, 6-7 wk of age and weighing 20-22 g, obtained from the Indian Institute of Chemical Biology (IICB, CSIR, Govt. of India) Kolkata, India, were used throughout the study. The animals were supplied with standard mouse pellet diet (Hindustan-Lever, Mumbai, India) and water ad libitum. The recommendations of Jadavpur University “Institutional Animal Ethics Committee” [“Committee for the Purpose of Control and Supervision of Experiment on Animals” (CPCSEA Regn. No. 0367/01/C/CPCSEA) India] for the care and use of laboratory animals were strictly followed throughout the study and the recommendations were in accordance with the National Institute of Health (NIH) guidelines.
Materials
All the chemicals were purchased from Sigma Chemicals Co. (St. Louis, MO) unless otherwise mentioned.
Plant material
Air-dried over-ground parts of the plant A ilicifolius were supplied by the United Chemicals and Allied Products (Calcutta, India). Dr. Alpana Bhattacharya, Department of Botany, Bethune College, Calcutta identified and authenticated the plant and a voucher specimen number [B.N.Chatterjee, 100A, 100B (CAL)] was deposited in the Central National Herbarium, Calcutta, India.
Preparation of the aqueous leaf extract (ALE) of the plant
50 g of air-dried powdered leaves of A ilicifolius were percolated with distilled water at room temperature until exhausted and filtered, and the filtrate was lyophilized and air-dried (quantity 5.3 g).
Tumour cell inoculation
EAC cells were maintained serially in ascites fluid in the peritoneal cavity of 7-wk-old Swiss albino mice. Viable cells were counted in a haemocytometer by the trypan blue dye exclusion method. An aliquot of 2 × 105 viable cells suspended in 0.2 mL of sterile phosphate buffered saline (PBS, pH 7.4, 0.2 mmol/L) was aseptically inoculated intraperitoneally (ip) into each mouse. The tumour growth was observed within 4-6 d following transplantation.
Experimental Long-term regimen
Mice were randomly divided into four different groups containing 10 mice in each group. Figure 1A shows the basic experimental regimen for haematology and immunohistology. Group A animals belonged to the normal (untreated) vehicle control that received a single, ip injection of 0.2 mL of PBS (pH 7.4) daily for 10, 20 and 30 consecutive days. Group B animals were the EAC-bearing mice (tumour-control group) that received serial ip inoculations of viable tumour cells in PBS. Group C animals were the ALE control group that received daily freshly prepared ALE of A ilicifolius in 0.2 mmol/L of PBS by ip administration at a dose of 2.5 mg/kg body weight for 10, 20 and 30 consecutive days of each phase of the study; while group D animals were the treatment group that had been transplanted with viable EAC cells followed by (24 h later) ip administration of the ALE at the same dose-regimen as in group C. The body weight of the animals was monitored after every five days. All the animals were fasted overnight and were sacrificed at three different time intervals separately, i.e. at 11, 21 and 31 d of tumour inoculation to carry out various experiments.
Figure 1.
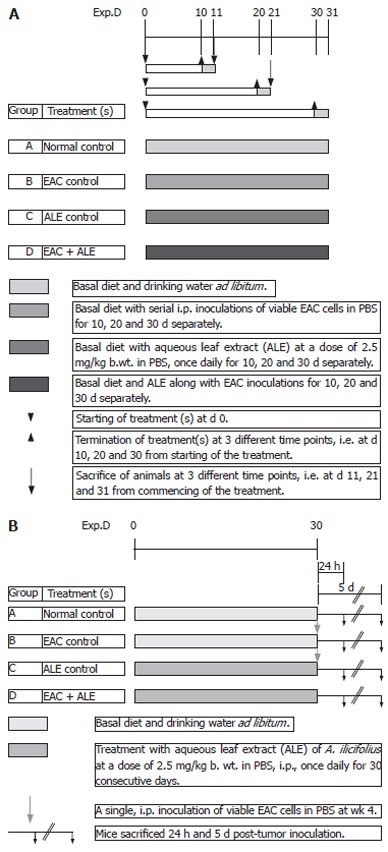
A: Basic experimental protocol for haematology and histology; B: Basic experimental protocol for DNA single-strand breaks assay.
The second set of animals (all the above four groups, A-D) with 20 mice/group was maintained with the same treatment schedule throughout without any prior sacrifice for carrying out survival study.
Short-term regimen
The third set of animals (groups A-D) with 10 mice/group was maintained for SCEs and DNA-chain break studies. In this regimen, the animals were divided into the following treatment schedule (Figure 1B): Group A = Untreated control; Group B = EAC control group that received a single ip EAC inoculation 30 d post-ALE treatment; Group C = ALE control that received the plant extract for 30 d continuously at the same dose-regimen mentioned earlier; Group D = EAC + ALE - treatment group.
Determination of tumour cell count and haematological indices
Tumour cell count in ascites fluid was recorded by the trypan blue dye exclusion method from EAC-bearing mice at a regular interval of 5 d. The total RBC, WBC and haematocrit (Htc) estimations from EDTA - treated blood samples were carried out on d 0, 5, 10 and 15 after tumour transplantation by an improved Neubauer haemocytometer.
Histopathology of liver tissue
Liver slices were taken from each lobe of the liver. After proper fixation and dehydration with graded ethanol solutions, sections of 5 μm in thickness were stained with haematoxylin and eosin (HE)[12]. The histopathological slides were observed under an ADCON-5591 (ADCON, Cleveland) photomicroscope.
Immunostaining of MT
Immunohistochemical detection of MT in cold acetone-fixed, paraffin-embedded liver sections was performed by the streptavidin-avidin-biotin-peroxidase-complex method[13]. After proper blocking with 1% H2O2 and normal goat serum separately, tissue sections were incubated overnight at 4°C with the primary antibody rabbit anti-rat MT-1 (polyclonal) using a 1:50 dilution. Sections were then incubated with a biotinylated secondary antibody goat anti-rabbit IgG (Sigma) at 37°C with 1:200 dilution. This was followed by incubation with streptavidin peroxidase (1:100) for 1 h and subsequent chromagen development with 0.5% 3, 3'-diaminobenzidine tetrahydrochloride (DAB) and 0.33% H2O2 in 0.5 mol/L Tris-NaCl as the substrate. The sections were then counterstained with Harris haematoxylin, dehydrated and mounted and served as positive control. Negative controls were prepared following all the above-mentioned steps omitting the primary antibody. MT immunostaining was considered positive when the nuclei and cytoplasm of the hepatocytes stained prominently purplish brown/reddish brown. MT immunoreactivity was expressed as percentage of immunopositive cells. A total of 10 high power fields were randomly chosen. The number of + ve cells was determined in relation to the total number of cells in that field[13].
SCE analysis
Mice were lightly ether anaesthetized and a vertical incision was made in the lower lateral abdominal region. The subcutaneous tissue was parted with forceps and 5-bromo-2'-deoxyuridine (BrdU) powder (1 mg/kg body wt.) was implanted by the method of Allen et al[14]. Approximately 24 h following BrdU implantation, and 1 h after ip injection of 0.04% colchicine at a rate of 1 mL/100 g body wt in 0.9% sodium chloride, mice were sacrificed and liver tissue was processed for chromosome preparation following the procedure of Horiuchi et al[15]. Staining for the detection of SCEs was accomplished by a modified fluorescence plus Giemsa (FPG) technique[16].
Scoring of SCEs
In order to determine the SCE levels, approximately 500 well-spread and differentially stained metaphase plates for second division mitosis were scanned from each treatment group, comprising of 10 animals. Each point of exchange was determined as SCE, including clear at the centromere. The SCE analysis was performed at five sequential time points.
Estimation of in vivo DNA damage by the Comet assay
Hepatic DNA damage was measured using the alkaline Comet assay, essentially as described by Olive et al[11]. The tissues were homogenized in phosphate-buffered saline (PBS; pH 8.0) under refrigeration and filtered. Cell viability was determined by the Trypan blue method. 4 μL of the homogenized tissue was then transferred to 50 μL of fresh PBS (pH 7.5), washed, suspended in 150 μL of 1% low melting point agarose at 37°C, and pipetted onto an agarose-precoated glass microscope slide. Slides were prepared in triplicate. The slides were immersed for 60 min in freshly-prepared, ice-cold lysis solution (2.5 mol/L NaCl, 0.1 mol/L Na2EDTA, 10 mmol/L Tris-HCl (pH 10), 10% DMSO and 1% Triton X-100) at 4°C in the dark, washed, and then subjected to horizontal electrophoresis using freshly made buffer (0.3 mol/L NaOH and 1 mmol/L Na2EDTA, pH > 13). After electrophoresis, the slides were stained with 5 μg/mL ethidium bromide and viewed under a Zeiss fluorescence microscope equipped with a green excitation filter and a 590 nm barrier filter. Routinely, 150 cells (50 cells/slide) were screened per liver sample from each animal. Nucleoid DNA extends under electrophoresis to form ‘comet tails’, and the length of the comets was evaluated for determination of the percentage of tailed DNA. This value is linearly related to the frequency of DNA breaks[11].
Assay of DNA unwinding
The principle of fluorimetric analysis of DNA unwinding (FADU) is that the fluorescent dye ethidium bromide (EtBr) binds selectively to double-stranded DNA (DS-DNA) in the presence of single-stranded DNA (SS-DNA) when short duplex regions in SS-DNA molecules are destabilized by alkali treatment[17].
Shearing of DNA, alkali treatment and neutralization
Whole liver genomic DNA was isolated from the frozen murine liver by a modification of the published criteria[18] with enzymatic RNA digestion before proteinase K treatment. After isolation, the purity of DNA solution was checked spectrophotometrically by determining the ratios of absorbance at A260/A280 and A260/A230. DNA was sheared by passing the DNA solution (20-25 times) through a 24-gauge needle using a hypodermic syringe. The optical density (OD) of the DNA sample was adjusted to 2.0 at 260 nm. For alkali denaturation, 2.0 mL of DNA solution in Tris-EDTA buffer (20 mmol/L Tris, 1 mmol/L M EDTA, pH 8.0) was mixed with an appropriate aliquot (about 2.4 mL) of alkali solution (0.1 mol/L NaOH, 0.001 mol/L M EDTA), so that the pH of the mixture becomes 12.8. After about 10 min (determined by trial experiments), the pH of the mixture was brought down to about 9.0 by addition of an approximate aliquot (about 1.3 mL) of an acid solution (0.025 mol/L Tris, 0.225 mol/L HCl).
FADU
DNA solutions from control and experimental groups were distributed into 12 test tubes[10]; in each time period (experiments were repeated four times) each tube contained 2.0 mL of the DNA solution of OD equal to 2.0 at 260 nm. The tubes were designated as T, P or B in each group. The DNA solution in tube B was sheared initially as described above. To the P and T tubes, alkali solutions were first added, mixed and then the tubes were incubated at 15°C for 10 min. Denaturation was stopped by chilling the solutions at 0°C and addition of acid solution, as described before.
The T tube differs from P tube in that the alkali and acid solutions, i.e., denaturing and neutralizing solutions were mixed together before addition of DNA solution. An aliquot (0.2 μL) of EtBr solution was added to each tube and the fluorescence was read in a spectrofluorimeter (LS-45, Perkin Elmer, USA) with excitation and emission wavelengths at 525 and 591 nm, respectively. The extent of DNA unwinding after a given time of exposure to alkali is calculated from the fluorescence of T, P and B samples. The fluorescence of the sample less than the fluorescence of the blank (P-B) provide an estimate of the amount of DS-DNA remaining. Percent D is given by the equation: Percent D (DS-DNA %) = (P-B)/(T-B) × 100.
Estimation of SSBs
It is assumed that the distribution of single-strand breaks in the DNA population follows a simple Poisson’s
Law. Under this circumstance, it is possible to make an approximate estimate of the average number of single-strand breaks (n) per DNA fragment from the simple equation given by Basak[19]: e-n = D/S + D; S = percentage DNA that remains single-stranded after alkali treatment; D = percentage remaining as DS-DNA. D/S+D represents the fraction (fo) of the molecules without strand-breaks. The values of ‘n’ corresponding to different DNA solutions isolated from different groups were then estimated.
Statistical analysis
The data were analyzed using the GraphPad Prism Software package, Version 4.01 (Barcode Softwares, Baltimore, MD). The results were expressed as the mean + SE and Student’s t-test was performed to compare sample means. Statistical significance was set at P < 0.05 for all comparisons. Percent inhibition was obtained by using the formula [(mean control - mean treatment)/mean control] × 100.
RESULTS
General observations
During the entire period of study, no differences in food and water consumptions were observed among the various groups of animals. Administration of the ALE at a dose of 2.5 mg/kg body weight to the group C animals indicates that the dose was well tolerated with adequate growth responsive effect, otherwise growth retardation or premature death would have occurred. Further, with this particular dose of ALE, there were no toxicologically significant changes in hematology, liver histology, clinical chemistry and clinical enzymology (data not shown), body and relative organ weights etc, suggesting that the administered dose of the plant extract was apparently devoid of toxicity.
The body weight of all groups of mice is recorded in Table 1. From the table, it is evident that there was no significant difference in the body weights of the normal control (Group A) and ALE control (Group C) mice suggesting that the plant extract does not have any adverse effect on the growth responses of the host. It can be seen that the body weights of all the four groups (Groups A-D) of animals were more or less the same and did not show any significant differences on the day of tumour transplantation (d 0). On d 10, the body weight of the EAC control (Group B) mice increased significantly (P < 0.05) from the normal control (Group A) mice. The body weight continued to increase on successive days (d 20 and 30; P < 0.01 and P < 0.001 respectively) when compared to the normal control (Group A). In the treatment group (Group D), there was no significant effect of the leaf extract on the reduction of the body weight on d 10 after tumour transplantation. In the following days, there was significant reduction in the body weights (P < 0.02 on d 20; P < 0.001 on d 30) compared to the EAC control (Group B). The body weights of Group D mice almost came down to normal on d 30 after continuous treatment with the leaf extract.
Table 1.
Body weight of different groups of mice
| Days following tumour transplantation |
Body weight (g) |
|||
| Normal control | EAC control | ALE control | EAC + ALE | |
| 0 | 15.6 ± 0.33 | 15.7 ± 0.46 | 15.5 ± 0.28 | 15.6 ± 0.38 |
| 10 | 16.9 ± 0.36 | 18.4 ± 0.58a | 17.2 ± 0.41 | 17.7 ± 0.29 |
| 20 | 18.3 ± 0.26 | 21.5 ± 0.51b | 18.5 ± 0.29 | 19.8 ± 0.35c |
| 30 | 20.6 ± 0.39 | 25.4 ± 0.66b | 21.1 ± 0.35 | 21.7 ± 0.49d |
Abbreviations: ALE: Aqueous leaf extract of A ilicifolius; EAC: Ehrlich ascites carcinoma. Values represent mean ± SE (n = 10).
P < 0.05,
P < 0.001 vs Normal control;
P < 0.02, and
P < 0.001 vs EAC control.
From Table 2, it is evident that there was no significant difference in liver and kidney weights between the normal control (Group A) and ALE control (Group C) groups suggesting that the aqueous extract probably do not have any adverse effect on the hosts’ physiology. Although, there was a slight decrease in the final liver and kidney weights of EAC-bearing mice (Group B) than that of the normal control (Group A) and on the other hand, there was a gradual increase in the organ weights in the treatment group (Group D) than that of Group B, however all these changes were not statistically significant.
Table 2.
Final weight of liver and kidney of different groups of animals sacrificed 30 d post-EAC inoculation
| Organ (s) |
Final weight (g) |
|||
| Normal control | EAC control | ALE control | EAC + ALE | |
| Liver | 1.25 ± 0.04 | 1.13 ± 0.06 | 1.26 ± 0.08 | 1.23 ± 0.03 |
| Kidney | 0.38 ± 0.03 | 0.33 ± 0.05 | 0.38 ± 0.05 | 0.37 ± 0.04 |
Abbreviations: ALE: Aqueous leaf extract of A. ilicifolius; EAC: Ehrlich ascites carcinoma. Values represent mean ± SE (n = 10). No significant differences between Normal control and EAC control and between EAC control and EAC + ALE.
On inoculation of the male Swiss albino mice with EAC (Group B), all mice died of carcinoma within 35 ± 3.46 d of inoculation (Figure 2); while the concurrent administration of ALE to the EAC-bearing hosts (Group D) resulted in an increase (P < 0.001) in mean survival of the animals, maximum life-span being 83 ± 2.69 d. Mice from the normal control (Group A) and ALE control (Group C) survived for the entire period of study (i.e. 110 ± 2.12 d and more).
Figure 2.
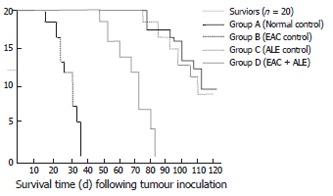
Survival curve of all the four groups of mice.
Effect of ALE on mean tumour cell count
The effect of the plant extract on mean tumour cell count is shown in Table 3. No tumour cell was detected on the animals for d 0. Viable tumour cells were observed from d 3/4. There was a gradual increase in cell count in tumour-bearing mice (Group B) from d 10 to d 30. Administration of the ALE to the EAC-bearing mice (Group D) resulted in a gradual significant decrease (P < 0.001) in the tumour-cell count from d 10 (37.91%) to d 30 (68.34%).
Table 3.
Effect of ALE on tumour cell count in Ehrlich ascites carcinoma-bearing mice
| Days following tumour transplantation |
Mean tumour cell count |
Decrease (%) | |
| EAC control | EAC + ALE | ||
| 10 | 18.2 × 106 ± 0.44 | 11.3 × 106 ± 0.35b | 37.91 |
| 20 | 52.1 × 106 ± 0.76 | 24.9 × 106 ± 0.41b | 52.20 |
| 30 | 228.7 × 106 ± 0.53 | 72.4 × 106 ± 0.49b | 68.34 |
Abbreviations: ALE: Aqueous leaf extract of A ilicifolius; EAC: Ehrlich ascites carcinoma. Values represent mean ± SE (n = 10).
P < 0.001 vs EAC control.
Effect of ALE on haematological indices
The total count (TC) of RBC, WBC, haemoglobin percent (Hb%) and haematocrit (Htc) of different groups of mice are shown in Table 4. From the table it is evident that, the TC of RBC gradually decreased from d 10 of tumour transplantation in EAC-bearing mice (Group B) and this reduction continued beyond d 30 until the death of the animals. Although the TC of RBC started to decrease from d 10, it showed statistical significance beyond d 10, i.e. on and from d 20 (P < 0.02) when compared to the normal vehicle control (Group A). Further reduction was observed on d 30 (P < 0.001) in the tumour-bearing animals. In the treatment group (group D), however, administration of ALE restored the TC of RBC significantly (P < 0.001 at d 30) almost to the normal value, when compared to Group B (EAC control).
Table 4.
Changes in total count (TC), Hb level and haematocrit in different groups of mice treated with or without ALE
| Days following EAC cell inoculation | Group | TC (RBC) (106/mm3) | TC (WBC) (106/mm3) | Hb (g%) | Haematocrit (%) |
| 0 | Normal control | 7.3 ± 0.12 | 5.8 ± 0.23 | 12.6 ± 0.16 | 33.43 ± 0.16 |
| EAC control | 7.2 ± 0.15 | 6.5 ± 0.52 | 12.0 ± 0.31 | 32.16 ± 0.27 | |
| ALE control | 7.3 ± 0.14 | 5.9 ± 0.22 | 12.5 ± 0.16 | 33.58 ± 0.25 | |
| EAC + ALE | 7.3 ± 0.13 | 6.2 ± 0.15 | 12.2 ± 0.27 | 33.11 ± 0.23 | |
| 10 | Normal control | 7.4 ± 0.14 | 5.8 ± 0.63 | 12.6 ± 0.65 | 33.51 ± 0.32 |
| EAC control | 6.6 ± 0.76 | 10.4 ± 0.72b | 10.0 ± 0.78a | 25.74 ± 0.53c | |
| ALE control | 7.6 ± 0.18 | 5.7 ± 0.55 | 12.8 ± 0.61 | 33.75 ± 0.35 | |
| EAC + ALE | 6.9 ± 0.53 | 7.7 ± 0.68e | 11.0 ± 0.49 | 29.08 ± 0.69d | |
| 20 | Normal control | 7.3 ± 0.54 | 5.7 ± 0.43 | 12.4 ± 0.78 | 33.46 ± 0.17 |
| EAC control | 5.1 ± 0.67a | 13.9 ± 0.56b | 8.2 ± 0.92f | 21.05 ± 0.26c | |
| ALE control | 7.5 ± 0.51 | 5.7 ± 0.38 | 12.6 ± 0.73 | 33.71 ± 0.18 | |
| EAC + ALE | 6.7 ± 0.12 | 7.9 ± 0.48d | 11.5 ± 0.57h | 28.87 ± 0.35d | |
| 30 | Normal control | 7.5 ± 0.14 | 5.8 ± 0.23 | 12.7 ± 0.84 | 33.49 ± 0.16 |
| EAC control | 4.3 ± 0.12b | 18.8 ± 0.54b | 6.4 ± 0.93b | 17.63 ± 0.72c | |
| ALE control | 7.7 ± 0.13 | 5.7 ± 0.24 | 12.9 ± 0.76 | 33.65 ± 0.21 | |
| EAC + ALE | 7.1 ± 0.13d | 8.4 ± 0.71d | 12.1 ± 0.77d | 30.23 ± 0.57d |
Abbreviations: ALE: Aqueous leaf extract of A. ilicifolius; EAC: Ehrlich ascites carcinoma; TC: Total count. Values represent mean ± SE (n = 10).
P < 0.02,
P < 0.001,
P < 0.05, and
P < 0.01 vs Normal control.
P < 0.001,
P < 0.02, and
P < 0.01 vs EAC control.
In case of the TC of WBC, the result was just reverse to that of the TC of RBC. The WBC content went on increasing significantly (P < 0.001) in Group B since d 10 of tumour inoculation, when compared to the normal control (Group A). Administration of the plant extract significantly reduced the TC of WBC (P < 0.001 at d 30) when compared to EAC control (Group B). Furthermore, the Hb% and Htc values were also decreased significantly (P < 0.001 at d 30) in EAC-bearing mice compared to the normal control and restored to the normal level in Group D mice after administration of the plant extract.
Effect of ALE on hepatic architecture
Phenotypically altered hepatocyte populations were found scattered in the livers of EAC-bearing mice (i.e. Groups B and D); but no such alterations were noticeable in untreated normal control (Group A; Figures 3A and 3B) or in the ALE control (Group C, Figure 3F). The HE- stained sections of liver slices revealed extensive hepatocellular lesions that were clearly distinguishable from the non-nodular surrounding normal parenchyma (NNSP).
Figure 3.
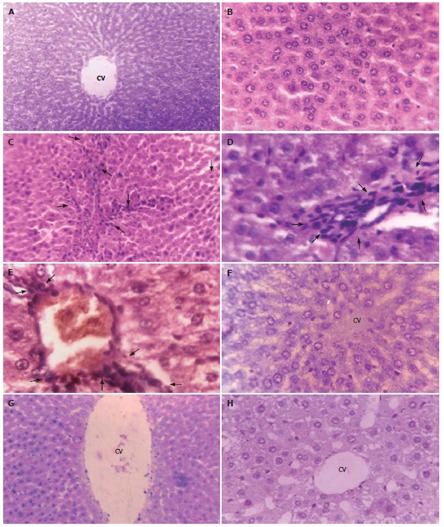
Contiguous liver sections from mice showing hepatocellular histological profiles. A, B, F: Normal hepatocellular architecture depicting hepatocytes radiating from the central vein; C, D, E: Aberrant hepatocellular phenotype with prominent basophilic focal lesions (black arrows) and the presence of eosinophilic and clear cell foci following serial, intraperitoneal (ip) inoculations of viable Ehrlich ascites carcinoma (EAC) cells; G, H: Almost normal hepatocellular architecture following simultaneous ip administrations of aqueous leaf extract (ALE) of A. ilicifolius and EAC. A and B: Untreated Control; C, D, and E: EAC Control; F: ALE Control; G and H: EAC + ALE. CV: Central vein. Magnification, A, C, and G: × 100; B, D, E, F, H: × 450.
In Group B (Figures 3C-3E), a gross alteration in hepatocellular architecture with neoplastic focal lesions was observed and hepatocytes appeared oval or irregular in shape upon EAC inoculation after 20 d and 30 d, with minimal histological changes being noticed after 10 d. The altered hepatocytes were found to be consistently enlarged with more than one nucleus, which were moreover largely vesiculated. They aggregated in clusters resulting in the appearance of prominent basophilic focal lesions. Some nuclei in the cells were large and hyperchromatic with clear and centrally located nucleoli (Figure 3D and 3E). Extensive vacuolation and necrosis were observed in the cytoplasm with masses of acidophilic (eosinophilic) material after 30 d. In contrast, the cellular architecture of hepatic lobules seemed to be almost like that of normal liver in Group D (Figures 3G and 3H) that received ALE treatment during the entire period of study. Liver sections from this group presented only a few altered hepatic cell foci. The cells were generally filled with cytoplasmic material and were less vacuolated. The size of the nuclei was essentially the same as that of normal cells and cells with two nuclei were considerably fewer than in group B.
Effect of ALE on liver MT expression
MT protein was detected in situ in the EAC-bearing liver tissue of mice (Group B) depicting a strong immunoexpression (Figures 4A and 4B). Generally, in sections (Figure 4B) with high MT-immunopositivity, the MT-positive cells formed contiguous foci or sheets and on some occasions isolated clusters of positive cells were seen. Figure 4A showed an intense staining of MT protein within the hepatic lesions indicating its focal expression. Semi-quantitative scoring of MT-positive cells showed that, there was substantial elevation of MT-immunoreactivity (P < 0.0001) (56.18% and 86.24% respectively at 20 and 30 d post-EAC inoculation; Table not shown) in Group B mice when compared to the basal expression level in normal control mice (Group A). In contrast, sections from ALE-treated murine liver (Group D) showed reduced MT-immunoreactivity [35.86% (P < 0.05) and 62.25% (P < 0.01) respectively at 20 and 30 d] with scattered MT-positive cells (Figure 4C).
Figure 4.
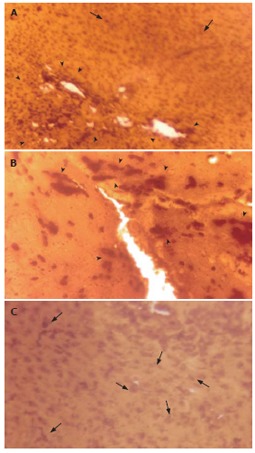
Light micrographs of tissue sections from murine liver (after 30 d of tumour transplantation) showing immunostaining of metallothionein (MT) with anti-rat MT-1 antibody and 3, 3'-diaminobenzidine tetrahydrochloride (DAB). A and B: Strong immunostaining of MT protein in EAC Control mice; C: Reduced immunostaining of MT in EAC + ALE-treatment mice. ALE: Aqueous leaf extract of A. ilicifolius; EAC: Ehrlich ascites carcinoma. Arrow Head ( ) indicates intense immunostaining of MT protein with prominent focal expression and isolated clusters of MT-positive cells. Arrow (↑) indicates scattered/individual MT immunopositive cells. Magnification, A: × 100; B and C: × 270.
Effect of ALE on the frequencies of SCEs in EAC-bearing mice
With transplantation of EAC, there was a moderate induction (P < 0.05) in mean SCE/cell in EAC-control mice (Group B) after 1 wk when compared to the untreated vehicle control (Group A) that showed spontaneous SCEs (2.89 ± 0.39) (Table 5). The induction showed steady increase (P < 0.02-0.001) in the frequency of SCEs on and from wk 2 with the progression of tumour development as a function of time and reached the peak (14.94 ± 2.14) prior to the death of the EAC-bearing animals at wk 5. In contrast, ALE administration to the EAC + ALE-treated mice offered a near complete inhibition of SCEs during the early phases of tumour growth. In this group, daily treatment with ALE resulted in a substantial decrement (P < 0.05-0.001) in the SCE frequency at all the time intervals (wk 2-5) when compared to EAC control animals at the same time points. Data of wk 2-5 showed 45.14%-65.73% reduction in SCE/cell from EAC + ALE-treated mice. As the mice from EAC control group died after wk 5, therefore, data obtained from the EAC + ALE-treatment group on and from wk 6 were compared with the last available data from EAC control mice at wk 5. Results showed that there were downhill trends in the SCE frequency in the ALE-treated EAC-bearing animals (Group D) until their survival up to wk 12 (data not shown). However, no significant difference between the normal control and ALE control in terms of SCE was noted.
Table 5.
Effect of ALE on sister-chromatid exchange (SCE) frequencies (500 plates/group) in different groups of mice killed at various time points
| Weeks after tumour inoculation | Group | Mean SCE/cell | Reduction in frequency (%) |
| 1 | EAC control | 4.59 ± 0.57 | - |
| EAC + ALE | 3.62 ± 0.29 | 21.13 | |
| 2 | EAC control | 6.89 ± 1.13 | - |
| EAC + ALE | 3.78 ± 0.78a | 45.14 | |
| 3 | EAC control | 7.83 ± 1.05 | - |
| EAC + ALE | 4.64 ± 0.54b | 40.74 | |
| 4 | EAC control | 10.26 ± 1.67 | - |
| EAC + ALE | 4.83 ± 0.69c | 52.92 | |
| 5 | EAC control | 14.94 ± 2.14 | - |
| EAC + ALE | 5.12 ± 1.16d | 65.73 |
Abbreviations: ALE: Aqueous leaf extract of A. ilicifolius; EAC: Ehrlich ascites carcinoma. Values represent mean ± SE (n = 10).
P < 0.05,
P < 0.02,
P < 0.01, and
P < 0.001 vs EAC control.
Effect of ALE on hepatic DNA damage
The mean length to width (L:W) ratio of the DNA mass, indicating the extent of DNA damage, was increased in the EAC control group 5 d post-EAC inoculation in comparison with the untreated control (P < 0.0001; Figure 5A). There was also a significant increase in the frequency of tailed DNA in EAC control mice compared to untreated control (P < 0.0001; Figure 5B). Treatment with ALE to the EAC-bearing hosts reduced the L:W ratio of DNA mass (65.34% reduction; P < 0.01), and the mean frequency of tailed DNA (53.59% reduction; P < 0.001) compared to that of the EAC control group.
Figure 5.
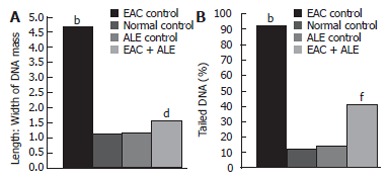
Effect of continuous treatment with ALE on DNA damage in the liver of mice 5 d post-EAC inoculation. A: Length:Width of DNA mass; B: Percentage of Tailed DNA. Each column and bar indicates the mean ± SE. n = 10. bP < 0.0001
Effect of ALE on hepatic SSBs
The protective effect of ALE on SSBs in EAC-bearing hosts 24 h and 5 d post-tumour transplantation is shown in Table 6. In EAC control mice, a significant increase (P < 0.001) in the number of SSBs/DNA could be observed following EAC transplantation when compared to that of normal control. The percentage of DS-DNA and SS-DNA in EAC control mice was 79.57% and 20.43%, suggesting that, the extent of host DNA lesions only after 24 h of tumour transplantation was not so severe. Moreover, the amount of DS-DNA (88.23%) in the EAC + ALE-treated group was found to be markedly close to that of normal control (94.75%); whereas, there was almost a 3-fold increase in SS-DNAs (82.79%) and a 4-fold decrease in DS-DNA (17.21%) in EAC control mice sacrificed 5 d post-tumour inoculation, than that of the same group (SS-DNA 31.43%, and DS-DNA 68.57%) being transplanted with tumours only 24 h prior to sacrifice. Treatment with ALE strictly abated (38.53%; P < 0.01) the generation of SSBs/DNA fragment in EAC + ALE-treated mice when compared to the EAC control. Moreover, there were no significant differences between normal control and ALE control in the percentage of DS-DNAs, suggesting that ALE apparently is non-genotoxic.
Table 6.
Effect of ALE on the generation of average number of single-strand breaks (SSBs)/DNA fragment in murine liver 24 h and 5 d post-EAC transplantation
| Time following EAC inoculation | Group | DS-DNA (%) | No. of SSBs/ DNA fragment | Inhibition (%) |
| 24 h | Normal control | 94.75 | 0.07 ± 0.01 | ---- |
| EAC control | 79.57 | 1.28 ± 0.22b | ---- | |
| ALE control | 94.87 | 0.07 ± 0.02 | ---- | |
| EAC + ALE | 88.23 | 1.14 ± 0.15 | ---- | |
| 5 d | Normal control | 95.12 | 0.08 ± 0.03 | ---- |
| EAC control | 17.21 | 3.14 ± 0.31b | ---- | |
| ALE control | 94.93 | 0.07 ± 0.3 | ---- | |
| EAC + ALE | 55.83 | 1.93 ± 0.23d | 38.53 |
ALE: Aqueous leaf extract of A. ilicifolius; EAC: Ehrlich ascites carcinoma. Values represent mean ± SE (n = 10).
P < 0.001 vs Normal control;
P < 0.01 vs EAC control.
DISCUSSION
The present study showed that administration of ALE reduced viable tumour cell count and brought about a marked increase in mean survival (average life-span) of tumour-bearing hosts, suggesting the tumour-combating efficacy of the said extract under investigation. Furthermore, ALE restored RBC count, Hb and Htc values, and reduced WBC count in EAC-bearing mice, thereby indicating its effectiveness in limiting haematological toxicity following tumour transplantation. There was substantial improvement in the hepatocellular architecture following daily administration of ALE over EAC control mice. We further report here the potential role of the ALE in controlling liver MT expression in mice. Finally, ALE treatment resulted in reduced SCEs and further prevented the generations of ‘tailed’ DNAs, SS-DNAs and SSBs in EAC-bearing mice hepatocytes as demonstrated by the Comet assay and FADU.
Analysis of the haematological toxicity during tumour transplantation and its possible alteration by ALE was monitored using a battery of haematological indices. Primarily, it resulted in an anemia probably due to severe red cell destruction, which was also reflected in the significantly lowered Htc value as compared to normal animals. The symptoms aggravated with time following tumour transplantation and the body weights of the EAC-bearing mice increased due to the accumulation of haemorrhagous ascitic fluid within the peritoneal cavity. The chemopreventive effects of the plant extract in reducing the severity of anaemia through improvement of RBC count and Htc value[20] not only maintained the normal body and organ weights of tumour-bearing mice, but also may account for the increased survival of the host as evident from our present findings. Administration of ALE further decreased viable tumour cell count in EAC-bearing animals. This may point toward the underlying chemopreventive potential of the plant extract in vivo. Although the mechanistic insights into the chemoprevention of the plant are unexplored, we may assume that ALE-mediated tumour cell apoptosis could be one possible pathway for the decreased number of viable tumour cells that warrants further investigation.
The beneficial effect of the plant extract was also apparent in elevating the Hb level, which was otherwise decreased in EAC-control animals. The exact mechanism of the ALE-mediated induction of Hb level during tumour growth is not clear at the present moment. We may speculate that it might somehow influence the process of haem synthesis, which may therefore account for the subsequent induction of Hb level. Whatever may be the mechanism, induction of Hb by ALE, as observed herein, could have a broad implication with respect to the antitumour efficacy of the extract if one considers the fact that high Hb level has been found to possess an inhibitory influence on tumour growth[20,21]. The data described herein showed a significant increase in total leukocyte count at different time intervals following tumour inoculation as compared to normal animals. Abnormal mitotic activity as evident from the appearance of different forms of abnormal and aberrant neutrophils in large numbers was essentially observed in EAC-bearing hosts. Now, the role of ALE in reducing the TC of WBC in the treatment group could be influenced through a reversal of lymphoid-myeloid ratio. Thus, the stabilization of leukocyte count with parallel retainments of normal Hb level and Htc value in tumour-bearing animals by ALE may have a paramount importance in limiting hematological toxicity in hosts.
The study further demonstrates an intense immuno-staining of MT in EAC-bearing liver tissue of mice in comparison to that of normal control. It is necessary to mention that the hyperbasophilic cells of the hepatocellular lesions stained more intensely for MT compared to that of the non-basophilic area of the same tissue, suggesting that the hyperbasophilic proliferative lesions consisting of rapidly proliferating cells may be considered to be the primary MT (-positive focal) expression zones. Up-regulation of MT expression in rapidly proliferating tissues appears to suggest its critical role in normal and neoplastic cell growth[13,22]. Treatment with ALE substantially reduced the expression of MT in the precancerous lesions along with decreased MT immunoreactivity. The precise mechanism of ALE-mediated down-regulation of MT expression is not clear at the moment. However, it is assumed that the antioxidant property[4] of A. ilicifolius may be involved in the elimination of reactive metabolites from the host cells, thereby posing low oxidative stress to the hosts and limiting MT expression thereby. Thus, control of liver MT expression, a cell-cycle dependent protein in EAC-transplanted mice by treatment with ALE might play an important role in controlling cell growth in vivo.
Inoculation of the hosts with EAC may act as primary agents producing secondary DNA cleavage and are potent inducers of chromosomal aberrations and SCEs[23]. Since a SCE represents the breakage of four strands of DNA (two double helices), a switch of these strands between chromatids of the same chromosome and the rejoining of these strands in their new location, it is important to know whether the breakage and rejoining events occur faithfully, that is without producing any modification in the genetic code. In early experiments using Chinese hamster cells in culture, it was found that the induction of SCEs was linearly related to the increase in single gene mutations when the cells were exposed to chemicals, each of which differed in the type of lesion produced in DNA[23,24]. Reports indicate that antioxidants suppress clastogenicity, and thus, many antioxidants are even anticarcinogens[25,26]. Studies have shown that A ilicifolius possesses antioxidant properties[4], and may therefore be effective in combating oxidative stress following tumour transplantation. In our present study, ALE-mediated suppression of SCEs indicates its anticlastogenicity and chemoprotection thereby. The decreased occurrence of SCEs may indirectly be related to increased cell survival or host’s anti-cancer surveillance, since studies in search of a relationship between SCE induction and other expressions of genotoxicity have shown a positive relationship between SCEs and reduced cell survival.
DNA damage in the form of SSBs may play an essential role in the pathogenesis of neoplasia. The SSBs detected herein by the Comet assay and FADU can be converted to double-strand DNA breaks (DSBs) upon replication. DSBs are a highly deleterious form of DNA damage, since they lead to mitotic cell death or mutations[27]. DNA DSBs are generated when the two complementary strands of the DNA double helix are broken simultaneously at sites that are sufficiently close to one another that base-pairing and chromatin structure are insufficient to keep the two DNA ends juxtaposed. As a consequence, the two DNA ends generated by a DSB may become physically dissociated from one another, resulting in error-prone repair and providing the opportunity for inappropriate recombination with other sites in the genome. Error-prone/inaccurate repair or lack of repair of DSBs may lead to mutations or to larger-scale chromosomal aberrations and genomic instability[28,29]. In addition, mutations in many of the factors involved in DSB signaling and repair lead to increased predisposition to cancer in humans and in animal models[30]. In the present study, ALE treatment resulted in a substantial decrease in the amount of SS-DNAs, SSBs, ‘tailed’ DNA and DNA ‘comets’ in the tumour-bearing murine livers. This reduction in DNA damage reflects the potential of the ALE to reduce the genotoxicity caused by EAC in host cells. The exact mechanism of action of the ALE in limiting DNA damage warrants a more robust investigation.
In conclusion, the results of our study clearly indicate that the ALE of A ilicifolius is able to inhibit the survival and proliferation of EAC cells in laboratory mice, and this observation is potentially useful in medical oncology. Our data show that, ALE treatment is beneficial in increasing the mean survival of the animals, in remodeling of the liver lesions and in limiting clastogenicity and genotoxicity in vivo. Although this study is at its preliminary stage, the results are promising. Future studies are planned in our laboratory to isolate and characterize the active fractions/principles of the plant and to elucidate the mechanistic basis of chemoprevention in a defined chemical carcino-genesis model.
ACKNOWLEDGMENTS
The authors sincerely acknowledge Professor J Geiser, University of Kansas Medical Center, Kansas City, Kansas for providing the antiserum of MT.
COMMENTS
Background
Herbal preparations/natural plant products constitute an important component of indigenous/traditional medicines. Herbs certainly have been an important source of many allopathic medicines. However, the way herbs are used in indigenous medical system is not supported by strong preclinical data with insights into the biological/pharmacological mechanisms. Ehrlich ascites carcinoma (EAC) is a strain-specific, rapidly proliferating transplantable epithelial tumour in mouse in which the neoplastic cells multiply and ascites fluid accumulates within the peritoneal cavity of the host and ultimately the host dies. Inhibition of the growth and proliferation of EAC cells by administration of plant products would be a safe and effective treatment strategy.
Research frontiers
The most significant approach to cancer chemoprevention seems to be the administration of chemopreventive agents in order to inhibit the pathogenesis of cancer or to delay or halt or limit the progression of neoplastic transformation. With the increasing trend in the incidence of cancers in our country, biomedical research directed at early detection and diagnosis, prognosis and survival as well as prevention of progression of malignancy is of prime importance. Since India is a rich source of indigenous herbs and several studies have reported potential therapeutic/chemopreventive utilities of these medicinal herbs, it is therefore of great importance for us to explore the chemopreventive efficacy of an Indian medicinal plant Acanthus ilicifolius in preventing DNA damage in an EAC model in mice.
Innovations and breakthroughs
In this paper, the primary chemopreventive mechanisms of the plant Acanthus ilicifolius have been investigated in an in vivo EAC model in Swiss albino mice. In contrast to meager previous reports demonstrating analgesic and antioxidant properties of the plant, the results of the present study clearly showed that, the aqueous leaf extract (ALE) was quite effective in preventing hepatic DNA alterations and sister-chromatid exchanges in EAC-transplanted murines. Our study further showed that ALE treatment was able to limit liver metallothionein expression, a potential marker for cell proliferation and increased the mean survival of animals to a significant extent. Results indicate that A ilicifolius could be used as a potential chemoprotector against neoplasia.
Applications
This study opens up a promising avenue in cancer chemoprevention with indigenous plants. Lack of toxicity favours further preclinical evaluation of A ilicifolius in a defined chemical carcinogenesis model. Elucidation of its mechanisms of action at the intricate molecular circuits, and isolation and characterization of the active principles, will provide a better understanding of the treatment strategy, and we would have the beginning of a new chemoprevention programme that could have a broader implication for the well-being of the society.
Peer review
In this experimental study, the authors showed the importance of Acanthus ilicifolius plant extract as chemoprotector on EAC-bearing murine model.
Footnotes
Supported by The Council of Scientific and Industrial Research, Government of India, No. 9/96(470)2K5-EMR-I
S- Editor Zhu LH L- Editor Roberts SE E- Editor Yin DH
References
- 1.Ananda K, Sridhar KR. Diversity of endophytic fungi in the roots of mangrove species on the west coast of India. Can J Microbiol. 2002;48:871–878. doi: 10.1139/w02-080. [DOI] [PubMed] [Google Scholar]
- 2.Kanchanapoom T, Kamel MS, Kasai R, Picheansoonthon C, Hiraga Y, Yamasaki K. Benzoxazinoid glucosides from Acanthus ilicifolius. Phytochemistry. 2001;58:637–640. doi: 10.1016/s0031-9422(01)00267-9. [DOI] [PubMed] [Google Scholar]
- 3.Kapil A, Sharma S, Wahidulla S. Leishmanicidal activity of 2-benzoxazolinone from Acanthus illicifolius in vitro. Planta Med. 1994;60:187–188. doi: 10.1055/s-2006-959449. [DOI] [PubMed] [Google Scholar]
- 4.Babu BH, Shylesh BS, Padikkala J. Antioxidant and hepatoprotective effect of Acanthus ilicifolius. Fitoterapia. 2001;72:272–277. doi: 10.1016/s0367-326x(00)00300-2. [DOI] [PubMed] [Google Scholar]
- 5.Stagos D, Karaberis E, Kouretas D. Assessment of antioxidant / anticarcinogenic activity of plant extracts by a combination of molecular methods. In Vivo. 2005;19:741–747. [PubMed] [Google Scholar]
- 6.Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993;362:709–715. doi: 10.1038/362709a0. [DOI] [PubMed] [Google Scholar]
- 7.Morgan WF, Day JP, Kaplan MI, McGhee EM, Limoli CL. Genomic instability induced by ionizing radiation. Radiat Res. 1996;146:247–258. [PubMed] [Google Scholar]
- 8.Stulp BK. An economic water-jacketed vacuum-enforced mini-column system for quick separation of double and single stranded DNA. J Biochem Biophys Methods. 1986;12:197–201. doi: 10.1016/0165-022x(86)90108-9. [DOI] [PubMed] [Google Scholar]
- 9.Vu VT, Møller ME, Grantham PH, Wirth PJ, Thorgeirsson SS. Association between DNA strand breaks and specific DNA adducts in murine hepatocytes following in vivo and in vitro exposure to N-hydroxy-2-acetylaminofluorene and N-acetoxy-2-acetylaminofluorene. Carcinogenesis. 1985;6:45–52. doi: 10.1093/carcin/6.1.45. [DOI] [PubMed] [Google Scholar]
- 10.Sarkar A, Basak R, Bishayee A, Basak J, Chatterjee M. Beta-carotene inhibits rat liver chromosomal aberrations and DNA chain break after a single injection of diethylnitrosamine. Br J Cancer. 1997;76:855–861. doi: 10.1038/bjc.1997.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olive PL, Wlodek D, Durand RE, Banáth JP. Factors influencing DNA migration from individual cells subjected to gel electrophoresis. Exp Cell Res. 1992;198:259–267. doi: 10.1016/0014-4827(92)90378-l. [DOI] [PubMed] [Google Scholar]
- 12.Stewart HL, Williams GM, Keysser CH, Lombard LS, Montail RJ. Histological typing of liver tumors of the rat. J Natl Cancer Inst. 1980;64:117–207. [Google Scholar]
- 13.Jin R, Chow VT, Tan PH, Dheen ST, Duan W, Bay BH. Metallothionein 2A expression is associated with cell proliferation in breast cancer. Carcinogenesis. 2002;23:81–86. doi: 10.1093/carcin/23.1.81. [DOI] [PubMed] [Google Scholar]
- 14.Allen JW, Shuler CF, Mendes RW, Latt SA. A simplified technique for in vivo analysis of sister-chromatid exchanges using 5-bromodeoxyuridine tablets. Cytogenet Cell Genet. 1977;18:231–237. doi: 10.1159/000130765. [DOI] [PubMed] [Google Scholar]
- 15.Horiuchi T, Ito K, Suzuki M, Umeda M. Sensitive induction of chromosome aberrations in the in vivo liver cells of rats by N-nitrosodiethylamine. Mutat Res. 1984;140:181–185. doi: 10.1016/0165-7992(84)90074-5. [DOI] [PubMed] [Google Scholar]
- 16.Perry P, Evans HJ. Cytological detection of mutagen-carcinogen exposure by sister chromatid exchange. Nature. 1975;258:121–125. doi: 10.1038/258121a0. [DOI] [PubMed] [Google Scholar]
- 17.Morgan AR, Pulleyblank DE. Native and denatured DNA, cross-linked and palindromic DNA and circular covalently-closed DNA analysed by a sensitive fluorometric procedure. Biochem Biophys Res Commun. 1974;61:396–403. doi: 10.1016/0006-291x(74)90970-x. [DOI] [PubMed] [Google Scholar]
- 18.Gupta RC. Nonrandom binding of the carcinogen N-hydroxy-2-acetylaminofluorene to repetitive sequences of rat liver DNA in vivo. Proc Natl Acad Sci USA. 1984;81:6943–6947. doi: 10.1073/pnas.81.22.6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basak J. Estimation of single-strand breaks induced in the dried film of DNA by high energy alpha particle from a cyclotron. Indian J Biochem Biophys. 1996;33:35–38. [PubMed] [Google Scholar]
- 20.Akase T, Hihara E, Shimada T, Kojima K, Akase T, Tashiro S, Aburada M. Efficacy of Tokishakuyakusan on the anemia in the iron-deficient pregnant rats. Biol Pharm Bull. 2007;30:1523–1528. doi: 10.1248/bpb.30.1523. [DOI] [PubMed] [Google Scholar]
- 21.Lee MK, Cho SY, Kim DJ, Jang JY, Shin KH, Park SA, Park EM, Lee JS, Choi MS, Lee JS, et al. Du-zhong (Eucommia ulmoides Oliv.) cortex water extract alters heme biosynthesis and erythrocyte antioxidant defense system in lead-administered rats. J Med Food. 2005;8:86–92. doi: 10.1089/jmf.2005.8.86. [DOI] [PubMed] [Google Scholar]
- 22.Huang GW, Yang LY. Metallothionein expression in hepatocellular carcinoma. World J Gastroenterol. 2002;8:650–653. doi: 10.3748/wjg.v8.i4.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carrano AV, Thompson LH, Lindl PA, Minkler JL. Sister chromatid exchange as an indicator of mutagenesis. Nature. 1978;271:551–553. doi: 10.1038/271551a0. [DOI] [PubMed] [Google Scholar]
- 24.Gomer CJ, Rucker N, Banerjee A, Benedict WF. Comparison of mutagenicity and induction of sister chromatid exchange in Chinese hamster cells exposed to hematoporphyrin derivative photoradiation, ionizing radiation, or ultraviolet radiation. Cancer Res. 1983;43:2622–2627. [PubMed] [Google Scholar]
- 25.Weitberg AB, Weitzman SA, Clark EP, Stossel TP. Effects of antioxidants on oxidant-induced sister chromatid exchange formation. J Clin Invest. 1985;75:1835–1841. doi: 10.1172/JCI111897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maxwell SR. Prospects for the use of antioxidant therapies. Drugs. 1995;49:345–361. doi: 10.2165/00003495-199549030-00003. [DOI] [PubMed] [Google Scholar]
- 27.Jackson SP. Sensing and repairing DNA double-strand breaks. Carcinogenesis. 2002;23:687–696. doi: 10.1093/carcin/23.5.687. [DOI] [PubMed] [Google Scholar]
- 28.Richardson C, Jasin M. Frequent chromosomal translocations induced by DNA double-strand breaks. Nature. 2000;405:697–700. doi: 10.1038/35015097. [DOI] [PubMed] [Google Scholar]
- 29.Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643–649. doi: 10.1038/25292. [DOI] [PubMed] [Google Scholar]
- 30.Khanna KK, Jackson SP. DNA double-strand breaks: signaling, repair and the cancer connection. Nat Genet. 2001;27:247–254. doi: 10.1038/85798. [DOI] [PubMed] [Google Scholar]


