Abstract
AIM: To evaluate the predictive value of D-dimer as a predictive indicator of portal vein thrombosis (PVT) after portal hypertension surgery in hepatitis B virus-related cirrhosis.
METHODS: A prospective study was carried out in 52 patients who had undergone surgery for portal hypertension in hepatitis B virus-related cirrhosis. Changes in perioperative dynamic D-dimer were observed. The sensitivity, specificity, positive predictive values and negative predictive values of D-dimer were calculated, and ROC curves were analyzed.
RESULTS: The D-dimer levels in the group developing postoperative PVT was significantly higher than those in the group not developing PVT (P = 0.001), and the ROC semi-quantitative and qualitative analysis of D-dimer showed a moderate predictive value in PVT (semi-quantitative value Az = 0.794, P = 0.000; qualitative analysis: Az = 0.739, P = 0.001).
CONCLUSION: Dynamic monitoring of D-dimer levels in patients with portal hypertension after surgery can help early diagnosis of PVT, as in cases where the D-dimer levels steadily increase and exceed 16 μg/mL, the possibility of PVT is very high.
Keywords: Portal hypertension, Portal vein thrombosis, Splenectomy, D-dimer, Diagnosis
INTRODUCTION
Portal vein thrombosis (PVT) is a significant postoperative complication in patients with portal hypertension, and procedures such as devascularization, portal systemic shunt and liver transplantation can cause PVT[1-3]. As the degree of obstruction and location vary greatly, the clinical manifestations of PVT are also highly variable[4-6]. Depending on the severity of the condition, it may even result in death of patients.
The incidence of PVT after portal hypertension surgery ranges from 22.2%-37.5%[1,7], depending on the surgical techniques used and patients selected. In China, the most common cause for portal hypertension is liver cirrhosis due to hepatitis B. There is an increasing trend in PVT after portal hypertension surgery for hepatitis B liver cirrhosis.
Currently, diagnosis of portal vein thrombosis is mainly based on imaging studies[8-10], especially colour Doppler ultrasound, because of its ability to provide not only information on blood vessel location, blood flow rate and direction, but also haemodynamic information, which aids diagnosis accuracy[11,12]. It is able to provide information on the thrombosed veins via echo analysis, detect the presence of post-stenotic dilatation, as well as flow defects and turbulence due to venous thrombosis[13,14]. Although portal vein thrombosis can be accurately diagnosed using this method, it produces side effects simultaneously. Therefore, the focus of clinicians is to prevent portal vein thrombosis after portal hypertension surgery as early as possible, the early predictive indicator of postoperative PVT is still lacking.
Currently, clinicians use the incrteased platelet count as the main deciding factor for initiating prophylactic treatment, but the prognostic value of platelet count in PVT is debatable[15,16]. Some studies showed that even at the time of portal vein thrombosis formation, the level of platelet count has not yet reached 500 × 109/L, and patients showing increased levels of platelet count do not have portal vein thrombosis[17]. Hence, the question of the indicator for initiation of preventive and therapeatic measures is still debatable.
Clotting factors are important in the assessment of liver function and have certain clinical significance, but have yet to show a good clinical value as a diagnostic indicator for thrombotic diseases. At present, D-dimer, the molecular marker in the fibrinolysis cycle, has shown a good negative and positive predictive value in thrombotic diseases such as deep vein thrombosis in the lower limbs and pulmonary embolism, and has therefore been used in studies on its clinical application[18,19]. However, since patients with portal hypertension due to liver cirrhosis have innate clotting and fibrinolytic disorders[20,21], more studies are required to show whether D-dimer has a better predictive value PVT after portal hypertension surgery due to the combined effects of portal vein thrombosis on platelets, clotting and fibrinolysis[22].
MATERIALS AND METHODS
Patient selection criteria
Patients who were admitted to the Third Affiliated Hospital of the Sun Yat-Sen University from September 2004 to March 2006, and diagnosed with portal hypertension due to liver cirrhosis (hepatitis B) were included in this study. The patients with splenomegalia and hypersplenia underwent splenectomy, and the patients with esophageal variceal bleeding underwent splenectomy with gastroesophageal devascularization, or with endoscopic variceal ligation (EVL). Patients who underwent portal hypertension shunting surgery, liver transplant patients, and patients having concurrent liver cirrhosis and hepatocellular carcinoma, and patients diagnosed with PVT before surgery, were excluded from this study.
General demographics
A total of 52 patients (46 males and 6 females) fulfilled the selection criteria. Their age ranged from 20-60 years, with a median age of 46 years (Table 1). Surgical procedures performed on patients included splenectomy and gastroesophageal devascularization (Hassab's operation) as previously described[23]. In brief, an extended left subcostal incision or a L incision of the left upper abdomen was used for extreme splenomegaly. After routine splenectomy, the gastric branch and 5-8 small branches of the gastric coronary veins were disconnected. The esophageal branch was disconnected and suture-ligated. The gastric posterior vein was ligated by suturing, and the left subphrenic vein was ligated as well. In cases of splenectomy with EVL, after splenectomy, an endoscope (GIF 240 or 260, Olympus Optical, Tokyo) was introduced during operation. Ligation was carried out 6-12 times by placing a single rubber band (Bard Interventional Products, Tewksbury, Mass.) over a varix.
Table 1.
Characteristics of 52 patients studied
| Thrombus | Non-thrombus | P value | ||
| Age ( mean ± SD ) | 41.45 ± 10.83 | 44.3 ± 8.65 | 0.388 | |
| Gender | Male | 15 | 31 | |
| Female | 2 | 4 | 0.649 | |
| Child's grade of Liver function | Grade A | 6 | 12 | |
| Grade B | 10 | 21 | 0.997 | |
| Grade C | 1 | 2 | ||
| Surgical procedure | Splenectomy | 7 | 13 | |
| Splenectomy + EVL1 | 9 | 19 | 0.923 | |
| Splenectomy + ligation2 | 1 | 3 |
Refers to endoscopic variceal ligation;
Refers to gastroesophageal devascularization.
Diagnosis of PVT
When colour Doppler ultrasonography imaging showed changes consistent with thrombus formation in the main portal vein, right portal vein, right anterior branch, right posterior branch, left portal vein, left horizontal branch, left sagittal portion, splenic vein, proximal and distal mesenteric vein, PVT was confirmed to be a complication after surgery.
Measurement of D-dimer levels
Dynamic D-dimer changes in 52 patients were monitored perioperatively, and the portal vein patency and the incidence of PVT were detected routinely by color Doppler ultrasonography from 7 to 14 d postoperatively. The 52 patients were divided into thrombosis group and non-thrombosis group, depending on the occurrence of PVT after surgery. Peripheral venous blood was collected perioperatively. Three mL blood was centrifuged at 1000 r/min for 15 min before plasma was prepared for D-dimer detection. D-dimer was detected by latex-agglutination assay (semi-quantitative method), and the reagent was provided by Shanghai Sun Company, and the procedure was performed following the instructions provided with the reagent pack.
Statistical analysis
After normality tests, D-dimer indicators showing a skewed distribution were subjected to signed rank test between the thrombosis and non-thrombosis groups, using the difference in the median and interquartile range to illustrate the central and disperse tendency. Chi-square test was used to analyze the dispersion. Sensitivity, specificity, positive and negative predictive value were calculated. ROC curve was plotted. Statistical analysis was performed using the SPSS software version 13.0 for Windows.
RESULTS
Characteristics of the 52 patients studied
Of the 52 patients, 17 developed PVT after surgery, and the incidence of PVT 7-14 d after portal hypertension surgery was 33.69%. There was no significant statistical difference in age, gender, liver function and surgical procedure between the thrombosis and non-thrombosis groups (Table 1).
Perioperative D-dimer level
The D-dimer level was significantly higher in the thrombosis group than in the non-thrombus group on day 5 after surgery (P = 0.001) (Table 2).
Table 2.
D-dimer level in the thrombosis and non-thrombosis groups (μg/mL)
| Before surgery | Day 1 after surgery | Day 5 after surgery | |
| Non-thrombus group | 1 (0.5, 2) | 4 (2, 8)1 | 4 (2, 4)12 |
| Thrombus group | 1 (0.5, 2) | 4 (2, 8)1 | 8 (4, 8)13 |
| Z value | 1.863 | 0.318 | 3.264 |
| P value | 0.062 | 0.751 | 0.001 |
Refers to the higher level that is statistically significant after surgery than that before surgery;
Refers to the lower level that is statistically significant before surgery than that one day after surgery;
Refers to the higher level that is statistically significant before surgery than that one day after surgery.
Analysis of predictive value for D-dimer in PVT after surgery
By using the change in D-dimer level post-surgery (used as the standard) and the D-dimer (used as semi-quantitative standard) as the diagnostic standard for PVT in liver cirrhosis patients after portal hypertension surgery, we achieved 37.5% accuracy in predicting PVT incidence, which is consistent with the reported data[1]. The sensitivity (Sen), specificity (Spe), positive predictive value (PPV) and negative predictive value (NPV) are listed in Table 3. It can be seen from Table 3 that the positive predictive value was 66.7% for the D-dimer one day after surgery, and the negative predictive value for the continuous increasing was 77.5%, which was significantly higher than the semi-quantitative value of 61.65% for PPV and 70.83% for NPV. Under the extreme circumstances, the semi-quantitative value for PPV and NPV was 100%, respectively.
Table 3.
Diagnostic value for different D-dimer diagnostic standards (%)
| Sensitivity | Specificity | PPV | NPV | ||
| Qualitative standard | No decrease Increase | 88.2 47.06 | 57.14 88.57 | 42.85 66.67 | 88.25 77.50 |
| Semi-quantitative standard | > 0.5 > 1 | 1 1 | 0 0.05714 | 37.50 38.88 | 0 100 |
| > 2 | 0.8235 | 0.4858 | 49 | 82.00 | |
| > 4 | 0.4118 | 0.8571 | 61.65 | 70.83 | |
| > 8 | 0.2353 | 1 | 100.00 | 33.89 | |
| > 16 | 0.2353 | 1 | 100.00 | 33.89 |
Based on the different changes in D-dimer levels and the false positive rates and sensitivity rates under different D-dimer levels, a ROC curve was plotted. The semi-quantitative and qualitative standards for ROC curve are shown in Figure 1. Using the ROC analysis, the area under the semi-qualitative ROC curve was Az = 0.794, P = 0.000, and the 95% confidence level was 0.794 ± 0.114. The area under the qualitative ROC curve was Az = 0.739, P = 0.001, and the 95% confidence level was 0.795 ± 0.146. Both standards had a median diagnostic value, and there was no statistical significant difference in the areas under the ROC curve (Z = 0.586, P = 0.719).
Figure 1.
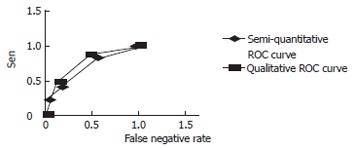
Semi-quantitative and qualititive ROC curves.
DISCUSSION
D-dimer is an antigenic determinant of fibrin, which, at the D-region, combines with factor XIIIa, and persists as an X-oligopolymer, as well as with other fibrin degradation products. However, it does not exist with profibrin breakdown products, and is a specific indicator of synthesis and breakdown of fibrin in the system. An increasing D-dimer level indicates active clotting and fibrinolysis in the system[24,25].
At present, D-dimer has a good negative and positive predictive value, which is used in the outpatient and emergency setting, to aid selective diagnosis of suspected thrombotic diseases[19,26-28]. Risch et al[29] reported that at D-dimer levels of 1 μg/mL and 10 μ/mL (VIDAS DD), the sensitivity is 83.3% and 23.8%, respectively, and the specificity is 65.8% and 98.7%, respectively. In our study, the D-dimer level was higher than the reported level in liver cirrhosis patients after portal hypertension surgery, measured at similar sensitivity conditions, but had a lower specificity than the reported one. This may be attributed to the hyperactive state of the coagulation pathway due to the high coagulability state in liver cirrhosis patients, activated fibrinolysis, blood products used perioperatively, and trauma due to surgery[20-22,30].
Dynamic D-dimer value monitoring during the early stages after portal hypertension surgery may be more useful in achieving a meaningful diagnosis of PVT due to thrombosis formation. By analyzing the D-dimer after surgery and its predictive value for PVT, our study proved that the positive predictive value was 100% at ≥ 16 μg/mL (Table 3). From the ROC curve (Figure 1), we could see that the qualitative standard was situated towards the left upper side of the semi-quantitative standard ROC curve, showing a higher sensitivity, but a lower rate of false positive response. Under the two extreme ends, the semi-quantitative standard curve was on the left-hand-side of the qualitative ROC curve, suggesting that the former may have a better diagnostic value. Therefore, combining semi-quantitative and qualitative measurement may be the best option for diagnosing PVT in liver cirrhosis patients after portal hypertension surgery.
In conclusion, D-dimer is an early predictive indicator of PVT after portal hypertension surgery in hepatitis B virus-related cirrhosis. Dynamic D-dimer monitoring should be performed perioperationly, and if elevation of the D-dimer levels persists or exceeds 16 μg/mL, PVT may occur, and anti-coagulation prevention and treatment should be considered.
COMMENTS
Background
Portal vein thrombosis (PVT) is a significant complication after portal hypertension surgery due to liver cirrhosis. The focus of clinicians is to prevent it as early as possible, but we still lack the early predictive indicator of postoperative PVT.
Research frontiers
PVT is a significant postoperative complication in patients with portal hypertension, especially after splenectomy. Since the mechanism of postoperative PVT remains unknown, it is difficult to prevent postoperative PVT. Most studies indicate that local operation may be the factor for postoperative PVT. Some studies indicate that D-dimer may be helpful in the diagnosis of deep vein thrombosis of lower extremity.
Innovations and breakthroughs
At present, most researchers and doctors diagnose PVT via imageology, especially color Doppler ultrasonography, and some reports indicate that platelets may be the predictive factor for PVT. D-dimer is a good predictive factor for deep vein thrombosis of lower extremity. The results of our study indicate that D-dimer may be a predictive factor for PVT after portal hypertension surgery in hepatitis B virus-related cirrhosis.
Applications
Since D-dimer is a predictive factor for PVT, PVT and its fatal complications can be prevented. By combining imageology, we can institute a scheme for the management of PVT, which helps to understand the mechanism of PVT.
Terminology
D-dimer: known as fragment D-dimer, fibrin degradation fragment; PVT: portal vein thrombosis. ROC curve: a graphic means for assessing the ability of a screening test to discriminate between healthy and diseased persons.
Peer review
This is a well-conducted study. It describes another way for the predictive diagnosis of PVT, but the relationship between the mechanisms of PVT and D-dimer remains unknown. D-dimer is only of a predictive diagnostic value.
Footnotes
Supported by Technology Support Fund of Guangdong Province, No. 2004B35001007
S- Editor Liu Y L- Editor Wang XL E- Editor Wang HF
References
- 1.Dai ZB, Peng ZH. Portal hypertension and portal venous thrombosis. Puwai Linchuang. 1993;8:150–152. [Google Scholar]
- 2.Mercado MA, Orozco H, Guillén-Navarro E, Acosta E, López-Martínez LM, Hinojosa C, Hernández J, Tielve M. Small-diameter mesocaval shunts: a 10-year evaluation. J Gastrointest Surg. 2000;4:453–457. doi: 10.1016/s1091-255x(00)80085-2. [DOI] [PubMed] [Google Scholar]
- 3.Settmacher U, Nüssler NC, Glanemann M, Haase R, Heise M, Bechstein WO, Neuhaus P. Venous complications after orthotopic liver transplantation. Clin Transplant. 2000;14:235–241. doi: 10.1034/j.1399-0012.2000.140309.x. [DOI] [PubMed] [Google Scholar]
- 4.Wang MC, Li S, Zhu JY, Leng XS, Du RY. [The reason and treatment of portal vein thrombosis in patients with portal hypertension postoperation] Zhonghua Waike Zazhi. 2004;42:269–271. [PubMed] [Google Scholar]
- 5.de Cleva R, Herman P, Saad WA, Pugliese V, Zilberstein B, Rodrigues JJ, Laudanna AA. Postoperative portal vein thrombosis in patients with hepatosplenic mansonic schistosomiasis: relationship with intraoperative portal pressure and flow. A prospective study. Hepatogastroenterology. 2005;52:1529–1533. [PubMed] [Google Scholar]
- 6.Condat B, Pessione F, Helene Denninger M, Hillaire S, Valla D. Recent portal or mesenteric venous thrombosis: increased recognition and frequent recanalization on anticoagulant therapy. Hepatology. 2000;32:466–470. doi: 10.1053/jhep.2000.16597. [DOI] [PubMed] [Google Scholar]
- 7.Okuda K, Ohnishi K, Kimura K, Matsutani S, Sumida M, Goto N, Musha H, Takashi M, Suzuki N, Shinagawa T. Incidence of portal vein thrombosis in liver cirrhosis. An angiographic study in 708 patients. Gastroenterology. 1985;89:279–286. doi: 10.1016/0016-5085(85)90327-0. [DOI] [PubMed] [Google Scholar]
- 8.Kreft B, Strunk H, Flacke S, Wolff M, Conrad R, Gieseke J, Pauleit D, Bachmann R, Hirner A, Schild HH. Detection of thrombosis in the portal venous system: comparison of contrast-enhanced MR angiography with intraarterial digital subtraction angiography. Radiology. 2000;216:86–92. doi: 10.1148/radiology.216.1.r00jl2386. [DOI] [PubMed] [Google Scholar]
- 9.Shah TU, Semelka RC, Voultsinos V, Elias J Jr, Altun E, Pamuklar E, Firat Z, Gerber DA, Fair J, Russo MW. Accuracy of magnetic resonance imaging for preoperative detection of portal vein thrombosis in liver transplant candidates. Liver Transpl. 2006;12:1682–1688. doi: 10.1002/lt.20873. [DOI] [PubMed] [Google Scholar]
- 10.Ikeda M, Sekimoto M, Takiguchi S, Kubota M, Ikenaga M, Yamamoto H, Fujiwara Y, Ohue M, Yasuda T, Imamura H, et al. High incidence of thrombosis of the portal venous system after laparoscopic splenectomy: a prospective study with contrast-enhanced CT scan. Ann Surg. 2005;241:208–216. doi: 10.1097/01.sla.0000151794.28392.a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yerdel MA, Gunson B, Mirza D, Karayalçin K, Olliff S, Buckels J, Mayer D, McMaster P, Pirenne J. Portal vein thrombosis in adults undergoing liver transplantation: risk factors, screening, management, and outcome. Transplantation. 2000;69:1873–1881. doi: 10.1097/00007890-200005150-00023. [DOI] [PubMed] [Google Scholar]
- 12.Tarantino L, Francica G, Sordelli I, Esposito F, Giorgio A, Sorrentino P, de Stefano G, Di Sarno A, Ferraioli G, Sperlongano P. Diagnosis of benign and malignant portal vein thrombosis in cirrhotic patients with hepatocellular carcinoma: color Doppler US, contrast-enhanced US, and fine-needle biopsy. Abdom Imaging. 2006;31:537–544. doi: 10.1007/s00261-005-0150-x. [DOI] [PubMed] [Google Scholar]
- 13.Tessler FN, Gehring BJ, Gomes AS, Perrella RR, Ragavendra N, Busuttil RW, Grant EG. Diagnosis of portal vein thrombosis: value of color Doppler imaging. AJR Am J Roentgenol. 1991;157:293–296. doi: 10.2214/ajr.157.2.1853809. [DOI] [PubMed] [Google Scholar]
- 14.Rossi S, Rosa L, Ravetta V, Cascina A, Quaretti P, Azzaretti A, Scagnelli P, Tinelli C, Dionigi P, Calliada F. Contrast-enhanced versus conventional and color Doppler sonography for the detection of thrombosis of the portal and hepatic venous systems. AJR Am J Roentgenol. 2006;186:763–773. doi: 10.2214/AJR.04.1218. [DOI] [PubMed] [Google Scholar]
- 15.Ziemski JM, Rudowski WJ, Jaskowiak W, Rusiniak L, Scharf R. Evaluation of early postsplenectomy complications. Surg Gynecol Obstet. 1987;165:507–514. [PubMed] [Google Scholar]
- 16.Lee JJ, Kim HJ, Chung IJ, Park MR, Seo KS, Jeong YY, Kim JK. Portal, mesenteric, and splenic vein thromboses after splenectomy in a patient with chronic myeloid leukemia variant with thrombocythemic onset. Am J Hematol. 1999;61:212–215. doi: 10.1002/(sici)1096-8652(199907)61:3<212::aid-ajh10>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 17.Winslow ER, Brunt LM, Drebin JA, Soper NJ, Klingensmith ME. Portal vein thrombosis after splenectomy. Am J Surg. 2002;184:631–635; discussion 631-635;. doi: 10.1016/s0002-9610(02)01095-4. [DOI] [PubMed] [Google Scholar]
- 18.Rathbun SW, Whitsett TL, Raskob GE. Negative D-dimer result to exclude recurrent deep venous thrombosis: a management trial. Ann Intern Med. 2004;141:839–845. doi: 10.7326/0003-4819-141-11-200412070-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardiner C, Pennaneac'h C, Walford C, Machin SJ, Mackie IJ. An evaluation of rapid D-dimer assays for the exclusion of deep vein thrombosis. Br J Haematol. 2005;128:842–848. doi: 10.1111/j.1365-2141.2005.05394.x. [DOI] [PubMed] [Google Scholar]
- 20.Violi F, Ferro D, Basili S, Saliola M, Quintarelli C, Alessandri C, Cordova C. Association between low-grade disseminated intravascular coagulation and endotoxemia in patients with liver cirrhosis. Gastroenterology. 1995;109:531–539. doi: 10.1016/0016-5085(95)90342-9. [DOI] [PubMed] [Google Scholar]
- 21.Colucci M, Binetti BM, Branca MG, Clerici C, Morelli A, Semeraro N, Gresele P. Deficiency of thrombin activatable fibrinolysis inhibitor in cirrhosis is associated with increased plasma fibrinolysis. Hepatology. 2003;38:230–237. doi: 10.1053/jhep.2003.50277. [DOI] [PubMed] [Google Scholar]
- 22.Hunt BJ, Parratt RN, Segal HC, Sheikh S, Kallis P, Yacoub M. Activation of coagulation and fibrinolysis during cardiothoracic operations. Ann Thorac Surg. 1998;65:712–718. doi: 10.1016/s0003-4975(97)01345-3. [DOI] [PubMed] [Google Scholar]
- 23.Yang Z, Qiu F. [Pericardial devascularization with splenectomy for the treatment of portal hypertension] Zhonghua Waike Zazhi. 2000;38:645–648. [PubMed] [Google Scholar]
- 24.Fimognari FL, De Santis A, Piccheri C, Moscatelli R, Gigliotti F, Vestri A, Attili A, Violi F. Evaluation of D-dimer and factor VIII in cirrhotic patients with asymptomatic portal venous thrombosis. J Lab Clin Med. 2005;146:238–243. doi: 10.1016/j.lab.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 25.Hafner JW, Gerdes E, Aldag JC. Combining clinical risk with D-dimer testing to rule out acute deep venous thrombosis (DVT) J Emerg Med. 2006;30:100–101. doi: 10.1016/j.jemermed.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 26.Dempfle C, Schraml M, Besenthal I, Hansen R, Gehrke J, Korte W, Risch M, Quehenberger P, Handler S, Minar E, et al. Multicentre evaluation of a new point-of-care test for the quantitative determination of D-dimer. Clin Chim Acta. 2001;307:211–218. doi: 10.1016/s0009-8981(01)00436-3. [DOI] [PubMed] [Google Scholar]
- 27.Legnani C, Fariselli S, Cini M, Oca G, Abate C, Palareti G. A new rapid bedside assay for quantitative testing of D-Dimer (Cardiac D-Dimer) in the diagnostic work-up for deep vein thrombosis. Thromb Res. 2003;111:149–153. doi: 10.1016/j.thromres.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 28.Wells PS, Anderson DR, Rodger M, Forgie M, Kearon C, Dreyer J, Kovacs G, Mitchell M, Lewandowski B, Kovacs MJ. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med. 2003;349:1227–1235. doi: 10.1056/NEJMoa023153. [DOI] [PubMed] [Google Scholar]
- 29.Risch L, Monn A, Lüthy R, Honegger H, Huber AR. The predictive characteristics of D-dimer testing in outpatients with suspected venous thromboembolism: a Bayesian approach. Clin Chim Acta. 2004;345:79–87. doi: 10.1016/j.cccn.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 30.Ogren M, Bergqvist D, Björck M, Acosta S, Eriksson H, Sternby NH. Portal vein thrombosis: prevalence, patient characteristics and lifetime risk: a population study based on 23,796 consecutive autopsies. World J Gastroenterol. 2006;12:2115–2119. doi: 10.3748/wjg.v12.i13.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]


