Abstract
AIM: To observe the expression of cyclooxygenase-2 (COX-2) and to investigate the association between COX-2 expression and infection with cytotoxic-associated gene A (cagA) positive strain Helicobacter pylori (Hp) in human gastric cancer, and subsequently to provide fresh ideas for the early prevention of gastric cancer.
METHODS: 32 Specimens of gastric cancer and corresponding adjacent normal gastric mucosa were obtained from patients who had undergone surgical operations of gastric cancer. All the samples including 1 case of stomach malignant lymphoma and 31 cases of gastric adenocarcinoma were confirmed by pathology diagnosis. The expression of COX-2 in 32 specimens of gastric cancer and corresponding adjacent normal gastric mucosa was quantitatively determined and analyzed with Flow Cytometry, and the levels of COX-2 protein were compared between specimens with cagA+ Hp infection and those without cagA+ Hp infection. The cagA gene in 32 specimens of gastric cancer was detected by polymerase chain reaction (PCR) method.
RESULTS: Twenty-seven of 32 (84%) specimens of gastric cancer showed over-expression of COX-2, compared with the adjacent normal gastric mucosa. cagA+ gene were detected from 19 specimens of gastric cancer, but not from the other 13 specimens. The levels of COX-2 protein in 19 specimens of gastric cancer with cagA+ Hp infection (the number of positive cells was 73.82 ± 18.2) were significantly higher than those in the 13 specimens without cagA+ Hp infection (the number of positive cells was 35.92 ± 22.1).
CONCLUSION: COX-2 is overexpressed in gastric cancer and cagA+ Hp infection could up-regulate the expression of COX-2 in gastric cancer in human. There may also exist another way or channel to regulate the expression of COX-2 in gastric cancer in addition to cagA+ Hp infection. Therefore, applying COX-2 selective inhibitors could be an effective and promising way to prevent gastric cancer.
INTRODUCTION
The mortality rate of gastric cancer still takes the first place in eastern Asia, particularly in China. So it is important to study the mechanism of gastric carcinogenesis and to explore the effective and reasonable methods for early prevention of gastric cancer, subsequently reducing the mortality rate of gastric cancer.
Epidemiological studies show that gastric cancer is closely linked to Helicobacter pylori (Hp) infection[1-5], because the incidence of gastric cancer increases 4-9 times after Hp infection, and more than 60% patients with gastric cancer had been infected with Hp[6]. Recent studies indicate that infection with cagA+ Hp possesses a potent toxin and high risk for causing gastric cancer[7,8]. There is a close association between cagA+ Hp and precancerous stage, even gastric cancer[9-12]. But the mechanism that how cagA+ Hp actually leads to gastric cancer still remains unclear.
Cyclooxygenase-2 (COX-2) is one isoform of COX enzyme family, which is the rate-limiting enzyme for prostaglandin H synthesis. It is an inducible enzyme, and normally absent in cells, but its expression is rapidly and transiently induced in response to growth factors, tumor promoters or cytokines[13,14]. Recent studies indicate that COX-2 not only involves in inflammatory responses but also relates to carcinogenesis. Since COX-2 is overexpressed in many tumors, such as colon-rectum cancer[15-17], esophageal cancer[18,19] etc., it may play an important role in the development and progression of cancers[20-22].
It is well documented that Hp infection can cause inflammation, and COX-2 is often involved in inflammatory responses and also related to cancinogenesis. However, it is not clear whether cagA+ Hp infection induces COX-2 expression and whether there is an association between them during gastric cancer development and progression.
To investigate the possible association between the COX-2 expression level and cagA+ Hp infection, in this study, Flow Cytometry technique was employed to quantitatively determine the expression level of COX-2 in specimens of gastric cancer and adjacent normal gastric mucosa and PCR method was used to amplify the cagA gene in specimens of gastric cancer.
MATERIALS AND METHODS
Materials
Specimens of gastric cancer and corresponding adjacent normal gastric mucosa were obtained from 32 patients who had undergone surgical operations of gastric cancer at the Department of Tumor Surgery of the first affiliated hospital of China Medical University. All samples including 1 case of stomach malignant lymphoma and 31 cases of gastric adenocarcinoma were confirmed by pathology diagnosis. The 32 patients were made up of 19 males and 13 females ranging in age from 46 to 73 years old.
Methods
Flow cytometry determination (1) Obtaining single cell solution: 0.5 cm3 specimen of gastric cancer and tissue of adjacent normal gastric mucosa were put on separate 400 pore cleaning copper nets on top of small cups, and the samples were cut into very small chips and washed with 0.9% NaCl, then single cell solution was collected and confirmed with microscope that over 90% cells were single cells; (2) Permeabilizing cells: After being washed 2 times with PBS, the single cells were resuspended with 2 mL 1 × FACS permeabilizing solution (Becton Dickson), and incubated at R.T. for 10 min before being washed 1 time with PBS containing 0.5% bovine serum albumin. The cells were then divided in 2 flow cytometer tubes, and made sure the number of cells in each tube is 106; (3) Immunofluorescence staining: COX-2 was indirectly immunofluorescence stained and 2 tubes were set for each specimens of gastric cancer and tissues of adjacent normal gastric mucosa. 1 tube of each set was stained with FITC (polyclonal anti-rabbit IgG Becton Dickson- Pharmingen company), which was a second fluorescent antibody, as a second antibody self contro l.2 μL anti-COX-2 rabbit polyclonal IgG (Santa Cruz Biotechnology, Inc.) were added to the other 1 tube of cells and mixed. The cells were incubated at R.T. for 30 min before being washed 1 time with PBS containing 0.5% bovine serum albumin. 2 μL secondary FITC antibody were then added and the cells were incubated in the dark at 2-8 °C for 30 min. After being washed 2 times with PBS, the cell pellet was resuspended in 500 μL PBS for COX-2 detection by Flow Cytometer (FACScan Becton Dickson); (4) FACS detection and analysis: Laser excitation was at 488nm, the data was obtained and analyzed with Cell Quest multiple function software, and the fragment interference was eliminated by drawing a gate.
PCR amplification The template from specimen of gastric cancer was amplified by polymerase chain reaction. The cagA -Hp primers used were: upstream: 5’-GTG CCT GCT AGT TTG TCA GCG; and the downstream: 5’-TTG GAA ACC ACC TTT TGT ATT AGC. (obtained from Dr Berg, Washington University). PCR reaction: the 20 μL reaction mixture including 5 μL template, 2 μL PCR buffer, 2 μL dNTP, 2 μL MgCl, 0.5 μL upstream and 0.5 μL downstream primers, 0.25 μL Taq DNA polymerase and dH2O was subjected to denaturation at 94 °C for 5 min; 35 cycles of 1 min at 94 °C, 1 min at 50 °C, 1 min at 72 °C; with a final extension at 72 °C for 10 min.The amplification reaction was proceeded in a PerkinElmer Cetus Thermocycler (PE-9600).
Assay of PCR production 10 μL of reaction mixture were loaded to 1.5% agarose gel containing 0.5 μg/mL ethidium bromide for electrophoresis, the gel was then placed under ultraviolet ray for detection. The amplified cagA gene product was about 390 bp.
Statistical analysis
The expression of COX-2 protein in gastric cancer was analyzed by paired T test with Excel 2000; the association between the levels of COX-2 expression and cagA -Hp infection in gastric cancer was analyzed with T test between the two groups.
RESULTS
Expression of COX-2 protein in gastric cancer
27 cases of gastric cancer (in 32 cases) expressed high levels of COX-2 with an average of 67.51% ± 21.11% positive cells and the range was between 30.36%-98.56% (Figure 1: A1, A2, A3), in contrast, the corresponding 27 cases of adjacent normal gastric mucosa only showed a weak expression of COX-2 with an average of 12.41% ± 8.16% positive cells and the range between 3.55%-29.16% (Figure 1: B1, B2, B3). The percentage of positive cells in gastric cancer was significantly higher than that in the normal gastric mucosa beside cancers (P < 0.001). Out of the 5 remaining cases, 3 did not show the expression of COX-2 in both specimen of gastric cancer and adjacent normal gastric mucosa, whereas in the other 2 cases, the percentage of positive cells in specimen of gastric cancer was not higher than that in the adjacent normal gastric mucosa. In total, 27 cases of 32 gastric cancer overexpressed COX-2 protein and the positive rate was 84.4%.
Figure 1.
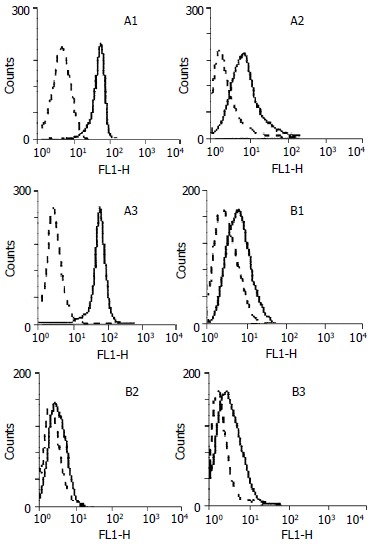
Analysis of COX-2 expressions in gastric cancer tissues and relevant adjacent normal gastric tissues by FACS. A1, A2, A3: Gastric cancer tissues; B1, B2, B3: Relevant adjacent normal gastric tissues. Dotted lines represent results generated with anti-rabbit-FITC; Solid lines represent results generated with rabbit-anti COX-2 and anti-rabbit-FITC.
The association between the levels of COX-2 expression and cagA -Hp infection in gastric cancer
The cagA+ Hp was detected in 19 out of 32 (59.3%) gastric cancers (Figure 2). According to the cagA detection results, 32 cases of gastric cancer were then divided into two groups, the cagA positive group and cagA negative group, so that we could analyze the difference in the levels of COX-2 expression between the two groups. As shown in Table 1, the levels of COX-2 expression in cagA positive group were much higher than those in cagA negative group, and cagA gene was not detected in the 5 cases with no obvious COX-2 expression. The results suggest that the expression of COX-2 is associated with the cagA+ Hp infection in gastric cancer and cagA+ Hp infection can up-regulate the expression of COX-2.
Figure 2.
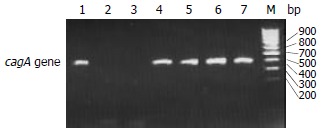
Agarose gel electrophoresis of PCR products of cagA gene in gastric cancer tissues. M: DNA marker; 1, 4, 5, 6 and 7: positive PCR products; 2 and 3: negative PCR products.
Table 1.
The expression of COX-2 protein in gastric cancer
| Grope | n | Cells expressed COX-2 (-x ± s)% |
| Gastric cancer | 32 | 67.51 ± 21.11a |
| Normal gastric mucosa | 32 | 12.41 ± 8.16 |
| Gastric cancer with cagA+ Hp | 19 | 73.82 ± 18.2b |
| Gastric cancer without cagA+ Hp | 13 | 35.92 ± 22.1 |
P < 0.001 vs normal gastric mucosa;
P < 0.001 vs without cagA+ Hp group.
DISCUSSION
Cyclooxgenases (COXs) are the key enzyme in arachidonate metabolism and catalyze the biosynthesis of prostaglandin H2, which is the precursor for prostanoids. COX family consists of the classical COX-1 enzyme, which is constitutively expressed in many tissues, and involved in the homeostasis of various physiologic functions, and its isozyme COX-2, which was discovered in 1991[13] and is involved in many inflammatory reactions with its expression rapidly induced by growth factors, tumor promoters or cytokines. Under normal conditions, COX-2 is absent in tissue cells. Since Hp infection causes inflammatory reaction, it may also induce the expression of COX-2. Romano and associates incubated MKN28 gastric mucosal cells with broth culture filtrates or bacterial suspensions from wild-type Hp, after 24 h COX-2 mRNA levels increased by 5-fold and the synthesis of PGE2 , the main product of COXs, increased by 3-fold, whereas COX-1 mRNA levels remained unchanged[23]. This effect was specifically related to Hp because it was not observed with Escherichia coli. This study indicates that Hp could induce the expression of COX-2 in gastric mucosal cells in vitro. However, whether Hp induces the expression of COX-2 in vivo, paticularly in gastric cancer, and whether there is an association between cagA+ Hp infection and the expression of COX-2 still remain unclear. Our results showed that 27 out of 32 cases (84%) of human gastric cancer expressed COX-2, which was similar to the status in colon-rectum cancer[24], and indicated that the expression of COX-2 was closely associated with gastric cancer. The average percentage of cells that positively express COX-2 was 73.82% in 19 cases of gastric cancer with cagA+ Hp infection; whereas it was 35.92% in 13 cases of gastric cancer without cagA+ Hp infection. The levels of COX-2 expression in gastric cancer with cagA+ Hp infection were much higher than those without cagA+ Hp infection (P < 0.001). 5 cases of gastric cancer did not show expression of COX-2 also were not detected for cagA gene PCR product. The result indicates that cagA+ Hp infection can up-regulate the expression of COX-2 in gastric cancer, and cagA+ Hp infection may play an important role during gastric carcinogenesis by mediating the expression of COX-2. McCarthy et al[25] analyzed the COX-2 expression in gastric antral mucosa before and after eradication of Hp infection by immunohistochemistry and the results indicate that acute and chronic antral inflammation is associated with Hp as well as the expression of COX-2 protein in epithelial cells. The expression of COX-2 was reduced, but not eliminated, in the epithelium after successful eradication of Hp. Despite the reduction in COX-2 expression after Hp eradication, expression of COX-2 in epithelial cells remained and strongly correlated with the extent of the chronic inflammatory cell infiltration. This conclusion supports our results that COX-2 was also expressed in some cases of gastric cancer without cagA+ Hp infection and that the expression of COX-2 in gastric cancer without cagA+ Hp infection is weaker than that with cagA+ Hp infection. Recent studies indicate that Hp can induce COX-2 expression in gastritis[26-30], and COX-2 expression in gastric cancer with Hp infection has also been reported recently[31,32], but most of the studies were conducted with immunohistochemistry, Western blot and RT-PCR methods[26-32], and to quantitatively determinate COX-2 expression and to divide Hp into subtypes for analysis has seldom been reported so far.
Recently, evidence has been presented that COX-2 is induced in human colorectal cancers and in the polyps of mouse FAP models. When the COX-2 gene is inactivated in FAP model mice, both the number and size of polyps are reduced dramatically. In addition, selective inhibitors of COX-2 cause results similar to those caused by COX-2 gene knockout mutations. These genetic and pharmacological data open up the possibility of effectively treating human FAP and various human cancers with COX-2 selective inhibitors[33-35].
The fact that COX-2 is overexpressed in gastric cancer and that the expression of COX-2 in gastric cancer infected by cagA+ Hp is much higher than that without cagA+ Hp infection indicates that cagA+ Hp infection up-regulate the expression of COX-2 in human gastric cancer, and the expression of COX-2 is closely associated with gastric cancer. But the fact that the specimens of gastric cancer without cagA+ Hp infection also express COX-2 at different levels implies that there may exist another way or channel to up-regulate the expression of COX-2 in gastric cancer besides cagA positive strain infection. Therefore, with the successful eradication of Hp infection, applying COX-2 selective inhibitors could be an effective and promising way to prevent gastric cancer.
Footnotes
Supported by The National Basic Research Program (973) of China, No. G1998051203
Edited by Pang LH
References
- 1.Correa P. A human model of gastric carcinogenesis. Cancer Res. 1988;48:3554–3560. [PubMed] [Google Scholar]
- 2.Tompkins LS, Falkow S. The new path to preventing ulcers. Science. 1995;267:1621–1622. doi: 10.1126/science.7886448. [DOI] [PubMed] [Google Scholar]
- 3.Gao HJ, Yu LZ, Bai JF, Peng YS, Sun G, Zhao HL, Miu K, L XZ, Zhang XY, Zhao ZQ. Multiple genetic alterations and behavior of cellular biology in gastric cancer and other gastric mucosal lesions: H.pylori infection, histological types and staging. World J Gastroenterol. 2000;6:848–854. doi: 10.3748/wjg.v6.i6.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhuang XQ, Lin SR. Research of Helicobacter pylori infection in precancerous gastric lesions. World J Gastroenterol. 2000;6:428–429. doi: 10.3748/wjg.v6.i3.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vandenplas Y. Helicobacter pylori infection. World J Gastroenterol. 2000;6:20–31. doi: 10.3748/wjg.v6.i1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quan J, Fan XG. The progress of experiment for Helicobacter pylori infection and gastric carcinogenesis. Shijie Huaren Xiaohua Zazhi. 1999;7:1068–1069. [Google Scholar]
- 7.You WC, Zhang L, Pan KF, Jiang J, Chang YS, Perez-Perez GI, Liu WD, MA JL, Gail MH, Blaser MJ, et al. Helicobacter pylori prevalence and CagA status among children in two counties of China with high and low risks of gastric cancer. Ann Epidemiol. 2001;11:543–546. doi: 10.1016/s1047-2797(01)00227-7. [DOI] [PubMed] [Google Scholar]
- 8.Hirai M, Azuma T, Ito S, Kato T, Kohli Y, Fujiki N. High prevalence of neutralizing activity to Helicobacter pylori cytotoxin in serum of gastric-carcinoma patients. Int J Cancer. 1994;56:56–60. doi: 10.1002/ijc.2910560111. [DOI] [PubMed] [Google Scholar]
- 9.Zhang ZW, Farthing MJ. Molecular mechanisms of H. pylori associated gastric carcinogenesis. World J Gastroenterol. 1999;5:369–374. doi: 10.3748/wjg.v5.i5.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blaser MJ, Perez-Perez GI, Kleanthous H, Cover TL, Peek RM, Chyou PH, Stemmermann GN, Nomura A. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111–2115. [PubMed] [Google Scholar]
- 11.Perez-Perez GI, Peek RM, Legath AJ, Heine PR, Graff LB. The role of CagA status in gastric and extragastric complications of Helicobacter pylori. J Physiol Pharmacol. 1999;50:833–845. [PubMed] [Google Scholar]
- 12.Guo XL, Wang LE, Wang L, Dong M, Yuan Y. The significence of examination on serum Hp-CagA in high risk population in gastric cancer high rate area. Shijie Huaren Xiaohua Zazhi. 2001;9:13–14. [Google Scholar]
- 13.Xie WL, Chipman JG, Robertson DL, Erikson RL, Simmons DL. Expression of a mitogen-responsive gene encoding prostaglandin synthase is regulated by mRNA splicing. Proc Natl Acad Sci USA. 1991;88:2692–2696. doi: 10.1073/pnas.88.7.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kujubu DA, Reddy ST, Fletcher BS, Herschman HR. Expression of the protein product of the prostaglandin synthase-2/TIS10 gene in mitogen-stimulated Swiss 3T3 cells. J Biol Chem. 1993;268:5425–5430. [PubMed] [Google Scholar]
- 15.Kargman SL, O'Neill GP, Vickers PJ, Evans JF, Mancini JA, Jothy S. Expression of prostaglandin G/H synthase-1 and -2 protein in human colon cancer. Cancer Res. 1995;55:2556–2559. [PubMed] [Google Scholar]
- 16.Sano H, Kawahito Y, Wilder RL, Hashiramoto A, Mukai S, Asai K, Kimura S, Kato H, Kondo M, Hla T. Expression of cyclooxygenase-1 and -2 in human colorectal cancer. Cancer Res. 1995;55:3785–3789. [PubMed] [Google Scholar]
- 17.Kutchera W, Jones DA, Matsunami N, Groden J, McIntyre TM, Zimmerman GA, White RL, Prescott SM. Prostaglandin H synthase 2 is expressed abnormally in human colon cancer: Evidence for a transcriptional effect. Proc Natl Acad Sci USA. 1996;93:4816–4820. doi: 10.1073/pnas.93.10.4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson KT, Fu S, Ramanujam KS, Meltzer SJ. Increased expression of inducible nitric oxide synthase and cyclooxygenase-2 in Barrett's esophagus and associated adenocarcinomas. Cancer Res. 1998;58:2929–2934. [PubMed] [Google Scholar]
- 19.Zimmermann KC, Sarbia M, Weber AA, Borchard F, Gabbert HE, Schrör K. Cyclooxygenase-2 expression in human esophageal carcinoma. Cancer Res. 1999;59:198–204. [PubMed] [Google Scholar]
- 20.van Rees BP, Saukkonen K, Ristimäki A, Polkowski W, Tytgat GN, Drillenburg P, Offerhaus GJ. Cyclooxygenase-2 expression during carcinogenesis in the human stomach. J Pathol. 2002;196:171–179. doi: 10.1002/path.1033. [DOI] [PubMed] [Google Scholar]
- 21.Alberts DS. Reducing the risk of colorectal cancer by intervening in the process of carcinogenesis: A status report. Cancer J. 2002;8:208–221. doi: 10.1097/00130404-200205000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Zhang H, Sun XF. Overexpression of cyclooxygenase-2 correlates with advanced stages of colorectal cancer. Am J Gastroenterol. 2002;97:1037–1041. doi: 10.1111/j.1572-0241.2002.05625.x. [DOI] [PubMed] [Google Scholar]
- 23.Romano M, Ricci V, Memoli A, Tuccillo C, Di Popolo A, Sommi P, Acquaviva AM, Del Vecchio Blanco C, Bruni CB, Zarrilli R. Helicobacter pylori up-regulates cyclooxygenase-2 mRNA expression and prostaglandin E2 synthesis in MKN 28 gastric mucosal cells in vitro. J Biol Chem. 1998;273:28560–28563. doi: 10.1074/jbc.273.44.28560. [DOI] [PubMed] [Google Scholar]
- 24.Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183–1188. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 25.McCarthy CJ, Crofford LJ, Greenson J, Scheiman JM. Cyclooxygenase-2 expression in gastric antral mucosa before and after eradication of Helicobacter pylori infection. Am J Gastroenterol. 1999;94:1218–1223. doi: 10.1111/j.1572-0241.1999.01070.x. [DOI] [PubMed] [Google Scholar]
- 26.Tatsuguchi A, Sakamoto C, Wada K, Akamatsu T, Tsukui T, Miyake K, Futagami S, Kishida T, Fukuda Y, Yamanaka N, et al. Localisation of cyclooxygenase 1 and cyclooxygenase 2 in Helicobacter pylori related gastritis and gastric ulcer tissues in humans. Gut. 2000;46:782–789. doi: 10.1136/gut.46.6.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fu S, Ramanujam KS, Wong A, Fantry GT, Drachenberg CB, James SP, Meltzer SJ, Wilson KT. Increased expression and cellular localization of inducible nitric oxide synthase and cyclooxygenase 2 in Helicobacter pylori gastritis. Gastroenterology. 1999;116:1319–1329. doi: 10.1016/s0016-5085(99)70496-8. [DOI] [PubMed] [Google Scholar]
- 28.Chan FK, To KF, Ng YP, Lee TL, Cheng AS, Leung WK, Sung JJ. Expression and cellular localization of COX-1 and -2 in Helicobacter pylori gastritis. Aliment Pharmacol Ther. 2001;15:187–193. doi: 10.1046/j.1365-2036.2001.00918.x. [DOI] [PubMed] [Google Scholar]
- 29.Akhtar M, Cheng Y, Magno RM, Ashktorab H, Smoot DT, Meltzer SJ, Wilson KT. Promoter methylation regulates Helicobacter pylori-stimulated cyclooxygenase-2 expression in gastric epithelial cells. Cancer Res. 2001;61:2399–2403. [PubMed] [Google Scholar]
- 30.Obonyo M, Guiney DG, Harwood J, Fierer J, Cole SP. Role of gamma interferon in Helicobacter pylori induction of inflammatory mediators during murine infection. Infect Immun. 2002;70:3295–3299. doi: 10.1128/IAI.70.6.3295-3299.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamagata R, Shimoyama T, Fukuda S, Yoshimura T, Tanaka M, Munakata A. Cyclooxygenase-2 expression is increased in early intestinal-type gastric cancer and gastric mucosa with intestinal metaplasia. Eur J Gastroenterol Hepatol. 2002;14:359–363. doi: 10.1097/00042737-200204000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Walker MM. Cyclooxygenase-2 expression in early gastric cancer, intestinal metaplasia and Helicobacter pylori infection. Eur J Gastroenterol Hepatol. 2002;14:347–349. doi: 10.1097/00042737-200204000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Dannenberg AJ, Altorki NK, Boyle JO, Dang C, Howe LR, Weksler BB, Subbaramaiah K. Cyclo-oxygenase 2: A pharmacological target for the prevention of cancer. Lancet Oncol. 2001;2:544–551. doi: 10.1016/S1470-2045(01)00488-0. [DOI] [PubMed] [Google Scholar]
- 34.Blanke CD. Celecoxib with chemotherapy in colorectal cancer. Oncology (Williston Park) 2002;16:17–21. [PubMed] [Google Scholar]
- 35.Koki AT, Masferrer JL. Celecoxib: A specific COX-2 inhibitor with anticancer properties. Cancer Control. 2002;9:28–35. doi: 10.1177/107327480200902S04. [DOI] [PubMed] [Google Scholar]


