Abstract
AIM: To elucidate the expression of E-cadherin and β-catenin correlating with its clinical outcome in patients with esophageal squamous cell carcinoma (ESCC), by analyzing their interrelationship with clinicopathological variables and their effects on progress and prognosis.
METHODS: Expression of E-cadherin and β-catenin was determined by SP immunohistochemical technique in patients with ESCC consecutively, their correlation with clinical characteristics was evaluated and analyzed by multivariate analysis.
RESULTS: The rate of expression of E-cadherin decreased to 66.03% (70/106) in ESCC and the protein level was negative correlated with histologic grade, tumor size, clinical staging, lymph node metastasis and venous invasion. Whereas the expression rate of β-catenin was reduced to 69.8% (74/106) and the level of protein expression correlated only with histologic grade. There obviously existed inverse correlation between level of E-cadherin protein and survival, especially in stage I, IIa, IIb (P = 0.0033), Patients with low-expressing tumors for β-catenin and non-expressing tumors for E-cadherin/β-catenin had lower survival period than those with normal-expressing ones (P = 0.0501 and P = 0.0080, respectively). Patients with diminished expression of E-cadherin as grade II or III had shorter survival period than those with normally expressing and grade I, no significance existed between grade I and grade II or III with respect to different status of E-cadherin expression. Furthermore, Correlation analysis showed level of E-cadherin correlated with that of β-catenin (P = 0.005). Cox proportional hazards model analysis suggested downregulation of E-cadherin was an important factor indicating poor prognosis.
CONCLUSION: As a probable independent prognostic factor, it correlates with overall and disease free survival period, expression of E-cadherin but not β-catenin may predict prognosis in patients with ESCC.
INTRODUCTION
Esophageal carcinoma is generally considered as one of the most extremely aggressive carcinomas with dismal prognosis identified thus far[1-5]. In recent years, postoperative survival of the patients with esophageal carcinoma have been improved, However, 5-year survival rate of operative advanced esophageal carcinoma is still 20%-25%. Early diagnosis and treatment are still important[2,3,6-9]. TNM system is always considered as a classic criterion to provide treatments and evaluate prognoses. Unfortunately tumor heterogeneity and individual differences influence its accurate estimation[10]. Therefore, to establish a “molecular staging” system based on materials combining biomarkers and clinical parameters including histologic grade and tumor stage may be helpful to guide individuated treatment and evaluate prognosis. Then some potential molecules remain to be identified[6,8,9,11-15].
Invasion and metastatic processes themselves consist of sequential multi-stage, multi-step involving host-tumor interactions[16]. Some studies showed status of intercellular adherens junction plays a pivotal role in tumor growth, invasion, metastasis and prognosis and the suppression of cell-cell adhesiveness may trigger the release of cancer cells from the primary cancer nests and confer invasive properties on a tumor[16-18]. Detecting expression of adhesion molecules may reflect biological behavior and characteristics of tumor and are conducive to predict and evaluate risk of relapse and metastasis of the patients with postoperative esophageal carcinoma, thus having realistic significance to guide individuated treatment.
The cadherins that exist in many kinds of cells, belong to a family of transmembrane glycoproteins responsible for homophilic interaction of calcium-dependent cell-cell adhesion. Among them, E-cadherin (120 kDa, chromosome 16q22.1) is a classical cadherin and forms the functional component of adherens junctions between epithelial cells, and β-catenin, a multifunctional cytoplasmic protein which links E-cadherin and α-catenin to cytoskeletoh constituted E-cadherin-catenin complex, both have important roles in maintaining integrity of cellular structure[16-20]. Reduction and loss of expression in cancer cells may destroy the junctional structure which can affect the intercellular adhesion, and facilitate tumor differentiation, infiltration and metastasis, Their expression are related to survival and prognosis in a portion of cancer. It had been formed that E-cadherin might be an independent predictor of occult lymph node or micrometastasis in nodes classified as N0 by routine histopathological methods. However, only few studies were available in ESCC[18,21,22].
β-catenin has recently been found as a member in Wnt signaling[23-25]. In the absence of a mitotic signal outside the cell, β-catenin is sequestered in a complex with the APC (adenomatous polyposis coli) gene product, a serine threonine glycogen synthetase kinase (GSK-3β) and an adapter protein axin (or a homologue conductin), enabling phosphorylation and degradation of free β-catenin by the ubiquitin-proteasome system[23-25]. When a mitotic signal is delivered by the Wnt pathway, by association of the Wg/Wnt family of secreted glycoproteins and their membrane receptors frizzled, it leads to activation of the dishevelled (Dsh) protein, which is recruited to the cell membrane. The activated Dsh downregulates the protein complex, so that it can no longer phosphorylate β-catenin, The release of β-catenin from the phosphorylation and degradation complex promotes β-catenin stabilization and signaling. This results in an increase of free cytosolic β-catenin which translocates to the nucleus and directly binds the transcription factors Lef and Tcf, leading to activation of gene expression[26,27].
Here, we performed E-cadherin and β-catenin expression analysis in a 106 ESCC in order to elucidate whether the expression of E-cadherin and β-catenin correlate with clinicopathological variables and clinical outcome, by analyzing their interrelationships and effects on progression of cancer and their prognostic evaluations in ESCC. Furthermore, may provide some considerable suggestions for clinical treatments.
MATERIALS AND METHODS
Clinicopathologic data
The specimens of cancer tissues and non-cancerous adjacent tissues were taken from 106 consecutive patients with esophageal squamous cell carcinoma who had undergone esophagectomy from January 1990 to December 1995 at Cancer Hospital of Tianjin Medical University, Tianjin, China. None of them received irradiation or chemotherapy preoperatively. The patients included 76 men and 30 women with a mean age of 59 (range 37-76) years. Ten tumors were in the upper segment, 68 in the middle segment and 28 in the lower segment. With respect to their growth pattern, they were 20 medullary type, 42 fungating, 34 ulcerative, 9 scirrhous and 1 other type. Of seventy-three patients underwent left thoracic approach esophagectomy, 60 cases had esophagogastric anastomosis above the aortic arch, 10 below the aortic arches and 3 cervical esophagogastric reconstruction, 25 patients underwent right thoracic posteriolateral, abdominal and cervical approach esophagectomy and 8 with other approach. Tumors were staged again according to the TNM classification (UICC1997): 2 patients with stage I, 31 IIa, 10 IIb, 60 III and 3 IV. Postoperative radiotherapy was performed in 12 patients and chemotherapy in 15, Survival periods were calculated from the date of operation to death from recurrence of the disease, Follow-up period ranged from 2 to 117 mo with an average of 32.4 mo.
Immunohistochemical staining
For parallel analyses of staining characteristic of the tumor and normal mucosa, representative tissue section comparing the most part of the tumor and adjacent normal mucosa were selected after the H&E stained slides were reviewed. In brief, Four micrometers thick sections prepared from 100 mL·L-1 neutral buffered formalin-fixed and paraffin-embedded tumor tissue on the slides were deparaffinized, dehydrated and blocked from removing endogenous peroxides activating with 3 mL·L-1 H2O2 in methanol for 30 min. The sections were microwaved in 0.l mol·L-1citrate buffer pH6.0 at 750 W for 12 min. After incubation with 10% normal goat serum to block non-specific binding and were then incubated with the primary antibodies such as mouse monoclonal anti-human E-cadherin (4A2C × 7, Santa Cruz Biotechnology, USA, ready to use) and polyclonal rabbit anti-β-catenin (Sigma Chemical Company, USA, diluted 1:1000 in PBS) overnight at 4 °C. After washing antibody with the SP kit (Zymed, USA) the peroxides reaction was performed using 3, 3’-diaminobenzidine tetrahydrochloride (DAB) (Zhongshan Biological Technology, Beijing, China) as chromogen. Tissues were counterstained with hematoxylin. The slides were washed between each step three times with PBS, Negative control was obtained by replacing the primary antibody with PBS. Adjacent normal squamous epithelium served as an internal positive control.
Evaluation of immunohistochemical staining
Tumor sections were scored by light microscopy by 3 independent observers (Chen H, Li H and Liu XS) without knowledge of the stage and patient profiles. Based on the previous immunohistochemical studies[28], the membranous staining of cells were positively stained by the antibody. Calculating the percentage of positively stained cells evaluated for each tissue section after counting 100 cells at more than 5 high-power (× 200) fields. The expression of E-cadherin and β-catenin were classified into 3 grades, as follows: -, If less than 10% of the tumor cells were positively stained; +, If more than 90% of the tumor cells were positively stained, the expression was judged to be positive; and ±, between 10% and 90% of the tumor cells were evaluated as heterogeneous. The specimens graded + stand for normal expression, ± and - were taken together and classified as reduced expression.
Statistical analysis
The χ2 test and Spearman rank correlation coefficient analysis were used to assess the univariate association between the immunohistochemical status and the clinicopathological characteristics. The survival rate was calculated by life table and cumulative survival curves were constructed according to the Kaplan-Meier method, and differences in survival were estimated with the Log-rank test Cox proportional hazards model was used for multivariate survival analysis. A P value less than 0.05 was considered significant. All the statistical analyses were performed using the SPSS 10.0 V for Windows software.
RESULTS
Expression of E-cadherin and β-catenin in normal esophagus
Normal esophageal epithelium showed strong expression of E-cadherin and β-catenin at the membrane of cell and the intercellular junctions. Expression of these molecules was more marked in the prickle cell layer and para-basal cell layers. No or weak cytoplasmic staining of E-cadherin and no cytoplasmic staining of β-catenin were seen in normal esophageal epithelium. Esophageal glands and their duct also showed expression of these molecules at cell membranes, Whereas non-epithelial cells such as muscular tissue and infiltrating lymphocytes did not.
Expression of E-cadherin and β-catenin in esophageal carcinoma
Reduced homogenous and heterogeneous staining of E-cadherin and β-catenin were found in esophageal carcinoma (Figure 1). In well/moderately differentiated carcinomas, cancer cells from the tumor nest peripheries showed weaker expression of these molecules than those in the centers showing zonal differentiation (Figure 2). Although reduced expression and loss of them were always present in poorly differentiated carcinomas, some showed normal expression of these molecules in neoplastic nests. Reduced membranous expression of E-cadherin and β-catenin were found in 66.0% (70/106) and 69.8% (74/106), respectively.
Figure 1.
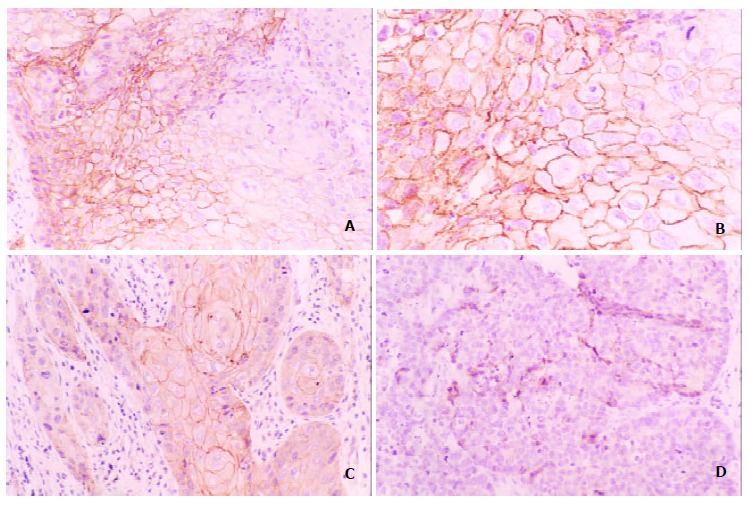
Expression of E-cadherin in esophageal squamous carcinoma tissue, brownish membrane and cytoplasm were shown in part of the tumor cells (A, B positive, C heterogeneous, D negative expression Original magnification × 200; B, Original magnification × 400).
Figure 2.
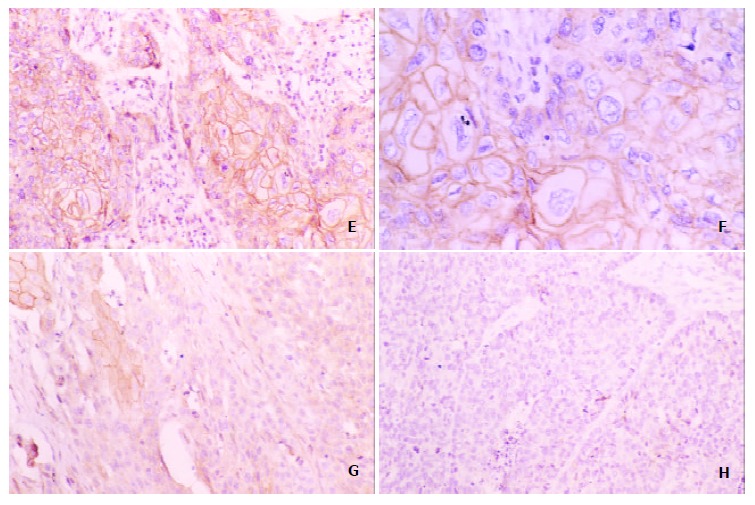
Expression of β-catenin in esophageal squamous carcinoma tissue ,brownish membrane and cytoplasm were shown in part of the tumor cells in the centers showing zonal differentiation (E, F positive, G heterogeneous, H negative expression Original magnification; H, Original magnification × 400)
Expression of E-cadherin and β-catenin and clinocopathologic variables
Significant inverse correlation existed between the intensity of E-cadherin expression and histologic grade, tumor size, clinical staging and venous invasiveness, No significant differences were seen in age, gender, location of tumor, growth pattern and invasion depth (rs = 0.020~0.156, P = 0.084~0.118). Expression of β-catenin correlated significantly only with histologic grade, Whereas no differences were observed in age, gender, location of tumor, tumor size, clinical staging, growth pattern, venous invasiveness and invasion depth (rs = 0.060~0.1569, P = 0.541~0.104), shown in Table 1. In carcinomas with lymph node metastasis, reduced expression of E-cadherin and β-catenin were seen in 76.59% (36/47) and 72.34% (34/47) vs 57.63% (34/59) and 67.79% (40/59) in carcinomas without lymph node metastasis, respectively. The staining of E-cadherin correlated only with lymph node status (P = 0.040) and no significant differences for β-catenin.
Table 1.
Relationships between espression of E-cadherin and β-catenin an clinicopathological parameters in patients with ESCC
| Group | Total |
E-cadherin |
P |
β-catenin |
P | ||||
| + | ± | - | rs | + | ± | - | rs | ||
| Histologic grade | |||||||||
| I | 17 | 8 | 8 | 1 | 9 | 6 | 2 | ||
| II | 69 | 25 | 33 | 11 | 0.001 | 21 | 28 | 20 | 0.014 |
| III | 20 | 3 | 7 | 10 | -0.309 | 2 | 12 | 6 | -0.238 |
| Tumor size (cm) | |||||||||
| < 3.0 | 10 | 6 | 2 | 2 | 0 | 5 | 5 | ||
| 3.0-5.0 | 41 | 16 | 18 | 7 | 15 | 15 | 11 | ||
| 5.0-7.0 | 44 | 11 | 24 | 9 | 0.054 | 12 | 24 | 8 | 0.213 |
| ≤ 7.0 | 11 | 3 | 4 | 4 | -0.187 | 5 | 2 | 4 | -0.212 |
| Clinical staging | |||||||||
| I | 2 | 2 | 0 | 0 | 0 | 2 | 0 | ||
| IIa | 31 | 16 | 11 | 4 | 13 | 13 | 5 | ||
| IIb | 10 | 2 | 5 | 3 | 0.013 | 3 | 2 | 5 | 0.124 |
| III | 60 | 15 | 31 | 14 | -0.241 | 6 | 27 | 17 | -0.150 |
| IV | 3 | 1 | 111 | 1 | 0 | 2 | 1 | ||
| Venous invasion | |||||||||
| Yes | 27 | 5 | 14 | 8 | 0.042 | 6 | 12 | 9 | 0.237 |
| No | 79 | 31 | 34 | 14 | -0.198 | 26 | 34 | 19 | -0.116 |
| Lymph nodemetastasis | |||||||||
| Yes | 47 | 11 | 22 | 14 | 0.014 | 13 | 21 | 13 | 0.646 |
| No | 59 | 25 | 26 | 8 | -0.239 | 19 | 25 | 15 | -0.045 |
Relationships between E-cadherin and β-catenin
As summarized in Table 2, the expression of both E-cadherin and β-catenin were evaluated in consecutive sections. The correlation between E-cadherin and β-catenin was statistically significant (P = 0.005). 17.92% (19/106) of tumors were E-cadherin (+)/β-catenin (± ~ -), and 14.15% (15/106) of tumors were E-cadherin (± ~ -) /β-catenin (+), statistically significant differences were observed in different expression of these molecules (Pearson X2 = 6.086, P = 0.014).
Table 2.
Relationships between E-cadherin and β-catenin expression in ESCC
| E-cadherin | Total |
β-catenin |
||
| + | ± | - | ||
| + | 36 | 17 | 13 | 6 |
| ± | 48 | 11 | 24 | 13 |
| - | 22 | 4 | 9 | 9 |
| Total | 106 | 32 | 46 | 28 |
rs = 0.270, P = 0.005.
Expression of E-cadherin and β-catenin and survival
The median survival was 28.64 mo and overall 1-, 3-, 5-year survival rates were 57.13%, 34.89% and 29.59%, respectively. There obviously existed inverse correlation between intensity of E-cadherin protein and survival (P = 0.0532), even in the group without receiving postoperative chemotherapy or radiotherapy (P = O.0067 and P = 0.0295, respectively). Patients with loss of E-cadherin expression had lower survival at 1-, 3-, 5-year than those with expression tumors, and Patients with reduced E-cadherin expression had shorter disease-free survival than those with normal expression (P = 0.0278). No significant correlation was apparent between abnormal expression of β-catenin and overall survival. But patients with low-expressing tumors for β-catenin and non-expressing tumors for E-cadherin/β-catenin had shorter median survival period than those with normal expressing tumors (P = 0.0501and P = 0.0080, respectively) in the group without receiving postoperative chemotherapy or radiotherapy (P = 0.0735 and P = 0.0205, respectively).
Expression of E-cadherin and survival stratified for tumor stage
In patients with stage I, IIa, IIb, the median survival for normal, heterogeneous and negative E-cadherin expressing groups were 106.59, 46.59 and 28.50 mo, respectively. Statistically significance existed in different level of E-cadherin and overall survival (P = 0.0033), Survival curves were shown in Figure 3, and disease-free survival (P = 0.0002) in different E-cadherin expressing groups whose median survival were 78,56, 32.00 and 14.00 mo. In contrast, as for stage III or IV, no significance existed in different level of E-cadherin (P = 0.2152 and P = 0.3397, respectively). Survival curves were shown in Figure 4.
Figure 3.
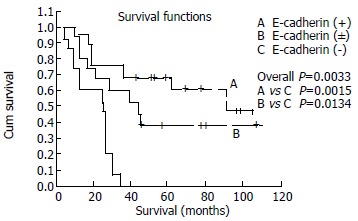
Kaplan-Meier survival curves for patients with stage I, II according to E-cadherin expression.
Figure 4.
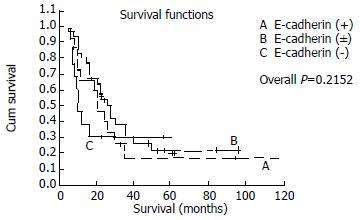
Kaplan-Meier survival curves for patients with stage III, IV according to E-cadherin expression.
Expression of E-cadherin and survival stratified for histoglogic grade
Patients with diminished expression of E-cadherin and grade II or III had shorter survival period than those with normally expressing ones and grade I, no significance existed between grade I and grade II or III with respect to different status of E-cadherin expression. In the patients with grade I, Statistically significant relationships existed between level of E-cadherin and overall survival (P = 0.0125). The survival curves were shown in Figure 5.
Figure 5.
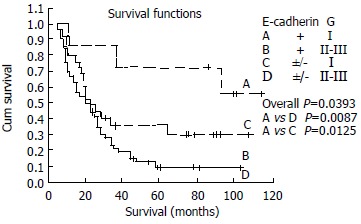
Kaplan-Meier survival curves for patients with ESCC stratified for histologic grade according to E-cadherin expression
Multivariate analysis of survival
Multivariate survival analysis was performed by means of the Cox proportional hazards model by entering the following covariates: Age, gender, location of tumor, tumor size, invasion depth, venous invasiveness, histologic grade, clinical stage, lymph node status, E-cadherin and β-catenin expression. Covariates were selected in a stepwise fashion by using method of Backward: Wald. The significant prognostic factors influencing survival were location of tumor, venous invasiveness, clinical stage, lymph node status, E-cadherin expression. The results were shown in Table 3.
Table 3.
Multivariate results in Cox proportional hazards analysis for patients with ESCC
| Variables | Category (Value) | β | Wald | P Value | HR | 95%CI |
| Overall survival (n = 106) | ||||||
| location (mid-thoracic) | No/Yes (0/l) | 0.650 | 5.665 | 0.017 | 1.916 | 1.112-3.272 |
| Lymph node metastasis | No/Yes (0/l) | 0.599 | 5.893 | 0.015 | 1.821 | 1.1222-953 |
| Venous invasion | No/Yes (0/l) | 1.147 | 18.633 | < 0.00l | 3.148 | 1.870-5.229 |
| Clinical staging | I/II/III/IV (0/1/2/3) | 0.420 | 8.475 | 0.004 | 1.521 | 1.147-2.018 |
| E-cadherin | -/ ± /+ (0/1/2) | -0.431 | 5.687 | 0.017 | 0.650 | 0.456-0.926 |
| Disease-free survival (n = 96) | ||||||
| Gender | M/F (0/1) | 0.675 | 4.786 | 0.029 | 1.965 | 1.073-3.598 |
| Tumor size | Continue | 0.179 | 4.302 | 0.038 | 1.196 | 1.010-1.417 |
| Clinical staging | I/II/III/IV (0/1/2/3) | 0.272 | 3.233 | 0.042 | 1.313 | 0.976-1.767 |
| Location (mid-thoracic) | No/Yes (0/l) | 0.615 | 4.111 | 0.043 | 1.849 | 1.021-3.349 |
| Venous invasion | No/Yes (0/l) | 0.982 | 9.797 | 0.002 | 2.671 | 1.444--4.942 |
| E-cadherin | -/ ± /+ (0/1/2) | -0.397 | 4.479 | 0.034 | 0.672 | 0.465-0.971 |
DISCUSSION
Previous studies reported that the reduction rate of E-cadherin expression was observed to be 18.2%-87% in specimens from the patients with ESCC[28-34], While 66% of ESCC examined showed reduced expression of E-cadherin in our investigation. This is not in accordance with references reported. It is our logical thoughts that selection of the patients entering into the study, immunohistochemical method, antibody origination, tumor heterogeneity and differences in staining evaluation may be individually or in combination held responsible. As a marker associated with squamous cell differentiation[35], the level of E-cadherin expression had inverse correlation with histologic grade. It suggested that normal function of E-cadherin molecule mediated cell-cell adherens junction to induce cell aggregates, which was consistent with histologic morphogenesis of tumor nest in well-differentiated tumors showed zonal differentiation. That phenomenon consists of a mechanical stretching and compression between adjacent cell and requires active contraction of the actin tethered to the cadherin intercellular contacts[36]. But normal expression of E-cadherin was found in poorly-differentiated tumors.,accounting for 15.0% in our study. Several studies were available about this phenomenon, Nakanishi et al[28] reported 14.58% of the patients with poorly-differentiated ESCC also appeared normal expression of E-cadherin, of catenins (α, β and γ), so did 3.22% of the patients with moderated or poorly-differentiated tumors from reports of Tamura et al[29]. Then this showed simply reduced expression of E-cadherin was not independent factor impact on adherens junction. On the other hand normal function of adherens junction relate to cytoplasm protein expression and its normal function. Expression of E-cadherin was closely associated with size of tumor, lymph node metastases and venous invasion in our study. These were not identical to the results of Nakanishi et al[28], Tamura et al[29]. However, diminished expression of E-cadherin facilitate tumor dedifferentiation, invasion and metastases, indeed, the tumor cells of solid tumor with high metastatic potentials are often focally dissociated at the invading fronts[28,29,31]. Of particular interest is the finding in some studies in recent years that expression of E-cadherin might be used to predict micrometastasis in lymph node of the patients without signs of lymph node or distant metastases classified as N0 and M0 by routine histopathological methods[22,37]. Based on these findings, in the present study, The relationship between reduced expression of E-cadherin and haematogenous metastasis in postoperative patients had also come under investigation. Hematogenous metastasis frequently occurred in the patients with reduced expression of E-cadherin, but muitivariate analysis revealed the finding need further evaluation (data not shown). It seemed to accept that more intercellular adhesion molecules such as integrins, selectins and immunoglobulin were involved in the process.
It appears to possess both irreversible and reversible mechanisms for inactivating the cell-cell adhesion system. Several studies revealed heterogeneous methylation patterns exhibit a remarkably unstable, allele-to-allele variability that, like the dynamic expression of E-cadherin during metastatic progression, can be modulated or selected for in relation to the tumor microenvironment[38]. There have been several reports on E-cadherin gene mutations in human cancer[16,18], however, mutations are rare in esophageal carcinoma, E-cadherin promoter hypermethylation is associated with decreased expression[39,40], moreover, recent studies showed that, despite the frequent LOH of the E-cadherin locus, mutations in the E-cadherin gene are rare events and can not be held responsible for down regulation of E-cadherin observed in the majority of adenocarcinomas of the esophagus[41]. Further studies demonstrated abnormal phosphorylation of cytoplasm protein regulated by tyrosine kinases and tyrosine phosphatases affected expression of E-cadherin and its function[42].
Significant difference in survival existed in patients with different level of E-cadherin protein and a trend towards better survival was found in patients having normal E-cadherin expression. But discrepancy resulted from studies on the kinds of tumors. Inada et al[30] reported 5-year survival rate of the patients with ESCC ranged from 87.8% in normal E-cadherin expression to 19.1% in absent expression of E-cadherin, but some coworkers did not acquire the same results[32,33]. Nakanishi et al[28] demonstrated no significant correlations between survival and reduced E-cadherin expression, however, in univariate analysis survival of the patients with reduced α-catenin expression was significantly shorter than that of those with normal expression, and the loss of α-catenin expression from cancer tissues might be attributable to loss of E-cadherin function, which reflected more serious dysfunction of the cadherin-mediated cell-cell adhesion system. In our study there obviously existed inverse correlation between the patients with reduced level of E-cadherin expression and survival, especially in tumors with stage I, II, and in group with absent expression of E-cadherin shorter survival period was observed. Multivariate analysis revealed E-cadherin expression was an independent factor impacted on prognosis of the patients with ESCC. These findings were consistent with the reports of Tamura et al[29], and recently a multicenter investigation supported this viewpoint[34]. In view of the association between E-cadherin and histologic grade, furthermore, we analyzed and tried to elucidate whether these factors played different roles in combination or individually. Although several studies approved the discrepancy of survival could be partly explained by the histologic grade, owing to other clinicopathologic features and grading categories not to be precisely defined, the results of studies remain controversial. In our present investigation, no significance existed between grade I and grade II or III with respect to different E-cadherin status. But in group with grade I, survival of the patients with normal E-cadherin expression was statistically significantly higher than that of those with diminished one, suggesting an apparent difference in the effect of downregulation of E-cadherin between different histologic grade. Therefore, E-cadherin expression as an independent prognostic factor had more significance to predict prognosis for the patients at early stage and with similar histologic grade, might provide some suggestions for clinical treatments.
β-catenin played an important role in the interactions between cadherins and other transmembrane receptor proteins and regulated cellular differentiation and proliferation. Reduced and absent expression of β-catenin were also frequently in tumors might disrupt stability and integrity of the E-cadherin-catenin complex and disturb cellular adherens junction, resulting in cell proliferation, migration, and invasion. Reduction of β-catenin expression was 73% in ESCC from Nakanishi et al[28] and 40.9% from Ninomiya et al[43]. Similarly, 69.8% of patients with β-catenin expression were judged to be reduced in our study, whereas reduced expression of β-catenin significantly correlated only with the histologic grade. Also, an association between abnormal expression of E-cadherin and β-catenin was found, suggesting that loss of E-cadherin binding may cause a redistribution of β-catenin from the cell membrane to the cytoplasm.
It remains unclear that β-catenin expression is associated with lymph node status, invasion depth, tumor stage, hematogenous metastasis and histologic grade. Many believe as a member of oncogenes and signaling molecules, β-catenin plays an important role in tumor progression and carcinogenesis, and there exists relation with cytoplasmic protein such as α-, γ-catenin, p120ctn, APC[23,24], Dsh, Tcf, Axin, β-Trcp, G12[44], PP2A[45], IQGAP1[23] and Smad4[46]. Since so intricate signaling network and multifunctional molecules are involved in regulating cellular function, it is difficult to explain abnormal expression of β-catenin and its functional abnormalities, thus negating correlation with clinicopathologic parameters[47]. But undoubtedly β-catenin as a member of adhesion molecule should be imprudently considered in prognostic value of ESCC. To some extent absent expression of β-catenin reflects dysfunction of E-cadherin mediated cellular adherens junction, which may cause weak cell-cell adhesion and confer invasive properties on a tumor[26,27,48].
To our knowledge, reduced β-catenin expression indicated poor prognosis in some kinds of tumor in addition to esophageal squamous cell carcinoma[49,50]. We found that patients with low-expression tumors for β-catenin and non-expression tumors for E-cadherin/β-catenin had shorter survival period than those with normally expressing ones. But in group with normal E-cadherin expression, no significant relation was found between β-catenin and prognosis. It was apparent that the prognostic value of β-catenin is lower than that of E-cadherin, this may be explained by their roles in the E-cadherin-catenin complex, E-cadhein influences redistribution and function of β-catenin, and γ-catenin might substitute for β-catenin as a component of the complex[51], in other tumors several studies showed aberrant expression of β-caienin or γ-catenin does not necessarily lead to a nonfunctional complex[52].
In conclusion, as a probable independent prognostic factor, it correlates with overall and disease free survival period, expression of E-cadherin but not β-catenin may predict prognosis in patients with ESCC, Further studies are needed to elucidate function of β-catenin by combinating other cytoplasmic proteins.
Footnotes
Edited by Wu XN
References
- 1.Ikeda G, Isaji S, Chandra B, Watanabe M, Kawarada Y. Prognostic significance of biologic factors in squamous cell carcinoma of the esophagus. Cancer. 1999;86:1396–1405. doi: 10.1002/(sici)1097-0142(19991015)86:8<1396::aid-cncr3>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 2.Ohashi K, Nemoto T, Nakamura K, Nemori R. Increased expression of matrix metalloproteinase 7 and 9 and membrane type 1-matrix metalloproteinase in esophageal squamous cell carcinomas. Cancer. 2000;88:2201–2209. [PubMed] [Google Scholar]
- 3.Miyazaki T, Kato H, Shitara Y, Yoshikawa M, Tajima K, Masuda N, Shouji H, Tsukada K, Nakajima T, Kuwano H. Mutation and expression of the metastasis suppressor gene KAI1 in esophageal squamous cell carcinoma. Cancer. 2000;89:955–962. doi: 10.1002/1097-0142(20000901)89:5<955::aid-cncr3>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 4.Gu ZP, Wang YJ, Li JG, Zhou YA. VEGF165 antisense RNA suppresses oncogenic properties of human esophageal squamous cell carcinoma. World J Gastroenterol. 2002;8:44–48. doi: 10.3748/wjg.v8.i1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao L, Zhou HY, Luo ZC, Liu J. Telomeric associations of chromosomes in patients with esophageal squamous cell carcinomas. World J Gastroenterol. 1998;4:231–233. doi: 10.3748/wjg.v4.i3.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qiao GB, Han CL, Jiang RC, Sun CS, Wang Y, Wang YJ. Overexpression of P53 and its risk factors in esophageal cancer in urban areas of Xi'an. World J Gastroenterol. 1998;4:57–60. doi: 10.3748/wjg.v4.i1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li J, Feng CW, Zhao ZG, Zhou Q, Wang LD. A preliminary study on ras protein expression in human esophageal cancer and precancerous lesions. World J Gastroenterol. 2000;6:278–280. doi: 10.3748/wjg.v6.i2.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shiozaki H, Doki Y, Kawanishi K, Shamma A, Yano M, Inoue M, Monden M. Clinical application of malignancy potential grading as a prognostic factor of human esophageal cancers. Surgery. 2000;127:552–561. doi: 10.1067/msy.2000.105028. [DOI] [PubMed] [Google Scholar]
- 9.Shimada Y, Imamura M, Watanabe G, Uchida S, Harada H, Makino T, Kano M. Prognostic factors of oesophageal squamous cell carcinoma from the perspective of molecular biology. Br J Cancer. 1999;80:1281–1288. doi: 10.1038/sj.bjc.6990499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helliwell TR. Molecular markers of metastasis in squamous carcinomas. J Pathol. 2001;194:289–293. doi: 10.1002/1096-9896(200107)194:3<289::AID-PATH912>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 11.Xu M, Jin YL, Fu J, Huang H, Chen SZ, Qu P, Tian HM, Liu ZY, Zhang W. The abnormal expression of retinoic acid receptor-beta, p 53 and Ki67 protein in normal, premalignant and malignant esophageal tissues. World J Gastroenterol. 2002;8:200–202. doi: 10.3748/wjg.v8.i2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hao MW, Liang YR, Liu YF, Liu L, Wu MY, Yang HX. Transcription factor EGR-1 inhibits growth of hepatocellular carcinoma and esophageal carcinoma cell lines. World J Gastroenterol. 2002;8:203–207. doi: 10.3748/wjg.v8.i2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang AH, Sun CS, Li LS, Huang JY, Chen QS. Relationship of tobacco smoking CYP1A1 GSTM1 gene polymorphism and esophageal cancer in Xi'an. World J Gastroenterol. 2002;8:49–53. doi: 10.3748/wjg.v8.i1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Igarashi M, Dhar DK, Kubota H, Yamamoto A, El-Assal O, Nagasue N. The prognostic significance of microvessel density and thymidine phosphorylase expression in squamous cell carcinoma of the esophagus. Cancer. 1998;82:1225–1232. doi: 10.1002/(sici)1097-0142(19980401)82:7<1225::aid-cncr3>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 15.Huang S, Li JY, Wu J, Meng L, Shou CC. Mycoplasma infections and different human carcinomas. World J Gastroenterol. 2001;7:266–269. doi: 10.3748/wjg.v7.i2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirohashi S. Inactivation of the E-cadherin-mediated cell adhesion system in human cancers. Am J Pathol. 1998;153:333–339. doi: 10.1016/S0002-9440(10)65575-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ilyas M, Tomlinson IP. The interactions of APC, E-cadherin and beta-catenin in tumour development and progression. J Pathol. 1997;182:128–137. doi: 10.1002/(SICI)1096-9896(199706)182:2<128::AID-PATH839>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 18.Wijnhoven BP, Dinjens WN, Pignatelli M. E-cadherin-catenin cell-cell adhesion complex and human cancer. Br J Surg. 2000;87:992–1005. doi: 10.1046/j.1365-2168.2000.01513.x. [DOI] [PubMed] [Google Scholar]
- 19.Yagi T, Takeichi M. Cadherin superfamily genes: functions, genomic organization, and neurologic diversity. Genes Dev. 2000;14:1169–1180. [PubMed] [Google Scholar]
- 20.Ivanov DB, Philippova MP, Tkachuk VA. Structure and functions of classical cadherins. Biochemistry (Mosc) 2001;66:1174–1186. doi: 10.1023/a:1012445316415. [DOI] [PubMed] [Google Scholar]
- 21.Sugio K, Kase S, Sakada T, Yamazaki K, Yamaguchi M, Ondo K, Yano T. Micrometastasis in the bone marrow of patients with lung cancer associated with a reduced expression of E-cadherin and beta-catenin: risk assessment by immunohistochemistry. Surgery. 2002;131:S226–S231. doi: 10.1067/msy.2002.119793. [DOI] [PubMed] [Google Scholar]
- 22.Natsugoe S, Mueller J, Stein HJ, Feith M, Höfler H, Siewert JR. Micrometastasis and tumor cell microinvolvement of lymph nodes from esophageal squamous cell carcinoma: frequency, associated tumor characteristics, and impact on prognosis. Cancer. 1998;83:858–866. [PubMed] [Google Scholar]
- 23.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- 24.Peifer M, Polakis P. Wnt signaling in oncogenesis and embryogenesis--a look outside the nucleus. Science. 2000;287:1606–1609. doi: 10.1126/science.287.5458.1606. [DOI] [PubMed] [Google Scholar]
- 25.Dale TC. Signal transduction by the Wnt family of ligands. Biochem J. 1998;329(Pt 2):209–223. doi: 10.1042/bj3290209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aoki M, Hecht A, Kruse U, Kemler R, Vogt PK. Nuclear endpoint of Wnt signaling: neoplastic transformation induced by transactivating lymphoid-enhancing factor 1. Proc Natl Acad Sci USA. 1999;96:139–144. doi: 10.1073/pnas.96.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hecht A, Litterst CM, Huber O, Kemler R. Functional characterization of multiple transactivating elements in beta-catenin, some of which interact with the TATA-binding protein in vitro. J Biol Chem. 1999;274:18017–18025. doi: 10.1074/jbc.274.25.18017. [DOI] [PubMed] [Google Scholar]
- 28.Nakanishi Y, Ochiai A, Akimoto S, Kato H, Watanabe H, Tachimori Y, Yamamoto S, Hirohashi S. Expression of E-cadherin, alpha-catenin, beta-catenin and plakoglobin in esophageal carcinomas and its prognostic significance: immunohistochemical analysis of 96 lesions. Oncology. 1997;54:158–165. doi: 10.1159/000227681. [DOI] [PubMed] [Google Scholar]
- 29.Tamura S, Shiozaki H, Miyata M, Kadowaki T, Inoue M, Matsui S, Iwazawa T, Takayama T, Takeichi M, Monden M. Decreased E-cadherin expression is associated with haematogenous recurrence and poor prognosis in patients with squamous cell carcinoma of the oesophagus. Br J Surg. 1996;83:1608–1614. doi: 10.1002/bjs.1800831138. [DOI] [PubMed] [Google Scholar]
- 30.Inada S, Koto T, Futami K, Arima S, Iwashita A. Evaluation of malignancy and the prognosis of esophageal cancer based on an immunohistochemical study (p53, E-cadherin, epidermal growth factor receptor) Surg Today. 1999;29:493–503. doi: 10.1007/BF02482343. [DOI] [PubMed] [Google Scholar]
- 31.de Castro J, Gamallo C, Palacios J, Moreno-Bueno G, Rodríguez N, Feliu J, González-Barón M. beta-catenin expression pattern in primary oesophageal squamous cell carcinoma. Relationship with clinicopathologic features and clinical outcome. Virchows Arch. 2000;437:599–604. doi: 10.1007/s004280000266. [DOI] [PubMed] [Google Scholar]
- 32.Jian WG, Darnton SJ, Jenner K, Billingham LJ, Matthews HR. Expression of E-cadherin in oesophageal carcinomas from the UK and China: disparities in prognostic significance. J Clin Pathol. 1997;50:640–644. doi: 10.1136/jcp.50.8.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pomp J, Blom J, van Krimpen C, Zwinderman AH, Immerzeel JJ. E-cadherin expression in oesophageal carcinoma treated with high-dose radiotherapy; correlation with pretreatment parameters and treatment outcome. J Cancer Res Clin Oncol. 1999;125:641–645. doi: 10.1007/s004320050328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Imamura M. Prognostic significance of CyclinD1 and E-Cadherin in patients with esophageal squamous cell carcinoma: multiinstitutional retrospective analysis. Research Committee on Malignancy of Esophageal Cancer, Japanese Society for Esophageal Diseases. J Am Coll Surg. 2001;192:708–718. [PubMed] [Google Scholar]
- 35.Wu H, Lotan R, Menter D, Lippman SM, Xu XC. Expression of E-cadherin is associated with squamous differentiation in squamous cell carcinomas. Anticancer Res. 2000;20:1385–1390. [PubMed] [Google Scholar]
- 36.Gottardi CJ, Wong E, Gumbiner BM. E-cadherin suppresses cellular transformation by inhibiting beta-catenin signaling in an adhesion-independent manner. J Cell Biol. 2001;153:1049–1060. doi: 10.1083/jcb.153.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsumoto M, Natsugoe S, Nakashima S, Sakamoto F, Okumura H, Sakita H, Baba M, Takao S, Aikou T. Clinical significance of lymph node micrometastasis of pN0 esophageal squamous cell carcinoma. Cancer Lett. 2000;153:189–197. doi: 10.1016/s0304-3835(00)00374-8. [DOI] [PubMed] [Google Scholar]
- 38.Graff JR, Gabrielson E, Fujii H, Baylin SB, Herman JG. Methylation patterns of the E-cadherin 5' CpG island are unstable and reflect the dynamic, heterogeneous loss of E-cadherin expression during metastatic progression. J Biol Chem. 2000;275:2727–2732. doi: 10.1074/jbc.275.4.2727. [DOI] [PubMed] [Google Scholar]
- 39.Si HX, Tsao SW, Lam KY, Srivastava G, Liu Y, Wong YC, Shen ZY, Cheung AL. E-cadherin expression is commonly downregulated by CpG island hypermethylation in esophageal carcinoma cells. Cancer Lett. 2001;173:71–78. doi: 10.1016/s0304-3835(01)00646-2. [DOI] [PubMed] [Google Scholar]
- 40.Corn PG, Heath EI, Heitmiller R, Fogt F, Forastiere AA, Herman JG, Wu TT. Frequent hypermethylation of the 5' CpG island of E-cadherin in esophageal adenocarcinoma. Clin Cancer Res. 2001;7:2765–2769. [PubMed] [Google Scholar]
- 41.Wijnhoven BP, de Both NJ, van Dekken H, Tilanus HW, Dinjens WN. E-cadherin gene mutations are rare in adenocarcinomas of the oesophagus. Br J Cancer. 1999;80:1652–1657. doi: 10.1038/sj.bjc.6690577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Müller T, Choidas A, Reichmann E, Ullrich A. Phosphorylation and free pool of beta-catenin are regulated by tyrosine kinases and tyrosine phosphatases during epithelial cell migration. J Biol Chem. 1999;274:10173–10183. doi: 10.1074/jbc.274.15.10173. [DOI] [PubMed] [Google Scholar]
- 43.Ninomiya I, Endo Y, Fushida S, Sasagawa T, Miyashita T, Fujimura T, Nishimura G, Tani T, Hashimoto T, Yagi M, et al. Alteration of beta-catenin expression in esophageal squamous-cell carcinoma. Int J Cancer. 2000;85:757–761. doi: 10.1002/(sici)1097-0215(20000315)85:6<757::aid-ijc3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 44.Meigs TE, Fields TA, McKee DD, Casey PJ. Interaction of Galpha 12 and Galpha 13 with the cytoplasmic domain of cadherin provides a mechanism for beta -catenin release. Proc Natl Acad Sci USA. 2001;98:519–524. doi: 10.1073/pnas.021350998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seeling JM, Miller JR, Gil R, Moon RT, White R, Virshup DM. Regulation of beta-catenin signaling by the B56 subunit of protein phosphatase 2A. Science. 1999;283:2089–2091. doi: 10.1126/science.283.5410.2089. [DOI] [PubMed] [Google Scholar]
- 46.Letamendia A, Labbé E, Attisano L. Transcriptional regulation by Smads: crosstalk between the TGF-beta and Wnt pathways. J Bone Joint Surg Am. 2001;83-A Suppl 1:S31–S39. [PubMed] [Google Scholar]
- 47.Kimura Y, Shiozaki H, Doki Y, Yamamoto M, Utsunomiya T, Kawanishi K, Fukuchi N, Inoue M, Tsujinaka T, Monden M. Cytoplasmic beta-catenin in esophageal cancers. Int J Cancer. 1999;84:174–178. doi: 10.1002/(sici)1097-0215(19990420)84:2<174::aid-ijc14>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 48.Cui J, Zhou XD, Liu YK, Tang ZY, Zile MH. Abnormal beta-catenin gene expression with invasiveness of primary hepatocellular carcinoma in China. World J Gastroenterol. 2001;7:542–546. doi: 10.3748/wjg.v7.i4.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krishnadath KK, Tilanus HW, van Blankenstein M, Hop WC, Kremers ED, Dinjens WN, Bosman FT. Reduced expression of the cadherin-catenin complex in oesophageal adenocarcinoma correlates with poor prognosis. J Pathol. 1997;182:331–338. doi: 10.1002/(SICI)1096-9896(199707)182:3<331::AID-PATH860>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 50.Huiping C, Kristjansdottir S, Jonasson JG, Magnusson J, Egilsson V, Ingvarsson S. Alterations of E-cadherin and beta-catenin in gastric cancer. BMC Cancer. 2001;1:16. doi: 10.1186/1471-2407-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhurinsky J, Shtutman M, Ben-Ze'ev A. Plakoglobin and beta-catenin: protein interactions, regulation and biological roles. J Cell Sci. 2000;113(Pt 18):3127–3139. doi: 10.1242/jcs.113.18.3127. [DOI] [PubMed] [Google Scholar]
- 52.Shimazui T, Bringuier PP, van Berkel H, Ruijter E, Akaza H, Debruyne FM, Oosterwijk E, Schalken JA. Decreased expression of alpha-catenin is associated with poor prognosis of patients with localized renal cell carcinoma. Int J Cancer. 1997;74:523–528. doi: 10.1002/(sici)1097-0215(19971021)74:5<523::aid-ijc8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]


