Abstract
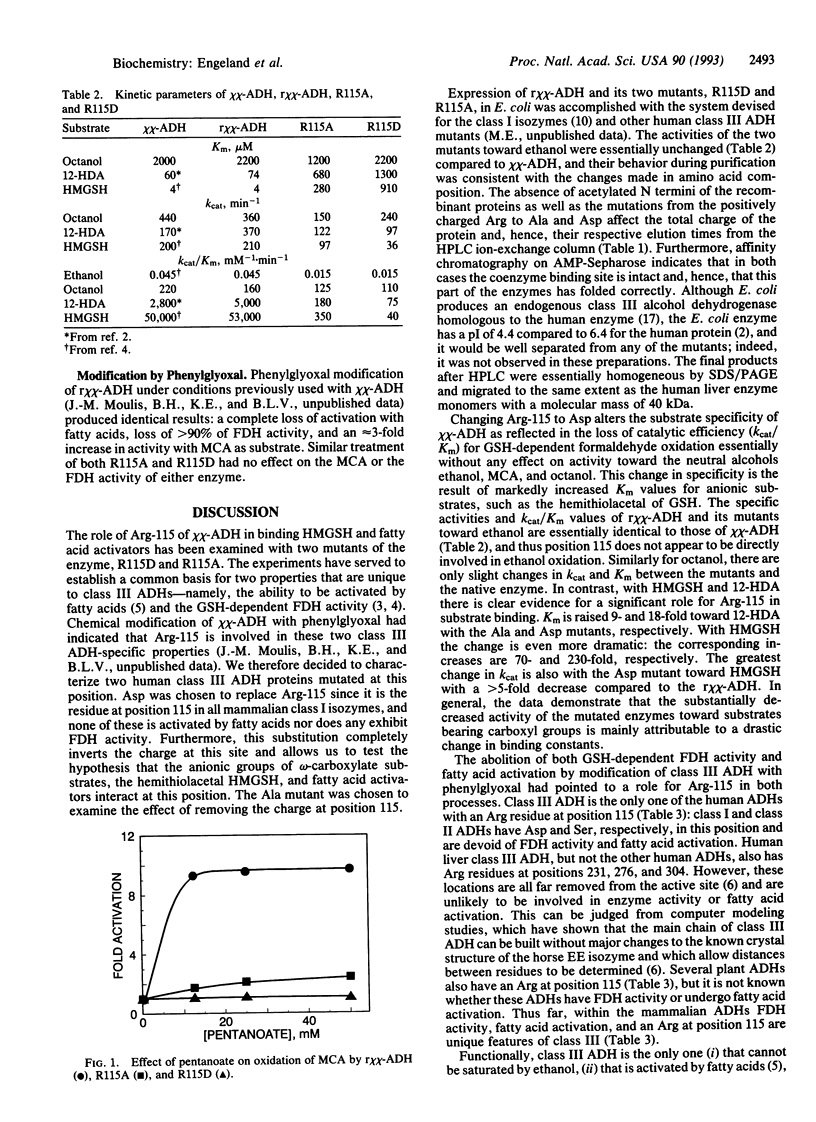
The origin of the fatty acid activation and formaldehyde dehydrogenase activity that distinguishes human class III alcohol dehydrogenase (alcohol:NAD+ oxidoreductase, EC 1.1.1.1) from all other alcohol dehydrogenases has been examined by site-directed mutagenesis of its Arg-115 residue. The Ala- and Asp-115 mutant proteins were expressed in Escherichia coli and purified by affinity chromatography and ion-exchange HPLC. The activities of the recombinant native and mutant enzymes toward ethanol are essentially identical, but mutagenesis greatly decreases the kcat/Km values for glutathione-dependent formaldehyde oxidation. The catalytic efficiency for the Asp variant is < 0.1% that of the unmutated enzyme, due to both a higher Km and a lower kcat value. As with the native enzyme, neither mutant can oxidize methanol, be saturated by ethanol, or be inhibited by 4-methylpyrazole; i.e., they retain these class III characteristics. In contrast, however, their activation by fatty acids, another characteristic unique to class III alcohol dehydrogenase, is markedly attenuated. The Ala mutant is activated only slightly, but the Asp mutant is not activated at all. The results strongly indicate that Arg-115 in class III alcohol dehydrogenase is a component of the binding site for activating fatty acids and is critical for the binding of S-hydroxymethylglutathione in glutathione-dependent formaldehyde dehydrogenase activity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Danielsson O., Eklund H., Jörnvall H. The major piscine liver alcohol dehydrogenase has class-mixed properties in relation to mammalian alcohol dehydrogenases of classes I and III. Biochemistry. 1992 Apr 21;31(15):3751–3759. doi: 10.1021/bi00130a004. [DOI] [PubMed] [Google Scholar]
- Danielsson O., Jörnvall H. "Enzymogenesis": classical liver alcohol dehydrogenase origin from the glutathione-dependent formaldehyde dehydrogenase line. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9247–9251. doi: 10.1073/pnas.89.19.9247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egestad B., Estonius M., Danielsson O., Persson B., Cederlund E., Kaiser R., Holmquist B., Vallee B., Parés X., Jefferey J. Fast atom bombardment mass spectrometry and chemical analysis in determinations of acyl-blocked protein structures. FEBS Lett. 1990 Aug 20;269(1):194–196. doi: 10.1016/0014-5793(90)81152-e. [DOI] [PubMed] [Google Scholar]
- Eklund H., Müller-Wille P., Horjales E., Futer O., Holmquist B., Vallee B. L., Hög J. O., Kaiser R., Jörnvall H. Comparison of three classes of human liver alcohol dehydrogenase. Emphasis on different substrate binding pockets. Eur J Biochem. 1990 Oct 24;193(2):303–310. doi: 10.1111/j.1432-1033.1990.tb19337.x. [DOI] [PubMed] [Google Scholar]
- Estonius M., Karlsson C., Fox E. A., Hög J. O., Holmquist B., Vallee B. L., Davidson W. S., Jörnvall H. Avian alcohol dehydrogenase: the chicken liver enzyme. Primary structure, cDNA-cloning, and relationships to other alcohol dehydrogenases. Eur J Biochem. 1990 Dec 12;194(2):593–602. doi: 10.1111/j.1432-1033.1990.tb15657.x. [DOI] [PubMed] [Google Scholar]
- Giri P. R., Linnoila M., O'Neill J. B., Goldman D. Distribution and possible metabolic role of class III alcohol dehydrogenase in the human brain. Brain Res. 1989 Feb 27;481(1):131–141. doi: 10.1016/0006-8993(89)90493-9. [DOI] [PubMed] [Google Scholar]
- Gutheil W. G., Holmquist B., Vallee B. L. Purification, characterization, and partial sequence of the glutathione-dependent formaldehyde dehydrogenase from Escherichia coli: a class III alcohol dehydrogenase. Biochemistry. 1992 Jan 21;31(2):475–481. doi: 10.1021/bi00117a025. [DOI] [PubMed] [Google Scholar]
- Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989 Apr 15;77(1):51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Holmquist B., Vallee B. L. Human liver class III alcohol and glutathione dependent formaldehyde dehydrogenase are the same enzyme. Biochem Biophys Res Commun. 1991 Aug 15;178(3):1371–1377. doi: 10.1016/0006-291x(91)91045-e. [DOI] [PubMed] [Google Scholar]
- Hög J. O., Eklund H., Jörnvall H. A single-residue exchange gives human recombinant beta beta alcohol dehydrogenase gamma gamma isozyme properties. Eur J Biochem. 1992 Apr 15;205(2):519–526. doi: 10.1111/j.1432-1033.1992.tb16808.x. [DOI] [PubMed] [Google Scholar]
- Hög J. O., Weis M., Zeppezauer M., Jörnvall H., von Bahr-Lindström H. Expression in Escherichia coli of active human alcohol dehydrogenase lacking N-terminal acetylation. Biosci Rep. 1987 Dec;7(12):969–974. doi: 10.1007/BF01122131. [DOI] [PubMed] [Google Scholar]
- Julià P., Pareś X., Jörnvall H. Rat liver alcohol dehydrogenase of class III. Primary structure, functional consequences and relationships to other alcohol dehydrogenases. Eur J Biochem. 1988 Feb 15;172(1):73–83. doi: 10.1111/j.1432-1033.1988.tb13857.x. [DOI] [PubMed] [Google Scholar]
- Jörnvall H., Persson B., Jeffery J. Characteristics of alcohol/polyol dehydrogenases. The zinc-containing long-chain alcohol dehydrogenases. Eur J Biochem. 1987 Sep 1;167(2):195–201. doi: 10.1111/j.1432-1033.1987.tb13323.x. [DOI] [PubMed] [Google Scholar]
- Kaiser R., Holmquist B., Hempel J., Vallee B. L., Jörnvall H. Class III human liver alcohol dehydrogenase: a novel structural type equidistantly related to the class I and class II enzymes. Biochemistry. 1988 Feb 23;27(4):1132–1140. doi: 10.1021/bi00404a009. [DOI] [PubMed] [Google Scholar]
- Kaiser R., Holmquist B., Vallee B. L., Jörnvall H. Characteristics of mammalian class III alcohol dehydrogenases, an enzyme less variable than the traditional liver enzyme of class I. Biochemistry. 1989 Oct 17;28(21):8432–8438. doi: 10.1021/bi00447a024. [DOI] [PubMed] [Google Scholar]
- Koivusalo M., Baumann M., Uotila L. Evidence for the identity of glutathione-dependent formaldehyde dehydrogenase and class III alcohol dehydrogenase. FEBS Lett. 1989 Oct 23;257(1):105–109. doi: 10.1016/0014-5793(89)81797-1. [DOI] [PubMed] [Google Scholar]
- Lutz R. A., Bull C., Rodbard D. Computer analysis of enzyme-substrate-inhibitor kinetic data with automatic model selection using IBM-PC compatible microcomputers. Enzyme. 1986;36(3):197–206. doi: 10.1159/000469292. [DOI] [PubMed] [Google Scholar]
- Moulis J. M., Holmquist B., Vallee B. L. Hydrophobic anion activation of human liver chi chi alcohol dehydrogenase. Biochemistry. 1991 Jun 11;30(23):5743–5749. doi: 10.1021/bi00237a016. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma C. P., Fox E. A., Holmquist B., Jörnvall H., Vallee B. L. cDNA sequence of human class III alcohol dehydrogenase. Biochem Biophys Res Commun. 1989 Oct 31;164(2):631–637. doi: 10.1016/0006-291x(89)91507-6. [DOI] [PubMed] [Google Scholar]
- Taylor J. W., Ott J., Eckstein F. The rapid generation of oligonucleotide-directed mutations at high frequency using phosphorothioate-modified DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8765–8785. doi: 10.1093/nar/13.24.8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner F. W., Parés X., Holmquist B., Vallee B. L. Physical and enzymatic properties of a class III isozyme of human liver alcohol dehydrogenase: chi-ADH. Biochemistry. 1984 May 8;23(10):2193–2199. doi: 10.1021/bi00305a014. [DOI] [PubMed] [Google Scholar]
- Yasunami M., Chen C. S., Yoshida A. A human alcohol dehydrogenase gene (ADH6) encoding an additional class of isozyme. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7610–7614. doi: 10.1073/pnas.88.17.7610. [DOI] [PMC free article] [PubMed] [Google Scholar]



